Abstract
Objective:
Determine the impact of HIV-1 seroconversion on inflammatory cytokines in the rectal mucosa.
Setting:
Secondary analysis of data from men who have sex with men (MSM) and transgender women who participated in a HIV prevention trial Lima, Peru
Methods:
From July to December 2017, 605 MSM and transgender women were screened for rectal gonorrhea/chlamydia (GC/CT). 50 GC/CT positive cases were randomly selected and matched with 52 GC/CT negative controls by age and number of receptive anal intercourse (RAI) partners in last month. All participants were HIV-negative at baseline and those with GC/CT at baseline and/or follow-up received appropriate antibiotic therapy. Participants underwent sponge collection of rectal secretions for measurement of inflammatory cytokines (IL-1β, IL-6, IL-8, and TNF-α) and were screened for rectal GC/CT and HIV at baseline, 3-months, and 6-months. Wilcoxon Rank-Sum tests compared inflammatory cytokine levels between participants diagnosed with HIV during follow-up compared to persons who remained HIV-negative.
Results:
Eight participants were diagnosed with HIV at the 3-month (n=6) or 6-month (n=2) visit. Median number of RAI partners in the month prior to HIV diagnosis was the same for those who acquired HIV and those who did not. There were no significant differences in inflammatory cytokine levels in rectal mucosa between participants who did and did not experience HIV seroconversion at any time point.
Conclusions:
Despite a surge in viral replication during acute infection, findings from this study suggest that there is no prolonged effect of HIV-1 seroconversion on inflammatory cytokine levels in the rectal mucosa.
Keywords: HIV seroconversion, rectal inflammation, rectal cytokines, MSM, Latin America, Peru
INTRODUCTION:
Men who have sex with men (MSM) and transgender women (TW) are disproportionately affected by the HIV and sexually transmitted infection (STI) epidemics1. Rectal Neisseria gonorrhoeae and/or Chlamydia trachomatis (GC/CT) are of particular public health importance as they are independently associated with HIV seroconversion, a relationship which is driven by rectal inflammation caused by rectal GC/CT infection2. Mucosal inflammation increases risk for HIV seroconversion through decreased mucosal barrier integrity and recruitment of HIV-susceptible cells, such as T-lymphocytes and macrophages3,4. Additionally, mucosal inflammation caused by STIs has been associated with increased viral shedding among individuals with HIV, independent of systemic HIV viral load5. With regard to the rectal compartment, these risks are potentiated by abundant levels of activated CD4+ T-lymphocytes, key target cells for HIV infection, that exist in the rectal mucosa and contribute to ongoing HIV transmission among MSM through condomless receptive anal intercourse (RAI)4.
While rectal mucosal inflammation is associated with increased risk of HIV transmission, the effects of HIV infection on mucosal inflammation remain largely unexplored. Given the surge in HIV replication associated with acute HIV infection, rectal inflammation in the setting of HIV seroconversion may represent an important driver of HIV transmission among serodiscordant partners. Cross-sectional studies have determined that chronic HIV infection does not result in elevated mucosal cytokine levels, regardless of HIV systemic viral load or antiretroviral therapy use6. However, the impact of acute HIV seroconversion on rectal cytokine levels remains unknown. To address this gap, we performed a secondary analysis of MSM and TW who participated in an intervention using rectal STI screening and treatment for HIV prevention in Lima, Peru to evaluate the association of HIV seroconversion on cytokine levels in the rectal mucosa.
