Abstract
There is growing evidence for a role of maternal diabetes in the pathogenesis of neurodevelopmental disorders. However, the specific association between gestational diabetes (GDM), as opposed to pre-gestational diabetes, has been poorly isolated. Thus the aim was to systematically review and meta-analyse literature pertaining to prevalence and risk for two neurodevelopmental disorders: autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD), when exposed to GDM. PubMed, Cochrane Library, EMBASE, PsycINFO and CINAHL were systematically searched for eligible literature, with forward and backward citation tracking. Screening for eligibility, risk of bias assessment and data extraction were performed by two independent reviewers. 18 studies measuring ASD and 15 measuring ADHD met inclusion criteria. On meta-analysis there was an increased risk of ASD (OR 1.42; 95% CI 1.22, 1.65) but not ADHD (OR 1.01; 95% CI 0.79, 1.28). We discuss potential mechanisms for these differing risks. Greater understanding of risk factors, including GDM, for these neurodevelopmental disorders and potential mechanisms may help inform strategies aimed at prevention of exposure to these adversities during pregnancy.
Subject terms: Risk factors, Endocrine system and metabolic diseases, Psychiatric disorders
Introduction
Gestational diabetes (GDM) is glucose intolerance that begins during pregnancy and has an estimated prevalence of between 1.8% and 22.3% in Europe, with higher rates in Africa, North and South America and the Middle East1. It is associated with adverse outcomes for mother and baby, including obstetric complications such as emergency Caesarean delivery and longer-term risks of Type 2 Diabetes in the mother and metabolic syndrome in offspring2.
There is also some emerging evidence for a relationship between GDM and adverse neurobehavioural outcomes in children. Several systematic reviews suggest an association between maternal diabetes and lower IQ scores, language impairment and symptoms of attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). However, many of these reviews group together women experiencing pregestational (Type 1 and Type 2) and gestational diabetes so do not investigate the effect of GDM specifically3–8. While GDM and pregestational diabetes share similar pathology of insulin resistance, in GDM this insulin resistance arises only during pregnancy, which is itself a state of insulin resistance. Therefore, while there may be some women diagnosed with GDM who have undiagnosed pregestational diabetes, the pathology is slightly different between the two conditions. There are many potential mechanisms that may underpin such an association between GDM and adverse offspring neurobehavioural outcomes. There may be mediating factors of obstetric and neonatal adversities such as pre-eclampsia or infants born large for gestational age9–11. There may also be epigenetic changes12 or oxidative stress13,14 resulting from a hyperglycaemic in-utero environment.
Thus, the aim of this study was to conduct a systematic review and meta-analysis of the prevalence and risk for ADHD and ASD in children of women affected specifically by GDM. Both ADHD and ASD are commonly diagnosed neurodevelopmental disorders encompassing a spectrum of neurobehavioural symptoms that are often diagnosed from a young age. ADHD has a global prevalence of around 5%15, is characterised by symptoms of inattention and hyperactivity16 and often has broad and enduring adverse impacts on quality of life and functioning17. ASD describes a range of conditions characterised by some or all of: impaired communication, impaired social interaction and repetitive, restricted and stereotyped behaviour16 and may also result in profound struggles in both personal and professional life. A range of pathophysiological mechanisms have been implicated for these neurodevelopmental disorders, including hyperglycaemia during pregnancy18. Thus, a greater understanding of the aetiology of these disorders could help to identify early life risk factors for their development.
Methods
The review followed ‘Meta-analysis of Observational Studies in Epidemiology’ (MOOSE)19 and ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA) guidelines20 and was registered with PROSPERO (CRD42019128376).
Search strategy
An electronic literature search was performed in the databases PubMed, Cochrane Library, EMBASE, PsycINFO and CINAHL from inception to 04/04/2019, with forward and backward citation tracking of eligible papers. Search terms were adapted from previous systematic reviews in the area (see supplementary material). Two separate searches were conducted for ASD and ADHD.
Study selection
Inclusion criteria were: published, peer-reviewed studies with children aged 18 and under, whose mothers had clinically diagnosed GDM during pregnancy and who were investigated for symptoms and/or diagnosis of ASD or ADHD. Report of symptoms of ASD and ADHD by questionnaires or other tests was accepted and clinical diagnosis was accepted through self-report, report from medical professionals or medical records. Either self-report of GDM, report from medical professionals, or medical records was accepted. Observational studies and baseline data from intervention studies were included, in any language.
Exclusion criteria were: case studies, editorials, reviews and conference abstracts. Non-human studies were also excluded. Studies which were known to include women with established pregestational diabetes were excluded, unless it was possible to extract data pertaining specifically to GDM. Studies in which there was some doubt surrounding this were included in the review but not included in the meta-analysis.
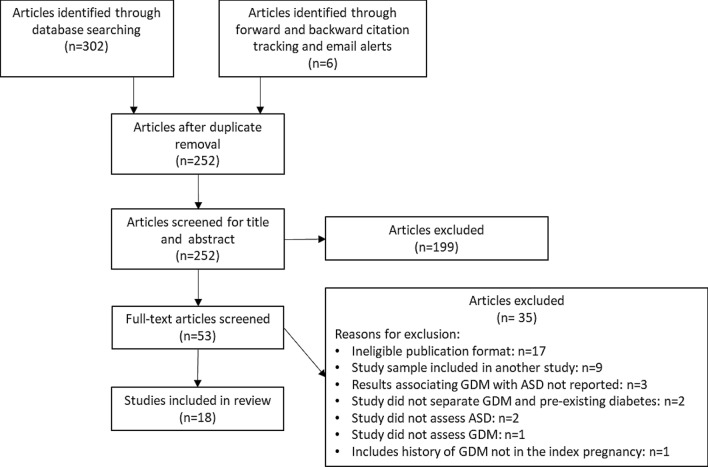
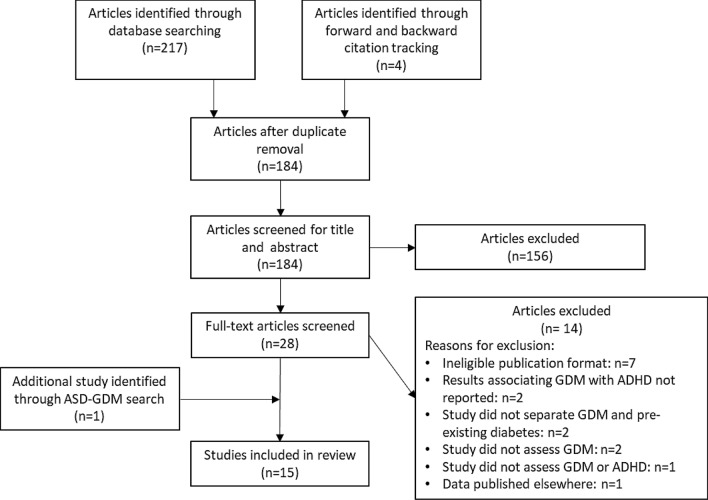
Two independent reviewers screened titles and abstracts then full texts for eligibility. Results of study selection are presented in Figs. 1 and 2.
Figure 1.
Flow diagram of ASD study selection.
Figure 2.
Flow diagram of ADHD study selection.
Data extraction and risk of bias assessment
Two independent reviewers extracted data, including study characteristics, prevalence and odds ratios (ORs) and any information on mechanisms for the associations. Study authors were e-mailed to request raw data if potentially relevant data may be available.
