Abstract
Air pollution from vehicle emissions is a major problem in developing countries. Consequently, the use of iron-based rechargeable batteries, which is an effective method of reducing air pollution, have been extensively studied for electric vehicles. The structures and morphologies of iron particles significantly affect the cycle performance of iron-based rechargeable batteries. The synthesis parameters for these iron materials also remarkably influence their structures, shapes, sizes, and electrochemical properties. In this study, we fabricated α-Fe2O3 materials with various shapes and sizes via a facile hydrothermal route and investigated the effects of raw materials on their structures, morphologies, and properties. The structural characteristics of the synthesized iron oxides were studied via X-ray diffraction using scanning electron microscopy. Results indicate that changing the concentration of raw materials modified the structure and morphology of the synthesized α-Fe2O3 particles, that is, the desired shape and size of α-Fe2O3 can be controlled. The effects of the structure and morphology of α-Fe2O3 particles on their electrochemical characteristics were investigated. The results show that the morphology and shape of the iron oxide particles remarkably affected the redox reaction rate and discharge capacity of the Fe2O3/C composite electrodes. Among the synthesized α-Fe2O3 materials, the cubic-shaped α-Fe2O3 exhibited the highest discharge capacity. This material is a potential candidate for application in iron-based aqueous batteries. Our results may facilitate not only the controlled synthesis of α-Fe2O3 nanoparticles for potential technical applications but also the production of electrode materials with high capacity and good cycle performance for iron-based rechargeable batteries.
Subject terms: Energy science and technology, Materials science
Introduction
Metal–air batteries have attracted the attention of researchers because they have a high theoretical energy, are environment-friendly, and cost lower than lithium-ion batteries1–5. Among the metal–air batteries, iron–air batteries are the most valued because iron has high theoretical specific energy and low cost and is abundant on earth and often non-toxic6–10. However, the actual energy of iron–air batteries has remained low due the limitations of iron electrodes, such as large overpotential, the passivation caused by the iron hydroxide formed during discharge, and low coulombic efficiency. In addition, hydrogen evolves simultaneously with reduction reaction of iron lowers the charging efficiency results in loss of water from the electrolyte11–13.
In aqueous alkaline solution, two plateaus can be observed in the discharge process. The first plateau at = − 0.975 V versus Hg/HgO corresponds to the oxidation of iron to Fe(OH)2. The second plateau at − 0.758 V versus Hg/HgO or − 0.658 V versus Hg/HgO corresponds to the further oxidation of Fe(OH)2 to Fe(III). Iron (III) can be formed as Fe3O4, FeOOH, and/or Fe2O311,14,15:
| 1 |
| 2 |
and/or
| 3 |
The low electrical conductivity of the iron oxides formed during the discharge processes can result in the low coulombic efficiency of iron electrodes.
The hydrogen evolution reaction is expressed as follows:
| 4 |
The standard reduction potential of the hydrogen evolution in alkaline electrolyte (Eq. 4) is slightly more positive than that of the iron electrode reaction (Eq. 1). Hydrogen evolution is possible even at open circuit on an iron electrode in alkaline solution. Consequently, hydrogen evolution (Eq. 4) occurs during charging (Eq. 1) in addition to the reduction in the iron electrode. Simultaneous hydrogen evolution can result in low faradaic efficiency during charging.
Previous studies16–20 reported the following reactions of Fe2O3 electrodes in alkaline solution at the first charge:
| 5 |
and
| 6 |
The composition and structure of iron electrodes have been modified to address their limitations. Furthermore, some additives for electrodes and/or electrolyte solutions are used to increase the redox reaction rate and reduce the rate of hydrogen evolution, resulting in the improved capacity and efficiency of iron electrodes21–29. However, the limitations of iron electrodes have not been overcome completely, so more investigations are needed. Previous studies have shown that the structure and morphology of iron particles strongly influence their electrochemical properties in alkaline solutions24–32. In the present study, we synthesized α-Fe2O3 materials with various structures and morphologies using a hydrothermal route to find the most suitable material for iron-based aqueous batteries. The desired shape and size of α-Fe2O3 particles were controlled by changing the concentration of raw materials and the hydrothermal parameters. Furthermore, we applied additives to the electrolyte to further improve the capacity and cycle performance of iron electrodes.
Results and discussion
Figure 1 shows the X-ray patterns of the as-prepared materials. The patterns of all the samples exhibit some typical diffraction peaks at 2 theta values of 24.17°, 33.19°, 35.66°, 40.9°, 49.51°, 54.13°, 62.49°, and 64.05°, which respectively correspond to the (012), (104) (110), (113), (024), (116), (214), and (300) planes. All the peaks can be easily indexed to hematite α-Fe2O3 and are consistent with the reported values in ICSD No. 82135. No other peaks for impurities can be observed in the XRD patterns. This result confirms that the synthesized products are pure α-Fe2O3.
Figure 1.
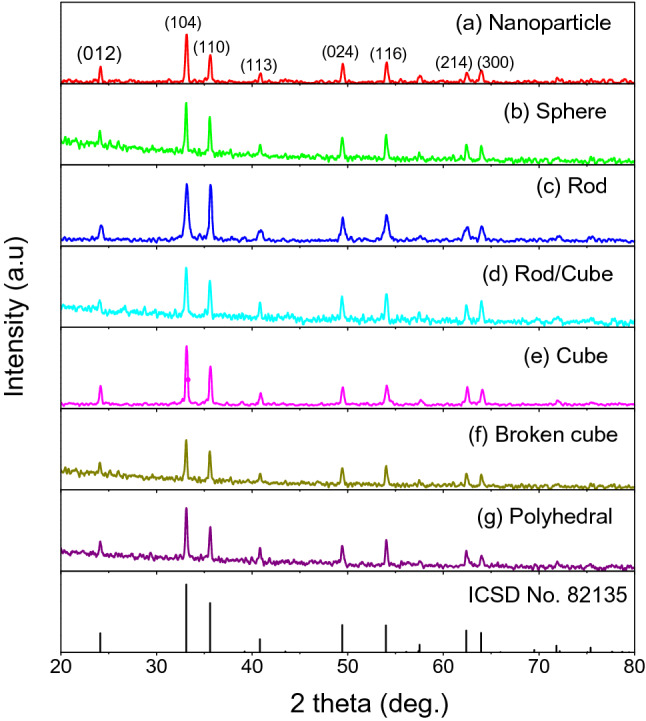
XRD patterns of α-Fe2O3 synthesized via hydrothermal route at various concentration ratios of FeCl3 and cetyltrimethylammonium bromide (CTAB) (/: (a) /, (b) , (c) , (d) , (e) , (f) , (g) .
