Abstract
Objectives:
We report the results of this phase I study to evaluate the maximally tolerated dose (MTD) and safety of veliparib, a poly(ADP-ribose) polymerase (PARP) inhibitor, combined with carboplatin and paclitaxel induction chemotherapy (IC) for locoregionally advanced head and neck squamous cell carcinoma (HNSCC).
Materials and methods:
In a 3+3 cohort design, patients with stage IVA-B human papillomavirus-negative HNSCC received 2 cycles of carboplatin (AUC 6, day 1), paclitaxel (100 mg/m2, days 1, 8, 15) and veliparib (days 1–7) every 21 days followed by standard curative-intent chemoradiotherapy. Primary endpoint: MTD and recommended phase II dose (RP2D) as determined by the first IC cycle.
Results:
Twenty patients enrolled. Two withdrew before treatment; 18 patients were analyzed. Median age was 63 years. Primary disease sites included hypopharynx (n=5), larynx (n=5), oral cavity (n=4), oropharynx (n=3), and nasal cavity (n=1). Through all of IC, the most common grade 3+ adverse events (AEs) were neutropenia (33%), thrombocytopenia (33%), anemia (11%), and white blood cell decrease (11%). One patient experienced a hematologic DLT at 350 mg BID. The RP2D for veliparib combined with carboplatin/paclitaxel is 350 mg BID. With 40.9 month median follow-up across dose levels for all patients, the 24-month overall and progression free survival was 77.8% (95% CI 60.8 – 99.6%) and 66.7% (95% CI 48.1 – 92.4%), respectively. Medians have not been reached.
Conclusion:
Addition of veliparib to carboplatin and paclitaxel IC was well tolerated in patients with advanced HNSCC. Hematologic toxicities were the most common AEs.
Keywords: Head and neck squamous cell carcinoma, induction therapy, veliparib, PARP inhibition
Introduction
For non-surgical patients with locally advanced head and neck squamous cell carcinoma (HNSCC), radiation therapy is the definitive treatment option. Adding concurrent chemotherapy, specifically cisplatin, to radiation improves survival.[1] For patients with human papillomavirus (HPV)-related HNSCC, prognosis is excellent.[2] However, patients with non-HPV and smoking-related HNSCCs continue to have a poor prognosis with a 40–50% 5-year survival rate.[3] Little advancement has been made in this area recently, and development of alternative treatment strategies are imperative.
Adding induction chemotherapy to definitive chemoradiation in HNSCC remains unsettled, as clinical trials with various designs have demonstrated conflicting results.[4–7] Only one large trial has demonstrated a statistical overall survival benefit with the addition of induction therapy.[5] Consistently, though, induction chemotherapy decreases the risk of distant failures and predicts response to subsequent chemoradiation. One current standard induction chemotherapy regimen consists of docetaxel, cisplatin, and 5-fluorouracil (TPF), although initial trials demonstrated high toxicity and frequent treatment discontinuation.[8, 9] Thus, a more tolerable induction regimen of a platinum/taxane-based regimen can be used.[10–12]
Poly(ADP-ribose) polymerase (PARP) is a nuclear enzyme that recognizes DNA damage and facilitates DNA repair.[13] PARP inhibitors have demonstrated therapeutic value in breast and ovarian cancers, particularly in BRCA-mutated cancers. Since BRCA mutations lead to the inability to repair double stranded breaks via homologous recombination, PARP inhibition is an ideal therapeutic strategy to further prevent DNA repair and cause tumor cell death. Multiple trials in breast and ovarian cancer demonstrated meaningful single agent efficacy using PARP inhibition in the presence of a germline BRCA mutation.[14–16]
Preclinical evidence shows that inhibition of PARP can also sensitize tumors to cytotoxic agents that induce DNA damage normally repaired through the base excision repair system.[17] Additional preclinical models also demonstrated efficacy with DNA altering agents including radiotherapy.[18, 19] PARP inhibition has demonstrated dose-dependent synergistic responses when combined with cisplatin in oral squamous cell in vitro and in vivo models.[20] Other tumor models have shown accelerated senescence with PARP inhibition in irradiated tumor cells.[21] Accelerated senescence may provide an alternative treatment pathway to achieve anti-tumor effects in tumors that may be resistant to necrotic or apoptotic death. In this study, we hypothesized that adding veliparib, a PARP inhibitor, to platinum-based induction chemotherapy for HNSCC would be feasible and lead to measurable differences in clinical outcome parameters. This was a designed phase I/II trial, and we report on the completed phase I portion.