Risk of bias in all included studies was also assessed by two independent reviewers using a pre-piloted modified Newcastle Ottawa Scale (see supplementary material). Scores for selection bias and measurement bias were of particular interest as most of the studies were of observational design. Each question in the tool had a score of zero to two (low to high risk of bias). A score of two on any item within the selection and measurement bias domains meant that the study was categorised as having a high risk of bias.
Data synthesis
Meta-analyses of ORs and prevalence were undertaken separately for ASD and ADHD if at least five studies were available21. In studies providing only prevalence data, ORs were calculated from this data (or raw data provided by authors). If there was any doubt as to whether or not pregestational diabetes had been excluded from the comparison group without GDM, these studies’ ORs were not included in meta-analysis.
Data were analysed using Stata 15. Metan and metaprop commands were used to produce pooled unadjusted ORs and prevalence and 95% confidence intervals (CIs) displayed as forest plots. Insufficient numbers of studies adjusted for similar characteristics to enable pooling of adjusted ORs. DerSimonian-Laird random effects meta-analysis22 was used as there was expected to be substantial heterogeneity between studies23,24. Heterogeneity was assessed using I2: proportion of total variation in study estimates that is due to heterogeneity25. It was decided a-priori that I2 > 75% would preclude meta-analysis as this represents considerable heterogeneity26. Both of the prevalence meta-analyses produced I2 > 75% so prevalence is presented as median with interquartile range (IQR) as a standard summary measure of non-parametric data. Sensitivity analyses on effect of risk of bias were conducted when sufficient studies were available. Publication bias was not assessed for the meta-analyses as there were insufficient numbers of studies (less than ten)27.
Results
Characteristics of ASD studies
18 studies measuring ASD were identified; three of these also measured ADHD. Table 1 provides a summary of their characteristics and findings. Nine of these studies were from North America. Four were from middle income countries; none were from low- income countries (according to World Bank classification at June 2019). All studies were observational. Most of the studies used medical records or parental report of ASD and GDM; diagnostic criteria for GDM were usually not reported. 11 of the studies were assessed as high risk of bias due to lack of information about how GDM or ASD was diagnosed, increasing the risk of measurement bias and/or lack of information about selection criteria preventing accurate assessment of risk of selection bias.
Table 1.
Summary of studies measuring ASD.
| Author and year | Study design | Country | Sample size and age of children at diagnosis | Ascertainment of GDM diagnosis | ASD measure | Findings [%, mean (SD), OR/HR/RR/β (95% CI)] |
Risk of bias score (low–high: 0–2) | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Alshaban et al. 201928 | Cross-sectional | Qatar |
176,960 children 844 with ASD and available clinical data Aged 5–12 years |
Medical records or parental interviews Diagnostic criteria unknown |
Parental report of diagnosis or formal assessment |
8.9% of children with autism were exposed to GDM 1.14% of total study population had ASD |
Selection bias: Representativeness: 0 Participation rates: 2 Measurement bias: GDM: 2 ASD: 0 |
High |
| Burstyn et al. 201050 | Prospective cohort (population-based) | Canada |
7453 children of GDM mothers 206,122 children of non-diabetic mothers Median age 36 months (IQR 47–65) |
Medical records Diagnostic criteria unknown |
Diagnosis from medical records |
0.72% of children of GDM mothers had autism 0.51% of children of non-diabetic mothers had autism RR: 1.24 (0.94–1.65) |
Selection bias: Representativeness: 0 Participation rates: 0 Measurement bias: GDM: 1 ASD: 0 |
Low to moderate |
| Chien et al. 201851 | Retrospective cohort | Taiwan |
323 children with ASD Mean age 10.7 years (SD 3.5) 257 unaffected siblings Mean age 11.7 years (SD 4.5) 1504 control children Mean age 8.9 years (SD 1.6) |
Self-report, 15% cases validated with medical records Diagnostic criteria defined by Carpenter-Coustan: 2 positive values on 100 g oral glucose tolerance test (OGTT) 95 (0 h), 180 (1 h), 155 (2 h), 140 mg/dL (3 h) |
Diagnosis from child psychiatrists |
1.2% children with ASD had mothers with GDM 0.4% of unaffected siblings had mothers with GDM 0.7% of control children had mothers with GDM OR (ASD vs control): 1.78 (0.52–6.12) OR (ASD vs unaffected sibling): 3.42 (0.57–20.82) Beta coefficient for ASD symptom severity with GDM: 3.43 (SD = 1.32). (Adjusted for child sex and age) |
Selection bias: Representativeness: 0 Participation rates: 2 Measurement bias: GDM: 2 ASD: 0 |
High |
| Connolly et al. 201641 | Retrospective cohort | USA |
503 children with ASD 38,810 control children Age of children not reported |
Medical records Diagnostic criteria unknown |
Diagnosis from medical records |
10.4% of children with ASD had mothers with GDM 6.6% of control children had mothers with GDM P = 0.0007 Unadjusted OR: 1.64 (1.22–2.22) Adjusted OR: 1.56 (1.14–2.11) (Adjusted for maternal age at birth, maternal race, year of birth and BMI) Sensitivity analysis restricting to births < 2011: OR: 1.74 (95% CI: 1.25–2.44) Adjusted OR: 1.44 (95% CI: 1.02–2.03) (adjusted for maternal age at birth, maternal race, year of birth and BMI) |
Selection bias: Representativeness: 0 Participation rates: 0 Measurement bias: GDM: 1 ASD: 0 |
Low to moderate |
| Dodds et al. 201152 | Retrospective cohort | Canada |
924 children with ASD 128,809 children without ASD Aged 1–17 years |
Medical records Diagnostic criteria unknown |
Diagnosis from medical records |
0.9% of children of mothers with GDM had autism 0.7% of children of non-GDM mothers had autism RR: 1.29 (0.90–1.83) 0.7% of children of mothers with no diabetes had autism* |
Selection bias: Representativeness: 0 Participation rates: 0 Measurement bias: GDM: 1 ADHD: 0 |
Low to moderate |
| George et al. 201453 | Case control | India |
143 children with autism Mean age 42 months 200 control children Mean age 41.6 months |
Self-report Diagnostic criteria unknown |
Diagnosis using Child Autism Rating Scale (method of report unknown) |
11.2% of children with autism had mothers with GDM 11.5% of controls had mothers with GDM |
Selection bias: Representativeness: 1 Participation rates: 2 Measurement bias: GDM: 2 ASD: 0 |
High |
| Hadjkacem et al. 201654 | Cross-sectional | Tunisia |
50 children with autism 51 control children Aged 3–7 years |
