Abstract
Despite the relevance of semantic fluency measures to risk for dementia and psychiatric disorders, little is known about their genetic and environmental architecture in mid-to-late life. Participants represent 21,684 middle-aged and older adult twins (M=60.84 years, SD=11.21; Range 40–89) from six studies from three countries participating in the Interplay of Genes and Environment across Multiple Studies (IGEMS) consortium. All completed the same measure of semantic fluency (naming animals in 60 seconds). Results revealed small-to-moderate phenotypic associations with age and education, with education more strongly and positively associated with fluency performance in females than males. Heritability and environmental influences did not vary by age. Environmental variance was smaller with higher levels of education, but this effect was observed only in males. This is the largest study to examine the genetic and environmental architecture of semantic fluency, and the first to demonstrate that environmental influences vary based on levels of education.
Keywords: verbal fluency, twin study, heritability, gene-by-environment interaction, aging
Introduction
Measures of semantic fluency are widely used in neuropsychological test batteries requiring individuals to name as many examples of a cued category (e.g., animals, boys’ names) as possible, typically within a minute. Semantic fluency tests are sensitive to a wide range of neurocognitive and psychiatric conditions. Impairments have been observed in Alzheimer’s disease, depression, schizophrenia, and a range of other clinical phenotypes (Henry & Crawford, 2004a, 2004b, 2005a, 2005b; Henry, Crawford, & Phillips, 2004). In meta-analyses, semantic fluency measures are often more strongly associated with these outcomes than corresponding letter fluency measures (e.g., naming words that start with a cued letter), suggesting they better capture impairments due to psychopathology or dementia. Thus, we focus on semantic fluency in this study. Semantic fluency measures are also associated with several other cognitive abilities including language, processing speed, executive function, and episodic memory (Gustavson et al., 2019; Shao, Janse, Visser, & Meyer, 2014; Whiteside et al., 2016). Genetic influences play a substantial role in many aspects of cognitive aging, Alzheimer’s disease, and psychiatric disorders (Kunkle et al., 2019), yet little is known about the genetic and environmental architecture of semantic fluency and its changes across the lifespan that might help explain the associations between semantic fluency and these conditions. Using data from six large twin samples, the current study firstly examined the heritability of semantic fluency, and then tested whether the magnitude of the genetic and environmental influences on semantic fluency vary based on age, sex, and level of education.
Existing studies examining the genetic and environmental architecture of verbal fluency in community samples of middle-aged or older adults have yielded a wide range of heritability estimates – suggesting that genetic influences explain between 20% and 77% of its total variance (Bratko, 1996; Giubilei et al., 2008; Gustavson et al., 2019; Lee et al., 2018). Some of this inconsistency may be based on the variety of fluency measures available (e.g., letter vs. semantic fluency) or the fact that higher heritability estimates are consistently obtained for studies that based analyses on multiple fluency measures or created latent variables (Gustavson et al., 2018; Lee et al., 2012). However, it is also possible that the genetic variance on semantic fluency differs as a function of age or other variables that varied across these studies.
In midlife, there is some evidence that the genetic influences on semantic fluency are highly stable, at least for short intervals. For example, we demonstrated a near perfect genetic correlation for a latent factor capturing letter and semantic fluency in a community sample of American male twins at mean ages 56 and 62 years (rgenetic =.94), with residual genetic influences on semantic fluency also showing perfect genetic stability (rgenetic = 1.0; Gustavson et al., 2018). However, focusing on genetic correlations can potentially mask important age-related changes in the genetic/environmental architecture of verbal fluency. For example, even if genetic variance remains perfectly correlated over time and does not decrease in magnitude, a decline in the magnitude of the environmental variance in older adults would result in higher heritability estimates over time. Similarly, an increase in the magnitude of the genetic variance would result in higher heritability estimates (providing the environmental variance remains the same). Thus, to more thoroughly elucidate the genetic and environmental architecture of verbal fluency, it is important to examine whether there are changes in genetic and environmental variance over the course of adulthood, especially in large samples with wide age ranges.
In addition to age-related changes, it is important to consider how other factors may affect the magnitude of genetic and/or environmental variance on semantic verbal fluency. Education is especially important because it is highly relevant to cognitive aging (Kremen et al., 2019) and is considered to be protective against cognitive decline and dementia (Boots et al., 2015; Dekhtyar, Wang, Fratiglioni, & Herlitz, 2016; Stern, 2012). Therefore, higher education may be related to better semantic fluency performance and/or less steep declines in fluency with age (e.g., age by education interaction). Genetic analyses can tease apart whether genetic and/or environmental variance on semantic fluency differ as a function of education and/or age, which may also contribute to the variability of existing heritability estimates.
Finally, it will be useful to explore whether the genetic or environmental variance differ by sex. This is important in studies of older adults as there are sex differences in life expectancy and incidence of dementia. That is, females live longer and may be at higher risk for Alzheimer’s disease (Beam et al., 2018; Mielke, Vemuri, & Rocca, 2014). There also may be sex differences in verbal fluency (Weiss et al., 2006) and in educational attainment, especially among older cohorts (Alexander & Eckland, 1974). This may map onto different trajectories of the magnitude of genetic and environmental variance in females compared to males, and different associations between education and genetic or environmental variance among females and males (e.g., sex by education interaction).
In the present study, we examined the genetic and environmental influences on semantic fluency in a sample of 21,684 individuals from six twin studies from the Interplay of Genes and Environment across Multiple Studies (IGEMS) consortium (Pedersen et al., 2013; Pedersen et al., 2019). This large sample allows us to obtain a fine-grained estimate of the phenotypic associations between semantic fluency and age, education, and sex, as well as potential interactions between these variables. We hypothesized that phenotypic associations with age would be non-linear, consistent with findings on other cognitive abilities that suggests that the rate of cognitive decline increases with advancing age (e.g., Alley, Suthers, & Crimmins, 2007; Nyberg et al., 2012; Wilson et al., 2002). Furthermore, we hypothesized that greater educational attainment would be associated with better semantic fluency performance, and possibly with a weaker age slope (age by education interaction). We did not make any a priori hypothesis regarding the effects of sex, but we examined whether it interacted with both age and education in phenotypic analyses. In genetic analyses, we examined the extent to which the genetic and environmental variance on semantic fluency vary as a function of age, education, or sex. We tested three competing alternatives for the moderation of genetic or environmental variance by education (increasing genetic or environmental variance, decreasing genetic or environmental variance, no moderation).
Method
Participants
Analyses were based on a total of 21,684 individual twins (10,831 males, 10,853 females), including 2,531 full monozygotic (MZ) twin pairs and 4,597 full dizygotic (DZ) twin pairs (including 1,805 opposite sex pairs). The full sample was drawn from six studies representing three countries (Australia, Denmark, and the United States) from the IGEMS consortium (Pedersen et al., 2019). Studies included the Older Australian Twins Study (OATS), Longitudinal Study of Aging Danish Twins (LSADT), Middle Age Danish Twin Studies (MADT/MIDT), Midlife in the United States (MIDUS), and the Vietnam Era Twin Study of Aging (VETSA; also from the United States). No studies had overlapping participants. Demographic characteristics displayed in Table 1 are separated by country and by sample (see supplement Figures S1 to S4 for histograms).