Materials and Methods
Patients
For this phase I portion of an initially planned phase I/II trial, patients across four Alliance for Clinical Trials in Oncology (Alliance) institutions were required to be 18 years of age or older with an Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0–1. Patients were required to have treatment-naïve stage IVA-IVB HPV-negative HNSCC per the American Joint Committee of Cancer (AJCC) 7th edition. Acceptable organ/marrow function was required. Exclusion criteria included M1 disease, impaired gastrointestinal function interfering with absorption, inability to swallow pills, co-morbidities interfering with therapy or survival, pregnancy, and malignancy within the past 3 years. The protocol was approved by the institutional review board of all participating institutions. All patients provided written informed consent prior to enrollment.
Treatment and Follow-Up
All patients were treated with two 21 day cycles of induction chemotherapy consisting of carboplatin (AUC 6, day 1), paclitaxel (100 mg/m2, days 1, 8, and 15), and veliparib (twice daily, days 1–7). Veliparib was dispensed in 50 mg tablets. The starting veliparib dose was 200 mg twice daily (4 pills twice daily) with dose level increments of +/− 50 mg to a maximum of 350 mg twice daily (7 pills twice daily). Based on previous trial design[6], two cycles of induction chemotherapy were planned. During the last week of induction chemotherapy (week 6), patients underwent repeat imaging with the same imaging technique utilized pre-treatment. RECIST v1.1 was used to evaluate for tumor response.
After completing induction therapy, patients were placed on one of two chemoradiotherapy regimens based on institutional preference. The first option consisted of chemoradiation to 72 Gy over 6 weeks with concurrent cisplatin 100 mg/m2 on days 1 and 22. The second option consisted of radiation to 75 Gy with concurrent 1.5 Gy twice-daily radiation and TFH (paclitaxel 100 mg/m2 on day 1, 5-fluorouracil continuous infusion at 600 mg/m2/day on days 0 – 5, and hydroxyurea 500 mg orally twice daily on days 0 – 5 with 11 doses per cycle) over five 14-day cycles. TFH has served as an institutional standard chemoradiation regimen demonstrating comparable oncologic outcomes to standard chemoradiation.[12, 22]
Patients were monitored during and after treatment by teams consisting of medical oncologists, radiation oncologists, and otolaryngologists. Patients underwent imaging and laryngopharyngoscopy at 3, 6, 12, 18, 24, 30, 36, 48, and 60 months following the completion of chemoradiation to evaluate clinical and radiographic outcomes. Imaging consisted of CT or MRI and PET scan when necessary.
Study Design and Statistical Considerations
The primary objective was to determine the maximum tolerated dose (MTD), recommended phase II dose (RP2D), and safety of veliparib in combination with induction chemotherapy using a conventional 3+3 phase I dose escalation design. Dose-limiting toxicities (DLTs) were assessed at each dose level only in the first induction cycle, where two or more DLTs in 3 or 6 patients would be considered too toxic and the next lower dose level would be considered the MTD. If multiple DLTs occurred in a single patient, the unit of decision would be based on the single patient. If the MTD was not reached, the highest dose level assessed would be considered the RP2D. A hematologic DLT was defined as any of the following during the first cycle of induction chemotherapy: grade 4 neutropenia lasting more than 14 days, febrile neutropenia, grade 4 thrombocytopenia, or a dose delay of greater than 3 weeks due to failed count recovery. Non-hematologic DLTs included any grade 3 or 4 toxicity excluding alopecia, fatigue, hypersensitivity reaction, nausea, vomiting, constipation, diarrhea, electrolyte abnormalities, or grade 3 hypertension. Any non-hematologic toxicity leading to a dose delay of greater than 3 weeks or any drug-related deaths were considered DLTs.
Adverse events were evaluated with National Cancer Institute Common Terminology Criteria for Adverse Events, version 4, and were summarized in a tabular manner as the maximum grade for a given type of event for each patient. Unless otherwise specified, the reporting of adverse events reflects both induction cycles. As stated earlier, DLT and dose-level determination only reflects the first cycle of induction. The commonly occurring grade 3+ adverse events, regardless of attribution, are reported. Kaplan-Meier methodology was used to describe the distribution of progression-free survival and overall survival. Descriptive statistics were used to describe the data.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. All analyses were based on the study database frozen on April 27, 2020.