Self-report Diagnostic criteria unknown |
Diagnosis from child psychiatrist |
8.0% of the autistic children had mothers with GDM 2.0% of the control children had mothers with GDM OR: 4.43 p = 0.2 |
Selection bias: Representativeness: 1 Participation rates: 2 Measurement bias: GDM: 2 ASD: 0 |
High |
| Kania et al. 201655 | Cross-sectional | Poland |
1007 children of GDM women (no control group) Median age at diagnosis 4.5 years (range 2.5–7 years) |
Medical records Diagnostic criteria from 1999- 2005: fasting glucose > 110 mg/dL or 140 mg/dL 2 h post OGTT From 2005–2011: fasting glucose > 100 mg/dL or > 140 mg/dL 2 h post OGTT |
Diagnosis from parental report | 0.08% of children were diagnosed with ASD |
Selection bias: Representativeness: 1 Participation rates: 2 Measurement bias: GDM: 0 ASD: 1 |
High |
| Khanom et al. 201556 | Case control | Bangladesh |
95 children with ASD 185 control children Aged 15–26 months |
Self-report Diagnostic criteria unknown |
Diagnosis Method of report unknown |
40.4% of children with ASD had mothers with GDM 22.7% of controls had mothers with GDM OR: 2.30 (1.36–3.91) |
Selection bias: Representativeness: 2 Participation rates: 2 Measurement bias: GDM: 2 ASD: 2 |
High |
| Kong et al. 201829 | Prospective cohort (population-based) | Finland |
101,696 children of GDM mothers 543,347 children of non-diabetic mothers Aged up to 11 years |
Medical records Diagnostic criteria unknown |
Diagnosis from medical records |
HR separated by BMI: Normal: 1.06 (0.88–1.28) Overweight: 1.27 (1.06- 1.52) Obese: 1.56 (1.26–1.93) Severely obese: 1.37 (1.04–1.81) |
Selection bias: Representativeness: 0 Participation rates: 0 Measurement bias: GDM: 1 ADHD: 0 |
Low to moderate |
| Krakowiak et al. 201257 | Case control | USA |
517 children with ASD 315 control children Aged 2–5 years |
Medical records or self-report | Diagnosis by diagnostic interview |
8.5% of children with ASD had mothers with GDM 6.0% of control children had mothers with GDM p = 0.18 |
Selection bias: Representativeness: 0 Participation rates: 2 Measurement bias: GDM: 2 ASD: 0 |
High |
| Li et al. 201658 | Prospective cohort | USA |
102 children with ASD 1748 typically developing children Median age 67 months |
Medical records Diagnostic criteria unknown |
Diagnosis from medical records |
8.8% of children with ASD had mothers with GDM 4.5% of typically developing children had mothers with GDM HR for ASD in GDM vs no diabetes: 1.86 (0.92–3.76) p = 0.08 HR for ASD in GDM and obesity vs neither condition: 3.04 (1.21–7.63) p = 0.02 |
Selection bias: Representativeness: 1 Participation rates: 2 Measurement bias: GDM: 1 ASD: 0 |
High |
| Maramara et al. 201430 | Retrospective cohort | USA |
268 children with autism 115,632 control children from general New Jersey population Age of children not reported |
Self-report, validated by medical records Diagnostic criteria unknown |
Diagnosis reported from paediatric neurologist |
4.7% of children with ASD had mothers with GDM 4.2% of the general New Jersey population had mothers with GDM P value not significant (not reported) |
Selection bias: Representativeness: 0 Participation rates: 0 Measurement bias: GDM: 2 ASD: 0 |
High |
| Raz et al. 201559 | Case control | USA |
245 children with ASD 1522 control children Age of children not reported |
Self-report Diagnostic criteria unknown |
Diagnosis from maternal report (validated in 50 cases) |
7% of children with ASD had mothers with GDM (missing data for 16%) 6% of control children had mothers with GDM (missing data 14%) |
Selection bias: Representativeness: 2 Participation rates: 0 Measurement bias: GDM: 2 ASD: 1 |
High |
| Sacks et al. 201635 | Prospective cohort (population-based) | Israel |
12,642 children of GDM mothers 218,629 children of non-diabetic mothers Age of children not reported |
Medical records Diagnostic criteria unknown |
Diagnosis from medical records |
0.04% children of GDM mothers had ASD 0.01% children of non-diabetic mothers had ASD Adjusted OR: 4.44 (1.55–12.69) p = 0.005 (Adjusted for maternal age, obesity, pre-eclampsia, fertility treatment, gestational week and time to event) |
Selection bias: Representativeness: 1 Participation rates: 0 Measurement bias: GDM: 1 ASD: 0 |
Low to moderate |
| Say et al. 201660 | Retrospective cohort | Turkey |
100 children with ASD Mean age 8.7 years (SD 3.86) 80 control children Mean age 8.5 years (SD 4.61) |
Self-report Diagnostic criteria unknown |
Diagnosis from expert child and adolescent psychiatrist |
3% of ASD group were exposed to GDM 1.3% of control group were exposed to GDM p = 0.717 |
Selection bias: Representativeness: 2 Participation rates: 2 Measurement bias: GDM: 2 ASD: 0 |
High |
| Straughen et al. 201761 | Case control | USA |
55 children with ASD 199 control children Age of children not reported |
Medical records Diagnostic criteria unknown |
Diagnosis from medical records |
10.9% of children with ASD had mothers with GDM (7.2% unknown) 7.0% of control children had mothers with GDM (3.5% unknown) |
Selection bias: Representativeness: 0 Participation rates: 0 Measurement bias: GDM: 1 ASD: 0 |
Low to moderate |
| Xiang et al. 201538 | Retrospective cohort | USA |
25,035 children with GDM mothers 290,792 children with non-GDM mothers Age of children not reported |
Medical records Diagnostic criteria: plasma glucose ≥ 200 mg/dL on glucose challenge test or defined by Carpenter-Coustan criteria on 100 g or 75 g OGTT |
Diagnosis from medical records |
1.2% of children of GDM mothers had autism 1.0% of children of non-GDM mothers had autism HR: 1.18 (1.04–1.33) p = 0.01 |
Selection bias: Representativeness: 0 Participation rates: 0 Measurement bias: GDM: 0 ASD: 0 |
Low to moderate |
% percentage, SD standard deviation, OR odds ratio, HR hazard ratio, RR risk ratio, β β coefficient, CI confidence interval.
*Calculated by subtracting data for mothers with pregestational diabetes from data for non-GDM mothers.
Pooled odds and prevalence of ASD in those exposed to GDM
Data on prevalence of ASD in those exposed to GDM were available for 15 studies; there were three studies which measured ASD and GDM but from which prevalence data could not be extracted28–30 (see Table 1). Heterogeneity on meta-analysis of these 15 studies was 98%, precluding meta-analysis. Median prevalence was 16.3% (IQR 0.9–48.8%).
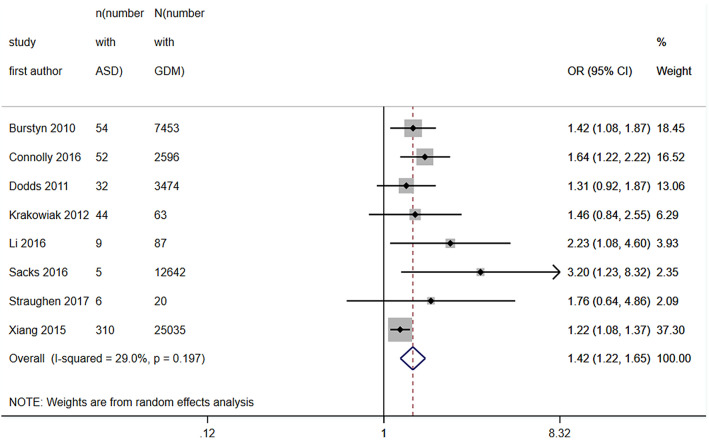
Eight of these studies were included in a meta-analysis of ORs. Studies were excluded in the absence of a control group or if there was doubt that pregestational diabetes had been excluded from the control group (not specifically mentioned in the exclusion criteria and no response to an e-mail to clarify). Pooled unadjusted OR was 1.42 (95% CI 1.22, 1.65) with heterogeneity 29% (see Fig. 3).