Table I:
Demographic Characteristics of the Sample
| Verbal Fluency | Sex | Age | Education (ISCED score) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Mean | SD | Range | % Female | Mean | SD | Range | N (ISCED) | Mean | SD | Range |
| Full Sample | 21684 | 22.24 | 6.93 | 0, 45.94 | 50.1 | 60.84 | 11.21 | 40, 89.16 | 20434 | 3.45 | 1.26 | 2, 6 |
| By Country | ||||||||||||
| Australia | 588 | 17.66 | 4.76 | 0, 32 | 65.1 | 71.18 | 5.34 | 65.08, 89.16 | 381 | 3.51 | 1.01 | 2, 5 |
| Denmark | 18511 | 22.89 | 7.01 | 1, 45.94 | 53.3 | 61.12 | 11.51 | 40, 89 | 17468 | 3.35 | 1.28 | 2, 6 |
| USA | 2585 | 18.65 | 5.06 | 4, 36.68 | 23.6 | 56.48 | 7.39 | 40, 82 | 2585 | 4.10 | 0.98 | 2, 6 |
| By Study | ||||||||||||
| OATS | 588 | 17.66 | 4.76 | 0, 32 | 65.1 | 71.18 | 5.34 | 65.08, 89.16 | 381 | 3.51 | 1.01 | 2, 5 |
| LSADT | 4232 | 18.18 | 6.72 | 1, 39.25 | 57.9 | 76.49 | 4.93 | 70, 89 | 4184 | 2.67 | 1.08 | 2, 6 |
| MADT | 4261 | 24.43 | 6.83 | 2.91, 45.94 | 49.0 | 56.39 | 6.33 | 45, 68 | 4254 | 3.21 | 1.17 | 2, 6 |
| MIDT | 10018 | 24.23 | 6.31 | 3, 44.56 | 53.1 | 56.64 | 9.39 | 40, 80 | 9030 | 3.73 | 1.26 | 2, 6 |
| MIDUS | 1105 | 18.03 | 5.79 | 4, 36.68 | 55.1 | 56.06 | 10.62 | 40, 82 | 1105 | 4.12 | 1.06 | 2, 6 |
| VETSA | 1480 | 19.12 | 4.38 | 6, 32.49 | 0.0 | 56.80 | 3.34 | 51.08, 66.92 | 1480 | 4.08 | 0.92 | 2, 6 |
Note: Fluency data is presented after trimming outlier scores greater/less than 3 SD from the mean to equal 3 SD from the mean (within sample, and within 10-year age bins). Only one subject received a score of 0, which was well within the 3 SD range of other 80-year-olds in OATS, so their data were not excluded. Raw age data are presented. Education data is shown after collapsing some ISCED to harmonize across studies (described in method). Australian Studies: OATS = Older Australian Twins Study; Danish Studies: LSADT = Longitudinal Study of Aging Danish Twins, MIDT/MADT = Middle Age Danish Twin Studies; USA Studies: MIDUS = Midlife in the United States, VETSA = Vietnam Era Twin Study of Aging.
For more information on each sample, see Pedersen et al., 2019. In summary, OATS is a longitudinal study of Australian twins born between 1919 and 1946 (Sachdev et al., 2009). LSADT is a study of older same-sex Danish twins born prior to 1920 (Christensen et al., 1999). MADT and MIDT are studies of middle-aged Danish twins recruited from the Danish Twin Registry born between 1931–1952 (MADT) or 1931–1969 (MIDT; Osler, McGue, Lund, & Christensen, 2008). MIDUS is a national telephone/mail survey carried out in 1995–1996 that included middle-aged twins (Barry, 2014). VETSA is a study of middle-aged male twins, all of whom served in the US military at some point between 1965 and 1975 (Kremen, Franz, & Lyons, 2013). All studies were of community-dwelling twins and not selected on the basis of any clinical diagnoses or health characteristics. For longitudinal studies, we used data from the first semantic fluency assessment only.
Measures
Semantic fluency.
In all studies, participants were asked to name as many animals as possible in 60 seconds. The dependent measure was the total number of correct responses (excluding repetitions). Although there were some administration differences (e.g., phone interviews for MIDUS, in-person assessment for all other studies), all procedures were similar enough to pool the raw scores (i.e., the total number of correct responses given in 60 seconds). However, to avoid the undue influence of outliers, we grouped participants into bins of 10 years within each study (e.g., age 40 to 49.99), then winsorized any scores greater or less than 3 standard deviations (SDs) from the mean to exactly 3 SDs (N=105; 0.48% of total observations). Winsorization was done within each bin of 10 years to ensure that we did not trim too much data (e.g., so 40-year-olds who performed well were not trimmed based on the population mean of 62 years).
Education.
Education was based on the International standard classification of education (ISCED; UNESCO Institute for Statistics, 2012). For all individuals, a score of 2 was given for those who completed up to a lower secondary education (grades 7–9; n = 5,569), a score of 3 was given to those who completed an upper secondary education (grades 10–12 or GED; n = 6,862), a score of 4 was given to those who completed post-secondary non-tertiary education or short-cycle tertiary education (e.g., vocational school, associate’s degree; n = 2,825), a score of 5 was given to those who completed a bachelor’s degree (or equivalent; n = 3,660), and a score of 6 was given to those who completed a master’s degree or higher (n = 1,518).
Some studies had more detailed interviews allowing for ISCED scores of 0 (no education, or less than primary education) or 1 (primary education, typically up to grade 6). Because these cases were very infrequent and specific to only some studies, we recoded all scores less than 2 to equal 2. Similarly, only some studies differentiated between masters and doctoral degrees, so we assigned a score of 6 to the 65 individuals who had doctoral degrees. Finally, our score of 4 is typically separated into two separate scores (post-secondary non-tertiary education, or short-cycle tertiary education), but these were collapsed because of low endorsement (to make the final measure more normally distributed). Alternate phenotypic analyses using non-adjusted ISCED scores resulted in nearly identical patterns of results.
Data Analysis
All analyses were performed using the R statistical software version 3.5.1. All phenotypic regression analyses were conducted using the lme4 package (Bates, Mächler, Bolker, & Walker, 2015), which used random intercepts to control for the nesting of data at the level of country, sample, and twin pair.
Genetic analyses were conducted using the OpenMx package (Neale et al., 2016), which accounts for missing observations using a full-information maximum likelihood approach. Model fit for genetic analyses was determined using −2 log-likelihood values (−2LL) and the Akaike information criterion (AIC). Good fitting models had the lowest −2LL and AIC values (Markon & Krueger, 2004).
Genetically informed models were also based on the standard assumptions in twin designs. Additive genetic influences (A) are correlated at 1.0 for MZ twin pairs and 0.5 for DZ twin pairs because MZ twins share 100% and DZ twins share, on average, 50% of their alleles identical-by-decent. Shared environmental influences (C) are correlated at 1.0 in both MZ and DZ twins. Non-shared environmental influences (E), which include measurement error, are set to not correlate for either MZ or DZ twin pairs. The standard twin design also assumes equal means and variances within pairs and across zygosity.