METHODS:
Participants and Recruitment
We conducted a secondary analysis of MSM and TW who participated in an intervention using rectal STI screening and treatment for HIV prevention in Lima, Peru. Participants were recruited from community venues by peer recruiters at Via Libre, a community-based organization that provides integrated sexual health services in Lima, Peru, from July to December 2017. Enrollment was limited to individuals who: 1) were ≥18 years old, 2) were assigned male sex at birth, 3) reported condomless RAI with an HIV-infected or unknown serostatus partner in the previous 3 months, and 4) were HIV-uninfected according to 4th generation rapid test. At the screening visit, participants underwent testing for rectal GC/CT, syphilis and HIV by 4th generation rapid testing. Of the 469 HIV-uninfected participants who underwent screening, 101 had rectal GC/CT, from whom 50 GC/CT positive cases were randomly selected, along with 52 GC/CT negative controls that were matched according to age and number of RAI partners during the last 30 days. Participants were matched for RAI as condomless RAI has been demonstrated to cause an increase in pro-inflammatory cytokines within the rectal mucosa for up to 24 hours7. Participants completed behavioral surveys, HIV/STI screening, and collection of rectal samples every 3 months (baseline, 3 and 6 months).
Study Procedures
All participants completed a computer-assisted self-interview at each visit, which included age, number of RAI partners in the last month, and condom use. At baseline, 3 and 6 months, participants were screened for rectal GC/CT, syphilis, and HIV and underwent physical examination for signs of symptomatic urethritis or proctitis. Rectal swabs were collected and tested for GC/CT infection with nucleic acid amplification testing (NAAT) using the Gen-Probe Aptima II assay (Hologic, San Diego, CA, USA). Blood was collected to test for syphilis by rapid plasma reagin (RPR) assay (RPRnosticon, Biomérieux, Marcy l’Etoile, France) with Treponema pallidum particle agglutination assay (TPPA) confirmation (Serodia TPPA, Fujirebio, Malvern, PA, USA). For the purpose of this analysis, RPR titers ≥16 were considered consistent with current syphilis infection and included in our findings. HIV screening was conducted on whole blood with rapid 4th generation testing (Alere Determine, Abbott, Chicago, IL, USA). Participants with clinically symptomatic urethritis, proctitis or with positive GC/CT NAAT were treated with ceftriaxone 250mg intramuscular injection and azithromycin 1g orally, according to CDC Guidelines8. All participants were provided with testing results within two weeks of result. Participants with syphilis received treatment according to the stage of their infection following physician review of previous RPR titers and treatment history. Participants were compensated 15 Nuevos soles (US $5) for screening, 25 Nuevos soles ($8) at enrollment, 35 Nuevos soles ($12) for 3-month follow-up, and 45 Nuevos soles ($15) for 6-month follow-up.
Rectal Sponge Collection and Cytokine Quantification
Rectal samples were collected at baseline, 3 and 6 months for cytokine analysis. Rectal secretions were collected using sterile polyvinyl acetate sponges (Merocel, Beaver Visitec, Waltham, MA, USA) introduced into the rectum via anoscopy and held against the rectal mucosa under direct visualization for 120 seconds9,10. Sponges were stored at −80°C until processing. For processing, sponges were thawed on ice, and sponge tips were transferred to a 2-mL Spin-X column (Corning, Corning, NY, USA) from which the acetate membrane was removed to prevent protein binding. Rectal secretions were eluted twice with 250μL of cold elution buffer [PBS containing 0.25% bovine serum albumin, 1% Igepal (Sigma Chemicals), and protease inhibitor cocktail (Sigma Chemicals)] by centrifugation (10,000 rpm for 30 minutes at 4°C). Interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α were then measured using a custom Milliplex High-Sensitivity multiplex panel (MilliporeSigma, Burlington, MA, USA) according to manufacturer’s instructions. Samples were run in duplicate and repeated if coefficient of variation (%CV) >25%.
Consent/Permissions
Written informed consent was obtained from all participants prior to enrollment. The study protocol was reviewed and approved by the Institutional Review Boards of the University of California, Los Angeles and Asociación Civil Via Libre. This study was registered on clinicaltrials.gov (NCT03010020).
Data Analysis
Bivariate analyses, with Fisher’s exact and Kruskal-Wallis tests when appropriate, were used to compare participant characteristics and sexual behaviors between individuals diagnosed with HIV during follow-up and those who remained HIV-negative. Wilcoxon Rank-Sum tests compared inflammatory cytokine concentrations between persons diagnosed with HIV during follow-up and those who remained HIV-negative. All analyses were conducted using Stata 15.0 (StataCorp, College Town, TX, USA). By completion of the study, 6 HIV-negative participants and 1 participant who experienced HIV seroconversion were lost to follow-up. Less than 5% of data were missing for any single variable.