Figure 3.
Forest plot showing pooled unadjusted odds ratios for ASD in those exposed to GDM versus those not exposed to GDM.
Characteristics of ADHD studies
15 studies measuring ADHD were identified. Table 2 provides a summary of their characteristics and findings. Compared to those studies measuring ASD, more of these studies were from European countries. All studies were observational; ten were prospective cohorts but there were less population-based cohorts than in the ASD literature so sample sizes were generally smaller. Moreover, measurement of symptoms using questionnaires were more frequently used in the ADHD literature (as opposed to diagnoses) and this in part led to only two studies being assessed as low to moderate risk of bias.
Table 2.
Summary of studies measuring ADHD
| Author and year | Study design | Country | Sample size and age of children at diagnosis | Ascertainment of GDM diagnosis | ADHD measure | Findings [%, mean (SD), OR/HR/RR, β (95% CI)] |
Risk of bias score (low–high: 0–2) | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Akaltun et al. 201962 | Case control | Turkey |
88 children of GDM mothers 128 children of non-diabetic mothers Aged 6–12 years |
Medical records and self-report Diagnostic criteria defined by Carpenter-Coustan on 100 g OGTT |
Diagnosis from medical records and parental report |
GDM group: 15.9% had ADHD Non-GDM group: 7.0% had ADHD p = 0.115 |
Selection bias: Representativeness: 2 Participation rates: 2 Measurement bias: GDM: 0 ADHD: 0 |
High |
| Chiu et al. 200963 | Cross-sectional | Taiwan |
11 children of GDM mothers 1380 children of non-GDM mothers Aged 4–9 years |
Self-report Diagnostic criteria unknown |
Symptoms of inattention using child behaviour checklist | OR not significant. No values given |
Selection bias: Representativeness: 1 Participation rates: 0 Measurement bias: GDM: 2 ADHD: 2 |
High |
| Daraki et al. 201764 | Prospective cohort | Greece |
55 children of GDM mothers 636 children of non-GDM mothers Aged 4 years |
Medical records Diagnostic criteria defined by Carpenter-Coustan on OGTT |
Symptoms using attention deficit hyperactivity disorder test (ADHDT) |
β: 1.75 (-2.14,5.66) (Adjusted for child sex and age) β: 2.41 (95% CI: -1.45,6.28) (Further adjusted for maternal age, maternal origin, maternal education level, parity and maternal smoking during pregnancy) β: 2.32 ( -1.52,6.16) (Further adjusted for maternal pre-pregnancy BMI) |
Selection bias: Representativeness: 0 Participation rates: 1 Measurement bias: GDM: 0 ADHD: 0 |
Low to moderate |
| Galera et al. 201865 | Prospective cohort | France |
84 children of GDM mothers 1158 children of non-diabetic mothers Aged 3–8 years |
Medical records or parental interviews Diagnostic criteria unknown |
Symptoms by the Strengths and Difficulties Questionnaire |
GDM group: 15.5% had high hyperactivity-impulsivity trajectories Non-GDM group: 14.9% had high hyperactivity-impulsivity trajectories |
Selection bias: Representativeness: 0 Participation rates: 2 Measurement bias: GDM: 2 ADHD: 2 |
High |
| Kong et al. 201829 |
Prospective cohort (population-based) |
Finland |
101,696 children of GDM mothers 543,347 children of non-diabetic mothers Aged up to 11 years |
Medical records Diagnostic criteria unknown |
Diagnosis from medical records |
HR separated by BMI: Normal: 1.15 (1.01–1.30) Overweight: 1.16 (1.02- 1.32) Obese: 1.64 (1.42–1.88) Severely obese: 2.15 (1.84–2.52) |
Selection bias: Representativeness: 0 Participation rates: 0 Measurement bias: GDM: 1 ADHD: 0 |
Low to moderate |
| Li et al. 201658 | Prospective cohort | USA |
301 children with ADHD 1748 typically developing children Age of children not reported |
Medical records Diagnostic criteria unknown |
Diagnosis from medical records |
4.0% of children with ADHD had mothers with GDM 4.5% of typically developing children had mothers with GDM HR for ADHD in GDM vs no diabetes: 0.99 (0.50–1.94) p = 0.98 HR for ADHD in GDM and obesity vs neither condition: 1.20 (0.49–2.93) p = 0.70 |
Selection bias: Representativeness: 1 Participation rates: 2 Measurement bias: GDM: 1 ASD: 0 |
High |
| Mina et al. 201743 | Prospective cohort | UK |
14 children of GDM mothers 96 children of non-GDM mothers Aged 3–5 years |
Medical records Diagnostic criteria unknown |
Symptoms using Conners hyperactivity scale |
Mean score for GDM children: 8.7 (6.0)* Mean score for non-GDM children: 7.3 (4.5)* |
Selection bias: Representativeness: 2 Participation rates: 2 Measurement bias: GDM: 1 ADHD: 0 |
High |
| Nomura et al. 201244 | Prospective cohort | USA |
21 children of GDM mothers Mean age 4.4 years (SD 0.48) 191 children of non-GDM mothers Mean age 4.3 years (SD 0.47) |
Self-report Diagnostic criteria unknown |
Diagnosis using a psychiatric interview and symptoms using ADHD rating scale-IV |
Mean inattention score at baseline (no standard deviations given): GDM group: 12.25 Non-GDM group: 9.50 p = 0.05 Mean hyperactivity/ impulsivity scores: GDM group: 12.58 Non GDM group: 11.29 p = 0.36 76.2% children exposed to GDM had ADHD* 61.3% children not exposed to GDM had ADHD* OR at baseline: 1.58 (0.77–3.27) p = 0.22 OR at age 6 years: 2.20 (1.00–4.82) p = 0.05 |
Selection bias: Representativeness: 2 Participation rates: 2 Measurement bias: GDM: 2 ADHD: 0 |
High |
| Ornoy et al. 199966 | Prospective cohort | Israel |
32 children of GDM mothers Mean age 8.5 years (SD 2.1) 57 children of non-diabetic mothers Mean age 8.3 years (SD 1.7) |
Medical records Diagnostic criteria: abnormal glucose tolerance test (≥ 190 mg % glucose at 90 min, ≥ 165 mg % at 120 min, ≥ 145 mg % at 180 minutes, or with ≥ 105 mg % fasting glucose blood concentrations) |
Symptoms using Conners parents’ questionnaire and Pollack Tapper Test |
Conners parents’ questionnaire: Children of GDM mothers had mean score of 8.0 (6.5) if young and 6.8 (6.3) if older Control children had a score of 7.9 (4.3) if young and 7.0 (4.3) if older 4 GDM children had abnormal scores (above 14) compared with only 2 controls. p = 0.06 Pollack general: Children of GDM mothers had a mean score of 19.0 (12.4) if young and 29.6 (10.5) if older Control children had a score of 28.0 (3.2) if young and 30.3 (6.9) if older Pollack’s sound: Children of GDM mothers had a score of 10.6 (6.6) if young and 14.9 (5.0) if older Control children had a score of 14.8 (6.5) if young and 15.6 (3.6) if older Pollack visual Children of GDM mothers had a score of 7.7 (5.9) if young and 14.1 (5.4) if older Control children had a score of 13.2 (2.0) if young and 14.7 (3.4) if older |