The genetic model fit here is displayed in the supplement (Figure S5). To test moderation by age and education, twins’ age and education were allowed to moderate the paths on their A, C, and E variances, as well as the mean (i.e., the phenotypic effect). Age was included as a family-level moderator (i.e., the mean age for each twin pair) because twins were essentially the same age at assessment (r=.99). Although we tested non-linear effects of age in phenotypic analyses, only the linear effect of age was included in the genetic model due to sparser representation after separating twins by zygosity and sex. Including a nonlinear effect of age in the genetic model appeared to overfit the data and did not alter the pattern of results for education. Additionally, because education differed within pair, we used the bivariate approach in which each twins’ level of education was formally included in the model (Purcell, 2002; van der Sluis, Posthuma, & Dolan, 2012). This approach simultaneously models the genetic and environmental associations between education and verbal fluency while testing whether education (and age) moderate the genetic and environmental influences on fluency.
Our genetic model also included a fixed effect of country on the mean (code 1: Australia = −1, Denmark = 0, USA = 1; code 2: Australia and USA = −1, Denmark = 2). Thus, the interpretation of the intercept reflects the mean of group means (rather than the grand mean). Parameters were significant if they could not be removed from the model without a significantly worse fit (using χ2 difference tests). Moderation of sex was tested by fitting separate groups and examining whether parameters in female pairs could be equated with parameters in male pairs (using χ2 difference tests). Analyses included opposite sex pairs.
Results
Descriptive Statistics and Preliminary Analyses
Demographic characteristics of the overall sample, and broken down by country and study, are displayed in Table 1. The average age of the full sample was 60.84 years. The full sample was evenly split between sexes (50.1% female). The mean for ISCED education scores (M=3.45) reflects greater than a high school education but not completion of any additional degrees (tertiary or bachelors) for the majority of participants. The average individual could name about 22 different animals in 1 minute. Phenotypic correlations between verbal fluency and the other key study variables (age, education, and sex) are displayed in Table 2 for the total sample and by country and study (see supplemental Figures S6 and S7 for scatterplots with trend lines). Effect sizes were similar across studies, with the largest variability for correlations with sex. Table 2 also displays the cross-twin correlations by country and study.
Table II:
Phenotypic and Cross-Twin Correlations for Semantic Fluency Measures
| Phenotypic Correlations with: | Cross-Twin Cross-Trait Correlations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Age | Education | Sex | MZ All | DZ All | MZ Male | DZ Male | MZ Female | DZ Female | DZ Opp Sex | |
| Full Sample | 21684 | −0.38 | 0.28 | 0.01 | 0.57 | 0.36 | 0.55 | 0.38 | 0.60 | 0.40 | 0.28 | |
| By Country | ||||||||||||
| Australia | 588 | −0.32 | 0.15 | 0.08 | 0.53 | 0.24 | 0.39 | 0.31 | 0.62 | 0.15 | 0.28 | |
| Denmark | 18511 | −0.43 | 0.34 | −0.02 | 0.54 | 0.32 | 0.50 | 0.34 | 0.57 | 0.38 | 0.23 | |
| USA | 2585 | −0.23 | 0.22 | −0.23 | 0.40 | 0.23 | 0.41 | 0.18 | 0.40 | 0.19 | 0.34 | |
| By Study | ||||||||||||
| OATS | 588 | −0.32 | 0.15 | 0.08 | 0.53 | 0.24 | 0.39 | 0.31 | 0.62 | 0.15 | 0.28 | |
| LSADT | 4232 | −0.33 | 0.27 | −0.16 | 0.44 | 0.30 | 0.42 | 0.36 | 0.45 | 0.24 | 0.37 | |
| MADT | 4261 | −0.12 | 0.27 | −0.07 | 0.40 | 0.27 | 0.42 | 0.30 | 0.40 | 0.19 | 0.34 | |
| MIDT | 10018 | −0.28 | 0.27 | 0.12 | 0.56 | 0.29 | 0.56 | 0.30 | 0.56 | 0.37 | 0.24 | |
| MIDUS | 1105 | −0.29 | 0.32 | −0.11 | 0.40 | 0.27 | 0.42 | 0.30 | 0.40 | 0.19 | 0.34 | |
| VETSA | 1480 | −0.13 | 0.13 | - | 0.41 | 0.17 | 0.41 | 0.17 | - | - | - | |
Note: Columns 3–5 display phenotypic correlations between semantic fluency and the other primary study variables: age, education, and sex. Columns 6–11 display the cross-twin correlations for verbal fluency among monozygotic (MZ) and dizygotic (DZ) twin pairs. Cross-twin correlations do not adjust for age, education, and sex effects. Significant correlations are displayed in bold (p < .05). Australian Studies: OATS = Older Australian Twins Study; Danish Studies: LSADT = Longitudinal Study of Aging Danish Twins, MIDT/MADT = Middle Age Danish Twin Studies; USA Studies: MIDUS = Midlife in the United States, VETSA = Vietnam Era Twin Study of Aging.
Phenotypic Analyses
First, we examined evidence for main effects of age, education, and sex, as well as evidence for 2-way interactions for combinations of these variables. We estimated non-linear effects of age using a spline regression with a single cut-point. Possible cut-points were estimated at 5-year intervals between age 50 and 75 (and the mean of 60.84 years). The best-fitting model had different linear effects of age before and after age 70 (see supplement Table S1 for model comparisons with alternate cut-points). In our final regression model, we also excluded all non-significant interaction terms (including these interaction terms did not alter the patterns of results).
Results of the regression analyses revealed significant effects of age both before age 70, β = −.207, p < .001, 95% CI [−.228, −.187], and after age 70, β = −.634, p < .001, 95% CI [−.689, −.578], with considerably larger effects of age in older adults. Even though these associations are cross-sectional, the unstandardized estimates suggested that individuals generate about 1 less word for every 7.7 years of aging before age 70 (b = −.13), and that this rate increases to about 1 less word for every 2.6 years of aging after age 70 (b = −.37).
There was no main effect of sex, β = .018, p = .169, 95% CI [−.007, .042]. There was a significant main effect of education, β = .215, p < .001, 95% CI [.197, 232], and a significant sex by education interaction, β = .033, p = .007, 95% CI [.009, .057]. Unstandardized estimates indicated that about 2 extra years of education (i.e., a 1-point higher ISCED score) corresponded to about one additional word generated (b = 1.18), with this beneficial effect about 15% stronger in females. Sex was only weakly correlated with ISCED education, with females receiving less education than males, β = −.068, p < .001, 95% CI [−.094, −.042], controlling for age, country, sample, and family. The pooled results described here are driven by the large size of the Danish sample, but results for Australia and the US largely follow the same patterns (see Figures S6 and S7).