From the Scherer equation, the mean size of hematite α-Fe2O3 crystallites is calculated, and the results are shown in Table 1. The concentration ratio of iron chloride and the CTAB surfactant (/) affects the average size of hematite crystallites. Without the CTAB (/), the mean size of hematite crystallites is approximately 29 nm (Fig. 1a). Using the CTAB with , if (/=0.02/0.01) (Fig. 1b), the mean size of hematite crystallites increases. However, if enhancing the concentration of FeCl3 to 0.05 M, the mean size of hematite crystallites decreases (Fig. 1c). The mean size of hematite crystallites increases when is maintained and the CTAB amount is increased to 0.02 M or 0.04 M, that is, / (Fig. 1d) or / (Fig. 1e). Similarly, the mean size of hematite crystallites increases when is maintained and the concentration of iron chloride is increased or decreased, that is, / (Fig. 1f) or / (Fig. 1g). Thus, the concentrations of iron chloride and the CTAB surfactant affect the mean size of hematite crystallites. The synthesized α-Fe2O3 hematite crystallites are polycrystalline materials whose particles contain several crystals.
Table 1.
Concentration ratios of precursors, mean size of the hematite crystallites synthesized via hydrothermal route.
| No. | Sample names | Concentration ratios of precursors (/ | Mean size of the hematite crystallites |
|---|---|---|---|
| 1 | Nanoparticle | / | 29.04 |
| 2 | Sphere | / | 36.36 |
| 3 | Rod | / | 20.52 |
| 4 | Rod/Cube | / | 30.68 |
| 5 | Cube | / | 33.08 |
| 6 | Broken cube | / | 36.41 |
| 7 | Polyhehral | / | 35.36 |
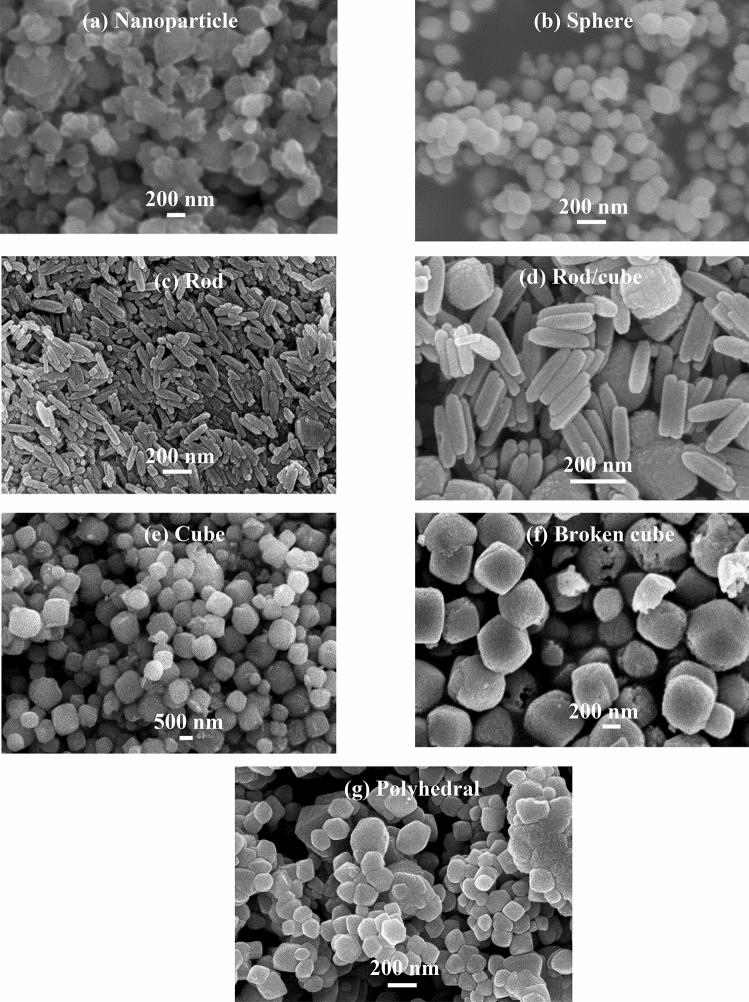
The SEM images of the α-Fe2O3 materials synthesized at various concentration ratios of FeCl3 and CTAB are shown in Fig. 2. All the samples were prepared under the same hydrothermal conditions, namely, at 120 °C for 14 h and a pH value of 10 in a typical hydrothermal process, but the concentration ratio of precursors FeCl3 and CTAB (/) was changed to obtain Fe2O3 materials with different morphologies, shapes, and sizes.
Figure 2.
SEM images of the α-Fe2O3 materials synthesized at various concentration ratios of FeCl3 and CTAB: (a) /(nanoparticle), (b) (sphere), (c) (rod), (d) (rod/cube), (e) (cube), (f) (broken cube), (g) (polyhedral).
In this study, CTAB acts as a cationic surfactant during synthesis. Thus, the CTAB amount affects the morphological characteristics of the synthesized iron oxides. Furthermore, the concentration of iron chloride also affects the morphology of the obtained iron oxides. By adjusting the concentration ratio of / in the reaction solution, different morphologies of α-Fe2O3 nanoparticles can be obtained. Pu et al.33 showed that the α-Fe2O3 formation in the hydrothermal process may proceed through two steps. First, Fe3+ is hydrolyzed in aqueous solution to form the β-FeOOH precursor. Second, the resulting β-FeOOH undergoes topotactic transformation to α-Fe2O3 via the dissolution/reprecipitation mechanism. They demonstrated that the morphologies of the β-FeOOH precursors change continuously from nanosphere, nanorod, to nanoraft with the increase of the FeCl3 concentration under the confinement of surfactants.