Selection bias: Representativeness: 2 Participation rates: 0 Measurement bias: GDM: 0 ADHD: 0 |
High |
| Pohlabeln et al. 201767 | Prospective cohort | Eight European countries |
435 children of GDM mothers 18% under 4 years of age, 27% aged 4–6, 38% aged 6–8 and 17% over 8 years of age |
Self-report Diagnostic criteria unknown |
Diagnosis from parental report |
1.8% of children exposed to GDM had ADHD 1.1% of children not exposed to GDM had ADHD OR: 1.42 (0.69–2.95) (adjusted for sociodemographics and country) OR: 1.28 (0.59–2.80) (further adjusted for pre-, peri- and postnatal influences) Unadjusted OR excluding pregestational diabetes: 1.149 (0.468–2.818) * Adjusted OR excluding pregestational diabetes: 1.032 (0.417–2.556) (adjusted for sex, age and country) * |
Selection bias: Representativeness: 0 Participation rates: 0 Measurement bias: GDM: 2 ADHD: 1 |
High |
| Say et al. 201660 | Retrospective cohort | Turkey |
100 children with ADHD Mean age 8.8 years (SD 1.98) 80 control children Mean age 8.5 years (SD 4.61) |
Self-report Diagnostic criteria unknown |
Diagnosis from expert child and adolescent psychiatrist |
2% of ADHD group were exposed to GDM 1.3% of control group were exposed to GDM p = 0.717 |
Selection bias: Representativeness: 2 Participation rates: 2 Measurement bias: GDM: 2 ADHD: 0 |
High |
| Schmitt and Romanos 201245 |
Cross-sectional (population-based) |
Germany |
280 children of GDM mothers 13,208 children of non-GDM mothers Aged 3–17 years |
Self-report Diagnostic criteria unknown |
Diagnosis from parental report |
8.6% of children of GDM mothers had ADHD 5.1% of children of non-GDM mothers had ADHD Unadjusted OR: 1.93 (1.26–2.95) Adjusted OR: 1.91 (1.21–3.01) (adjusted for child sex, age, socioeconomic position, maternal smoking during pregnancy, maternal alcohol consumption during pregnancy, perinatal health problems, breastfeeding and atopic eczema) |
Selection bias: Representativeness: 0 Participation rates: 1 Measurement bias: GDM: 2 ADHD: 1 |
High |
| Veena et al. 201032 | Prospective cohort | India |
32 children of GDM mothers 515 children of non-diabetic mothers Aged 9–10 years |
Medical records Diagnostic criteria defined by Carpenter-Coustan on OGTT |
Symptoms using Coding-Wechsler Intelligence Scale for Children—3rd Edition (Coding WISC-III) score |
Mean score for GDM group: 36.8 (8.0) Mean score for non-GDM group: 32.4 (8.1) p = 0.003 β: 0.4 (0.09–0.75) p = 0.01 (adjusted for child’s sex, gestation and age) β: 0.3 (0.01, 0.67) p = 0.04 (further adjusted for SES, parents’ education and rural/urban residence) β: 0.3 (-0.04, 0.67) p = 0.08 (further adjusted for maternal age, BMI and parity in pregnancy and child’s weight and head circumference at birth) |
Selection bias: Representativeness: 0 Participation rates: 0 Measurement bias: GDM: 0 ADHD: 2 |
High |
| Wolford et al. 201731 | Prospective cohort | Finland |
176 children of GDM mothers 1,603 children of non-GDM mothers (9 with T1DM) Mean 3.8 years (SD 0.5) |
Medical records Diagnostic criteria unknown |
Symptoms using Conners hyperactivity index (CHI) | Mean difference in CHI sum score for GDM vs no GDM: -0.05 (p = 0.08) |
Selection bias: Representativeness: 0 Participation rates: 1 Measurement bias: GDM: 1 ADHD: 0 |
Low to moderate |
| Xiang et al. 201836 | Retrospective cohort | USA |
29,534 children of GDM mothers 295,304 children of non-diabetic mothers Age of children not reported |
Medical records Diagnostic criteria: ≥ 200 mg/dL blood glucose on 1 h 50 g glucose challenge test or 3 h 100 g or 2 h 75 g OGTT defined by Carpenter-Coustan criteria |
Diagnosis from medical records |
4.8% children of GDM mothers diagnosed with ADHD 5.2% children of non-diabetic mothers diagnosed with ADHD HR: 0.94 (0.88–1.00) p = 0.04 (adjusted for random sibling effect and birth year) HR: 1.02 (0.96–1.09) p = 1.50 (further adjusted for maternal age at delivery, parity, education, household income, maternal race/ethnicity, history of comorbidity, history of maternal ADHD and sex of the child) HR: 1.00 (0.94–1.06) (further adjusted for smoking, alcohol and pre-pregnancy BMI) HR: 1.01 (0.95, 1.08) (Adjusted for variables in first multivariate adjusted HR (1.02), plus pre-eclampsia, eclampsia, congenital anomalies, birth weight, and gestational age at delivery) |
Selection bias: Representativeness: 1 Participation rates: 0 Measurement bias: GDM: 0 ADHD: 0 |
Low to moderate |
%: percentage, SD standard deviation, OR odds ratio, HR hazard ratio, RR risk ratio, β β coefficient, CI confidence interval.
*Data provided by study author
Pooled odds and prevalence of ADHD in those exposed to GDM
Data on prevalence of ADHD in those exposed to GDM were available for eight studies. Heterogeneity on meta-analysis of prevalence from these eight studies was 93.7%, precluding meta-analysis. Median prevalence was 14.4% (IQR 6.7–41.3%).
Studies not included in meta-analysis were those presenting numerical scores on a symptom-based questionnaire, as opposed to numbers scoring above or below a defined threshold, precluding the calculation of prevalence or odds (see Table 2). Relatively small numbers of children are included in these studies, with only one study including over 100 children exposed to GDM31. All but one of these studies found no evidence of differences in scores between GDM exposed and unexposed children; one study found some evidence for greater concentration and inattention symptoms in children of mothers with versus those without GDM32, which was attenuated on adjustment for a range of obstetric, neonatal and sociodemographic confounders (see Table 2)..
Five of the eight studies providing information on prevalence were included in a meta-analysis of ORs; three studies were excluded for the same reasons as in the ASD meta-analysis i.e. unable to verify that pregestational diabetes had been excluded from the control population. Pooled unadjusted OR was 1.01 (95% CI 0.79, 1.28) with heterogeneity 26.2% (see Fig. 4).
Figure 4.
Forest plot showing pooled unadjusted odds ratios for ADHD in those exposed to GDM versus those not exposed to GDM.
Sensitivity analyses
In the meta-analysis of ORs for ASD, it appeared that effect sizes were slightly larger for those studies at low to moderate risk of bias. However, removal of the two studies at high risk of bias from the meta-analysis resulted in little change. Indeed, the pooled OR was slightly reduced at 1.39 (95% CI 1.19, 1.63). There were insufficient numbers of studies to facilitate the same sensitivity analysis for ADHD.