Results
Demographics
A total of 20 patients were enrolled to this phase I study across four Alliance treating sites from February 2015 through October 2017. Of these 20 patients, two withdrew prior to receiving treatment, leaving 18 evaluable patients. One patient withdrew prior to treatment initiation due to symptomatic tumor burden leading to difficulty swallowing pills. The second withdrew due to patient preference. Patient characteristics are shown in Table 1. These 18 patients were predominantly white (83%) and had a median age of 63 years (range: 24 – 75). Half the patients were female, and all had an ECOG performance status 0–1. Primary sites of disease included laryngeal (28%), hypopharyngeal (28%), mucosal lip and oral cavity (22%), oropharyngeal (17%), and nasal cavity (6%).
Table 1.
Patient Baseline Characteristics
| Total (N=18) | |
|---|---|
| Dose Level (twice daily) | |
| 200 mg | 3 (16.7%) |
| 250 mg | 3 (16.7%) |
| 300 mg | 5 (27.8%) |
| 350 mg | 7 (38.9%) |
| Age (years) | |
| N | 18 |
| Mean (SD) | 59.1 (11.9) |
| Median | 62.5 |
| Q1, Q3 | 58.0, 67.0 |
| Range | (24.0–75.0) |
| Race | |
| White | 15 (83.3%) |
| Black or African American | 1 (5.6%) |
| American Indian or Alaska Native | 1 (5.6%) |
| Unknown: Patient unsure | 1 (5.6%) |
| Gender | |
| Female | 9 (50.0%) |
| Male | 9 (50.0%) |
| ECOG Performance Status | |
| 0 | 10 (55.6%) |
| 1 | 8 (44.4%) |
| Primary Tumor Site | |
| Hypopharynx | 5 (27.8%) |
| Larynx | 5 (27.8%) |
| Lip and oral cavity | 4 (22.2%) |
| Nasal cavity and ethmoid sinus | 1 (5.6%) |
| Oropharynx | 3 (16.7%) |
Dosing
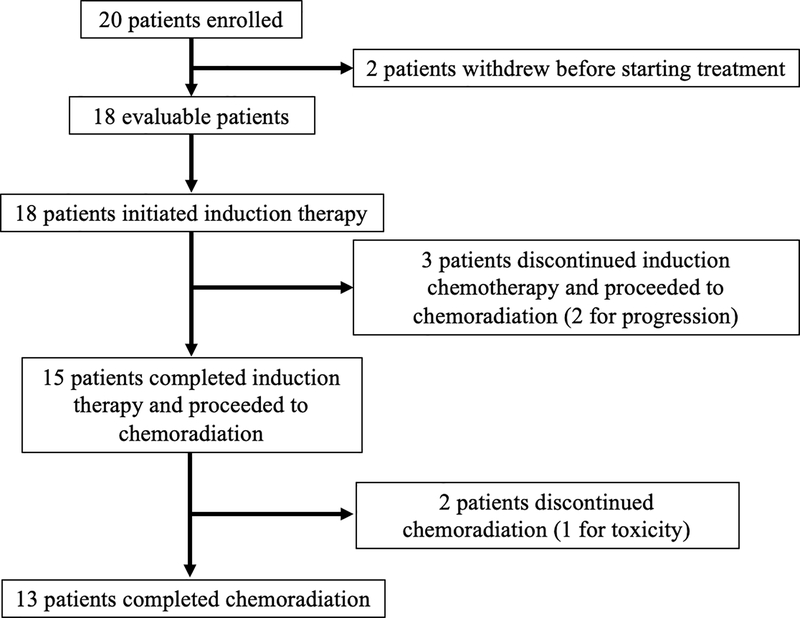
Patients were enrolled across four dose levels - four patients at 200 mg twice daily (BID), three patients at 250 mg BID, six patients at 300 mg BID, and seven patients at 350 mg BID. Of the two patients who withdrew prior to treatment initiation, one was in the 200 mg cohort and the other in the 300 mg cohort. Of the 18 evaluable patients, thirteen patients completed both induction and definitive treatment per protocol (Figure 1). Three patients discontinued induction therapy after one cycle. One patient at the 300 mg dose level discontinued due to the required number (6) and size of veliparib pills. Two patients, both at the 350 mg dose level, developed progressive disease during induction therapy. As designed, these three patients were removed from the study, but all went on to receive definitive chemoradiation and are included in the clinical outcomes data.