Genetic Analyses
Next, we examined whether the genetic and/or environmental variance on semantic fluency varied as a function of age (Table 3, Figure 1), as well as by education and sex (Table 4, Figure 2). We conducted these analyses in the context of the same full genetic model (i.e., with moderation effects of age and education on A, C, and E paths estimated separately for males and females) but present our evaluation of submodels for the effects of age and education separately for clarity. Age was standardized, then re-centered at age 70 and education (ISCED score) was standardized. Thus, effects of age are interpreted at the mean level of education and effects of education are interpreted at age 70 (i.e., the point of the phenotypic regression spline). Parameter estimates and standard errors of this full model are displayed in supplemental Table S2.
Table III:
Model Comparisons for Genetic and Environmental Moderation by Age
| Model | ep | neg 2LL | df | AIC | diff −2LL | diff df | p |
|---|---|---|---|---|---|---|---|
| Full Model: Mean Effects & ACE Variance Moderation | 64 | 64474.09 | 25716 | 13042 | - | - | - |
| Test ACE Moderation for Age | |||||||
| No A Moderation by Age for Females | 62 | 64474.26 | 25718 | 13038 | 0.17 | 2 | 0.919 |
| No C Moderation by Age for Females | 62 | 64477.02 | 25718 | 13041 | 2.93 | 2 | 0.231 |
| No E Moderation by Age for Females | 62 | 64475.30 | 25718 | 13039 | 1.21 | 2 | 0.546 |
| No A Moderation by Age for Males | 62 | 64476.54 | 25718 | 13041 | 2.45 | 2 | 0.294 |
| No C Moderation by Age for Males | 62 | 64474.13 | 25718 | 13038 | 0.04 | 2 | 0.980 |
| No E Moderation by Age for Males | 62 | 64474.71 | 25718 | 13039 | 0.62 | 2 | 0.733 |
| Equate ACE Moderation of Age Across Sex | |||||||
| Equate A Moderation of Age Across Sex | 62 | 64476.95 | 25718 | 13041 | 2.86 | 2 | 0.239 |
| Equate C Moderation of Age Across Sex | 62 | 64475.07 | 25718 | 13039 | 0.98 | 2 | 0.613 |
| Equate E Moderation of Age Across Sex | 62 | 64474.69 | 25718 | 13039 | 0.60 | 2 | 0.741 |
Note: The model described in the results (and displayed Figure 1) is listed in bold font, which included moderation effects of age and education on the genetic (A), shared environmental (C), and nonshared environmental (E) influences on semantic fluency separately for each sex, as well as the phenotypic effects of age and country in each sex on the mean (and independent means for each sex). Moderation of age was evaluated by fixing ACE moderation parameters to zero in each sex. Tests have 2 df because age is allowed to moderate the genetic/environmental paths from education to semantic fluency as well as the unique genetic/environmental influences on semantic fluency.
Figure 1:
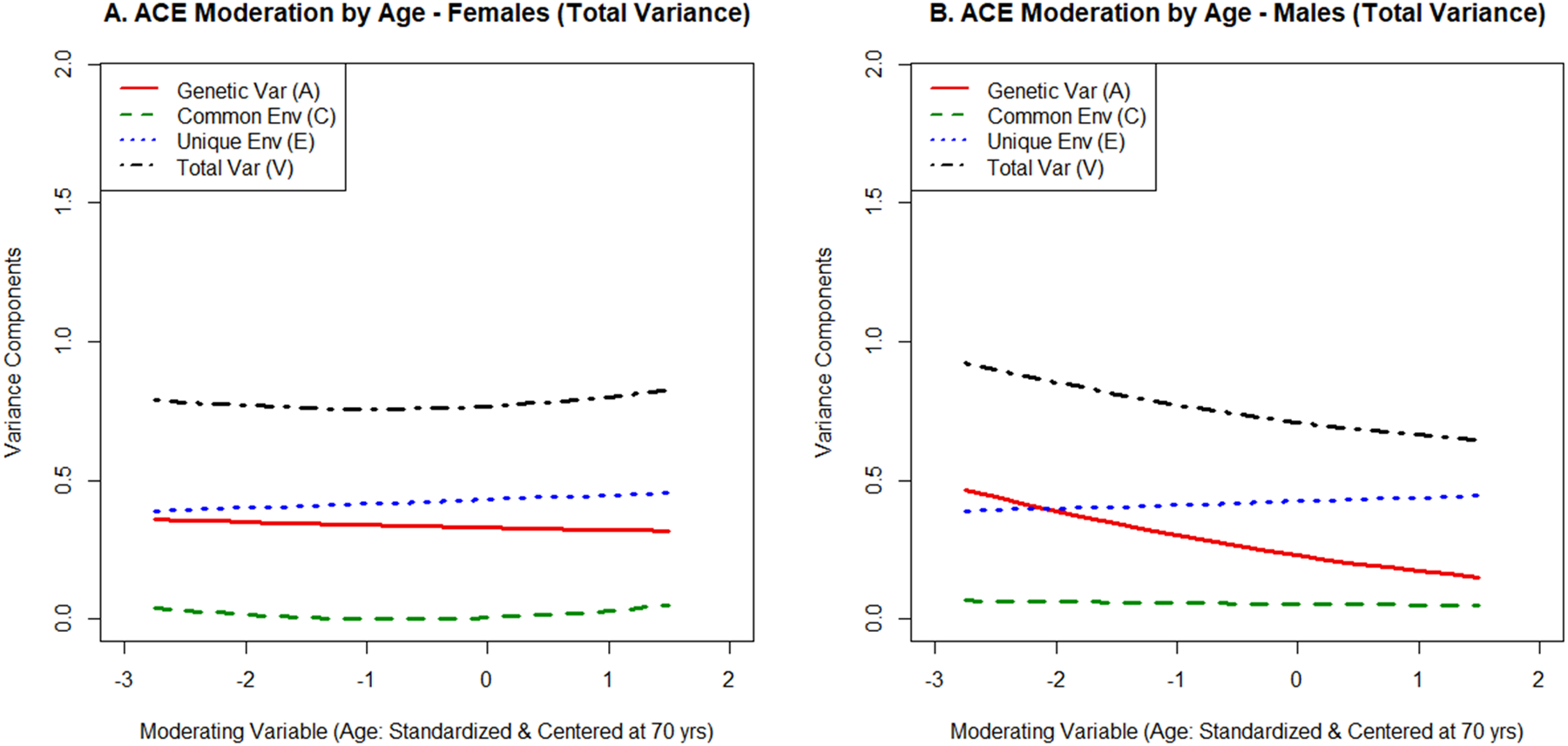
Moderation of the genetic (A), shared environmental (C), and nonshared environmental (E) influences on verbal fluency by age. The total variance (V) is also displayed.