Discussion
Main findings
Pooled OR for risk of ASD following exposure to GDM was 1.42 (95% CI 1.22, 1.65) and for ADHD was 1.01 (0.79, 1.28). In general, studies measuring ADHD more often utilised screening tools of symptoms in smaller populations than the studies measuring ASD, which more often measured clinical diagnoses within larger population-based cohorts. Median prevalence of ASD of 16.3% and ADHD of 14.4% in those exposed to GDM is higher than that estimated in the general population15,33. However, there was substantial heterogeneity between studies included in these estimates, also reflected in wide IQRs for these medians and indicative of the broad range of study designs, populations and measures of both exposure and outcomes. That ORs in these studies when comparing risk in the GDM exposed versus unexposed were only modestly elevated also suggests that rates of ASD and/or ADHD were elevated in the study population as a whole, either due to selection of at-risk samples or due to systematic measurement of symptoms. Nonetheless, pooled unadjusted OR for risk of ASD in those exposed to GDM of 1.42 provides some evidence for a slightly increased risk, not seen to the same extent for ADHD (OR 1.01). Two previous meta-analyses investigating only risk for ASD found an increased risk; one with a pooled relative risk (RR) of 1.63 had substantially more heterogeneity (I2 75%)7 and the other with RR between 1.48 and 1.72 did not separate pregestational and gestational diabetes6. In contrast to our meta-analysis, in a meta-analysis of risk for ADHD following exposure to GDM across four studies, RR was 2.0 (95% CI 1.42, 2.81)5. However, as previously discussed, these meta-analyses did not specifically exclude pregestational diabetes from their control populations, which may explain the difference in results.
Strengths and limitations
This is the first study to our knowledge that has rigorously reviewed the literature and meta-analysed prevalence and risk from studies pertaining to both diagnoses and symptoms of ASD and ADHD. Using the same review strategy for more than one neurodevelopmental disorder allows a direct comparison of risk across a range of disorders. Another unique strength of this review is the exclusion from meta-analysis those studies in which pregestational diabetes was not removed from the control population. As previously discussed, pregestational and gestational diabetes differ somewhat in their pathology which could have implications for the degree of risk for adverse neurobehavioural outcomes and potential mechanistic pathways discussed below. However, just as the degree of glucose intolerance may differ between pregestational and gestational diabetes, it can also differ between populations with GDM due to widespread variation in diagnostic criteria. A significant limitation of the studies included within this review is that most of them do not provide information on GDM diagnostic criteria or any other indicators of GDM severity such as use of insulin or medication. Yet there is now evidence that maternal hyperglycaemia even below that of diagnostic threshold for GDM may be associated with adverse obstetric and neonatal outcomes34. It may be useful for future studies to investigate the impact of severity of maternal hyperglycaemia on risk for neurodevelopmental disorders; for example, whether or not there is a dose response relationship between maternal glucose levels and risk for disorder.
Lack of reporting on GDM diagnostic criteria within the included studies is one of the reasons why over half of the studies were assessed as at high risk of bias, although removal of studies at high risk of bias in the ASD meta-analysis of ORs resulted in minimal change to the effect estimate. However, there was also substantial diagnostic heterogeneity in the outcome of neurodevelopmental disorders, particularly in ADHD, where a broad range of questionnaires measuring levels of symptoms of ADHD were measured, which may not have met diagnostic threshold. Insufficient numbers of studies were available for ADHD to explore the impact that this may have had within a sensitivity analysis. A further limitation of the studies included within this review is that only some investigated the influence of other factors on the GDM and neurodevelopmental disorders association. This is discussed further below.
Potential mechanisms
The differences in risk between ASD and ADHD found in this review could be due to differing causal pathways, although clearly there are limitations to inferring any causality from observational studies. It could also be due to differences in the exposure, specifically degree of hyperglycaemia, although as previously discussed, this is often difficult to assess as so few studies consider it. Another possibility is that smaller sample sizes in the ADHD studies have failed to provide sufficient power to detect a difference in risk.
There were a few studies which looked at possible indicators of severity of GDM and degree of hyperglycaemia. For example, studies comparing GDM treated with medication versus without suggested an increased risk in medication-treated groups for both ASD35 and ADHD36. The pathway through which hyperglycaemia may impact neurodevelopment may be mediated by oxidative stress, which has been associated with adverse neurobehavioural outcomes such as motor deficits13. It may also influence epigenetic changes in the offspring, such as reduced DNA methylation seen in neurodevelopmental disorders such as ASD14. Moreover, hyperglycaemia can lead to systemic inflammation and pro-inflammatory cytokines are able to cross the placenta and the foetal blood–brain barrier, which may affect neurodevelopment37. However, there may be critical periods of exposure to hyperglycaemia during pregnancy for the different neurodevelopmental conditions. Xiang et al. have conducted analyses in a large population-based cohort on risk for both ASD38 and ADHD36 following exposure to maternal diabetes. They found that the later the GDM is diagnosed, the lesser the risk of ASD but saw no association with ADHD which may indicate differing critical periods.
Women with GDM are at a greater risk of several adverse obstetric outcomes, such as pre-eclampsia, foetal macrosomia, perinatal mortality, Caesarean delivery and preterm delivery9,39, which may also increase the risk of neurodevelopmental disorders40. While some studies presented data on gestational age at birth and birthweight, none explored their role as a potential mediator. A number of studies also investigated the role of obesity and socioeconomic status (SES) as effect modifiers of the association between GDM and neurodevelopmental disorders. Higher body mass index (BMI) increased the risk of both ASD29,41 and ADHD29,42,43 following exposure to GDM. Likewise, low SES has been shown to further increase the risk of ADHD44,45 following exposure to GDM, although this has been less explored in ASD.
Implications and conclusions
Therefore, future potential areas for research include an investigation of these mechanistic pathways underlying the association between maternal hyperglycaemia across the spectrum of subclinical, gestational and pregestational diabetes, and adverse neurobehavioural outcomes. Baseline risk for neurodevelopmental disorders in the general population is relatively low so absolute risk for a neurodevelopmental disorder in the offspring of mothers with GDM is still relatively low and there are many children exposed to GDM during pregnancy who do not develop a neurodevelopmental disorder. This supports an approach to measuring risk on a continuum and is one of the reasons that we chose to include symptoms of disorder in addition to clinical diagnoses.
A greater understanding of the early determinants of a child’s cognitive, social and emotional wellbeing would add support to interventions aimed at better management of these adversities, such as GDM, during pregnancy. Access to information about their condition has been identified as an enabler for women with GDM to manage their condition46. Such information could include sensitively informing women about potential risks to their baby. There is now evidence that effective management results in reductions in obstetric morbidities such as shoulder dystocia and pre-eclampsia47. However, there is also some evidence to support an inverse correlation between level of hyperglycaemia in pregnancy and longer-term neurobehavioral outcomes in offspring, such as verbal IQ48.
Furthering knowledge of these early predictors of adverse neurobehavioural outcomes would also underscore the importance of interventions aimed at prevention of such adverse pregnancy exposures by targeting their broader determinants in early pregnancy or even earlier in the preconception period. For example, there is some evidence that diet and physical activity interventions in early pregnancy reduce gestational weight gain and may be associated with a reduced risk of GDM49. That a number of studies included in the review found that socioeconomic status was an effect modifier of the association between GDM and neurodevelopmental disorders also highlights the importance of considering the broader determinants of health within healthcare. Thus there are a number of points at which healthcare professionals and policy makers involved in the care of women and children affected by GDM may usefully intervene.
In conclusion, there may be an association between GDM and the neurodevelopmental disorders of ASD and ADHD, with potentially differing levels of risk and mechanistic pathways for different neurodevelopmental disorders. Greater understanding of these risks and mechanisms may help to modify potential adverse developmental trajectories from becoming established in children.