Figure 1 –
Consort Flow Diagram
Twenty patients were enrolled on the trial. Eighteen evaluable patients initiated treatment. Fifteen patients completed induction therapy and proceeded to definitive chemoradiation. Thirteen patients completed the entirety of treatment.
Safety
Across the four dose levels, the most common grade 3+ adverse events during the two cycles of induction therapy included neutropenia (33%), thrombocytopenia (33%), anemia (11%), and white blood cell decrease (11%). Ten patients experienced at least one grade 3 or worse adverse event. Three patients experienced at least one grade 4 event, all of which were hematologic. Table 2 lists all of the grade 3+ toxicities experienced during the two cycles of the induction phase of treatment. One patient suffered a myocardial infarction that was felt to be unrelated to study treatment. Only one DLT occurred during the first cycle of induction therapy, such that 350 mg BID was found to be the RP2D. The one patient who experienced a DLT developed both grade 4 neutropenia (>14 days) and grade 4 thrombocytopenia at 350 mg BID.
Table 2.
Grade 3+ Adverse Events during Induction Therapy
| Grade 3 | Grade 4 | Grade 5 | ||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Hematologic Adverse Events | ||||||
| Blood/Bone Marrow | ||||||
| Anemia | 2 | (11%) | 0 | (0%) | 0 | (0%) |
| Lymphocyte count decrease | 1 | (6%) | 0 | (0%) | 0 | (0%) |
| Neutrophil count decrease | 4 | (22%) | 2 | (11%) | 0 | (0%) |
| Platelet count decrease | 4 | (22%) | 2 | (11%) | 0 | (0%) |
| White blood cell decrease | 2 | (11%) | 0 | (0%) | 0 | (0%) |
| Non-Hematologic Adverse Events | ||||||
| Cardiac disorders | ||||||
| Myocardial infarction | 1 | (6%) | 0 | (0%) | 0 | (0%) |
| Gastrointestinal disorders | ||||||
| Hernia | 1 | (6%) | 0 | (0%) | 0 | (0%) |
| Nausea | 1 | (6%) | 0 | (0%) | 0 | (0%) |
| Infections and infestations | ||||||
| Parotitis/cellulitis | 1 | (6%) | 0 | (0%) | 0 | (0%) |
| Metabolic abnormalities | ||||||
| Hypomagnesemia | 1 | (6%) | 0 | (0%) | 0 | (0%) |
The seven patients at 350 mg BID experienced grade 3+ anemia (14%), lymphocyte count decrease (14%), neutrophil count decrease (28%), thrombocytopenia (28%), myocardial infarction (14%), hernia (14%), nausea (14%), facial cellulitis/parotitis (14%), and hypomagnesemia (14%).
Chemoradiation
All fifteen patients who completed induction chemotherapy proceeded to definitive chemoradiation. Among these 15 patients, five patients received concurrent cisplatin and ten patients received TFH. Of the five patients who received concurrent cisplatin, four received the planned two doses. Nine of ten patients who received TFH underwent the planned five cycles of treatment. Two patients discontinued treatment during chemoradiation. One patient, who received TFH, stopped due to diarrhea which was related to an exacerbation of a chronic medical condition. The other patient, who received cisplatin, discontinued due to a treatment regimen change per patient preference.
During the chemoradiation phase, 80% of patients experienced a grade 3+ AE. Five patients (33%) had grade 3+ neutrophil count decrease, three patients (20%) had grade 3+ anemia, and three patients (20%) had grade 3+ thrombocytopenia. Grade 3+ mucositis and radiation dermatitis occurred in 47% and 20%, respectively. No additional grade 3+ side effects occurred in more than one patient.
Outcomes
Ten patients had a response after induction therapy (all partial responses) (Table 3). Of the 18 eligible patients, 14 had a clinical or radiographic response and 7 had a complete radiographic response across all treatment including definitive chemoradiation.
Table 3.