Figure 2a presents the sample prepared with = 0.02 M without CTAB (/) is nanostructured particles, free shape, non-uniform with diameters from a few hundred nanometers to one micrometer. When and (/=0.02/0.01) (Fig. 2b), sphere-shaped iron oxides are formed with diameters less than 200 nm. When and the iron chloride amount is increased to 0.05 M, that is, /, the product comprises rod-like nanoparticles (Fig. 2c). The diameters of the α-Fe2O3 nanorods are approximately 50 nm, and their lengths range from 100 to 200 nm. When and the CTAB concentration is increased to , nanorods aggregate and thus form some nanocubes in the product (Fig. 2d). Notably, when , perfect cubic-shaped structures are observed in the sample (Fig. 2e), but their dimensions are not uniform, with edges of a few hundred nanometers to one micrometer. Thus, when is low at , the product mainly consists of zero-dimensional nanoparticles, such as nanospheres (Fig. 2b). According to Pu et al.33, the precursors in this case might be sphere-like particles of several nanometers. The shapes of the synthesized products turn to rods (Fig. 2c) and cubes (Fig. 2e) with the gradual increase of the FeCl3 concentration. The cubes are attributed to be transformed from raft-like precursors or aggregation of nanorods. The product containing both rods and cubes (Fig. 2d) is an intermediate step of turning from rods to cubes, suggesting that the precursor morphologies change from sphere to rod to cube with the increase of . This transformation is like that observed by Pu et al.33. When and the iron chloride amount is increased to 0.07 M, that is, /, the product still consists of nanocubes, and some of them are broken (Fig. 2f). When the iron chloride concentration is decreased to while (/), the perfect cubic-shaped structures of Fe2O3 are destroyed, the sample contains non-uniform polyhedral particles, and the particle sizes are less than one micrometer.
Consequently, when the FeCl3 concentration is low, sphere-like β-FeOOH precursors of several nanometers and capped completely by CTAB are formed and transformed into sphere-shaped α-Fe2O3 during precursor dissolution/reprecipitation. By increasing the amount of FeCl3 to moderate, α-Fe2O3 nanorods form via the transformation of rod-like β-FeOOH precursors. When the FeCl3 concentration is high, the precursors turn to larger raft-like particles, which aggregate and transform to cubic-shaped α-Fe2O3particles. These results indicate that the shapes of the β-FeOOH precursors have a significant effect on the morphologies of the synthesized α-Fe2O3, and CTAB plays an important role in mediating the hydrolysis of FeCl3. CTAB-the cationic surfactant-capping agents can confine the growth of iron(III) oxides in the nanometer regime. The concentration ratios of raw materials FeCl3 and CTAB (/, the sample names, and the preparation conditions are listed in Table 2.
Table 2.
Preparation conditions and samples synthesized via hydrothermal route.
| No. | Sample names | Concentration ratios of precursors (/ | Hydrothermal treatment |
|---|---|---|---|
| 1 | Nanoparticle | / | 120 °C—14 h, pH 10 |
| 2 | Sphere | / | 120 °C—14 h, pH 10 |
| 3 | Rod | / | 120 °C—14 h, pH 10 |
| 4 | Rod/Cube | / | 120 °C—14 h, pH 10 |
| 5 | Cube | / | 120 °C—14 h, pH 10 |
| 6 | Broken cube | / | 120 °C—14 h, pH 10 |
| 7 | Polyhehral | / | 120 °C—14 h, pH 10 |
In this case, the hydrothermal parameters, namely, pH value, time, and temperature reaction, were kept constant with the changing concentrations of the raw materials, namely, and . In the presence of CTAB, α-Fe2O3 nanoparticles with different shapes were obtained when was changed. When , α-Fe2O3 nanospheres formed. The shapes of the synthesized α-Fe2O3 turned to rods (Fig. 2c) and then cubes (Fig. 2e) with the gradual increase of the FeCl3 concentration. When CTAB was not used, the particles grew randomly to form nanoparticles with different shapes and sizes (Fig. 2a). At , α-Fe2O3 nanospheres appeared when . When the CTAB concentration was increased to 0.04 and , the morphologies of α-Fe2O3 changed from spheres to rods and then to cubes. When , the morphology of the α-Fe2O3 particles changed to imperfect cubes, that is, broken (Fig. 2f) or polyhedral (Fig. 2g) cubes, with the further increase or decrease of the FeCl3 concentration. This result confirms that CTAB can confine the growth of synthesized products in the nanometer regime, while the FeCl3 concentration can change the shape of the α-Fe2O3 nanoparticles. These results are consistent with those in the literature33. Therefore, the morphological characteristics of α-Fe2O3 are controlled by changing the concentration of raw materials. The synthesized α-Fe2O3 nanoparticles with various morphologies can be applied in various technologies. The α-Fe2O3 particles with perfect cubic-shaped structures are expected to improve the electrochemical properties of the iron-based battery anode.
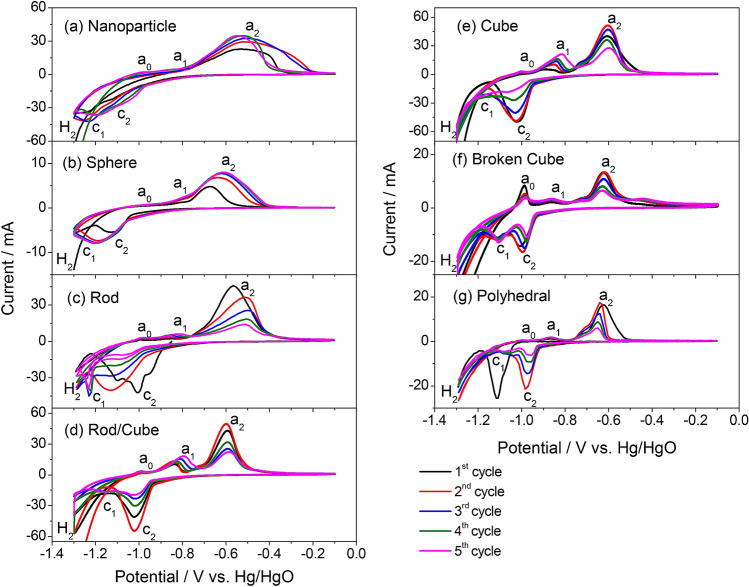
The cyclic voltammetry (CV) measurements of the Fe2O3/AB electrodes in base electrolyte were taken to investigate the electrochemical properties of the α-Fe2O3 materials fabricated via a hydrothermal route, and the results are presented in Fig. 3. The CV profiles of the samples are similar, except that of the Fe2O3/AB electrodes using the Fe2O3 nanoparticle obtained at / (Fig. 3a). The CV results of the samples depict two pairs of redox peaks, namely, Fe/Fe(II) (a1/c1) and Fe(II)/Fe(III) (a2/c2), observed at approximately − 0.8 V (a1)/ − 1.1 V (c1) and − 0.6 V (a2)/ − 1.0 V (c2). Anodic peaks a1 and a2 can be attributed to the oxidation of Fe to Fe(II) and Fe(II) to Fe(III), while cathodic peaks c2 and c1 correspond to the reduction of Fe(III)/Fe(II) and Fe(II)/Fe, respectively. Thus, a1 and c1 correspond to the Fe/Fe(II) redox couple (Eq. 1), while a2 and c2 correspond to the Fe(II)/Fe(III) redox couple (Eqs. 2 or 3). Furthermore, we also observed a small oxidation peak (a0) around − 1.0 V and hydrogen evolution around − 1.2 V. According to Cerny et al.15, the first oxidation peak (a0) corresponds to iron oxidation to form [Fe(OH)]ads before Fe(OH)2 is produced. Oxidation peak a2 is relatively larger and broader than a1, especially for the Fe2O3 nanoparticles (Fig. 3a), and the redox current under the a2/c2 couple is higher than that under the a1/c1 couple, suggesting that anodic peak a2 covers the oxidation of the Fe/Fe(II) and Fe(II)/Fe(III) couples. Remarkably, the reduction peaks (c1) of the Fe2O3/AB electrodes with Fe2O3 nanoparticles (Fig. 3a) and sphere (Fig. 3b) are completely covered by hydrogen evolution peaks, which may result in low charge efficiency.