Table IV:
Model Comparisons for Genetic and Environmental Moderation by Education and Sex
| Model | ep | neg 2LL | df | AIC | diff −2LL | diff df | p |
|---|---|---|---|---|---|---|---|
| Full Model: Mean Effects & ACE Variance Moderation | 64 | 64474.09 | 25716 | 13042 | - | - | - |
| Equate ACE Variance Across Sex | |||||||
| Equate A Variances Across Sex | 61 | 64486.75 | 25719 | 12948 | 12.66 | 3 | 0.005 |
| Equate C Variances Across Sex | 61 | 64481.02 | 25719 | 13043 | 6.93 | 3 | 0.074 |
| Equate E Variances Across Sex | 61 | 64476.13 | 25719 | 13038 | 2.04 | 3 | 0.361 |
| Test ACE Moderation for Education | |||||||
| No A Moderation of Education for Females | 62 | 64476.13 | 25718 | 13040 | 2.04 | 2 | 0.361 |
| No C Moderation of Education for Females | 62 | 64477.31 | 25718 | 13041 | 3.22 | 2 | 0.200 |
| No E Moderation of Education for Females | 62 | 64475.12 | 25718 | 13039 | 1.03 | 2 | 0.598 |
| No A Moderation of Education for Males | 62 | 64474.54 | 25718 | 13039 | 0.45 | 2 | 0.799 |
| No C Moderation of Education for Males | 62 | 64478.07 | 25718 | 13042 | 3.98 | 2 | 0.137 |
| No E Moderation of Education for Males | 62 | 64486.98 | 25718 | 13051 | 12.89 | 2 | 0.002 |
| Equate ACE Moderation of Education Across Sex | |||||||
| Equate A Moderation of Education Across Sex | 62 | 64477.53 | 25718 | 13042 | 3.44 | 2 | 0.179 |
| Equate C Moderation of Education Across Sex | 62 | 64478.08 | 25718 | 13042 | 3.99 | 2 | 0.136 |
| Equate E Moderation of Education Across Sex | 62 | 64484.13 | 25718 | 13048 | 10.04 | 2 | 0.007 |
Note: The model described in the results (and displayed in Figure 2) is listed in bold font, which included moderation effects of age and education on the genetic (A), shared environmental (C), and nonshared environmental (E) influences on semantic fluency separately for each sex, as well as the phenotypic effects of age and education in each sex on the mean (and independent means for each sex). Tests have 2 df because education is allowed to moderate the genetic/environmental paths from education to semantic fluency as well as the unique genetic/environmental influences on semantic fluency.
Figure 2:
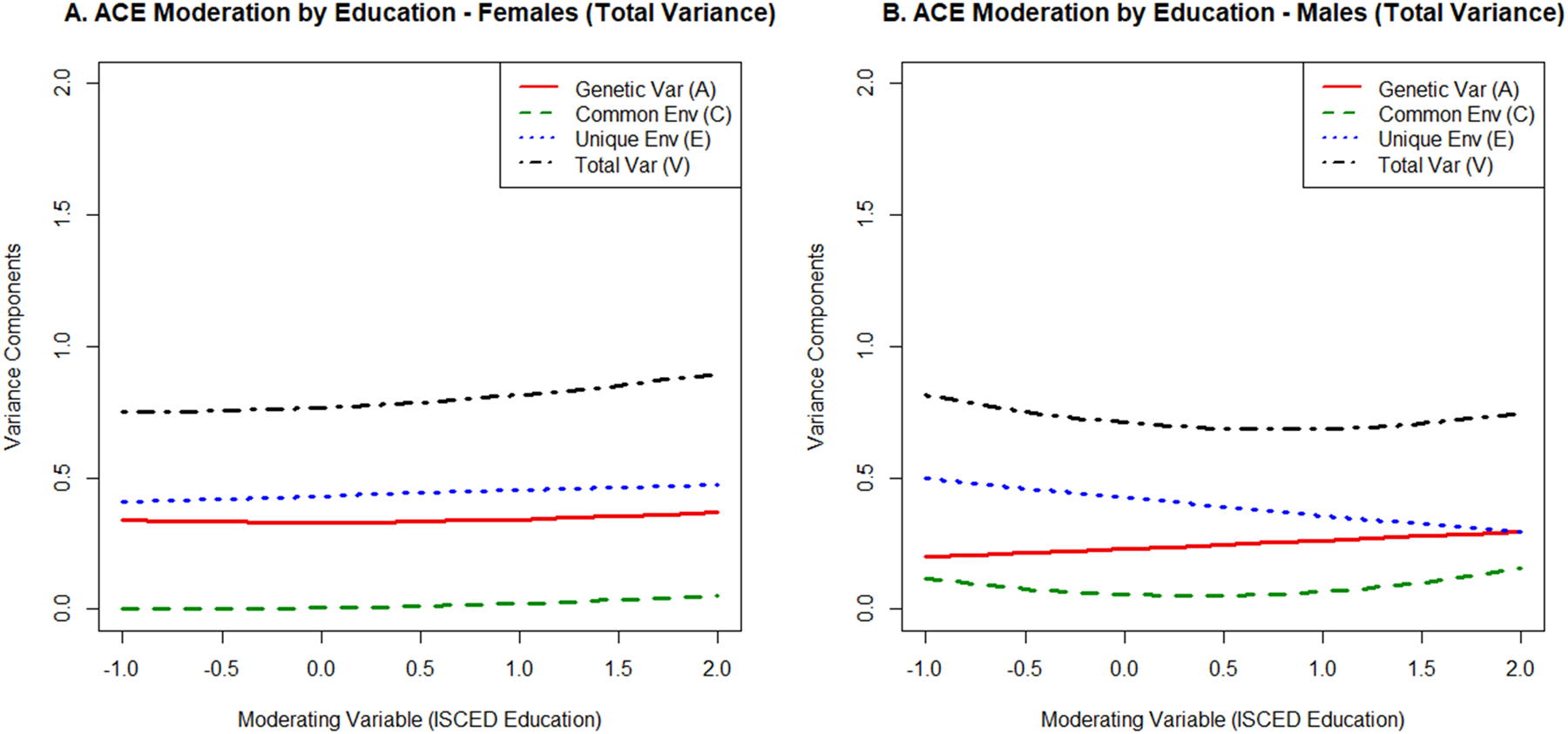
Moderation of the genetic (A), shared environmental (C), and nonshared environmental (E) influences on verbal fluency by education. The total variance (V) is also displayed.
Moderation by age.
As shown in Table 3 and Figure 1, there was no evidence for moderation of semantic fluency by age. A, C, and E moderation by age could be dropped without poorer model fit for both females, all χ2(2)<2.93, p>.231, and males, all χ2(2)<2.45, p>.294. Additionally, there was no evidence that the A, C, and E moderation effects differed across sex, all χ2(2)<2.86, p>.239.
Assuming a mean level of education, heritability estimates for semantic fluency ranged from a2=.46 (age 45) to a2=.39 (age 85) in females and from a2=.47 (age 45) to a2=.24 (age 85) in males. Evidence for shared environmental influences was weak in the entire sample, with c2 < .10 in all individuals. Nonshared environmental influence estimates ranged from e2=.51 (age 45) to e2=.56 (age 85) in females and from e2=.45 (age 45) to e2=.68 (age 85) in males.
Moderation by education.
