Abstract
Purpose:
Prior studies, predominantly in women, reported long and short self-reported sleep duration are associated with lower BMD. Associations between actigraphy-determined sleep duration and BMD or bone turnover markers (BTMs) in older men are unknown.
Methods:
Men in The Osteoporotic Fractures in Men (MrOS) Study with wrist actigraphy and concurrent BMD assessment but without comorbidities affecting bone health were included. Sleep duration was considered as a continuous (N = 1,926) and dichotomized variable where men were classified as getting the recommended (7-8 h/night; N = 478) or short (<6 h/night; N = 577) sleep. The cross-sectional association between BMD, BTMs, and sleep duration was examined using a t-test or linear regression, where appropriate, in unadjusted and adjusted models.
Results:
There were no clinically or statistically significant differences in BMD at the L-spine, total hip or femoral neck between men getting the recommended vs. short sleep duration, using actigraphy or self-reported sleep duration (all p ≥ 0.07). When sleep duration was considered as a continuous variable, femoral neck BMD was higher in men with longer self-reported sleep duration (β=0.006±0.003, p=0.02), but this was not significant after further adjustment. In men with low 25OHD (<20 ng/mL), longer actigraphy-determined sleep duration was associated with higher total hip BMD (β=0.016±0.008; p=0.04). Sleep duration and BTMs were not associated.
Conclusion:
Sleep duration was not associated with hip or L-spine BMD or BTMs in older men. Future research should determine if vitamin D status or other factors modify this relationship.
Keywords: Sleep duration, bone mineral density (BMD) bone turnover markers (BTMs), actigraphy, older men
Mini-Abstract
The associations between objective measures of sleep duration and bone outcomes in older men are unknown. No consistent, significant association was identified between sleep duration and bone mineral density (BMD) in the current analysis. However, future research should determine if vitamin D status modifies this relationship.
Introduction
In the U.S., one in five men over the age of 50 years will experience a fracture[1]. After hip fracture, men have a higher mortality rate than women [2] and many fail to regain functional independence[3]. Although men are more likely than women to have a secondary, underlying cause for osteoporosis identified [4], approximately one third are diagnosed with idiopathic or age-related osteoporosis[4]. Identification of novel risk factors for osteoporosis and fracture could lead to better disease prevention, lower mortality, and healthier, more independent aging.
Shortened sleep duration is prevalent, with approximately one-third of U.S. adults getting less than the recommended amount of sleep per night [5]. Three weeks of sleep restriction combined with circadian disruption (e.g., shift work, jet lag) caused a significant (18-28%) decrease in a bone formation marker, N-terminal propeptide of procollagen type I (P1NP) in men, with no change in the bone resorption marker C-telopeptide of type I collagen (CTX)[6]. In another controlled laboratory study of ten male soldiers exposed to 72 hours of sleep restriction (2 hours of sleep opportunity per night for 3 nights), bone formation marker levels declined after 24 hours and bone resorption markers increased after 48-72 hours [7]. Similar changes in bone turnover markers (BTMs) were observed in men before and after an 8-week U.S. Army Ranger Training Program that included sleep restriction[8]. Over time, these BTM changes (lower levels of bone formation markers and unchanged or higher levels of bone resorption) could lead to bone loss, osteoporosis, and fracture.
Findings from animal studies showed similar BTM changes in response to sleep restriction and subsequent changes in bone mineral density (BMD) and bone microarchitecture. Male rats exposed to 10 days of sleep restriction followed by 2 days of ad libitum sleep repeatedly over 72 days had similar BTM changes as those described above in humans[9]. In that study, the lower levels of bone formation markers and higher levels of bone resorption markers resulted in lower BMD in sleep restricted rats compared to controls[9]. Chronically sleep deprived female rats had lower bone formation marker levels after 1 month and lower bone resorption markers after 3 months[10]. Over time, the chronically sleep restricted rats had lower BMD and poorer bone microarchitecture compared to controls[10].
Evidence that acute BTM changes in response to sleep restriction translate into long-term BMD changes in humans are more difficult to ascertain. Human data are limited to mostly cross-sectional studies showing no association[11,12], or that both short[13–23] and long [13–17,24–30] sleep durations are associated with low BMD (as reviewed in [31]). Of the 20 studies published to date on the association between sleep duration and BMD in humans[11–30], women comprised the majority of participants. All studies of men used subjective (self-reported) sleep duration and focused primarily on middle-aged adults when it is older men who are at highest risk of bone loss and fracture, and potential effect modifiers, such as vitamin D status were not considered. For the current study, we used the Osteoporotic Fractures in Men (MrOS) Study to evaluate the association between bone outcomes (BMD, BTMs) and objectively determined sleep duration, assessed by wrist actigraphy, in older men. For hypothesis generation, we explored whether 25-hydroxyvitamin D (25OHD) levels affected the association between sleep duration and BMD.
Methods:
Study Design and Participant Selection
The study design and cohort characteristics of the Osteoporotic Fractures in Men (MrOS) Study have been previously described [32,33]. In short, 5,994 community dwelling, ambulatory men ≥ 65 years were recruited between March 2000 and April 2002 from 6 clinical sites in the United States (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Monongahela Valley near Pittsburgh, PA; Portland, OR; and San Diego, CA). Men had to be without bilateral hip replacements and able to walk unassisted to participate.
Of the initial 5,994 men recruited for the MrOS study, 3,135 (56% of active survivors, exceeding the recruitment goal) participated in the ancillary MrOS Sleep Study between 2003 and 2005. Actigraphy data were collected on 3,058 men who were asked to wear the wrist device for a minimum of five consecutive 24-hour periods. Actigraphy was typically initiated on the same day of the clinical exam when the Epworth Sleepiness Scale and Pittsburgh Sleep Quality Index (PSQI) were performed, along with other medical questionnaires and clinical assessments including fasted morning serum and urine collection.
As depicted in Figure 1, men were included in the sleep duration/BMD analytical cohort if they had actigraphy data and had concurrent BMD assessments of the total hip, femoral neck, and lumbar spine as previously described[32,34]. Men were excluded from the analysis if they had concurrent medical and/or sleep conditions that could confound the relationship under investigation, specifically, current tobacco use, current excess alcohol intake (≥14 drinks/week), use of oral glucocorticoids or bisphosphonates, Diabetes Mellitus, hyperthyroidism, self-reported sleep apnea and/or chronic kidney disease with eGFR <30 ml/min/1.73m2 (Figure 1). A total of 1,926 men met the eligibility criteria and were included in the analysis between continuous sleep duration and BMD (Figure 1). For the primary analysis that compared BMD between men with short vs. recommended sleep duration, men were subsequently categorized using actigraphy-scored nocturnal sleep time as “short” or “recommended” sleepers if they had <6 hours (N = 577) or 7-8 hours (N = 478) of sleep per night, respectively, according to NIH sleep duration recommendations[35] (Figure 1).