RESULTS:
Median age of participants was 24 years (IQR 21–29). Median number of last month RAI partners was 4 (IQR 2–7; range 0–20). At 3 months, 6 participants experienced HIV seroconversion, followed by an additional 2 participants at 6 months, resulting in a total of 8 individuals who experienced HIV seroconversion by study completion. No differences were observed in baseline age or number of last month RAI partners between participants who experienced HIV seroconversion compared to those who remained HIV-negative (Table 1). At baseline, 75% (6/8) of participants who experienced seroconversion had rectal GC/CT infection, compared to 43.6% (41/94) of HIV-negative participants (p=0.14). Among participants who experienced HIV seroconversion, one participant (12.5%) had recurrent GC/CT at 3 months. Among HIV-negative participants, 15.7% (14/89) and 10.2% (9/88) had recurrent GC/CT at 3 and 6 months, respectively. Median number of RAI partners in the month prior to HIV diagnosis was 2 for both those with HIV seroconversion (IQR 0.5–3; range 0–4) and participants who remained HIV-negative (IQR 1–3; range 0–10; p=0.79). In the month prior to HIV diagnosis, the median number of condomless RAI partners was 1 for both HIV seroconverters (IQR 0–1.5; range 0–4) and those who remained HIV-negative (IQR 0–2; range 0–10; p= 0.93).
Table 1:
Participant characteristics and sexual risk behaviors among men who have sex with men and transgender women stratified by HIV-1 seroconversion; Lima, Peru 2018–19 (N=102)
| HIV-1 Seroconverters | HIV-1 Uninfected Controls | p-value† | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Baseline | n=8 | n=94 | |||||
| Age‡ | 25 (22–31) | 24 (21–29) | 0.62 | ||||
| Gender | |||||||
| Cisgender male | 6 | 75 | 76 | 80.9 | 0.69 | ||
| Transgender female | 2 | 25 | 18 | 19.2 | |||
| Rectal GC/CT | |||||||
| Negative | 2 | 25 | 53 | 56.4 | 0.14 | ||
| Positive | 6 | 75 | 41 | 43.6 | |||
| Syphilis | |||||||
| Negative | 7 | 87.5 | 91 | 96.8 | 0.28 | ||
| Positive | 1 | 12.5 | 3 | 3.2 | |||
| Sexual partners last month‡ | 4.5 (2.5–5.5) | 6 (2.5–10) | 0.39 | ||||
| RAI partners last month‡ | 4.5 (2.5–8) | 4 (2–7) | 0.71 | ||||
| CRAI partners last month‡ | 2.5 (1–4) | 3 (1–4) | 0.93 | ||||