Supplementary Information
Acknowledgements
The authors wish to thank those who contributed raw data: Yoko Nomura (Queens College, City University of New York, USA), Hermann Pohlabeln (Leibniz Institute for Prevention Research and Epidemiology- BIPS, Germany), Rebecca Reynolds (University of Edinburgh, UK) and Marius Lahti-Pulkkinen (University of Helsinki, Finland). CAW carried out this work as part of a Medical Research Council (MRC) funded Clinical Research Training Fellowship (MR/P019293/1).
Author contributions
Both authors contributed to the study conception and design. Literature search was performed by J.R. Study selection, data collection and risk of bias assessment was performed by J.R. and C.A.W. Meta-analysis was conducted by C.A.W. The first draft of the manuscript was written by J.R. and C.A.W. Both authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jennifer Rowland and Claire A. Wilson.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-84573-3.
References
- 1.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: A global perspective. Curr. Diab. Rep. 2016;16:7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kampmann U, et al. Gestational diabetes: A clinical update. World J. Diabetes. 2015;6:1065–1072. doi: 10.4239/wjd.v6.i8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilar Cordero MJ, et al. Diabetes mellitus materna y su influencia en el neurodesarrollo del niño; revisión sistemática. Nutr. Hosp. 2015;32:2484–2495. doi: 10.3305/nh.2015.32.6.10069. [DOI] [PubMed] [Google Scholar]
- 4.Camprubí Robles, M. et al. Maternal diabetes and cognitive performance in the offspring: A systematic review and meta-analysis. PLoS One.10, e0142583, 10.1371/journal.pone.0142583 (2015). [DOI] [PMC free article] [PubMed]
- 5.Zhao L, et al. The association of maternal diabetes with attention deficit and hyperactivity disorder in offspring: A meta-analysis. Neuropsychiatr. Dis. Treat. 2019;15:675–684. doi: 10.2147/NDT.S189200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu G, Jing J, Bowers K, Liu B, Bao W. Maternal diabetes and the risk of autism spectrum disorders in the offspring: A systematic review and meta-analysis. J. Autism Dev. Disord. 2014;44:766–775. doi: 10.1007/s10803-013-1928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan, H., Zhang, C., Li, H., Luan, S. & Liu, C. Association of maternal diabetes with autism spectrum disorders in offspring: A systematic review and meta-analysis. Medicine. 97, e9438, 10.1097/MD.0000000000009438 (2018). [DOI] [PMC free article] [PubMed]
- 8.Perna R, Loughan AR, Le J, Tyson K. Gestational diabetes: Long-term central nervous system developmental and cognitive sequelae. Appl. Neuropsychol. Child. 2015;4:217–220. doi: 10.1080/21622965.2013.874951. [DOI] [PubMed] [Google Scholar]
- 9.Wendland EM, et al. Gestational diabetes and pregnancy outcomes - A systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childb. 2012;12:23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore GS, Kneitel AW, Walker CK, Gilbert WM, Xing G. Autism risk in small-and large-for-gestational-age infants. Am. J. Obstet. Gynecol. 2012;206(314):e1–314.e9. doi: 10.1016/j.ajog.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maher GM, et al. Association of hypertensive disorders of pregnancy with risk of neurodevelopmental disorders in offspring: A systematic review and meta-analysis. JAMA Psychiatry. 2018;78:809–819. doi: 10.1001/jamapsychiatry.2018.0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latham KE, Sapienza C, Engel N. The epigenetic lorax: Gene–environment interactions in human health. Epigenomics. 2011;4:383–402. doi: 10.2217/epi.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells PG, et al. Oxidative stress in developmental origins of disease: Teratogenesis, neurodevelopmental deficits, and cancer. Toxicol. Sci. 2009;108:4–18. doi: 10.1093/toxsci/kfn263. [DOI] [PubMed] [Google Scholar]
- 14.Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology. 2008;29:190–201. doi: 10.1016/j.neuro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organisation. International Statistical Classification of Disease and Related Health Problems (10th revision). https://icd.who.int/browse10/2016/en (2016).
- 17.Harpin VA. The effect of ADHD on the life of an individual, their family, and community from preschool to adult life. Arch. Dis. Child. 2005;90:2–7. doi: 10.1136/adc.2004.059006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hami J, et al. Some of the experimental and clinical aspects of the effects of the maternal diabetes on developing hippocampus. World J. Diabetes. 2015;15:412–422. doi: 10.4239/wjd.v6.i3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Moher, D. et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med.6, e1000097, 10.1371/journal.pmed.1000100 (2009). [DOI] [PMC free article] [PubMed]
- 21.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int. J. Evid. Based Healthc. 2015;13:196–207. doi: 10.1097/XEB.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt FL, Oh IS, Hayes TL. Fixed- versus random-effects models in meta-analysis: Model properties and an empirical comparison of differences in results. Br. J. Math. Stat. Psychol. 2009;62:97–128. doi: 10.1348/000711007X255327. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne, J.A.C. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ.343, d4002, 10.1136/bmj.d4002 (2011). [DOI] [PubMed]
- 28.Alshaban F, et al. Prevalence and correlates of autism spectrum disorder in Qatar: A national study. J. Child Psychol. Psychiatry. 2019;60:1254–1268. doi: 10.1111/jcpp.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong, L., Norstedt, G., Schalling, M., Gissler, M. & Lavebratt, C. The risk of offspring psychiatric disorders in the setting of maternal obesity and diabetes. Pediatrics. 142, e20180776, 10.1542/peds.2018-0776 (2018). [DOI] [PubMed]
- 30.Maramara LA, He W, Ming X. Pre- and perinatal risk factors for autism spectrum disorder in a New Jersey cohort. J. Child Neurol. 2014;29:1645–1651. doi: 10.1177/0883073813512899. [DOI] [PubMed] [Google Scholar]
- 31.Wolford E. et al. Maternal depressive symptoms during and after pregnancy are associated with attention-deficit/hyperactivity disorder symptoms in their 3- to 6-year-old children. PLoS One. 12, e0190248, 10.1371/journal.pone.0190248 (2017). [DOI] [PMC free article] [PubMed]
- 32.Veena SR, et al. Childhood cognitive ability: Relationship to gestational diabetes mellitus in India. Diabetologia. 2010;53:2134–2138. doi: 10.1007/s00125-010-1847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baxter AJ, et al. The epidemiology and global burden of autism spectrum disorders. Psychol. Med. 2015;45:601–613. doi: 10.1017/S003329171400172X. [DOI] [PubMed] [Google Scholar]
- 34.Metzger BE, et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 35.Sacks, K.N. et al. Prenatal exposure to gestational diabetes mellitus as an independent risk factor for long-term neuropsychiatric morbidity of the offspring. Am. J. Obstet. Gynecol.215, 380.e.1–380.e7, 10.1016/j.ajog.2016.03.030 (2016). [DOI] [PubMed]
- 36.Xiang AH, et al. Maternal gestational diabetes mellitus, type 1 diabetes, and type 2 diabetes during pregnancy and risk of ADHD in offspring. Diabetes Care. 2018;41:2502–2508. doi: 10.2337/dc18-0733. [DOI] [PubMed] [Google Scholar]