Figure 1:
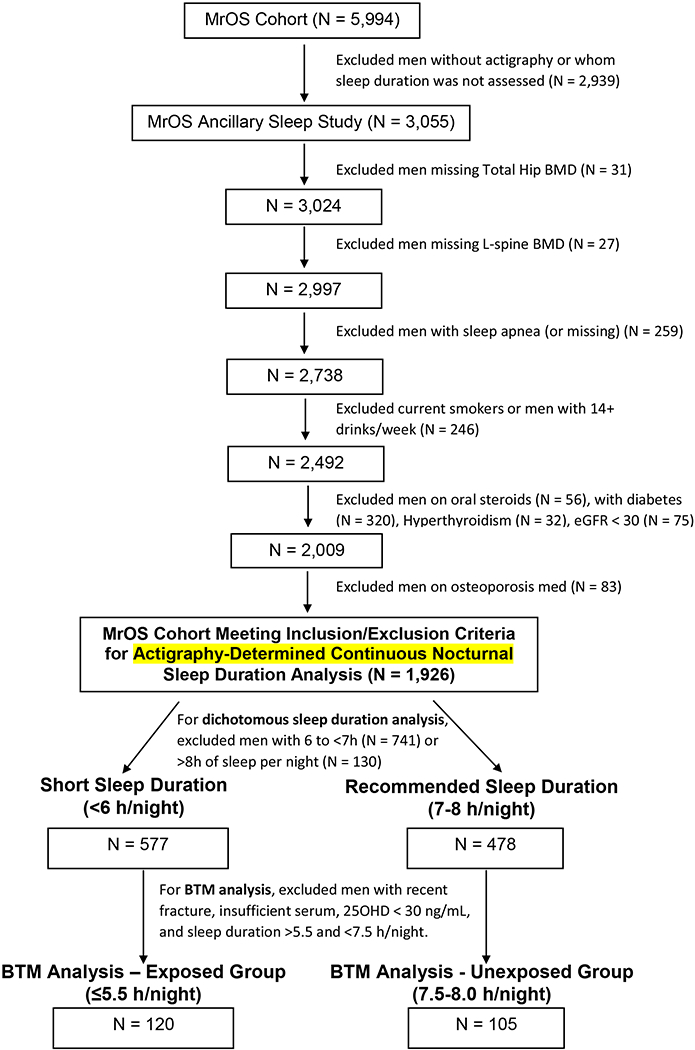
Subject Flow Diagram
A preplanned subgroup of men with sufficient serum was selected to assess bone turnover marker (BTM) levels in those with “short” vs. “recommended” actigraphy-determined sleep duration. For this subgroup analysis, men were excluded if they had a 25OHD level < 30ng/mL and/or a fracture within 3 months of their clinic visit, as this would be expected to alter serum BTM levels (Figure 1). We previously identified no difference in BTM levels in postmenopausal women sleeping <6 vs 7-8 hours/night[24]. Therefore, for the current BTM analysis we chose sleep durations that would create a larger gap between the groups to minimize the risk of misclassification and make the short sleep duration more similar to the sleep restriction imposed in prior intervention studies[6]. BTM levels in men with ≤5.5 hours of sleep per night were compared to those in men getting 7.5-8 hours/night. These cutoffs widened the difference in sleep duration between the two groups without sacrificing sample size. A total of 225 men met criteria for this BTM subgroup analysis including 120 with ≤5.5 hours/night and 105 with 7.5-8 hours/night (Figure 1). CTX and P1NP (see below) were measured on fasted morning serum from these 225 men. All primary and subgroup analyses are described in the statistical analysis.
The Institutional Review Board at each clinical site approved the study, and all participants provided written consent. The current analysis used de-identified serum and data and was deemed non-human subjects research by the Colorado Multiple Institution Review Board.
Wrist Actigraphy (Objective Sleep Duration)
The SleepWatch-O (Ambulatory Monitoring, Inc, Ardsley, NY) was worn on the non-dominant wrist to collect actigraphy data. Clinic staff in charge of collecting actigraphy data underwent centralized training and certification. The San Francisco Coordinating Center (San Francisco, CA) processed the actigraphy data centrally using Action W-2 software to score the data [34]. The University of California, San Diego (UCSD) scoring algorithm [36] was used for data collected in the Proportional Integration Mode (PIM) mode, which calculates a moving average that takes into account the activity levels immediately prior to and after the current minute to determine if each time point should be coded as sleep or wake. The PIM mode was used to assess total sleep time as this mode has been reported to have the best accuracy for older cohorts. The sleep diary was used in editing the data to determine time in and out of bed and time the actigraph was removed. Inter-scorer reliability was high (intra-class coefficient = 0.95) [37] and had good concordance with polysomnography-determined total sleep time [34]. On average, MrOS men wore the actigraph for 5.2 ± 0.9 nights. As a part of MrOS centralized data cleaning, the first day’s data was deleted so participants could get accustomed to wearing the watch. In addition, if the watch was removed for over 10% of the day or for over 2 hours at night, data for that day or night were excluded since their wake/sleep status could not be reliably determined. Actigraphy data were averaged across all included nights to reduce day-to-day variability. Nocturnal sleep durations were primarily used for all analyses. However, daytime naps were included for 24-h sleep duration analyses.
Subjective, Self-Reported Sleep Duration and Other Subjective Sleep Variables
Self-reported sleep duration and amount of sleep needed were ascertained on questionnaire by asking “On most nights, how many hours do you sleep each night?” and “How many hours of sleep do you need each night to feel rested?” Presence of daily naps was established by self-report by asking “Do you take naps regularly?” and, if respondents answered “yes” subsequently asking “How many days per week do you usually nap?”. Nocturia information was obtained by asking “Over the past month, how many times did you most typically get up to urinate from the time you went to bed at night until the time you got up in the morning?”. The Epworth Sleepiness Scale (ESS)[38,39] was performed at the sleep visit to measure daytime sleepiness where a value >10 indicates excessive daytime sleepiness. The Pittsburgh Sleep Quality Index (PSQI) was performed at the sleep visit to measure sleep patterns and problems where a PSQI >5 was used to define poor sleepers.
Bone Mineral Density (BMD) by Dual Energy X-ray Absorptiometry (DXA)
BMD was measured at the proximal femur (total hip, femoral neck) and lumbar spine using DXA (Hologic, Inc., MA)[32]. DXA was performed by certified operators using standardized procedures across all MrOS clinical sites. A central lab was used to ensure quality control, including phantom scans at baseline across all clinical sites.
Falls and Fractures
Falls were determined by self-reported questionnaire that asked “During the past 12 months, have you fallen and landed on the floor or ground, or fallen and hit an object like a table or chair?”. If the participant indicated “yes,” he was asked how many times he had fallen in the last 12 months. Confirmed fractures occurring after the baseline MrOS visit in 2000-2002 but before their MrOS sleep visit in 2003-2005 were included.
Bone Turnover Marker Assays
C-telopeptide of type I collagen (CTX) and intact N-terminal propeptide of procollagen type I (P1NP) were measured in morning, fasted serum and were used to assess bone resorption and formation, respectively. All assays were run at the Colorado Clinical and Translational Sciences Institute (CCTSI) Clinical and Translational Research Center (CTRC) laboratory, which provided inter- and intra-assay coefficients of variation (CV). Samples were run in singleton on serum that had been stored at <−70°C after one previous freeze-thaw cycle. CTX was measured by ELISA (Immunodiagnostics Systems, United Kingdom). Interassay CV was 8.6% at 0.196 ng/mL and 6.4% at 0.406 ng/mL and 5.8% at 2.08 ng/mL. Intra-assay CV was 7.7% at 0.201 ng/mL, 4.6% at 0.446 ng/mL, and 3.4% at 2.05 ng/mL. P1NP was measured by ELISA (Immunodiagnostics Systems, United Kingdom). Interassay CV was 5.6% at 20.36 ng/mL, 3.5% at 51.23 ng/mL and 1.7% at 175.21 ng/mL. Intraassay CV was 4.5% at 2.77 ng/mL, 1.9% at 37.24 ng/mL and 2.0% at 175.84 ng/mL.
25-hydroxyvitamin D (25OHD) and Melatonin
25OHD (both D2 and D3 isoforms) was measured on fasted serum samples by liquid chromatography-tandem mass spectrometry (LC-MS/MS; ThermoFisher Scientific, Franklin, MA and Applied Biosystems-MDS Sciex, Foster City, CA). Total 25OHD was calculated by adding D2 and D3 isoforms. Intra-assay CVs for 25OHD2 were 4.4% at 14 ng/mL, 3.3% at 41 ng/mL and 4.2% at 124 ng/mL and for 25OHD3 were 3.8% at 25 ng/mL, 2.4% at 54 ng/mL and 4.7% at 140 ng/mL. Inter-assay CVs for 25OHD2 were 6.1% at 15 ng/mL, 6.2% at 43 ng/mL and 4.7% at 128 ng/mL and for 25OHD3 were 6.4% at 24 ng/mL, 6.8% at 52 ng/mL and 5.0% at 140 ng/mL.