| 3-month follow-up | n=8 | n=89 | |||||
| Rectal GC/CT | |||||||
| Negative | 7 | 87.5 | 75 | 84.3 | 1.00 | ||
| Positive | 1 | 12.5 | 14 | 15.7 | |||
| Sexual partners‡ | 3 (0.5–3.5) | 2 (1–4) | 0.69 | ||||
| RAI partners last month‡ | 2 (0.5–3) | 2 (1–3) | 0.79 | ||||
| CRAI partners last month‡ | 0.5 (0–1.5) | 1 (0–2) | 0.71 | ||||
| 6-month follow-up | n=7 | n=88 | |||||
| Rectal GC/CT | |||||||
| Negative | 7 | 100 | 79 | 89.8 | 0.49 | ||
| Positive | 0 | 0 | 9 | 10.2 | |||
| Sexual partners last month‡ | 3 (0–4) | 2 (1–5) | 0.94 | ||||
| RAI partners last month‡ | 3 (0–4) | 1 (0.5–3) | 0.72 | ||||
| CRAI partners last month‡ | 0 (0–1) | 0 (0–1) | 0.29 | ||||
| Month prior to seroconversion | n=8 | n=88 | |||||
| Sexual partners‡ | 3 (0.5–3.5) | 2 (1–4) | 0.69 | ||||
| RAI partners‡ | 2 (0.5–3) | 2 (1–3) | 0.79 | ||||
| CRAI partners‡ | 1 (0–1.5) | 1 (0–2) | 0.93 | ||||
Calculated using Fisher’s exact or Kruskal-Wallis tests
Median (interquartile range)
GC/CT = gonorrhea/chlamydia infection; RAI = receptive anal intercourse; CRAI = condomless receptive anal intercourse
At baseline, no differences were observed in median rectal mucosal cytokine concentrations for all cytokines measured (IL-1β, IL-6, IL-8, and TNF-α) between participants who experienced HIV seroconversion and those who remained HIV-negative (Figure 1). At 3 months, where most participants (6/8) were diagnosed with HIV infection, there were no differences in median rectal mucosal cytokines (IL-1β, IL-6, IL-8, and TNF-α) between those who seroconverted and participants who remained HIV-negative. No differences in median rectal mucosal cytokines were observed between HIV seroconverters and those who remained HIV-negative at 6 months. Median and mean rectal mucosal cytokine concentrations are in Supplemental Table 1. Rectal mucosal cytokine levels for participants who experienced HIV seroconversion are in Supplemental Table 2.
Figure 1: Median rectal mucosal cytokine levels of men who have sex with men who experienced HIV-1 seroconversion compared to men who have sex with men and transgender women who remained HIV-negative; Lima, Peru 2018–19.
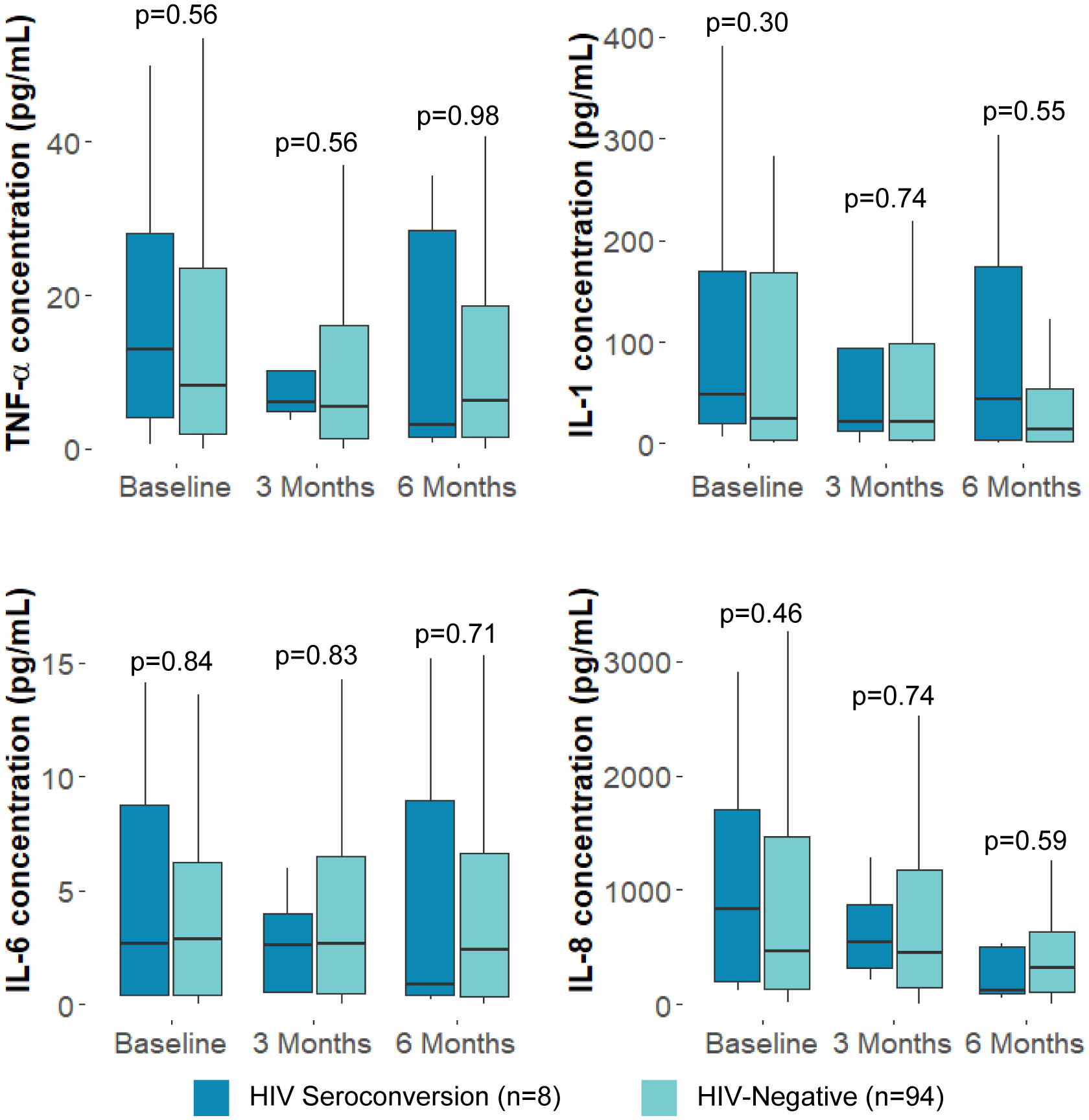
IL = Interleukin; TNF = Tumor Necrosis Factor