As shown in Figure 2 and Table 4, nonshared environmental influences on semantic fluency were moderated by levels of education in males only, χ2(2)=12.89, p=.002. Specifically, nonshared environmental variance was lower in males with higher education, driven by education moderating the nonshared environmental variance unique to semantic fluency (e22 path, supplemental Table S2d). This moderation effect was significantly larger in males than in females, χ2(2)=10.04, p=.007, where it was nonsignificant (see Table 4). Additionally, the shared environmental variance was higher in males with the highest or lowest levels of education (c22 path, supplemental Table S2d). The corresponding 2 df test in Table 4 was nonsignificant, χ2(2)=3.98, p=.137, again because education specifically moderated the shared environmental influences unique to semantic fluency (the c22 path) rather than moderating the shared environmental association between education and fluency (c12 path). There was no evidence for moderation of genetic variance by education in either sex.
Model estimates suggested that nonshared environmental influences for individuals with less than a high school education (ISCED score of 2) were e2=.61 (males) and e2=.54 (females) whereas nonshared environmental estimates for individuals with a master’s degree or higher (ISCED score of 6) were e2=.39 (males) and e2=.53 (females). Finally, as shown in supplemental Table S2a and S2b, levels of education were associated with semantic fluency through a significant genetic correlation in females, rg=.51 (rc=.75 and re=.02 were both nonsignificant) and through a significant shared environmental correlation in males, rc=.91 (rg=.21 and re=.07 were both nonsignificant).
Sex differences.
Sex differences in ACE variances are displayed at the top of Table 4, evaluated at the mean education and at age 70. The genetic variance matrix could not be equated across sex, χ2(3)=12.66, p=.005, driven by a significantly stronger genetic overlap between education and semantic fluency in females than males, χ2(1)=5.74, p=.017. In contrast, there were no differences in the shared environmental, χ2(3)=6.93, p=.074, or nonshared environmental influences, χ2(3)=2.04, p=.361, between females and males. However, these effects should be interpreted within the context of the previous interactions. For example, males show smaller nonshared environmental influences (and therefore higher heritability) at higher levels of education.
Discussion
We examined, in a very large sample of adult twins ranging in age from 40 to 89, the associations between semantic fluency, age, education, and sex. Results of the cross-sectional phenotypic analyses revealed that through about age 70, individuals produce about one less word for every seven to eight years of aging, but the rate of decline may increase substantially over time (e.g., one less word per 2.6 years of aging after age 70). Each additional point of education on the ISCED scale (which corresponds to about 2 years of education after high school) related to about one additional word produced, and this effect was slightly larger for females than males. However, the lack of interaction between age and education suggests that although an individual’s level of semantic fluency performance is associated with their level of education, education may not be associated with a slower rate of decline in performance.
Cross-sectional genetic moderation analyses revealed no evidence that genetic or environmental influences on semantic verbal fluency varied by age in these middle-aged to older participants. Heritability estimates ranged from a2=.46 to a2=.39 in females and from a2=.47 to a2=.24 in males, with the apparent decrease in heritability in males driven by increasing nonshared environmental variance (rather than decreasing genetic variance). Prior research provided a wide range of heritability estimates, between 20% and 77% (Bratko, 1996; Giubilei et al., 2008; Gustavson et al., 2019; Lee et al., 2018). Our results suggest that it is unlikely that the heterogeneity in these estimates is explained by the age of the sample (although participants from the two largest prior studies – OATS and VETSA – were included in the current analysis). Instead, the range in heritability estimates may reflect administration differences or the use of latent variables in some studies (Gustavson et al., 2018) which tend to yield higher heritability. Nevertheless, these findings highlight the importance of examining total genetic variance rather than focusing on the total percent variance, as there was some evidence for moderation of environmental influences by education (discussed below). A previous publication from IGEMS considered age moderation of specific cognitive abilities but did not include semantic fluency (Pahlen et al., 2018). Pahlen et al. reported decreasing genetic variance with age for digit span forward and backward but not for vocabulary, synonyms, block design, or symbol digit. This suggests these patterns of moderation effects may be ability-specific.
In contrast to the results for age, there was evidence that environmental influences on semantic fluency varied based on levels of education. In males, nonshared environmental variance in semantic fluency decreased with higher education, and shared environmental influences were largest at the tails of the distribution. Females showed no significant moderation but demonstrated stronger positive phenotypic associations between semantic fluency and education than males. There has been substantial previous attention to how socioenvironmental context moderates individual differences in cognitive tests during childhood and adolescence. Generally, at least in US samples, additive genetic influences in cognition have been higher with more favorable parental socioeconomic status (Tucker-Drob, Briley, & Harden, 2013; Turkheimer & Horn, 2014), including earlier analysis of intelligence in MIDUS based on a composite of 5 tests including semantic fluency (Bates, Lewis, & Weiss, 2013). However, similar work on adolescent Australian twins revealed no moderation of intelligence by parental socioeconomic status (Bates, Hansell, Martin, & Wright, 2016). There has been little prior work with older adults, but what is available suggests that findings based on parental socioeconomic status in children and adolescents may not hold true for attained socioeconomic status in older adults. A previous IGEMS publication that did not include verbal fluency found that genetic variance on cognitive tests was generally smaller with higher attained socioeconomic status, with perceptual speed (i.e., digit-symbol or symbol-digit tests) the one exception (Zavala et al., 2018). Our findings were most similar to the Bates et al. (2016) study, revealing no genetic moderation by age. However, heritability appeared to increase in males, similar to the earlier US studies including analyses of MIDUS, only because environmental variances decreased. Like our conclusion for age, it is also possible that moderation effects of education may depend on the specific cognitive ability being studied.
Finally, females exhibited greater variance in semantic fluency scores than males at the zero point for our moderating variables (i.e. mean education of 3.45 and at age 70). Specifically, although unique genetic influences on semantic fluency were similar in females and males (path a22=.49 in females and .47 in males in Table S2a and S2b), additional overlapping genetic influences between semantic fluency and education were observed only in females (path a12=.30 in females and .10 in males in Table S2a and S2b), resulting in a stronger genetic correlation between education and fluency for females (rg=.51) than males (rg=.21). These results suggest that although educational attainment may not influence the magnitude of genetic influences on semantic fluency in either sex, the genetic influences underlying educational attainment appear to play a stronger role in fluency performance in females than males (at least for middle-aged and older females born in the early to mid-20th century). There were no sex differences in the magnitude of shared or nonshared environmental influences on semantic fluency at the zero point for our moderating variables. However, because environmental variance in semantic fluency was lower in the male group with higher education, females had larger nonshared environmental influences (and therefore smaller heritability than males) at higher levels of education, but smaller nonshared environmental influences (and higher heritability than males) at lower levels of education.
Strengths and Limitations
This is the largest study to date to examine the genetic and environmental architecture of semantic verbal fluency, and the first to demonstrate that the magnitude of environmental influences varies by level of education in males. The sample was drawn from 6 studies representing Denmark, the United States, and Australia, making it more representative than most studies that focus on individuals from only one population. However, these samples remain predominantly individuals of European ancestry and do not represent the global population. All studies administered a similar measure of semantic fluency, and although there were some minor administration differences, there was no need to harmonize measures. Although verbal fluency scores were winsorized to address outliers, no other steps were taken to remove anyone who might have met clinical diagnosis for dementia, as almost all of the studies lacked formal assessment for dementia and those with formal assessments identified only a few cases. Moreover, these results inform the association between age and verbal fluency performance, but all analyses were cross-sectional rather than longitudinal.