6-sulphatoxymelatonin (aMT6s), the primary metabolite of melatonin, was measured using the Buhlmann ELISA (ALPCO Diagnostics Windham, NH) on first void morning urine samples at the Oregon Clinical and Translational Research Institute (OCTRI) core laboratory at Oregon Health and Sciences University in June 2010. Samples were refrigerated after collection and then stored at −80°C until assayed. Samples were run in duplicate with the average taken as the final result. aMT6s values were then adjusted for creatinine and are expressed as ng per mg creatinine. Inter- and intra-assay coefficients of variation from pooled controlled were 12.5% and 5.0%, respectively.
Participant Characteristics Including Past Medical History and Medication Use
Race/Ethnicity were self-reported on questionnaire at baseline. Body mass index (BMI) was calculated using height and weight measured at the sleep visit. Standing height was measured using Harpenden Sadiometer (Holtain Ltd., Crymych, Dyfed, UK) and weight using a balance beam scale at all sites except Portland where a digital scale was used. Self-reported alcohol use was asked at the sleep visit. Presence or absence of various medical conditions (osteoporosis, hypertension, COPD) were established by self-reported physician diagnosis. The Geriatric Depression Scale (GDS) was performed at the sleep visit with a GDS ≥ 6 on a 15-point scale used to define depression. Medication use was collected using a medication inventory form. All prescription medications recorded by the clinics were stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA). Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA). The Physical Activity Scale for the Elderly (PASE) questionnaire was administered at the sleep visit and summary score calculated from weights and frequencies for each of the 12 types of activities described in the questionnaire. Participants categorized their overall health status into one of five categories (Excellent, Good, Fair, Poor, or Very Poor). C-reactive protein (CRP) was measured using the ELISA assay kit from ALPCO with inter-assay CV range 11.6-13.8%. Estimated glomerular filtration rate (eGFR) was calculated using the MDRD equation [40] based on serum creatinine measured at the sleep visit using the Roche Modular P chemistry analyzer at the University of Minnesota. The inter-assay CV was 3.7% at 0.82 mg/dL and 2.3% at 3.62 mg/dL.
Statistical Analysis
In the primary analysis, BMD at the lumbar spine, total hip and femoral neck were compared in men with short nocturnal actigraphy-determined sleep duration (<6 hours/night) and those getting the recommended amount of sleep (7-8 hours/night) using a t-test (unadjusted, “Model A”). A regression model was used to assess the effect of nocturnal actigraphy-determined sleep duration (short vs. recommended) on BMD in both minimally adjusted (“Model B”, adjusted for age, race, clinic site, and BMI) and fully adjusted (“Model C”, additionally adjusted for alcohol use, 25OHD level, eGFR, number of hours of sleep needed to feel rested, daily naps, PASE score, overall health, aMT6s level, and use of calcium, androgens, anti-androgens) analyses. Methods for the collection of these additional covariates has been described above and previously[32]. Reference values for covariates in dichotomized analyses were as follows: “excellent” for overall health because it was the highest in ordinal arrangement and was the second largest group; “Caucasian” and “Pittsburgh” for race and clinical site, respectively, because they were the largest (race) and second largest (clinical site) groups in the cohort and to be consistent with prior analyses[24]; “No” for all medication use and naps; continuous covariates were centered on the mean value. An individual was excluded from an analysis if a covariate was missing (<10%), therefore the analyzed sample size is noted for each model. Covariates were selected based on clinical relevance and notable clinical or statistical differences identified in baseline characteristics (Table 1).
Table 1: Baseline characteristics of MrOS Participants by Actigraphy-Determined Nocturnal Sleep Duration Group.
Results presented as N (%) or Mean ± SD as appropriate.
| Short Sleep Duration (<6h/night; N = 577) | Recommended Sleep Duration (7-8h/night; N = 478) | p-value | |
|---|---|---|---|
| Age (years) | 76.7 (5.6) | 76.5 (5.8) | 0.69 |
| Race/Ethnicity | |||
| Caucasian | 518 (89.8%) | 447 (93.5%) | 0.03 |
| African American | 23 (4.0%) | 8 (1.7%) | |
| Asian | 21 (3.6%) | 8 (1.7%) | |
| Hispanic | 13 (2.3%) | 10 (2.1%) | |
| Other | 2 (0.3%) | 5 (1.0%) | |
| Study Site | |||
| Birmingham | 80 (13.9%) | 78 (16.3%) | <0.001 |
| Minneapolis | 107 (18.5%) | 102 (21.3%) | |
| Palo Alto | 71 (12.3%) | 93 (19.5%) | |
| Pittsburgh | 142 (24.6%) | 66 (13.8%) | |
| Portland | 84 (14.6%) | 62 (13.0%) | |
| San Diego | 93 (16.1%) | 77 (16.1%) | |
| BMI (kg/m2) | 27.7 ± 4.0 | 26.3 ± 3.3 | <0.001 |
| Alcohol Drinks/Week | 1.7 ± 1.6 | 2.0 ± 1.6 | 0.008 |
| Osteoporosis | 21 (3.6%) | 15 (3.1%) | 0.66 |
| Calcium Use | 147 (25.5%) | 145 (30.3%) | 0.08 |
| Vitamin D Use | 359 (62.2%) | 310 (64.9%) | 0.38 |
| 25OHD Level (ng/mL) | 27.7 ± 8.4 | 29.7 ± 8.7 | <0.001 |
| Season of Vitamin D level | |||
| Winter | 179 (31.0%) | 162 (33.9%) | 0.04 |
| Spring | 149 (25.8%) | 105 (22.0%) | |
| Summer | 151 (26.2%) | 104 (21.8%) | |
| Fall | 98 (17.0%) | 107 (22.4%) | |
| History of Fracture | 26 (4.5%) | 18 (3.8%) | 0.55 |
| History of Falls | 187 (32.4%) | 129 (27.0%) | 0.06 |
| Hypertension | 267 (46.3%) | 222 (46.4%) | 0.96 |
| COPD | 28 (4.9%) | 15 (3.1%) | 0.16 |
| Depression | 34 (5.9%) | 26 (5.4%) | 0.74 |
| PASE Score | 149 ± 73 | 146 ± 72 | 0.51 |
| Health Quality | |||
| Excellent | 192 (33.3%) | 188 (39.3%) | 0.12 |
| Good | 323 (56.0%) | 236 (49.4%) | |
| Fair | 60 (10.4%) | 49 (10.3%) | |
| Poor | 1 (0.2%) | 4 (0.8%) | |
| Very Poor | 1 (0.2%) | 1 (0.2%) | |
| Testosterone Use | 6 (1.0%) | 3 (0.6%) | 0.47 |
| Androgen Deprivation Therapy Use | 7 (1.2%) | 2 (0.4%) | 0.16 |
| CRP Level (μg/mL) | 3.0 ± 5.1 | 2.6 ± 6.8 | 0.30 |
| eGFR (mL/min/1.73 m2) | 74.3 ± 16.6 | 72.1 ± 15.7 | 0.03 |
| Epworth Sleepiness Scale | 6.7 ± 3.8 | 5.3 ± 3.3 | <0.001 |
| Global PSQI Score | 5.9 ± 3.4 | 5.2 ± 3.2 | <0.001 |
| Taking a Sleep Medication | 71 (12.3%) | 49 (10.3%) | 0.30 |
| Melatonin Use | 7 (1.2%) | 10 (2.1%) | 0.26 |
| aMT6s Level (ng/mg Cr) | 10.2 ± 8.4 | 11.1 ± 8.5 | 0.11 |
| Sleep Duration (hours) | |||
| By Actigraphy | 5.1 ± 0.8 | 7.4 ± 0.3 | <0.001 |
| By Self-Report | 6.5 ± 1.2 | 7.2 ± 1.1 | <0.001 |
| Sleep Duration Needed to Feel Rested (hours) | 6.7 ± 1.1 | 7.3 ± 1.0 | <0.001 |
| Takes Naps Daily | 122 (21.1%) | 45 (9.4%) | <0.001 |
| Nap Duration by Actigraphy (minutes) | 54.9 ± 49.5 | 53.3 ± 51.2 | 0.62 |
| Nocturia | |||
| None | 31 (5.4%) | 21 (4.4%) | 0.54 |
| Once per Night | 170 (29.5%) | 149 (31.2%) | |
| Twice per Night | 193 (33.4%) | 141 (29.5%) | |
| Three or More Times per Night | 183 (31.7%) | 167 (34.9%) | |
As a pre-planned analysis, the association between nocturnal actigraphy-determined sleep duration and BMD was also examined using a linear regression model with sleep duration as a continuous variable to estimate the effect of each additional hour of sleep on BMD in g/cm2 (β). Multivariate regression models were also used to estimate the same effect in both the minimally (Model B) and fully adjusted (Model C) models using the same covariates described above. Visual inspection of sleep duration-BMD data plots indicated nonlinear models were not needed. To confirm, a quadratic term was tested in Model C and was non-significant for all three anatomical sites (all p-values > 0.25). To determine if 25OHD level was an effect modifier, the association between continuous actigraphy-determined nocturnal sleep duration and BMD was also analyzed by 25OHD level (<20 ng/mL, 20-29 ng/mL and ≥30 ng/mL) and an interaction between 25OHD and sleep duration was investigated in Model C (adjusted for season of vitamin D level instead of 25OHD level). Analyses between continuous actigraphy-determined sleep duration (per 1 hour increase) and BMD were repeated using actigraphy-determined 24-h sleep duration (calculated as actigraphy assessed nocturnal sleep duration plus daytime nap duration). To facilitate comparison to prior literature, sleep duration-BMD analyses were also repeated using subjective, self-reported, nocturnal sleep duration as the exposure variable. Finally, the association between nocturnal actigraphy-determined sleep duration and BMD was evaluated by sleep duration quartile using unadjusted ANOVA.