Figure 3.
CVs of Fe2O3/AB electrodes with various morphologies of Fe2O3 synthesized via hydrothermal route in KOH solution: (a) nanoparticle, (b) sphere, (c) rod, (d) rod/cube, (e) cube, (f) broken cube, and (g) polyhedral.
Further cycling, the oxidation peaks shifted toward a more positive potential, while the reduction peaks moved toward a more negative potential with the Fe2O3 nanoparticles (Fig. 3a), sphere (Fig. 3b), and rod (Fig. 3c) samples; this shift increased the overpotentials, leading to the disappearance of redox peaks. In contrast, the overpotentials gradually decreased with the Fe2O3 rods/cubes (Fig. 3d), cubes (Fig. 3e), broken cubes (Fig. 3f), and polyhedral particles (Fig. 3g). The increase in overpotential may cause negative effects on the capacity and cycling efficiency of Fe2O3/AB electrodes. In addition, the redox currents of the electrodes with Fe2O3 nanoparticles (Fig. 3a) and sphere (Fig. 3b) increased upon cycling and decreased with the remaining Fe2O3 samples (Fig. 3c–f).
The CV profiles of the electrodes with Fe2O3 rode/cube, cube, broken cube, and polyhedral samples are similar (Fig. 3d–g); for instance, the redox current increased and then decreased, and their reduction peaks (c1) are separated from the hydrogen evolution peaks. This behavior is quite different from that of the Fe2O3 nanoparticle and sphere samples (Fig. 3a,b), that is, the redox current gradually increased, and their reduction peaks (c1) disappeared. In contrast, the Fe2O3 rod (Fig. 3c) seems to be the intermediate state of the two types of CV curves. These CV results are consistent with the observation of the SEM results as evidenced by the Fe2O3 rode/cube, cube, broken cube, and polyhedral samples (Fig. 2d–g), which have similar morphologies, while the Fe2O3 nanoparticle and sphere samples (Fig. 2a,b) have similar morphologies, and the rod (Fig. 2c) is in intermediate shape. However, the cubic-shaped structures of the Fe2O3 in the polyhedral sample (Fig. 2g) are destroyed, exhibiting random polyhedral particle shapes. Therefore, they provide smaller redox peaks (Fig. 3g) than other cubic-shaped Fe2O3 samples (Fig. 3d–f). These findings suggest that the morphological characteristics of the Fe2O3 synthesized via a hydrothermal route significantly affect the electrochemical properties of Fe2O3/AB electrodes.
A comparison of the CV results of all samples Fe2O3 nanoparticle, sphere, rod, rod/cube, cube, broken cube and polyhedral shows that cubic-shaped Fe2O3 provides better cyclability than the other samples as clearly evidenced by the well-defined redox peaks. This phenomenon is acceptable because the morphological characteristics of iron oxide affect its redox behaviors. At similar cubic shapes, the Fe2O3 particles in the electrode are close-packed, and the contact between cubic-shaped Fe2O3 and AB should be tighter than that between other-shaped Fe2O3 and AB. Thus, the internal resistance of the cubic-shaped Fe2O3/AB electrode should be smaller than that of the other-shaped Fe2O3/AB electrode. Consequently, the reaction rate of the cubic-shaped Fe2O3 particles was higher than that of other-shaped Fe2O3 particles. The SEM images of the synthesized Fe2O3 materials show that Fe2O3 nanoparticles (Fig. 2a), spheres (Fig. 2b), and rods (Fig. 2c) have a smaller size than cubic-shaped Fe2O3 (Fig. 2d–g) and thus need more binders to prepare Fe2O3/AB electrodes. However, all the electrodes were prepared at the same ratio of Fe2O3:AB:PTFE = 45:45:10 wt%. The number of binders was insufficient to tightly bind to iron oxide and the AB particles in the electrodes when a smaller Fe2O3 size was used, resulting in the high internal resistance of the Fe2O3/AB electrode. Consequently, Fe2O3/AB electrodes with Fe2O3 nanoparticles, spheres, and rods exhibit a low redox reaction rate, leading to increased overpotentials and unobservable peaks, whereas the cubic-shaped Fe2O3 provides better cyclability and observable oxidation peaks (c1).
Thus, the Fe2O3 materials synthesized at different hydrothermal conditions exhibited different morphologies and consequently provided different electrochemical characterizations. In other words, hydrothermal parameters affected the particle sizes and shapes of the Fe2O3 materials and strongly influenced their electrochemical properties. Therefore, by changing the preparation conditions, we can control the desired morphologies, sizes, and shapes of the Fe2O3 materials to provide the best electrochemical characterizations.
The CV results show that the shape, size, and morphology of the Fe2O3 particles strongly affected the electrochemical properties of Fe2O3/AB electrodes, and the cubic-shaped Fe2O3, including rods/cubes, cubes, and broken cubes, exhibited better cyclability than the Fe2O3 nanoparticles, spheres, and rods.