Box plots display the 25th percentile, median, and 75th percentile of values. Lower whiskers represent the minimum value of data within 1.5 times of the interquartile range (IQR) below the 25th percentile. Upper whiskers represent the maximum value of data within 1.5 times the IQR above the 75th percentile. P-values calculated using Wilcoxon Rank-Sum tests.
DISCUSSION:
This is the first study to evaluate inflammatory cytokine levels in the rectal mucosa following HIV seroconversion. In this analysis of MSM and TW who participated in an intervention using rectal STI screening and treatment for HIV prevention in Lima, Peru, individuals who experienced HIV seroconversion did not have any differences in rectal mucosal cytokine levels compared to participants who remained HIV seronegative. Despite a surge in viral replication during acute infection, our findings suggest that HIV-1 acquisition does not appear to result in a prolonged inflammatory process. These results are in stark contrast to the dramatic increase in mucosal inflammation that is observed with GC/CT infection4,11.
Our findings are initially surprising, particularly as acute HIV-1 infection results in a surge in viral replication, immune activation, and destruction of gut-associated lymphoid tissue CD4+ T-cells12,13. HIV infection has also been associated with increased mRNA expression of inflammatory cytokines, such as IL-4, IL-6, IL-10, and TNF-α, in colonic mucosa regardless of antiretroviral therapy use or viral load14,15. However, at least one other study has compared inflammatory cytokine levels in the rectal mucosa during HIV infection and found that chronic HIV infection is not associated with elevated rectal mucosal cytokine levels6, congruent with our findings. The discrepancy observed between cytokine mRNA expression documented in previous studies and cytokine levels measured in the rectal mucosa may be related to anatomic location of sampling, different regulatory mechanisms between mRNA expression and protein, and host factors contributing to localized inflammation. Better understanding of the mechanisms leading to and regulating mucosal inflammation in HIV is an important area for future study.