Both CTX and P1NP levels were positively skewed so values were log transformed for all analyses. Bone turnover marker (CTX, P1NP) levels were compared between men with objective, actigraphy-assessed short (≤ 5.5 hours/night) or the recommended (7.5-8 hours/night) sleep duration using an independent t-test. Regression models were used to test the association between these two sleep duration groups and BTM levels, adjusted for BMI and time between usual wake time and time of blood draw to account for any acute circadian disruptions due to the MrOS visit. Regression models were also used to test the association between log transformed CTX and P1NP levels and aMT6s. Pearson correlations were used to determine the associations between aMT6s and actigraphy-determined sleep duration and BMD. p-values < 0.05 were considered statistically significant. All analyses were conducted using SAS Software version 9.4 (Cary, NC).
A priori power calculations indicated that with 53 participants per group there was 80% power to detect a 0.055 g/cm2 difference in BMD (assuming a standard deviation (SD) = 0.1), 85% power to detect a 10 mcg/L difference in P1NP (SD = 17.0), and 80% power to detect a 0.250 ng/mL difference in CTX (SD = 0.448; all with two-sided α = 0.05). Our final, larger, sample size provided the test with 90% power to detect a 7.40 mcg/L difference in P1NP between groups, a 0.195 ng/mL difference in CTX, and a 0.020 g/cm2 difference in BMD, all with the same SD and two-sided α as above.
Results:
Overall, the mean age was 77 years old and ≥90% of men were Caucasian (Table 1). More men had short (<6 h/night) compared to the recommended (7-8 h/night) sleep duration and averaged 5.1 ± 0.8 h/night and 7.4 ± 0.3 h/night of actigraphy measured sleep, respectively. Men with actigraphy-determined short sleep had a higher BMI than men with the recommended sleep duration (27.7 vs. 26.3 kg/m2, p < 0.001) and lower 25-hydroxyvitamin D levels (27.7 ng/mL vs. 29.7 ng/mL, p < 0.001). On average, men with actigraphy-determined short sleep reported requiring less sleep to feel rested than men who got the recommended amount of sleep (6.7. ± 1.1 h/night vs. 7.3 ± 1.0 h/night). Short sleepers tended to over-estimate their sleep duration by more than one hour whereas men who got the recommended amount of actigraphy-determined sleep underestimated their sleep duration by only a few minutes (Table 1). Short sleepers by actigraphy had higher scores on both Epworth sleepiness Scale and global PSQI (both p < 0.001) reflecting more daytime sleepiness and worse sleep quality (Table 1). Significantly more men with short nocturnal actigraphy-determined sleep reported daily naps (21.1% vs. 9.4%, p < 0.001). When naps occurred, the actigraphy-determined duration of the nap was similar between groups at ~54 minutes (p = 0.62). Nocturia was prevalent to a similar extent in both groups (p = 0.54).
There were no clinically or statistically significant differences in BMD at the L-spine, total hip or femoral neck between men getting the recommended vs. short sleep duration in unadjusted and adjusted models, using actigraphy-determined or self-reported nocturnal sleep durations (Table 2, all p ≥ 0.07). When sleep duration was considered as a continuous variable, results were largely unchanged with one exception (Table 3). First, femoral neck BMD was significantly higher with longer self-reported sleep durations in the minimally adjusted model (β = 0.006 ± 0.003 g/cm2 p = 0.02). This relationship was no longer statistically significant in the fully adjusted model (β = 0.002 ± 0.004 g/cm2, p = 0.51). In the actigraphy-determined continuous nocturnal sleep duration cohort (N = 1,926), aMT6s was not correlated with sleep duration (r = 0.04, p = 0.08). aMT6s had a weak, inverse association with BMD, but this was significant only for total hip (r = −0.06, p < 0.01) and not femoral neck (r = −0.04, p = 0.13) or L-spine (r = −0.02, p = 0.32). BMD at the total hip, femoral neck or L-spine did not differ by actigraphy-determined continuous sleep duration quartile (all p ≥ 0.22).
Table 2: BMD (g/cm2) in Older Men with the Recommended (7-8 hours/night) vs. Short (<6 hours/night) Sleep Duration Using Objective (Actigraphy) and Subjective (Self-Report) Sleep Durations.
Data are presented as adjusted means (95%CI).