Induction Phase Response
| Dose Level | 200 mg BID (N = 3) | 250 mg BID (N = 3) | 300 mg BID (N =5) | 350 mg BID (N = 7) | Total (N=18) | p value (Chi-square, 2-sided) |
|---|---|---|---|---|---|---|
| Best Response | 0.5826 | |||||
| Partial Response | 2 | 3 | 2 | 3 | 10 (55.6%) | |
| Stable Disease | 1 | 0 | 2 | 1 | 4 (22.2%) | |
| Progression | 0 | 0 | 0 | 2 | 2 (11.1%) | |
| Not Assessed | 0 | 0 | 1 | 1 | 2 (11.1%) | |
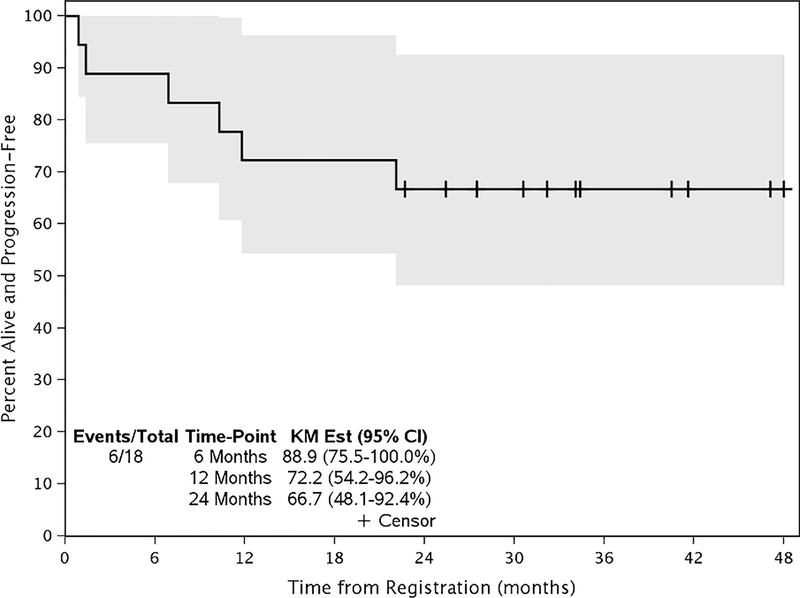
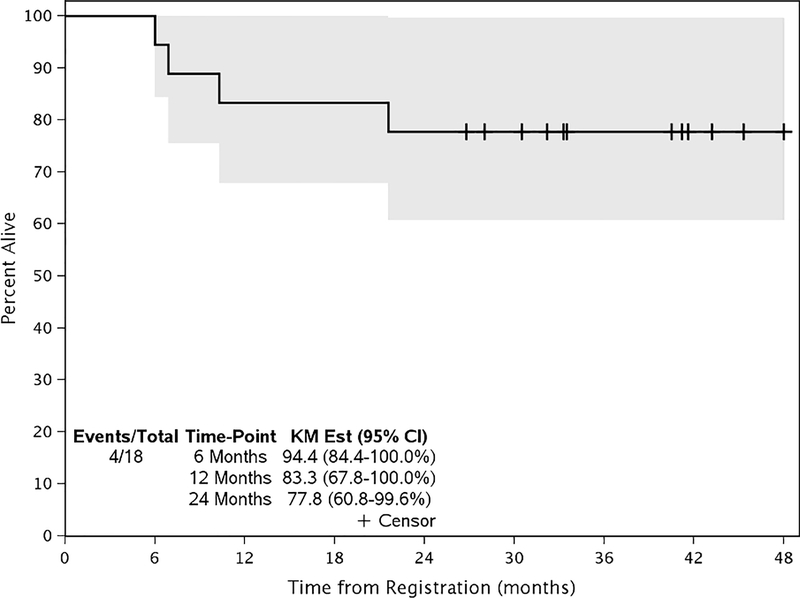
The median follow-up is 40.9 months (26.8 – 53.6 months). The overall 24-month progression-free survival (PFS) rate is 66.7% (95% CI: 48.1% - 92.4%) (Figure 2). PFS was similar between the dose levels. Of the patients who progressed, all developed locoregional recurrences. No distant failures were noted. The overall 24-month survival rate across all patients is 77.8% (95% CI: 60.8% - 99.6%) and was similar between the dose levels (Figure 3). The medians have not been reached.
Figure 2 –
Progression Free Survival
At a median follow up of 40.9 months, the 24-month progression free survival rate is 66.7%. Median has not been reached. All incidents of progression were locoregional recurrences. The tick marks indicate censoring of a patient for survival. The gray bands indicate the 95% confidence interval for the curve.
Figure 3 –
Overall Survival
At a median follow up of 40.9 months, the 24-month overall survival is 77.8%. Median has not been reached. The tick marks indicate censoring of a patient for survival. The gray bands indicate the 95% confidence interval for the curve.