- 37.Buehler MR. A proposed mechanism for autism: an aberrant neuroimmune response manifested as a psychiatric disorder. Med Hypotheses. 2011;76:863–870. doi: 10.1016/j.mehy.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 38.Xiang AH, et al. Association of maternal diabetes with autism in offspring. JAMA. 2015;313:1425–1434. doi: 10.1001/jama.2015.2707. [DOI] [PubMed] [Google Scholar]
- 39.Billionnet C, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60:636–644. doi: 10.1007/s00125-017-4206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schieve LA, et al. Population impact of preterm birth and low birth weight on developmental disabilities in US children. Ann Epidemiol. 2016;26:267–274. doi: 10.1016/j.annepidem.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connolly N, et al. Maternal metabolic risk factors for autism spectrum disorder—An analysis of electronic medical records and linked birth data. Autism Res. 2016;9:829–837. doi: 10.1002/aur.1586. [DOI] [PubMed] [Google Scholar]
- 42.Li, M. et al. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics. 137, e20152206, 10.1542/peds.2015-2206 (2016). [DOI] [PMC free article] [PubMed]
- 43.Mina TH, et al. Prenatal exposure to very severe maternal obesity is associated with adverse neuropsychiatric outcomes in children. Psychol Med. 2017;47:353–362. doi: 10.1017/S0033291716002452. [DOI] [PubMed] [Google Scholar]
- 44.Nomura Y, et al. Exposure to gestational diabetes mellitus and low socioeconomic status: Effects on neurocognitive development and risk of attention-deficit/hyperactivity disorder in offspring. Arch. Pediatr. Adolesc. Med. 2012;166:337–343. doi: 10.1001/archpediatrics.2011.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitt J, Romanos M. Prenatal and perinatal risk factors for attention-deficit/hyperactivity disorder. Arch. Paediatr. Adolesc. Med. 2012;166:1074–1075. doi: 10.1001/archpediatrics.2012.1078. [DOI] [PubMed] [Google Scholar]
- 46.Martis, R., Brown, J., McAra-Couper, J. & Crowther, C.A. Enablers and barriers for women with gestational diabetes mellitus to achieve optimal glycaemic control—A qualitative study using the theoretical domains framework. BMC Pregnancy Childb. 18, 91, 10.1186/s12884-018-1710-8 (2018). [DOI] [PMC free article] [PubMed]
- 47.Hartling, L. et al. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. preventive services task force and the national institutes of health office of medical applications of research. Ann. Intern. Med. 159, 123–129 (2013). [DOI] [PubMed]
- 48.Ornoy A. Growth and neurodevelopmental outcome of children born to mothers with pregestational and gestational diabetes. Pediatr. Endocrinol. Rev. 2005;3:104–113. [PubMed] [Google Scholar]
- 49.Oteng-Ntim, E., Varma, R., Croker, H., Poston, L. & Doyle, P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: Systematic review and meta-analysis. BMC Med.10, 47, 10.1186/1741-7015-10-47 (2012). [DOI] [PMC free article] [PubMed]
- 50.Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis. Can. 2010;30:125–134. doi: 10.24095/hpcdp.30.4.04. [DOI] [PubMed] [Google Scholar]
- 51.Chien YL, et al. Prenatal and perinatal risk factors and the clinical implications on autism spectrum disorder. Autism. 2018;23:783–791. doi: 10.1177/1362361318772813. [DOI] [PubMed] [Google Scholar]
- 52.Dodds L, et al. The role of prenatal, obstetric and neonatal factors in the development of autism. J. Autism Dev. Disord. 2011;41:891–902. doi: 10.1007/s10803-010-1114-8. [DOI] [PubMed] [Google Scholar]
- 53.George B, Padmam MS, Nair MK, Leena ML, Russell PS. CDC Kerala 13: Antenatal, natal and postnatal factors among children (2–6 y) with autism—A case control study. Indian J Pediatr. 2014;81:133–137. doi: 10.1007/s12098-014-1594-1. [DOI] [PubMed] [Google Scholar]
- 54.Hadjkacem I, et al. Prenatal, perinatal and postnatal factors associated with autism spectrum disorder. J. Pediatr. (Rio J). 2016;92:595–601. doi: 10.1016/j.jped.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 55.Kania M, et al. The association of maternal gestational diabetes mellitus with autism spectrum disorders in the offspring. Clin. Diabetol. 2016;5:147–151. doi: 10.5603/DK.2016.0026. [DOI] [Google Scholar]
- 56.Khanom F, Chowdhury S, Ahmed S, Moniruzzaman M, Ahmed MSAM. Association of autism spectrum disorder and gestational diabetes mellitus of mothers in Bangladesh. Indian J. Commun. Health. 2015;27:391–397. [Google Scholar]
- 57.Krakowiak P. et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics.129, e1121, 10.1542/peds.2011-2583 (2012). [DOI] [PMC free article] [PubMed]
- 58.Li M. et al. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics.137, e20152206, 10.1542/peds.2015-2206 (2016). [DOI] [PMC free article] [PubMed]
- 59.Raz R, et al. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: A nested case-control analysis within the Nurses’ Health Study II cohort. Environ. Health Perspect. 2015;123:264–270. doi: 10.1289/ehp.1408133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Say GN, Karabekiroğlu K, Babadağı Z, Yüce M. Maternal stress and perinatal features in autism and attention deficit/hyperactivity disorder. Pediatr. Int. 2016;58:265–269. doi: 10.1111/ped.12822. [DOI] [PubMed] [Google Scholar]
- 61.Straughen JK, et al. The association between placental histopathology and autism spectrum disorder. Placenta. 2017;57:183–188. doi: 10.1016/j.placenta.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 62.Akaltun İ., Yapça Ö.E., Ayaydin H. & Kara T. An evaluation of attention deficit hyperactivity disorder and specific learning disorder in children born to diabetic gravidas: A case control study. Anadolu Psikiyatr De.20, 10.5455/apd.10445 (2019).
- 63.Chiu YN, Gau SSF, Tsai WC, Soong WT, Shang CY. Demographic and perinatal factors for behavioral problems among children aged 4–9 in Taiwan. Psychiatry Clin. Neurosci. 2009;63:569–576. doi: 10.1111/j.1440-1819.2009.01979.x. [DOI] [PubMed] [Google Scholar]
- 64.Daraki V, et al. Effect of parental obesity and gestational diabetes on child neuropsychological and behavioral development at 4 years of age: The Rhea mother–child cohort, Crete, Greece. Eur. Child Adolesc. Psychiatry. 2017;26:703–714. doi: 10.1007/s00787-016-0934-2. [DOI] [PubMed] [Google Scholar]
- 65.Galera C, et al. Prenatal diet and children's trajectories of hyperactivity-inattention and conduct problems from 3 to 8 years: The EDEN mother-child cohort. J. Child Psychol. Psychiatry. 2018;59:1003–1011. doi: 10.1111/jcpp.12898. [DOI] [PubMed] [Google Scholar]
- 66.Ornoy A, Wolf A, Ratzon N, Greenbaum C, Dulitzky M. Neurodevelopmental outcome at early school age of children born to mothers with gestational diabetes. Arch. Dis. Child Fetal Neonatal Ed. 1999;81:F10–14. doi: 10.1136/fn.81.1.F10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pohlabeln H, et al. Further evidence for the role of pregnancy-induced hypertension and other early life influences in the development of ADHD: Results from the IDEFICS study. Eur. Child Adolesc. Psychiatry. 2017;26:957–967. doi: 10.1007/s00787-017-0966-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.