| Objective Nocturnal Sleep Duration, Determined by Actigraphy | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model A (Unadjusted)a | Model B (Minimally Adjusted)b | Model C (Fully Adjusted)c | |||||||
| Recommended Sleep Duration | Short Sleepers | Difference (Recommended - Short) in g/cm2 | Recommended Sleep Duration | Short Sleepers | Difference (Recommended - Short) in g/cm2 | Recommended Sleep Duration | Short Sleepers | Difference (Recommended - Short) in g/cm2 | |
| N | 478 | 577 | 478 | 577 | 442 | 519 | |||
| L-spine | 1.210 (1.187, 1.233) | 1.214 (1.194, 1.235) | 0.004 p = 0.79 | 1.213 (1.172, 1.253) | 1.204 (1.167, 1.241) | 0.009 p = 0.60 | 1.230 (1.180, 1.280) | 1.229 (1.181, 1.277) | 0.001 p = 0.96 |
| Total Hip | 0.952 (0.940, 0.964) | 0.957 (0.946, 0.968) | 0.005 p = 0.54 | 0.965 (0.946, 0.984) | 0.955 (0.937, 0.973) | 0.010 p = 0.20 | 0.990 (0.966, 1.014) | 0.981 (0.958, 1.004) | 0.008 p = 0.33 |
| Femoral Neck | 0.781 (0.769, 0.792) | 0.781 (0.771, 0.791) | 0.001 p = 0.92 | 0.780 (0.761, 0.799) | 0.770 (0.753, 0.787) | 0.011 p = 0.15 | 0.798 (0.776, 0.820) | 0.791 (0.769, 0.813) | 0.007 p = 0.40 |
| Self-Reported Nocturnal Sleep Duration, Determined by Questionnaire | |||||||||
| Model A (Unadjusted)a | Model B (Minimally Adjusted)b | Model C (Fully Adjusted)c | |||||||
| Recommended Sleep Duration | Short Sleepers | Difference (Recommended - Short) in g/cm2 | Recommended Sleep Duration | Short Sleepers | Difference (Recommended - Short) in g/cm2 | Recommended Sleep Duration | Short Sleepers | Difference (Recommended - Short) in g/cm2 | |
| N | 1191 | 638 | 1191 | 638 | 1099 | 580 | |||
| L-spine | 1.206 (1.191, 1.220) | 1.203 (1.183, 1.223) | −0.003 p = 0.84 | 1.204 (1.175, 1.232) | 1.199 (1.168, 1.231) | 0.004 p = 0.74 | 1.222 (1.186, 1.259) | 1.224 (1.182, 1.266) | −0.002 p = 0.92 |
| Total Hip | 0.954 (0.947, 0.962) | 0.947 (0.936, 0.957) | −0.007 p = 0.26 | 0.958 (0.944, 0.972) | 0.949 (0.934, 0.964) | 0.009 p = 0.13 | 0.980 (0.962, 0.997) | 0.974 (0.953, 0.994) | 0.006 p = 0.42 |
| Femoral Neck | 0.780 (0.773, 0.787) | 0.772 (0.762, 0.781) | −0.008 p = 0.18 | 0.776 (0.763, 0.789) | 0.765 (0.751, 780) | 0.011 p = 0.07 | 0.790 (0.774, 0.807) | 0.786 (0.767, 0.804) | 0.005 p = 0.50 |
Model A = Unadjusted
Model B = Minimally adjusted: age, race, clinical site, and BMI
Model C = Fully adjusted: age, race, clinical site, BMI, alcohol, 25-hydroxyvitamin D level, eGFR, daily naps, hours of sleep needed to feel rested, melatonin level, calcium use, androgen use, anti-androgen use, PASE Score, self-reported health status.
Table 3: Association Between BMD and Sleep Duration (Continuous Variable) Using Objective (Actigraphy; Nocturnal and Total 24-h) and Subjective (Self-Report) Nocturnal Sleep Duration.
Data presented as the difference in BMD (g/cm2) per additional hour of sleep (β) ± standard error of the estimate (SEE), p-value.
| Objective Nocturnal Sleep Duration, Determined by Actigraphy | |||
|---|---|---|---|
| Model A - Unadjusteda (N = 1,926) | Model B - Minimally Adjustedb (N = 1,926) | Model C - Fully Adjustedc (N = 1,764) | |
| L-spine | −0.001 ± 0.005 p = 0.91 | 0.006 ± 0.005 p = 0.26 | 0.007 ± 0.006 p = 0.24 |
| Total Hip | −0.003 ± 0.003 p = 0.22 | 0.003 ± 0.003 p = 0.20 | 0.005 ± 0.003 p = 0.07 |
| Femoral Neck | −0.002 ± 0.002 p = 0.38 | 0.003 ± 0.002 p = 0.24 | 0.003 ± 0.003 p = 0.22 |
| Objective Total 24-h Sleep Duration (including daytime naps), Determined by Actigraphy | |||
| Model A - Unadjusteda (N = 1,926) | Model B – Minimally Adjustedb (N = 1,926) | Model C - Fully Adjustedc (N = 1,764) | |
| L-spine | 0.002 ± 0.004 p = 0.70 | 0.003 ±0.004 p = 0.47 | 0.004 ± 0.004 p = 0.43 |
| Total Hip | −0.002 ± 0.002 p = 0.45 | 0.002 ± 0.002 p = 0.24 | 0.004 ± 0.002 p = 0.06 |
| Femoral Neck | −0.001 ± 0.002 p = 0.71 | 0.002 ± 0.002 p = 0.19 | 0.003 ± 0.002 p = 0.11 |
| Self-Reported Nocturnal Sleep Duration, Determined from Questionnaire | |||
| Model A Unadjusteda (N = 1,829) | Model B Minimally Adjustedb (N = 1,829) | Model C Fully Adjustedc (N = 1,679) | |
| L-spine | 0.004 ± 0.006 p = 0.52 | 0.005 ± 0.006 p = 0.41 | −0.001 ± 0.008 p = 0.88 |
| Total Hip | 0.004 ± 0.003 p = 0.21 | 0.005 ± 0.003 p = 0.06 | 0.003 ± 0.004 p = 0.43 |
| Femoral Neck | 0.004 ± 0.003 p = 0.11 | 0.006 ± 0.003 p = 0.02 | 0.002 ± 0.004 p = 0.51 |
Model A = Unadjusted
Model B = Minimally adjusted: age, race, clinical site, and BMI
Model C = Fully adjusted: age, race, clinical site, BMI, alcohol, 25-hydroxyvitamin D level, eGFR, daily naps, hours of sleep needed to feel rested, melatonin level, calcium use, androgen use, anti-androgen use, PASE Score, self-reported health status.
In an exploratory analysis, the association between BMD and continuous actigraphy-determined nocturnal sleep duration was examined by 25-hydroxyvitamin D level (<20, 20-29 and ≥30 ng/mL) in the fully adjusted model (Table 4). In those with 25OHD < 20 ng/mL (N = 228), total hip BMD was higher with each additional hour of sleep (β= 0.016 ± 0.008 g/cm2; p = 0.04). A similar trend was seen at the L-spine (β = 0.023 ± 0.015 g/cm2; p = 0.12) and femoral neck (β = 0.011 ± 0.007 g/cm2; p = 0.12) but these were not statistically significant. There was no interaction between 25OHD level and actigraphy-determined sleep duration (p = 0.29).
Table 4: Association between actigraphy-determined continuous nocturnal sleep duration and BMD by 25OHD level in fully adjusted model.
Data presented as the difference in BMD (g/cm2) per additional hour of sleep (β) ± SEE (p-value). Adjusted for age, race, clinical site, BMI, alcohol, season of 25-hydroxyvitamin D blood draw, eGFR, daily naps, hours of sleep needed to feel rested, melatonin level, calcium use, androgen use, anti-androgen use, PASE Score, self-reported health status
| 25OHD <20 ng/mL | 25OHD 20-29 ng/mL | 25OHD ≥30 ng/mL | |
|---|---|---|---|
| N (%) | 228 | 744 | 792 |
| L-spine | 0.023 ± 0.015 (p = 0.12) | 0.011 ± 0.009 (p = 0.25) | −0.003 ± 0.008 (p = 0.67) |
| Total Hip | 0.016 ± 0.008 (p = 0.04) | 0.003 ± 0.004 (p = 0.43) | 0.003 ±0.004 (p = 0.44) |
| Femoral Neck | 0.011 ± 0.007 (p = 0.12) | 0.0002 ± 0.004 (p = 0.97) | 0.004 ± 0.004 (p = 0.31) |
In the BTM subgroup analysis, mean P1NP and CTX levels were higher in men who got the recommended actigraphy-determined sleep duration (N = 105) versus those with short actigraphy-determined sleep duration (N = 120; P1NP 49.6 ± 32.0 ng/mL vs. 43.5 ± 16.3 ng/mL; CTX 0.284 ± 0.207 ng/mL vs. 0.231 ± 0.172 ng/mL). However, after adjustment, there were no statistically significant differences between the two sleep duration groups for P1NP (p = 0.35) or CTX (p = 0.08). aMT6s was weakly correlated with log transformed P1NP (r = 0.14, p = 0.04), but not significantly correlated with log transformed CTX (r = 0.08, p = 0.25).