Our study has several strengths. This is the first longitudinal study evaluating the effects of HIV seroconversion on rectal inflammation among a group of MSM and TW in a study with high rates of retention. However, the study does have some limitations. Given the small sample size of participants who experienced HIV seroconversion (n=8), our analysis may not be adequately powered to detect small differences in cytokine concentrations, particularly given substantial variation in cytokine levels in both groups. Additionally, HIV viral load among those who experienced HIV seroconversion was not known, as this data was not collected as part of this study. However, it is likely that HIV viremia at time of diagnosis was high, as all participants who experienced HIV seroconversion had received their diagnosis through this study and were not receiving antiretroviral therapy. Most importantly, the timing of rectal sampling to acute HIV infection is unknown, particularly as HIV was diagnosed with antibody test and not PCR. Since study visits occurred every 3 months, it is probable that HIV infection and mucosal sampling may be separated by several weeks to months. For this reason, we can conclude that HIV seroconversion does not associate with prolonged rectal inflammation but may still result in inflammation limited to the more acute period. As rectal inflammation is associated with increased risk of HIV/STI transmission, future studies evaluating the impact of acute HIV-1 seroconversion on rectal mucosal cytokine levels is warranted. The absence of rectal mucosal inflammation associated with HIV seroconversion, if confirmed in subsequent studies, may have important implications for research on HIV prevention and transmission.
Supplementary Material
Acknowledgements:
We would like to thank the participants and their families. In addition, we acknowledge Williams Gonzales, the study teams and clinical research staff at the study sites.
Conflicts of Interest and Source of Funding:
Reagents for GC/CT testing were donated by Hologic (San Diego, CA, USA). The authors have no conflicts of interest to declare. This work was supported by the National Institute of Mental Health of the National Institutes of Health [R34 MH105272 to JLC and T32MH080634].
REFERENCES
- 1.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2014–2018. HIV Surveillance Supplemental Report 2020. Web site. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-25-1.pdf. Published 2020. Accessed November 10, 2020. [Google Scholar]
- 2.Barbee LA, Khosropour CM, Dombrowksi JC, et al. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis. 2017;44(7):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilen CB, Tilton JC, Doms RW. HIV: cell binding and entry. Cold Spring Harb Perspect Med. 2012;2(8):a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Curr Opin Immunol. 2015;36:22–30. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Council OD, Chen JS. Sexually transmitted infections and HIV in the era of antiretroviral treatment and prevention: the biologic basis for epidemiologic synergy. J Int AIDS Soc. 2019;22(S6):e25355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heiligenberg M, Lutter R, Pajkrt D, et al. Effect of HIV and chlamydia infection on rectal inflammation and cytokine concentrations in men who have sex with men. Clin Vaccine Immunol. 2013;20(10):1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley CF, Kraft CS, de Man TJ, et al. The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: implications for HIV transmission and prevention. Mucosal Immunol. 2017;10(4):996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Workowski KA. Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis. 2015;61(suppl_8):S759–S762. [DOI] [PubMed] [Google Scholar]
- 9.Kozlowski PA, Lynch RM, Patterson RR, et al. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr. 2000;24(4):297–309. [DOI] [PubMed] [Google Scholar]
- 10.McGowan I, Elliott J, Cortina G, et al. Characterization of baseline intestinal mucosal indices of injury and inflammation in men for use in rectal microbicide trials (HIV Prevention Trials Network-056). J Acquir Immune Defic Syndr. 2007;46(4):417–425. [DOI] [PubMed] [Google Scholar]
- 11.Passmore JA, Jaspan HB, Masson L. Genital inflammation, immune activation and risk of sexual HIV acquisition. Curr Opin HIV AIDS. 2016;11(2):156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMichael AJ, Borrow P, Tomaras GD, et al. The immune response during acute HIV-1 infection: clues for vaccine development. Nature Rev Immun. 2010;10(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pastor L, Parker E, Carrillo J, et al. A cytokine pattern that differentiates preseroconversion from postseroconversion phases of primary HIV infection. J Acquir Immune Defic Syndr. 2017;74(4):459–466. [DOI] [PubMed] [Google Scholar]
- 14.Schulbin H, Bode H, Stocker H, et al. Cytokine expression in the colonic mucosa of human immunodeficiency virus-infected individuals before and during 9 months of antiretroviral therapy. Antimicrob Agents Chemother. 2008;52(9):3377–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mait-Kaufman J, Fakioglu E, Mesquita PM, et al. Chronic HIV infection is associated with upregulation of proinflammatory cytokine and chemokine and alpha defensin gene expression in colorectal mucosa. AIDS Res Hum Retroviruses. 2015;31(6):615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