Discussion
This phase I trial adding veliparib to induction carboplatin and paclitaxel demonstrated an acceptable toxicity rate with a recommended veliparib dose of 350 mg twice daily. To our knowledge, only two other studies have demonstrated equal or higher dosing of veliparib in combination with cytotoxic chemotherapy. Veliparib at 400 mg BID was tolerated in combination with capecitabine and radiation therapy in rectal cancer patients and in combination with cisplatin and paclitaxel on 21 day cycles in cervical cancer patients.[23, 24] On our trial, there was one hematologic dose-limiting toxicity and no significant delays to definitive chemoradiation attributed to induction therapy. With an adequate follow-up interval, the survival data appears promising.
The addition of PARP inhibition to cytotoxic chemotherapy raises the concern for increased or intolerable adverse events. This trial demonstrates the ability to combine these two treatment modalities without significant toxicity. When compared to carboplatin and paclitaxel, overall grade 3+ hematologic AEs were relatively similar with the addition of veliparib. Thrombocytopenia was more common with the addition of veliparib; anemia and neutropenia occurred at similar rates with or without veliparib.[25, 26] Several trials have combined carboplatin, paclitaxel, and veliparib; hematologic abnormalities were the most common grade 3+ AEs.[27–29] Other combinations of chemotherapy and PARP inhibitors have led to similar hematologic toxicity profiles.[23, 24] A phase II trial randomized small cell lung cancer patients to cisplatin and etoposide with or without veliparib 100 mg twice daily on days 1–7. Lymphopenia and neutropenia were more common on the veliparib arm.[30] Similarly, anemia and neutropenia were the most common grade 3+ events in a phase II trial randomizing ovarian cancer patients to platinum chemotherapy with or without PARP inhibition.[31] Most importantly, on our trial, toxicities due to induction therapy did not delay definitive chemoradiation.
All patients who started induction therapy went on to receive definitive chemoradiation including the three patients who were removed from the study during induction treatment. Other induction trials have demonstrated a significant percentage of patients who did not receive any radiotherapy after starting induction chemotherapy – 12% in Vermorken et al., 10% in Ghi et al., 28% in Hitt et al.[4, 5, 9] Similarly, among the 15 patients who started chemoradiation after completing induction therapy, 87% remained compliant to the entirety of the definitive chemoradiation course which is comparable to these historical induction trials. Furthermore, adherence to chemoradiation after induction in our trial is similar to patients receiving only definitive chemoradiation using modern treatment techniques[32, 33]
The response rates seen in this trial are numerically less compared to previous induction chemotherapy trials.[8, 9, 34] Patients enrolled on TAX324 received induction chemotherapy followed by definitive concurrent chemoradiation – a paradigm similar to this trial. The overall and complete response rates for patients receiving TPF were 72% and 17%, respectively.[8] On this small phase I trial, 55% of patients had a response to induction therapy and no complete responses were observed. However, it should be noted that the TAX324 trial included approximately 50% of patients with oropharynx cancer (14% HPV-positive, 55% unknown).[34] Many of these early induction chemotherapy trials did not isolate HPV-related disease for which we would expect increased response rates to chemotherapy. In addition, pre-clinical models demonstrated a dose-dependent synergy between PARP inhibition and chemotherapy.[20] We observed evidence of response across all dose levels with no apparent correlation to increasing dose level.
Although advances have been made in HNSCC, HPV-negative disease consistently demonstrates 3-year survival rates nearly 30% below HPV-positive tumors.[2, 35] Despite the addition of novel agents, such as cetuximab and avelumab, to definitive chemoradiation, outcomes have not improved.[36, 37] Acknowledging the small sample size, limited follow-up, and various chemoradiation regimens, the early survival data are promising. In addition, no distant failures were seen. Notably, all patients had HPV-negative HNSCC. Despite the rising incidence of HPV-positive disease, HPV-negative HNSCC remains a highly morbid and fatal disease. Thus, continued strategies for increased efficacy and disease control are necessary.
The ability to prevent DNA repair with the addition of PARP inhibition also makes this an attractive strategy in the definitive treatment setting combined with radiation therapy. Early preclinical data demonstrated synergistic effects when combining PARP inhibition with radiotherapy.[38] A phase I trial combining olaparib, cetuximab, and radiation in heavy smokers with advanced HNSCC showed tolerability with respectable two-year outcomes.[39] The toxicity profiles among these patients were reflective of concurrent radiation therapy. Hematologic toxicities, primarily leukopenia, were uncommon within the cohort. However, PARP inhibitor dosing seemed limited by increasing mucositis and dermatitis with increasing dose levels. Despite known myelosuppressive agents used in our induction regimen, we were able to reach the maximum dose specified prior to trial initiation.