To find the most suitable Fe2O3 among the synthesized materials, the discharge capacities of the Fe2O3/AB electrodes with all the synthesized Fe2O3 were calculated from the CV profiles, and the results are shown in Fig. 4. Among the synthesized materials, the Fe2O3 cube provided the largest discharge capacities. This result is acceptable from the perspective that among the cubic-shaped Fe2O3, the Fe2O3 cubes possessed a perfect cubic shape, whereas the Fe2O3 rods/cubes contained rods and the broken cubes fragments of cubes. This result confirms that Fe2O3 cube is the most suitable material. However, the discharge capacity of the Fe2O3/AB electrode with an Fe2O3 cube gradually decreased with repeated cycling. The electrolyte additive was used to overcome this problem.
Figure 4.
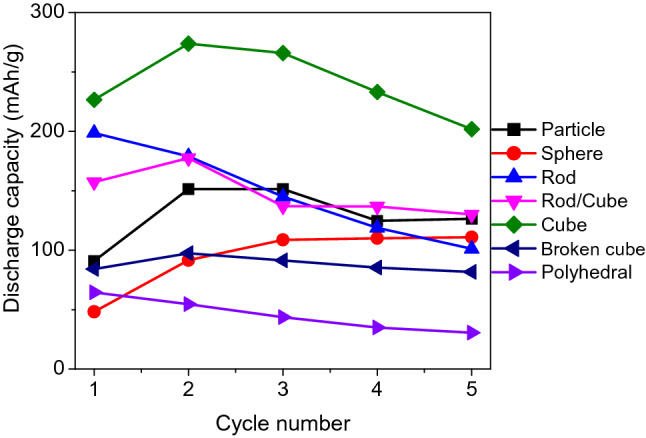
Discharge capacities of Fe2O3/AB electrodes in KOH solution.
The effects of the K2S additive on the electrochemical properties of the Fe2O3/AB composite electrode were investigated with the Fe2O3 cube, showing the highest capacity (Fig. 4), and the result is shown in Fig. 5. The Fe2O3 cube sample in the additive electrolyte has larger discharge capacity than that in the base electrolyte. The positive effects of the S2− additive have been reported in previous studies as follows. Ion sulfide is incorporated into the oxide lattice and interacts with Fe(I), Fe(II), or Fe(III) in the oxide film to promote the dissolution of iron24,28, thus preventing the rapid passivation of iron electrodes during discharge, and increasing the hydrogen evolution overpotential and extending the discharge curve13,24–29, thereby improving the cyclability and the discharge rates.
Figure 5.

Discharge capacities of Fe2O3/AB electrodes with Fe2O3 cube in KOH and KOH + K2S solutions.
The charge–discharge measurement was taken in KOH aqueous solution containing K2S additive to evaluate the applicability of α-Fe2O3 cube materials synthesized via a hydrothermal route in rechargeable batteries. Figure 6 presents the discharge capacities as a function of the number of cycles for the Fe2O3/AB electrode using α-Fe2O3 cube in the additive electrolyte. The discharge capacities increased from 418 mAh g−1 in the first cycle to 457, 478, and 481 mAh g−1 in the second, third, and fourth cycles, respectively, and then gradually decreased to 478 mAh g−1 in the fifth cycle. The discharge capacity of the Fe2O3/AB electrode was approximately 365 mAhg−1 at the 30th cycle. Thus, the discharge capacities gradually decreased with the number of cycles. This capacity decline is ascribed to the passivation of the Fe(OH)2 layer formed during the discharge process even in presence of K2S additive in the electrolyte. Yang et al.28 investigated sintered iron electrodes and found that the electrode surface is packed closely, and the pores are also filled with the discharge products. In the inner part of the electrode, the discharge products appeared less tightly packed, and a considerable number of pores remained available. They also demonstrated that with small pores, a larger surface of the active material is available for the electrode reaction during discharge. In our case, the Fe2O3/AB composite electrode was prepared by pressing a mixture of the Fe2O3 particles, AB, and PTFE binder, resulting in high porosity and small pores. It also has a large active material surface for better electrode reaction during discharge. However, capacity decline still occurs and is attributed to the passivation of the Fe(OH)2 layer formed during the discharge process. At the first cycle, the discharged product (Fe(OH)2) is formed on the outer parts of the electrode, and the interior parts of the electrode become inaccessible to the electrolyte. Consequently, the iron oxide particles in the interior of the electrode remain under-utilized. Given the small pores of the electrode, in the next cycles, the electrolyte diffuses into the inner parts of the electrode, and redox reactions occur, thereby increasing the capacity. However, the redox reactions of the inner portions of the porous electrodes are poor. With further cycling, the pores are filled with the Fe(OH)2 product, blocking the electrolyte access. Consequently, the capacity gradually decreases.
Figure 6.
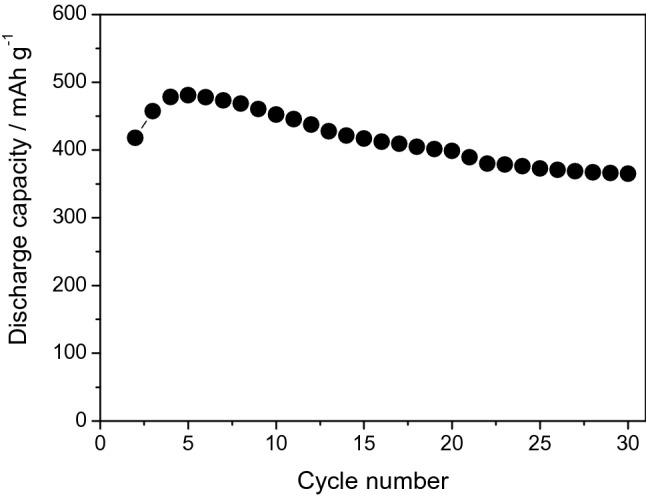
Discharge capacities of Fe2O3/AB electrode with cubic-shaped α-Fe2O3 in KOH + K2S solution.