Discussion:
This study represents the first evaluation of the relationship between sleep duration and BMD in older men with objectively-determined sleep duration measured by wrist actigraphy. Findings showed that sleep duration was not associated with BMD or BTMs. This was true when sleep duration was determined objectively by wrist actigraphy or by self-report, and analyzed as a dichotomous variable according to NIH-recommended sleep duration[35] or as a continuous variable, with or without daytime naps. Although a few analyses that used continuous sleep duration had statistically significant results, the clinical significance of those differences is likely minimal. However, in vitamin D deficient men, longer actigraphy-determined nocturnal sleep duration was associated with higher total hip BMD. Although there was no statistically significant interaction between sleep duration and vitamin D, it remains possible that the skeletal effects of insufficient sleep duration are more pronounced in the vitamin D-deficient state because it exacerbates BTM changes in a high bone turnover state. This would be consistent with a prior MrOS report, which found additive effects (e.g., low sex steroid levels and vitamin D deficiency) increased risk for low BMD and bone loss[41].
The current literature on the association between self-reported sleep duration and BMD in men is mixed. Some studies found both long[16,25–27,30] and short[12,15,16,20,23] sleep duration are associated with low BMD, however, some studies did not show any association[11,13,29]. The lack of a significant association between BMD and sleep duration in older men in the current study is similar to findings from three prior studies in middle-aged and older men. An analysis of a similar-aged cohort of 2,438 older men from the AGES-Reykjavik Study[11] identified no association between self-reported sleep duration (including naps) and volumetric BMD. No association was identified between self-reported sleep duration and BMD by calcaneal ultrasound in 3,950 middle aged Chinese men, where 8-9 h/night was used as the reference group[13]. Similarly, no association was identified between self-reported sleep duration and BMD by DXA in approximately 1,400 Korean men (average age 68 years)[29]. The current study was the first to consider vitamin D status when examining the relationship between sleep duration and BMD. Vitamin D levels likely varied between study populations of prior studies based on geography, season of study, etc., and therefore may help to explain different findings in the various cohorts. In other words, significant associations between sleep duration and BMD may only be observed in vitamin D deficient populations. In addition, prior sleep/circadian intervention studies in men suggest the magnitude of effect may be greatest at younger age[6], which may make it harder to detect differences in a cohort with an average age of 76 years. The lack of an association between BMD and sleep duration in this cohort was also consistent with the lack of an association between sleep duration and BTMs in the subgroup analysis (which excluded men with 25OHD < 30 ng/mL). A prior MrOS analysis that included all sleep durations showed that sleep disturbances, including short sleep duration measured by actigraphy (but not self-reported), were associated with an increased risk of falls [42], but no statistically significant association was identified in this cohort.
The current findings were also consistent with those in older women from the Study of Osteoporotic Fractures (SOF)[24], which used objective sleep duration determined by wrist actigraphy, with and without naps. In that study, the only significant association was between longer 24h sleep duration and lower BMD at the total hip [24]. The cohort of older men in the current analysis was younger and more ethnically diverse than the post-menopausal women in SOF[24]. In addition, more women in SOF reported 8+ h/night of sleep than men in MrOS, potentially limiting the ability to detect associations with longer sleep durations. The current cohort was also significantly smaller than the cohort used for the Women’s Health Initiative (WHI) analysis, which identified a risk of lower BMD with shorter sleep duration [43].
In unadjusted analyses, the BTM results in short vs. recommended sleep duration groups were consistent with prior intervention studies of men [6] and male rats [9], which found reduced bone formation marker levels in response to shortened sleep duration. The association between sleep duration and P1NP became non-significant after adjustment for BMI and difference between time of blood draw and wake time. Obesity has been associated with lower BTM levels compared to normal BMI controls[44], and BMI was higher in short sleepers, consistent with prior literature [45]. Urinary aMT6s, which reflects nocturnal melatonin levels [46,47], was weakly correlated with serum P1NP. aMT6s levels correlated with sleep duration in other studies [46,48], possibly due to lower levels of light exposure (and subsequently more melatonin production) with longer sleep duration. Therefore, the correlation between aMT6s and P1NP could also be consistent with shorter sleep duration (i.e., lower aMT6s) being associated with lower P1NP level. Taken together with other studies, it is possible, at least in men, that bone formation may be more affected by short sleep duration than bone resorption. The weak inverse correlation between aMT6s and total hip BMD may be a chance finding since no other significant aMT6s correlations were identified, and is likely of little clinical significance.
The current study was the largest to examine the association between objective sleep duration and BMD in men but had limitations. Despite the relatively large sample size, it was a cross-sectional analysis of a mostly Caucasian cohort and results may not be generalizable to other race/ethnicities. In addition, the analyses may have been underpowered to detect small BMD differences or associations with longer sleep durations since a relatively small number of men (N = 130) slept 8+ h/night. For example, the trend towards higher total hip BMD with longer actigraphy-determined 24-h and nocturnal sleep duration, and the higher percentage of short sleepers reporting falls and fractures, may suggest a weak relationship that we were underpowered to detect. Sex steroid levels were not available to determine if and how they affect the sleep-bone relationship. However, exogenous testosterone use in this cohort was low (~1%) and similar between short and recommended sleepers. The BTM subgroup analysis may have included some men with residual BTM elevation after sustaining a fracture >3 months prior to the blood draw. If this did occur, the effect was likely minimal and unlikely to materially change the results as the overall number of fractures was low and men were also excluded for osteoporosis medication use, which may have excluded those with more distant fractures who were subsequently started on pharmacotherapy. Furthermore, the high prevalence of nocturia in the cohort and potential underreporting or underdiagnosis of sleep apnea may have adversely affected the quality of sleep, regardless of the quantity of sleep, thereby limiting our ability to detect consequences of short sleep duration. In addition, other aspects of sleep (e.g., longer sleep latency) or nocturia causing wake after sleep onset may have led to misclassification of actigraphy-determined sleep duration. However, results were similar when analyzed by self-reported sleep duration.
In conclusion, sleep duration, determined objectively with wrist actigraphy or by self-report, was not associated with BMD in older men. However, in vitamin D deficient men, longer sleep duration was associated with higher total hip BMD. This report was the first sleep-BMD study to use objectively determined sleep duration in men, and contributes to the growing body of literature showing mixed associations between BMD and sleep duration. Future studies should determine if vitamin D status plays a role in the sleep duration-BMD relationship.
Acknowledgements:
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 1K23AR070275, the Eastern Colorado VA Geriatric, Research, Education, and Clinical Center (GRECC), and the funding sources for the MrOS and MrOS Sleep Studies (as below). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. This research is also supported by NIH/NCATS Oregon Health and Science University CTSA Grant Number UL1 TR000128 and NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The Osteoporotic Fractures in Men (MrOS) Study is supported by NIH funding via the following institutes: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research, under the following grant numbers: U01AG027810, U01AG042124, U01AG042139, U01AG042140, U01 AG042143, U01 AG042145, U01 AG042168, and U01 AR066160.
The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. The National Institute on Aging (NIA) provided funding for Vitamin D and melatonin assays under R01 AG030089
CMS was supported by NIH grant T32DK007674-20, NIH grant T32DK007446-34, and NIH grant K23AR070275.
SR was partially supported by NIH HL R35 135818.
KPW received support from NIH grants R01 HL135598, R01 HL131458, R01 HD087707, R01 DK114272, R01 DK115502, U01 HL150596, a Pac-12 Grant Application, and ONR MURI N00014-15-1-2809 during the time of this research.