There are multiple ongoing trials combining PARP inhibition with radiation and/or chemotherapy. As much of the current research involving PARP inhibitors revolves around concurrent damage to DNA repair mechanisms, their future role in HNSCC remains unclear. For the HPV negative HNSCC population, the combination of carboplatin, paclitaxel, and veliparib in the induction setting was safe and feasible. With high two year survival rates, further studies to this approach should be considered. Further investigations into this combination are in discussion.
Highlights:
Adding veliparib to induction chemotherapy was well tolerated in head/neck cancer
Hematologic toxicities were most common when combining veliparib and chemotherapy
Adding veliparib to induction chemotherapy did not delay time to curative treatment
Acknowledgments
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), and UG1CA233327. Also supported in part by AbbVie. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
https://acknowledgements.alliancefound.org
The following institutions participated in this study:
Northwestern University Robert H. Lurie Comprehensive Cancer Center, supported by Priya Kumthekar, 1UG1CA233247
University of Chicago Comprehensive Cancer Center, supported by Hedy Kindler, 1UG1CA233327
University of North Carolina Lineberger Comprehensive Cancer Center, supported by Matthew Milowsky, 1UG1CA233373
Washington University in St. Louis Siteman Cancer Center, supported by Nancy Bartlett, 1UG1CA233339
Footnotes
Conflict of Interest
EVV holds a consultant/advisory role with AbbVie.
ClinicalTrials.gov Identified: NCT01711541
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pignon JP, le Maître A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. [DOI] [PubMed] [Google Scholar]
- [2].O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17:440–51. [DOI] [PubMed] [Google Scholar]
- [3].Howlander N, Noone A, Krapcho M, Miller D, Bishop K, Kosary C, et al. SEER Cancer Statistics Review, 1975–2014. Bethesda, MD: National Cancer Institute. [Google Scholar]
- [4].Hitt R, Grau JJ, López-Pousa A, Berrocal A, García-Girón C, Irigoyen A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014;25:216–25. [DOI] [PubMed] [Google Scholar]
- [5].Ghi MG, Paccagnella A, Ferrari D, Foa P, Alterio D, Codecà C, et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncol. 2017;28:2206–12. [DOI] [PubMed] [Google Scholar]
- [6].Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32:2735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Geoffrois L, Martin L, De Raucourt D, Sun XS, Tao Y, Maingon P, et al. Induction Chemotherapy Followed by Cetuximab Radiotherapy Is Not Superior to Concurrent Chemoradiotherapy for Head and Neck Carcinomas: Results of the GORTEC 2007–02 Phase III Randomized Trial. J Clin Oncol. 2018:JCO2017762591. [DOI] [PubMed] [Google Scholar]
- [8].Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15. [DOI] [PubMed] [Google Scholar]
- [9].Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704. [DOI] [PubMed] [Google Scholar]
- [10].Kies MS, Holsinger FC, Lee JJ, William WN, Glisson BS, Lin HY, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. 2010;28:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Marur S, Li S, Cmelak AJ, Gillison ML, Zhao WJ, Ferris RL, et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx-ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017;35:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Haraf DJ, Rosen FR, Stenson K, Argiris A, Mittal BB, Witt ME, et al. Induction chemotherapy followed by concomitant TFHX chemoradiotherapy with reduced dose radiation in advanced head and neck cancer. Clin Cancer Res. 2003;9:5936–43. [PubMed] [Google Scholar]
- [13].Schreiber V, Amé JC, Dollé P, Schultz I, Rinaldi B, Fraulob V, et al. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J Biol Chem. 2002;277:23028–36. [DOI] [PubMed] [Google Scholar]
- [14].Robson M, Goessl C, Domchek S. Olaparib for Metastatic Germline BRCA-Mutated Breast Cancer. N Engl J Med. 2017;377:1792–3. [DOI] [PubMed] [Google Scholar]
- [15].Domchek SM, Aghajanian C, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Donawho CK, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–37. [DOI] [PubMed] [Google Scholar]