As mentioned above, when K2S was added to the electrolyte, S2- additives interacted with Fe(I), Fe(II), and Fe(III) in the oxide film to promote the dissolution of iron, increasing the hydrogen evolution overpotential and prolonging the discharge curve, thereby improving the cyclability and discharge rate of iron electrodes. From the charge–discharge curves, we can estimate the hydrogen evolution produced during the charge and the contribution of the Fe/Fe(II) and Fe(II)/Fe(III) redox couples to the discharge capacity of the electrode. Therefore, the charge–discharge measurement of the Fe2O3/AB electrode additive was taken in the additive electrolyte, and the results are shown in Fig. 7.
Figure 7.
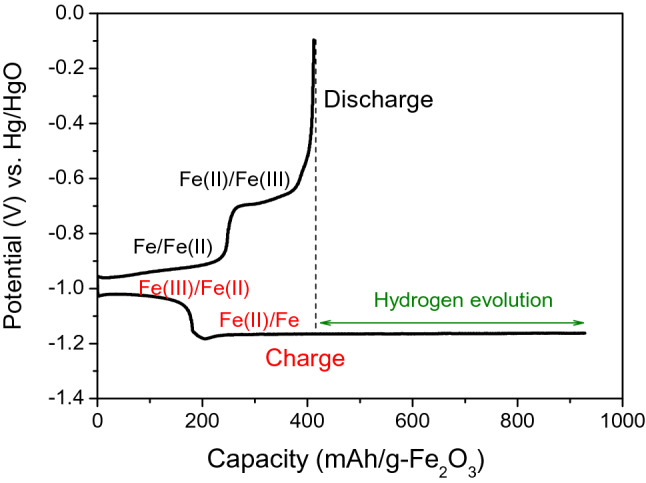
Charge–discharge curves of Fe2O3/AB electrode with cubic-shaped α-Fe2O3 in KOH + K2S.
The charge capacity is much larger than the discharge capacity. Two plateaus are observed at approximately − 0.95 and − 0.70 V (vs. Hg/HgO) in the discharge curves corresponding to the Fe/Fe(II) (Eq. 1) and Fe(II)/Fe(III) (Eqs. 2 and/or 3) reactions, whereas they occur at approximately − 0.95 and − 1.15 V (vs. Hg/HgO) in the charge curves. During the charging process, the valence of iron changes from Fe(III) to Fe(II) and then Fe(II) to Fe. The length of the first and second plateaus in the discharge curves is nearly the same. In the charge curves, the second plateau is longer than the first plateau, suggesting that the second plateau covered the reduction reaction of Fe(II)/Fe (Eq. 1) and hydrogen evolution (Eq. 4). The difference between the discharge and charge capacities is ascribed to the hydrogen evolution reaction. This result demonstrates that hydrogen evolution was only partly suppressed by the S2- additive. The simultaneous hydrogen evolution with the iron deposition reaction results in a low faradaic efficiency during charging and discharge capacity deterioration. Thus, capacity decline is related to the passivation of the Fe(OH)2 formed during the discharge process and the hydrogen evolution reaction during the charge process.
With the further improvement in the capacity retention of Fe2O3/AB composite electrodes, the synthesized cubic-shaped α-Fe2O3 material can be a potential candidate for energy storage systems. Furthermore, the α-Fe2O3 nanoparticles with various morphologies synthesized via a facile hydrothermal route can also be potential materials for other technical applications.
Conclusion
In the present study, various morphologies of α-Fe2O3, including nanoparticle, sphere, rod, rod/cube, cube, broken cube, and polyhedral shapes, were successfully synthesized via a facile hydrothermal route in which the morphology is controllable by changing the concentration of raw materials FeCl3 and CTAB. CTAB acts as a surfactant and capping agent and FeCl3 acts as the iron source during synthesis; thus, their concentrations affect the morphological characteristics of the synthesized iron oxides. CTAB can confine the growth of the synthesized α-Fe2O3 in the nanometer regime, while the concentration of FeCl3 changes the shape of the α-Fe2O3 nanoparticles. By adjusting the concentration ratio of / in the reaction solution, various morphologies of α-Fe2O3 materials could be produced. These α-Fe2O3 nanoparticles present potential applications in various technological areas.
The investigation of the electrochemical behaviors of the Fe2O3/AB composite electrodes using synthesized α-Fe2O3 revealed that the morphology, shape, and size of Fe2O3 particles strongly affect the performance of Fe2O3/AB electrodes. Among the various synthesized α-Fe2O3, the cubic shape displayed the highest discharge capacity, but the discharge capacity gradually decreased with prolonged cycling. The K2S additive in the electrolyte was used to improve the capacity decline. The positive effect of the K2S additive on the Fe2O3/AB electrodes was confirmed by the good cyclability and large capacity of the Fe2O3/AB electrodes in the additive electrolyte. Through the optimization of the fabrication conditions and the further improvement in capacity retention, cubic-shaped α-Fe2O3 synthesized via hydrothermal treatment is a potential material for iron-based battery anodes. The controllable synthesis of α-Fe2O3 nanoparticles via a facile hydrothermal route is a potential method of producing a large number of nanomaterials for various applications.
Experimental
Synthesis of α-Fe2O3 nanoparticles with various morphologies
We prepared α-Fe2O3 nanoparticles with different sizes and shapes using the method reported in literature33 with modifications. The chemicals used in this work, including iron chloride (FeCl3.6H2O) and cetyltrimethylammonium bromide (CTAB), were of analytical grade. CTAB was used as a surfactant and iron chloride as the iron source. Initially, CTAB aqueous solutions with various concentrations () of 0.01, 0.02, and 0.04 M were prepared by dissolving corresponding CTAB amounts in deionized water, stirring the mixture until the solution became transparent. Iron chloride aqueous solutions with various concentrations () of 0.02, 0.03, 0.05, and 0.07 M were prepared using the same process. Then, 80 ml of iron chloride aqueous solution was dissolved in 80 ml of the above CTAB aqueous solution with various concentration ratios of FeCl3/CTAB (/), namely, 0.02/0, 0.02/0.01, 0.05/0.01, 0.05/0.02, 0.05/0.04, 0.07/0.04, and 0.03/0.04. The resultant mixtures were stirred for 60 min to form homogeneous solutions, and the pH value was adjusted to 10 using NH3 before they were transferred into a Teflon-lined stainless-steel autoclave, sealed, and maintained at 120 °C for 14 h in a typical hydrothermal process. The autoclave was then gradually cooled down to room temperature. The red brown precipitates were collected through centrifugation, washed several times with distilled water and ethanol, subsequently dried at 60 °C for 12 h, and finally annealed at 400 °C for 2 h in air to obtain α-Fe2O3 powder.