Funding: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 1K23AR070275, the Eastern Colorado VA Geriatric, Research, Education, and Clinical Center (GRECC), and the funding sources for the MrOS and MrOS Sleep Studies (as detailed in acknowledgements). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. This research is also supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. The National Institute on Aging (NIA) provided funding for Vitamin D and melatonin assays under R01 AG030089. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- (BMD)
Bone mineral density
- (CTX)
C-telopeptide of Type I Collagen
- (P1NP)
N-terminal propeptide of procollagen type I
- (BTMs)
bone turnover markers
- (PSQI)
Pittsburgh Sleep Quality Index
- (PSG)
polysomnography
- (DXA)
dual energy x-ray absorptiometry
- (MrOS) Study
Osteoporotic Fractures in Men
- (25OHD)
25-hydroxyvitamin D
- (CV)
coefficient of variation
- (SD)
standard deviation
- (SEE)
standard error of the estimate
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Disclosure Statement:
In the interest of full disclosure, we report the following, however, we do not believe any of these pertain to the current work.
CMS, PJB, JAC, NEL, TSRS, DCB, MEW, WMK have nothing to disclose KLS has received grant funding from Merck.
SR has received consulting fees from Jazz Pharma, Respircardia and Eisa Inc and grant support from Jazz Pharma (unrelated to this paper)
KPW reports research support from the NIH, Office of Naval Research, Pac-12; Financial relationships: consulting fees Circadian Therapeutics, LTD., Circadian Biotherapies, Philips Respironics. Board of Directors: Sleep Research Society.
ESO has received research support from or consulting for Amgen, Mereo and Bayer.
Conflicts of Interest: CM Swanson, PJ Blatchford, KL Stone, JA Cauley, NE Lane, TS Rogers-Soeder, S Redline, DC Bauer, KP Wright, ME Wierman, WM Kohrt and ES Orwoll declare they have no conflicts of interest pertaining to the current work. However, in the interest of full disclosure, we have made a detailed disclosure statement below.
Ethics Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Institutional Review Board at each clinical site approved the study, and all participants provided written consent. The current analysis used de-identified serum and data and was deemed non-human subjects research by the Colorado Multiple Institution Review Board.
Availability of Data and Material: Data from the MrOS study are available online https://mrosdata.sfcc-cpmc.net/ after registration and acceptance of the Data Use Agreement.
Code Availability: The SAS Code used to generate these results are not publicly available but are available from the corresponding author on reasonable request.
REFERENCES
- 1.Foundation IO What is Osteoporosis? International Osteoporosis Foundation. https://www.iofbonehealth.org/what-is-osteoporosis. Accessed February 7, 2020 2020 [Google Scholar]
- 2.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR (2009) Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. Jama 301 (5):513–521. doi: 10.1001/jama.2009.50 [DOI] [PubMed] [Google Scholar]
- 3.Wolinsky FD, Fitzgerald JF, Stump TE (1997) The effect of hip fracture on mortality, hospitalization, and functional status: a prospective study. American journal of public health 87 (3):398–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudec SM, Camacho PM (2013) Secondary causes of osteoporosis. Endocr Pract 19 (1):120–128. doi: 10.4158/EP12059.RA [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB (2016) Prevalence of Healthy Sleep Duration among Adults - United States, 2014. MMWR Morbidity and mortality weekly report 65 (6):137–141. doi: 10.15585/mmwr.mm6506a1 [DOI] [PubMed] [Google Scholar]
- 6.Swanson C, Shea SA, Wolfe P, Cain SW, Munch M, Vujovic N, Czeisler CA, Buxton OM, Orwoll ES (2017) Bone Turnover Markers After Sleep Restriction and Circadian Disruption: A Mechanism for Sleep-Related Bone Loss in Humans. The Journal of clinical endocrinology and metabolism 102:3722–3730. doi: 10.1210/jc.2017-01147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staab JS, Smith TJ, Wilson M, Montain SJ, Gaffney-Stomberg E (2019) Bone turnover is altered during 72 h of sleep restriction: a controlled laboratory study. Endocrine 65 (1):192–199. doi: 10.1007/s12020-019-01937-6 [DOI] [PubMed] [Google Scholar]
- 8.Hughes JM, Smith MA, Henning PC, Scofield DE, Spiering BA, Staab JS, Hydren JR, Nindl BC, Matheny RW Jr. (2014) Bone formation is suppressed with multi-stressor military training. European journal of applied physiology. doi: 10.1007/s00421-014-2950-6 [DOI] [PubMed] [Google Scholar]
- 9.Everson CA, Folley AE, Toth JM (2012) Chronically inadequate sleep results in abnormal bone formation and abnormal bone marrow in rats. Experimental biology and medicine 237 (9):1101–1109. doi: 10.1258/ebm.2012.012043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X, Wang L, Chen L, Su T, Zhang Y, Wang T, Ma W, Yang F, Zhai W, Xie Y, Li D, Chen Q, Fu X, Ma Y, Zhang Y (2016) Effects of chronic sleep deprivation on bone mass and bone metabolism in rats. J Orthop Surg Res 11 (1):87. doi: 10.1186/s13018-016-0418-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marques EA, Figueiredo P, Gudnason V, Lang T, Sigurdsson G, Sigurdsson S, Aspelund T, Siggeirsdottir K, Launer L, Eiriksdottir G, Harris TB (2017) Associations of 24-hour sleep duration and CT-derived measurements of muscle and bone: The AGES-Reykjavik Study. Exp Gerontol 93:1–6. doi: 10.1016/j.exger.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucassen EA, de Mutsert R, le Cessie S, Appelman-Dijkstra NM, Rosendaal FR, van Heemst D, den Heijer M, Biermasz NR, group NEOs (2017) Poor sleep quality and later sleep timing are risk factors for osteopenia and sarcopenia in middle-aged men and women: The NEO study. PloS one 12 (5):e0176685. doi: 10.1371/journal.pone.0176685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G, Chen L, Wen J, Yao J, Li L, Lin L, Tang K, Huang H, Liang J, Lin W, Chen H, Li M, Gong X, Peng S, Lu J, Bi Y, Ning G (2014) Associations between sleep duration, daytime nap duration, and osteoporosis vary by sex, menopause, and sleep quality. The Journal of clinical endocrinology and metabolism 99 (8):2869–2877. doi: 10.1210/jc.2013-3629 [DOI] [PubMed] [Google Scholar]
- 14.Wang K, Wu Y, Yang Y, Chen J, Zhang D, Hu Y, Liu Z, Xu J, Shen Q, Zhang N, Mao X, Liu C (2015) The associations of bedtime, nocturnal, and daytime sleep duration with bone mineral density in pre- and post-menopausal women. Endocrine 49 (2):538–548. doi: 10.1007/s12020-014-0493-6 [DOI] [PubMed] [Google Scholar]
- 15.Cunningham TD, Di Pace BS (2015) Is Self-Reported Sleep Duration Associated with Osteoporosis? Data from a 4-Year Aggregated Analysis from the National Health and Nutrition Examination Survey. Journal of the American Geriatrics Society 63 (7):1401–1406. doi: 10.1111/jgs.13477 [DOI] [PubMed] [Google Scholar]
- 16.Lima MG, Bergamo Francisco PM, de Azevedo Barros MB (2012) Sleep duration pattern and chronic diseases in Brazilian adults (ISACAMP, 2008/09). Sleep medicine 13 (2):139–144. doi: 10.1016/j.sleep.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 17.Wang D, Ruan W, Peng Y, Li W (2018) Sleep duration and the risk of osteoporosis among middle-aged and elderly adults: a dose-response meta-analysis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 29 (8):1689–1695. doi: 10.1007/s00198-018-4487-8 [DOI] [PubMed] [Google Scholar]
- 18.Specker BL, Binkley T, Vukovich M, Beare T (2007) Volumetric bone mineral density and bone size in sleep-deprived individuals. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 18 (1):93–99. doi: 10.1007/s00198-006-0207-x [DOI] [PubMed] [Google Scholar]