Furthermore, although we controlled for the nesting of data within country, sample, and family, using hierarchical linear modelling techniques, it is important to note that the majority of the data came from the three large Danish studies that had considerably higher verbal fluency performance compared to the other 3 samples (see Table 1). It is unclear what factors led to this higher performance, but it may stem from language differences or other differences between samples (e.g., administration, urbanization, cohort). Interestingly, Danish twins had the lowest level education, suggesting education differences could not account for their higher fluency performance. Indeed, there may be cohort effects on cognition (including semantic fluency), in part due to education differences across countries not captured by the ISCED scores (Ahrenfeldt et al., 2018; Briley, Harden, Bates, & Tucker-Drob, 2015). The structure of the sample precluded identification of similar birth cohorts within each country. Nevertheless, our post-hoc analyses split by country or sample suggest that the effects of age and education do not appear to differ based on country (see supplemental Figures S2 and S3).
Concluding Remarks
Semantic fluency is an important construct interwoven with a network of other cognitive abilities and highly relevant to both clinical disorders and aging/dementia. However, there is much to learn about its genetic and environmental structure and the way these influences vary based on other biological and environmental factors. This study is the first to demonstrate that the environmental variance in semantic fluency performance may depend on level of education. The effect of education on verbal fluency may differ across sexes, both at the phenotypic level and at the level of moderation of environmental influences. These findings highlight the dynamic interplay of genetic and environmental influences on cognitive function, and highlight the need to examine the genetic underpinnings of semantic fluency (and other cognitive abilities) using very large samples that can capture these complex associations.
Supplementary Material
Funding
IGEMS is supported by the National Institutes of Health Grants No. R01 AG037985, R56 AG037985, R01 AG059329, R01 AG060470, RF1 AG058068. The Danish Twin Registry has been supported by grants from The National Program for Research Infrastructure 2007 from the Danish Agency for Science and Innovation, the Velux Foundation and the US National Institute of Health (P01 AG08761). VETSA was supported by National Institute of Health grants NIA R01 AG050595, R01 AG022381, and R01 AG022982. The Cooperative Studies Program of the Office of Research & Development of the United States Department of Veterans Affairs (VA) has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. The MIDUS study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development and by National Institute on Aging Grant AG20166. We acknowledge the contribution of the OATS research team (https://cheba.unsw.edu.au/project/older-australian-twins-study) to this study. The OATS study has been funded by a National Health & Medical Research Council (NHMRC) and Australian Research Council (ARC) Strategic Award Grant of the Ageing Well, Ageing Productively Program (ID No. 401162) and NHMRC Project Grants (ID 1045325 and 1085606). OATS participant recruitment was facilitated through Twins Research Australia, a national resource in part supported by a Centre for Research Excellence Grant (ID: 1079102), from the National Health and Medical Research Council. We thank the participants for their time and generosity in contributing to this research. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA/NIH, or the VA. The first author was also supported by NIH grants R03 AG065643, R01 DC016977, and DP2 HD098859.
Footnotes
Conflicts of interests
The authors report no conflicts of interests
Ethics approval
All studies included within IGEMS received approval from relevant Institutional Review Boards at participating institutions. Analyses of de-identified data presented here was approved by Vanderbilt University Medical Center.
References
- Ahrenfeldt LJ, Lindahl-Jacobsen R, Rizzi S, Thinggaard M, Christensen K, & Vaupel JW (2018). Comparison of cognitive and physical functioning of Europeans in 2004–05 and 2013. International Journal of Epidemiology, 47, 1518–1528. doi: 10.1093/ije/dyy094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KL, & Eckland BK (1974). Sex differences in the educational attainment process. American Sociological Review, 39, 668–682. doi: 10.2307/2094313 [DOI] [Google Scholar]
- Alley D, Suthers K, & Crimmins E (2007). Education and Cognitive Decline in Older Americans: Results From the AHEAD Sample. Research on Aging, 29, 73–94. doi: 10.1177/0164027506294245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry TR (2014). The Midlife in the United States (MIDUS) series: A national longitudinal study of health and well-being. Open Health Data, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, 67. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bates TC, Hansell NK, Martin NG, & Wright MJ (2016). When does socioeconomic status (SES) moderate the heritability of IQ? No evidence for g × SES interaction for IQ in a representative sample of 1176 Australian adolescent twin pairs. Intelligence, 56, 10–15. doi: 10.1016/j.intell.2016.02.003 [DOI] [Google Scholar]
- Bates TC, Lewis GJ, & Weiss A (2013). Childhood socioeconomic status amplifies genetic effects on adult intelligence. Psychological Science, 24, 2111–2116. doi: 10.1177/0956797613488394 [DOI] [PubMed] [Google Scholar]
- Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, & Gatz M (2018). Differences between women and men in incidence rates of dementia and Alzheimer’s disease. Journal of Alzheimer’s Disease, 64, 1077–1083. doi: 10.3233/JAD-180141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots EA, Schultz SA, Almeida RP, Oh JM, Koscik RL, Dowling MN, …Bendlin BB (2015). Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Archives of Clinical Neuropsychology, 30, 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratko D (1996). Twin study of verbal and spatial abilities. Personality and Individual Differences, 21, 621–624. doi: 10.1016/0191-8869(96)00091-8 [DOI] [Google Scholar]
- Briley DA, Harden KP, Bates TC, & Tucker-Drob EM (2015). Nonparametric estimates of gene x environment interaction using local structural equation modeling. Behavior Genetics, 45, 581–596. doi: 10.1007/s10519-015-9732-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Holm NV, McGue M, Corder L, & Vaupel JW (1999). A Danish population-based twin study on general health in the elderly. Journal of Aging and Health, 11, 49–64. doi: 10.1177/089826439901100103 [DOI] [PubMed] [Google Scholar]
- Dekhtyar S, Wang HX, Fratiglioni L, & Herlitz A (2016). Childhood school performance, education and occupational complexity: A life-course study of dementia in the Kungsholmen Project. International Journal of Epidemiology, 45, 1207–1215. doi: 10.1093/ije/dyw008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giubilei F, Medda E, Fagnani C, Bianchi V, De Carolis A, Salvetti M, … Stazi MA (2008). Heritability of neurocognitive functioning in the elderly: Evidence from an Italian twin study. Age and Ageing, 37, 640–646. doi: 10.1093/ageing/afn132 [DOI] [PubMed] [Google Scholar]