Characterization
The crystal structure of the synthesized powder was identified using an X-ray diffractometer (XRD; Rigaku) with Cu Kα radiation (k = 0.1542 nm) at 40 kV and 150 mA. The diffraction pattern was recorded in the 2-theta range of 20°–80° with a scanning rate of 5°/min. The morphology and shape of the as-synthesized products were observed by scanning electron microscopy (SEM, JEOL JSM-6060LA/VI, Japan) with an accelerating voltage of 25 kV.
Electrode preparation
The electrode sheet was prepared by mixing 45 wt% synthesized Fe2O3 powder as an active material, 45 wt% acetylene black carbon (AB, Denki Kagaku Co.) as an additive, and 10 wt% polytetrafluoroethylene (PTFE; Daikin Co.) binder, followed by rolling. The Fe2O3/AB composite electrodes were punched from the electrode sheets into the pellets of 1-cm diameter. The electrode was then pressed onto the conductive Titanium mesh with a pressure of approximately 150 kg cm−2.
Electrochemical measurements
The electrochemical measurements are described in our previous work32,34. In this study, we applied the charge current density of 50 mA cm−2 instead of 5 mA cm−2 in the cycling performance measurement. The details of the electrochemical measurements are as follows. CV and charge/discharge measurements were taken on a three-electrode glass cell assembly with an Fe2O3/AB composite as the working electrode, Pt mesh as the counter electrode, and Hg/HgO as the reference electrode. The electrolyte was either a base electrolyte or an additive electrolyte. The base electrolyte was 8 mol dm−3 of aqueous KOH solution, and the additive electrolyte was a KOH solution containing 0.01 M K2S. The CV measurements were taken at a scan rate of 5 mV s-1 and within the range of − 1.3 V to − 0.1 V.
For the cycling performance measurement, the galvanostatic process with a cutoff capacity of 1007 mAh g−1 Fe2O3 was carried out in the charge course. The applied charge current density was 50 mA cm−2. In the discharge course, a constant current density of 2.0 mAcm−2 was applied to the electrode with a cutoff potential of − 0.1 V. In all the electrochemical measurements, fresh electrodes were used without precycling.
Acknowledgments
This research was funded by Vietnam National Foundation for Science and Technology Development under Grant number 103.02-2018.04.
Author contributions
B.T.H. designed the experiments, analyzed the experimental results and wrote the manuscript. T.T.A. performed the measurements. All authors discussed the research and reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dobley A., Robak J., FinalReport on Research of Air Cathodes for Aluminum Air Batteries, 16 (2004).
- 2.Zhang T, Tao Z, Chen J. Magnesium–air batteries: From principle to application. Mater. Horiz. 2014;1(2):196–206. doi: 10.1039/C3MH00059A. [DOI] [Google Scholar]
- 3.Wang K, Pei P, Ma Z, Chen H, Xu H, Chen D, Wang X. Dendrite growth in the recharging process of zinc–air batteries. J. Mater. Chem. A. 2015;3(45):22648–22655. doi: 10.1039/C5TA06366C. [DOI] [Google Scholar]
- 4.Higashi S, Lee SW, Lee JS, Takechi K, Cui Y. Avoiding short circuits from zinc metal dendrites in anode by backside-plating configuration. Nat. Commun. 2016;7:11801. doi: 10.1038/ncomms11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKerracher RD, Ponce de Leon C, Wills RGA, Shah AA, Walsh FC. A review of the iron-air secondary battery for energy storage. ChemPlusChem. 2015;80(2):323–335. doi: 10.1002/cplu.201402238. [DOI] [Google Scholar]
- 6.Narayanan SR, Prakash GKS, Manohar A, Yang B, Malkhandi S, Kindler A. Materials challenges and technical approaches for realizing inexpensive and robust iron–air batteries for large-scale energy storage. Solid State Ion. 2012;216:105–109. doi: 10.1016/j.ssi.2011.12.002. [DOI] [Google Scholar]
- 7.Manohar AK, Malkhandi S, Yang B, Yang C, Surya Prakash GK, Narayanan SR. A high-performance rechargeable iron electrode for large-scale battery-based energy storage. J. Electrochem. Soc. 2012;159(8):A1209–A1214. doi: 10.1149/2.034208jes. [DOI] [Google Scholar]
- 8.McKerracher RD, Figueredo-Rodriguez HA, Alegre C, Arico AS, Baglio V, Ponce de Leon C. Improving the stability and discharge capacity of nanostructured Fe2O3/C anodes for iron-air batteries and investigation of 1-octhanethiol as an electrolyte additive. Electrochim. Acta. 2019;318:625–634. doi: 10.1016/j.electacta.2019.06.043. [DOI] [Google Scholar]
- 9.Ohmori H, Iwai H. Simulation of solid oxide iron-air battery: Effects of heat and mass transfer on charge/discharge characteristics. J. Power Sources. 2015;286:264–275. doi: 10.1016/j.jpowsour.2015.03.149. [DOI] [Google Scholar]
- 10.Um T, Wilke SK, Choe H, Dunand DC. Effects of pore morphology on the cyclical oxidation/reduction of iron foams created via camphene-based freeze casting. J. Alloy. Compd. 2020;845:156278. doi: 10.1016/j.jallcom.2020.156278. [DOI] [Google Scholar]
- 11.Micka K, Zábranský Z. Study of iron oxide electrodes in an alkaline electrolyte. J. Power Sources. 1987;19(4):315–323. doi: 10.1016/0378-7753(87)87007-6. [DOI] [Google Scholar]
- 12.Vijayamohanan K, Balasubramanian TS, Shukla AK. Rechargeable alkaline iron electrodes. J. Power Sources. 1991;34(3):269–285. doi: 10.1016/0378-7753(91)80093-D. [DOI] [Google Scholar]