- 19.Fu X, Zhao X, Lu H, Jiang F, Ma X, Zhu S (2011) Association between sleep duration and bone mineral density in Chinese women. Bone 49 (5):1062–1066. doi: 10.1016/j.bone.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 20.Kuriyama N, Inaba M, Ozaki E, Yoneda Y, Matsui D, Hashiguchi K, Koyama T, Iwai K, Watanabe I, Tanaka R, Omichi C, Mizuno S, Kurokawa M, Horii M, Niwa F, Iwasa K, Yamada S, Watanabe Y (2017) Association between loss of bone mass due to short sleep and leptin-sympathetic nervous system activity. Arch Gerontol Geriatr 70:201–208. doi: 10.1016/j.archger.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 21.Casazza K, Hanks LJ, Fernandez JR (2011) Shorter sleep may be a risk factor for impaired bone mass accrual in childhood. J Clin Densitom 14 (4):453–457. doi: 10.1016/j.jocd.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochs-Balcom HM, Hovey KM, Andrews C, Cauley JA, Hale L, Li W, Bea JW, Sarto GE, Stefanick ML, Stone KL, Watts NB, Zaslavsky O, Wactawski-Wende J (2020) Short Sleep Is Associated With Low Bone Mineral Density and Osteoporosis in the Women’s Health Initiative. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 35 (2):261–268. doi: 10.1002/jbmr.3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foley D, Ancoli-Israel S, Britz P, Walsh J (2004) Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. Journal of psychosomatic research 56 (5):497–502. doi: 10.1016/j.jpsychores.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 24.Swanson CM, Blatchford PJ, Orwoll ES, Cauley JA, LeBlanc ES, Fink HA, Wright KP Jr., Wierman ME, Kohrt WM, Stone KL, Study of Osteoporotic F (2019) Association between objective sleep duration and bone mineral density in older postmenopausal women from the Study of Osteoporotic Fractures (SOF). Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 30 (10):2087–2098. doi: 10.1007/s00198-019-05007-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T (2012) Association between osteoporosis and sleep duration in healthy middle-aged and elderly adults: a large-scale, cross-sectional study in Japan. Sleep & breathing = Schlaf & Atmung 16 (2):579–583. doi: 10.1007/s11325-011-0545-6 [DOI] [PubMed] [Google Scholar]
- 26.Niu J, Sahni S, Liao S, Tucker KL, Dawson-Hughes B, Gao X (2015) Association between Sleep Duration, Insomnia Symptoms and Bone Mineral Density in Older Boston Puerto Rican Adults. PloS one 10 (7):e0132342. doi: 10.1371/journal.pone.0132342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian Y, Shen L, Wu J, Xu G, Yang S, Song L, Zhang Y, Mandiwa C, Yang H, Liang Y, Wang Y (2015) Sleep duration and timing in relation to osteoporosis in an elderly Chinese population: a cross-sectional analysis in the Dongfeng-Tongji cohort study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 26 (11):2641–2648. doi: 10.1007/s00198-015-3172-4 [DOI] [PubMed] [Google Scholar]
- 28.Moradi S, Shab-Bidar S, Alizadeh S, Djafarian K (2017) Association between sleep duration and osteoporosis risk in middle-aged and elderly women: A systematic review and meta-analysis of observational studies. Metabolism 69:199–206. doi: 10.1016/j.metabol.2017.01.027 [DOI] [PubMed] [Google Scholar]
- 29.Kim N, Choi HR, Kim SW, Kim BS, Won CW, Kim SY (2014) Association between Bone Mineral Density and Sleep Duration in the Korean Elderly Population. Korean J Fam Med 35 (2):90–97. doi: 10.4082/kjfm.2014.35.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saint Martin M, Labeix P, Garet M, Thomas T, Barthelemy JC, Collet P, Roche F, Sforza E (2016) Does Subjective Sleep Affect Bone Mineral Density in Older People with Minimal Health Disorders? The PROOF Cohort. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 12 (11):1461–1469. doi: 10.5664/jcsm.6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanson CM, Kohrt WM, Buxton OM, Everson CA, Wright KP Jr., Orwoll ES, Shea SA (2018) The importance of the circadian system & sleep for bone health. Metabolism 84:28–43. doi: 10.1016/j.metabol.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K (2005) Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26 (5):569–585. doi: 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 33.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR (2005) Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26 (5):557–568. doi: 10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 34.Blackwell T, Ancoli-Israel S, Redline S, Stone KL, Osteoporotic Fractures in Men Study G (2011) Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 7 (4):357–367. doi: 10.5664/JCSM.1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Explore Sleep Deprivation and Deficiency: How Much Sleep Is Enough? (2012) National Heart, Lung, and Blood Institute. http://www.nhlbi.nih.gov/health/health-topics/topics/sdd/howmuch. [Google Scholar]
- 36.Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD (2001) Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods 105 (2):185–191 [DOI] [PubMed] [Google Scholar]
- 37.Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL (2005) Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep 28 (12):1599–1605 [DOI] [PubMed] [Google Scholar]
- 38.Johns MW (2000) Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. Journal of sleep research 9 (1):5–11 [DOI] [PubMed] [Google Scholar]
- 39.Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14 (6):540–545 [DOI] [PubMed] [Google Scholar]
- 40.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology C (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of internal medicine 145 (4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 41.Barrett-Connor E, Laughlin GA, Li H, Nielson CM, Wang PY, Dam TT, Cauley JA, Ensrud KE, Stefanick ML, Lau E, Hoffman AR, Orwoll ES, Osteoporotic Fractures in Men Research G (2012) The association of concurrent vitamin D and sex hormone deficiency with bone loss and fracture risk in older men: the osteoporotic fractures in men (MrOS) study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 27 (11):2306–2313. doi: 10.1002/jbmr.1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone KL, Blackwell TL, Ancoli-Israel S, Cauley JA, Redline S, Marshall LM, Ensrud KE, Osteoporotic Fractures in Men Study G (2014) Sleep disturbances and risk of falls in older community-dwelling men: the outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. Journal of the American Geriatrics Society 62 (2):299–305. doi: 10.1111/jgs.12649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cauley JA, Hovey KM, Stone KL, Andrews CA, Barbour KE, Hale L, Jackson RD, Johnson KC, LeBlanc ES, Li W, Zaslavsky O, Ochs-Balcom H, Wactawski-Wende J, Crandall CJ (2019) Characteristics of Self-Reported Sleep and the Risk of Falls and Fractures: The Women’s Health Initiative (WHI). Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 34 (3):464–474. doi: 10.1002/jbmr.3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maimoun L, Garnero P, Mura T, Nocca D, Lefebvre P, Philibert P, Seneque M, Gaspari L, Vauchot F, Courtet P, Sultan A, Piketty ML, Sultan C, Renard E, Guillaume S, Mariano-Goulart D (2020) Specific Effects of Anorexia Nervosa and Obesity on Bone Mineral Density and Bone Turnover in Young Women. The Journal of clinical endocrinology and metabolism 105 (4). doi: 10.1210/clinem/dgz259 [DOI] [PubMed] [Google Scholar]
- 45.Bjorvatn B, Sagen IM, Oyane N, Waage S, Fetveit A, Pallesen S, Ursin R (2007) The association between sleep duration, body mass index and metabolic measures in the Hordaland Health Study. Journal of sleep research 16 (1):66–76. doi: 10.1111/j.1365-2869.2007.00569.x [DOI] [PubMed] [Google Scholar]
- 46.Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB (2006) A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res 66 (10):5521–5525. doi: 10.1158/0008-5472.CAN-05-4652 [DOI] [PubMed] [Google Scholar]
- 47.Graham C, Cook MR, Kavet R, Sastre A, Smith DK (1998) Prediction of nocturnal plasma melatonin from morning urinary measures. Journal of pineal research 24 (4):230–238. doi: 10.1111/j.1600-079x.1998.tb00538.x [DOI] [PubMed] [Google Scholar]
- 48.Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA (2003) A longer biological night in long sleepers than in short sleepers. The Journal of clinical endocrinology and metabolism 88 (1):26–30. doi: 10.1210/jc.2002-020827 [DOI] [PubMed] [Google Scholar]