- [18].Palma JP, Wang YC, Rodriguez LE, Montgomery D, Ellis PA, Bukofzer G, et al. ABT-888 confers broad in vivo activity in combination with temozolomide in diverse tumors. Clin Cancer Res. 2009;15:7277–90. [DOI] [PubMed] [Google Scholar]
- [19].Tuli R, Surmak AJ, Reyes J, Armour M, Hacker-Prietz A, Wong J, et al. Radiosensitization of Pancreatic Cancer Cells In Vitro and In Vivo through Poly (ADP-ribose) Polymerase Inhibition with ABT-888. Transl Oncol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yasukawa M, Fujihara H, Fujimori H, Kawaguchi K, Yamada H, Nakayama R, et al. Synergetic Effects of PARP Inhibitor AZD2281 and Cisplatin in Oral Squamous Cell Carcinoma in Vitro and in Vivo. Int J Mol Sci. 2016;17:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Efimova EV, Mauceri HJ, Golden DW, Labay E, Bindokas VP, Darga TE, et al. Poly(ADP-ribose) polymerase inhibitor induces accelerated senescence in irradiated breast cancer cells and tumors. Cancer Res. 2010;70:6277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vokes EE, Stenson K, Rosen FR, Kies MS, Rademaker AW, Witt ME, et al. Weekly carboplatin and paclitaxel followed by concomitant paclitaxel, fluorouracil, and hydroxyurea chemoradiotherapy: curative and organ-preserving therapy for advanced head and neck cancer. J Clin Oncol. 2003;21:320–6. [DOI] [PubMed] [Google Scholar]
- [23].Czito BG, Deming DA, Jameson GS, Mulcahy MF, Vaghefi H, Dudley MW, et al. Safety and tolerability of veliparib combined with capecitabine plus radiotherapy in patients with locally advanced rectal cancer: a phase 1b study. Lancet Gastroenterol Hepatol. 2017;2:418–26. [DOI] [PubMed] [Google Scholar]
- [24].Thaker PH, Salani R, Brady WE, Lankes HA, Cohn DE, Mutch DG, et al. A phase I trial of paclitaxel, cisplatin, and veliparib in the treatment of persistent or recurrent carcinoma of the cervix: an NRG Oncology Study (NCT#01281852). Ann Oncol. 2017;28:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Han S, Hong Y, Liu T, Wu N, Ye Z. The efficacy and safety of paclitaxel and carboplatin with versus without bevacizumab in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2018;9:14619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Griesinger F, Korol EE, Kayaniyil S, Varol N, Ebner T, Goring SM. Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: A meta-analysis. Lung Cancer. 2019;135:196–204. [DOI] [PubMed] [Google Scholar]
- [27].Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N Engl J Med. 2019;381:2403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Han HS, Diéras V, Robson M, Palácová M, Marcom PK, Jager A, et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann Oncol. 2018;29:154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ramalingam SS, Blais N, Mazieres J, Reck M, Jones CM, Juhasz E, et al. Randomized, Placebo-Controlled, Phase II Study of Veliparib in Combination with Carboplatin and Paclitaxel for Advanced/Metastatic Non-Small Cell Lung Cancer. Clin Cancer Res. 2017;23:1937–44. [DOI] [PubMed] [Google Scholar]
- [30].Owonikoko TK, Dahlberg SE, Sica GL, Wagner LI, Wade JL, Srkalovic G, et al. Randomized Phase II Trial of Cisplatin and Etoposide in Combination With Veliparib or Placebo for Extensive-Stage Small-Cell Lung Cancer: ECOG-ACRIN 2511 Study. J Clin Oncol. 2019;37:222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16:87–97. [DOI] [PubMed] [Google Scholar]
- [32].Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lorch JH, Goloubeva O, Haddad RI, Cullen K, Sarlis N, Tishler R, et al. Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol. 2011;12:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].EMD Serono IaP, Inc. EMD Serono and Pfizer provide update on phase III javelin head and neck 100 study. Rockland, Massachusetts and New York, New York3/13/2020.
- [38].Lesueur P, Chevalier F, Austry J, Waissi W, Burckel H, Noel G, et al. Poly-(ADP-ribose)-polymerase inhibitors as radiosensitizers: a systematic review of pre-clinical and clinical human studies. Oncotarget. 2017;8:69105–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Karam SD, Reddy K, Blatchford PJ, Waxweiler T, DeLouize AM, Oweida A, et al. Final Report of a Phase I Trial of Olaparib with Cetuximab and Radiation for Heavy Smoker Patients with Locally Advanced Head and Neck Cancer. Clin Cancer Res. 2018;24:4949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]