- Gustavson DE, Panizzon MS, Elman JA, Beck A, Franz CE, Reynolds CA, …Kremen WS (2018). Genetic and environmental influences on verbal fluency in middle age: A longitudinal twin study. Behavior Genetics, 48, 361–373. doi: 10.1007/s10519-018-9910-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson DE, Panizzon MS, Franz CE, Reynolds CA, Corley RP, Hewitt JK, …Friedman NP (2019). Integrating verbal fluency with executive functions: Evidence from twin studies in adolescence and middle age. Journal of Experimental Psychology: General, 148, 2104–2119. doi: 10.1037/xge0000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, & Crawford JR (2004a). A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology, 18, 284–295. doi: 10.1037/0894-4105.18.2.284 [DOI] [PubMed] [Google Scholar]
- Henry JD, & Crawford JR (2004b). Verbal fluency deficits in Parkinson’s disease: A meta-analysis. Journal of the International Neuropsychological Society, 10, 608–622. doi: 10.1017/S1355617704104141 [DOI] [PubMed] [Google Scholar]
- Henry JD, & Crawford JR (2005a). A meta-analytic review of verbal fluency deficits in depression. Journal of Clinical and Experimental Neuropsychology, 27, 78–101. doi: 10.1080/138033990513654 [DOI] [PubMed] [Google Scholar]
- Henry JD, & Crawford JR (2005b). A meta-analytic review of verbal fluency deficits in schizophrenia relative to other neurocognitive deficits. Cognitive Neuropsychiatry, 10, 1–33. doi: 10.1080/13546800344000309 [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR, & Phillips LH (2004). Verbal fluency performance in dementia of the Alzheimer’s type: A meta-analysis. Neuropsychologia, 42, 1212–1222. doi: 10.1016/j.neuropsychologia.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Kremen WS, Beck A, Elman JA, Gustavson DE, Reynolds CA, Tu XM, …Franz CE (2019). Influence of young adult cognitive ability and additional education on later-life cognition. Proceedings of the National Academy of Sciences, 116, 2021–2026. doi: 10.1073/pnas.1811537116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Franz CE, & Lyons MJ (2013). VETSA: The Vietnam Era Twin Study of Aging. Twin Research and Human Genetics, 16, 399–402. doi: 10.1017/thg.2012.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, …Environmental Risk for Alzheimer’s Disease, C. (2019). Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nature Genetics, 51, 414–430. doi: 10.1038/s41588-019-0358-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Mosing MA, Henry JD, Trollor JN, Ames D, Martin NG, …Team OR (2012). Genetic influences on four measures of executive functions and their covariation with general cognitive ability: The Older Australian Twins Study. Behavior Genetics, 42, 528–538. doi: 10.1007/s10519-012-9526-1 [DOI] [PubMed] [Google Scholar]
- Lee T, Thalamuthu A, Henry JD, Trollor JN, Ames D, Wright MJ, …Team OR (2018). Genetic and environmental influences on language ability in older adults: Findings from the Older Australian Twins Study. Behavior Genetics, 1–11. doi: 10.1007/s10519-018-9897-z [DOI] [PubMed] [Google Scholar]
- Markon KE, & Krueger RF (2004). An empirical comparison of information-theoretic selection criteria for multivariate behavior genetic models. Behavior Genetics, 34, 593–610. doi: 10.1007/s10519-004-5587-0 [DOI] [PubMed] [Google Scholar]
- Mielke MM, Vemuri P, & Rocca WA (2014). Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clinical Epidemiology, 6, 37–48. doi: 10.2147/CLEP.S37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM, …Boker SM (2016). OpenMx 2.0: Extended structural equation and statistical modeling. Psychometrika, 81, 535–549. doi: 10.1007/s11336-014-9435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Lovden M, Riklund K, Lindenberger U, & Backman L (2012). Memory aging and brain maintenance. Trends in Cognitive Sciences, 16, 292–305. doi: 10.1016/j.tics.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Osler M, McGue M, Lund R, & Christensen K (2008). Marital status and twins’ health and behavior: An analysis of middle-aged Danish twins. Psychosom Med, 70, 482–487. doi: 10.1097/PSY.0b013e31816f857b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlen S, Hamdi NR, Dahl Aslan AK, Horwitz BN, Panizzon MS, Petersen I, …McGue M (2018). Age-moderation of genetic and environmental contributions to cognitive functioning in mid- and late-life for specific cognitive abilities. Intelligence, 68, 70–81. doi: 10.1016/j.intell.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL, Christensen K, Dahl AK, Finkel D, Franz CE, Gatz M, …Reynolds CA (2013). IGEMS: The consortium on Interplay of Genes and Environment across Multiple Studies. Twin Research and Human Genetics, 16, 481–489. doi: 10.1017/thg.2012.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL, Gatz M, Finch BK, Finkel D, Butler DA, Dahl Aslan A, …Whitfield KE (2019). IGEMS: The Consortium on Interplay of Genes and Environment Across Multiple Studies - An update. Twin Research and Human Genetics, 22, 809–816. doi: 10.1017/thg.2019.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S (2002). Variance components models for gene-environment interaction in twin analysis. Twin Research, 5, 554–571. doi: 10.1375/136905202762342026 [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Lammel A, Trollor JN, Lee T, Wright MJ, Ames D, …team O. r. (2009). A comprehensive neuropsychiatric study of elderly twins: the Older Australian Twins Study. Twin Res Hum Genet, 12, 573–582. doi: 10.1375/twin.12.6.573 [DOI] [PubMed] [Google Scholar]
- Shao Z, Janse E, Visser K, & Meyer AS (2014). What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Frontiers in Psychology, 5, 772. doi: 10.3389/fpsyg.2014.00772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurology, 11, 1006–1012. doi: 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Briley DA, & Harden KP (2013). Genetic and Environmental Influences on Cognition Across Development and Context. Current Directions in Psychological Science, 22, 349–355. doi: 10.1177/0963721413485087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, & Horn EE (2014). Interactions Between Socioeconomic Status and Components of Variation in Cognitive Ability Behavior Genetics of Cognition Across the Lifespan (pp. 41–68): Springer. [Google Scholar]
- UNESCO Institute for Statistics. (2012). International standard classification of education: ISCED 2011: UNESCO Institute for Statistics Montreal.
- van der Sluis S, Posthuma D, & Dolan CV (2012). A note on false positives and power in G x E modelling of twin data. Behavior Genetics, 42, 170–186. doi: 10.1007/s10519-011-9480-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EM, Ragland JD, Brensinger CM, Bilker WB, Deisenhammer EA, & Delazer M (2006). Sex differences in clustering and switching in verbal fluency tasks. Journal of the International Neuropsychological Society, 12, 502–509. [DOI] [PubMed] [Google Scholar]
- Whiteside DM, Kealey T, Semla M, Luu H, Rice L, Basso MR, & Roper B (2016). Verbal fluency: Language or executive function measure? Applied Neuropsychology: Adult, 23, 29–34. doi: 10.1080/23279095.2015.1004574 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, & Bennett DA (2002). Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging, 17, 179–193. [PubMed] [Google Scholar]
- Zavala C, Beam CR, Finch BK, Gatz M, Johnson W, Kremen WS, …Reynolds CA (2018). Attained SES as a moderator of adult cognitive performance: Testing gene-environment interaction in various cognitive domains. Developmental Psychology, 54, 2356–2370. doi: 10.1037/dev0000576 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