- 13.Caldas CA, Lopes MC, Carlos IA. The role of FeS and (NH4)2CO3 additives on the pressed type Fe electrode. J. Power Sources. 1998;74(1):108–112. doi: 10.1016/S0378-7753(98)00039-1. [DOI] [Google Scholar]
- 14.Chakkaravarthy C, Perasamy P, Jegannathan S, Vasu KI. The nickel/iron battery. J. Power Sources. 1991;35:21–35. doi: 10.1016/0378-7753(91)80002-F. [DOI] [Google Scholar]
- 15.Cerny KMJ. Voltammetric study of an iron electrode in alkaline electrolytes. J. Power Sources. 1989;25:111–122. doi: 10.1016/0378-7753(89)85003-7. [DOI] [Google Scholar]
- 16.Salkind AJ, Venuto CJ, Falk SU. The reaction at the iron alkaline electrode. J. Electrochem. Soc. 1964;111:493–495. doi: 10.1149/1.2426166. [DOI] [Google Scholar]
- 17.Ojefors L. SEM studies of discharge products from alkaline iron electrodes. J. Electrochem. Soc. 1976;123:1691–1696. doi: 10.1149/1.2132669. [DOI] [Google Scholar]
- 18.Armstrong RD, Baurhoo I. The dissolution of iron in concentrated alkali. J. Electroanal. Chem. 1972;40:325–338. doi: 10.1016/S0022-0728(72)80377-2. [DOI] [Google Scholar]
- 19.Periasamy P, Babu BR, Iyer SV. Cyclic voltammetric studies of porous iron electrodes in alkaline-solutions used for alkaline batteries. J. Power Sources. 1996;58:35–40. doi: 10.1016/0378-7753(95)02274-0. [DOI] [Google Scholar]
- 20.Periasamy P, Babu BR, Iyer SV. Electrochemical behaviour of Teflon-bonded iron oxide electrodes in alkaline solutions. J. Power Sources. 1997;63:79–85. doi: 10.1016/S0378-7753(96)02449-4. [DOI] [Google Scholar]
- 21.Hang BT, Eashira M, Watanabe I, Okada S, Yamaki JI, Yoon SH, Mochida I. The effect of carbon species on the properties of Fe/C composite for metal-air battery anode. J. Power Sources. 2005;143:256–264. doi: 10.1016/j.jpowsour.2004.11.044. [DOI] [Google Scholar]
- 22.Hang BT, Yoon S, Okada S, Yamaki J. Effect of metal-sulfide additives on electrochemical properties of nano-sized Fe2O3-loaded carbon for Fe/air battery anodes. J. Power Sources. 2007;168:522–532. doi: 10.1016/j.jpowsour.2007.02.067. [DOI] [Google Scholar]
- 23.Manohar AK, Yang C, Narayanan SR. The role of sulfide additives in achieving long cycle life rechargeable iron electrodes in alkaline batteries. J. Electrochem. Soc. 2015;162(9):A1864–A1872. doi: 10.1149/2.0741509jes. [DOI] [Google Scholar]
- 24.Ravikumar MK, Rajan AS, Sampath S, Priolkar KR, Shukla AK. In situ crystallographic probing on ameliorating effect of sulfide additives and carbon grafting in iron electrodes. J. Electrochem. Soc. 2015;162:A2339–A2350. doi: 10.1149/2.0721512jes. [DOI] [Google Scholar]
- 25.McKerracher RD, Figueredo-Rodriguez HA, Dimogiannis K, Alegre C, Villanueva-Martinez NI, Lázaro MJ, Aricò AS, Ponce de Leόn CP. Effect of 1-octanethiol as an electrolyte additive on the performance of the iron-air battery electrodes. J. Solid State Electrochem. 2021;25(1):225–230. doi: 10.1007/s10008-020-04738-4. [DOI] [Google Scholar]
- 26.Gil Posada JO, Hall PJ. Post-hoc comparisons among iron electrode formulations based on bismuth, bismuth sulphide, ironsulphide, and potassium sulphide. J. Appl. Electrochem. 2016;46:451–458. doi: 10.1007/s10800-015-0911-3. [DOI] [Google Scholar]
- 27.Wang Q, Wang Y. Overcoming the limiting step of Fe2O3 reduction via in situ sulfide modification. ACS Appl. Mater. Interfaces. 2016;8:10334–10342. doi: 10.1021/acsami.6b01737. [DOI] [PubMed] [Google Scholar]
- 28.Yang C, Manohar AK, Narayanan SR. A high-performance sintered iron electrode for rechargeable alkaline batteries to enable large-scale energy storage. J. Electrochem. Soc. 2017;164(2):A418–A429. doi: 10.1149/2.1161702jes. [DOI] [Google Scholar]
- 29.Chamoun M, Skarman B, Vidarsson H, Smith RI, Hull S, Lelis M, Milcius D, Noreus D. Stannate increases hydrogen evolution overpotential on rechargeable alkaline iron electrodes. J. Electrochem. Soc. 2017;164(6):A1251–A1257. doi: 10.1149/2.1401706jes. [DOI] [Google Scholar]
- 30.Bharathi S, Nataraj D, Mangalaraj D, Masuda Y, Senthil K, Yong K. Highly mesoporous α-Fe2O3 nanostructures: Preparation, characterization and improved photocatalytic performance towards Rhodamine B (RhB) J. Phys. D Appl. Phys. 2009;43(1):15501. doi: 10.1088/0022-3727/43/1/015501. [DOI] [Google Scholar]
- 31.Long NV, Yang Y, Thi CM, Hang BT, Cao Y, Nogami M. Controlled synthesis and characterization of iron oxide micro-particles for Fe-air battery electrode material. Colloid Polym. Sci. 2014;293(1):49–63. doi: 10.1007/s00396-014-3363-0. [DOI] [Google Scholar]
- 32.Hang BT, Anh TT, Thang DH. Effect of morphology on the electrochemical properties of Fe/C composite electrode as Fe-air battery anode. J. Nanosci. Nanotechnol. 2016;16(8):7999–8006. doi: 10.1166/jnn.2016.12760. [DOI] [Google Scholar]
- 33.Pu Z, Cao M, Yang J, Huang K, Hu C. Controlled synthesis and growth mechanism of hematite nanorhombohedra, nanorods and nanocubes. Nanotechnology. 2006;17(3):799–804. doi: 10.1088/0957-4484/17/3/031. [DOI] [Google Scholar]
- 34.Hang BT, Thang DH, Nga NT, Minh PTL, Kobayashi E. Nanoparticle Fe2O3-loaded carbon nanofibers as iron-air battery anodes. J. Electrochem. Soc. 2013;160(9):A1442–A1445. doi: 10.1149/2.066309jes. [DOI] [Google Scholar]