Abstract
Obesity is associated with complex perturbations to whole-body and tissue iron homeostasis. Recent evidence suggests a potentially important influence of iron storage in skeletal muscle on whole-body iron homeostasis, but this association is not clearly resolved. The primary aim of this study was to assess the relationship between whole-body and skeletal muscle iron stores by measuring the abundance of the key iron storage (ferritin) and import (transferrin receptor) proteins in skeletal muscle, as well as markers of whole-body iron homeostasis in men (n=19) and women (n=43) with obesity. Plasma ferritin concentration (a marker of whole-body iron stores) was highly correlated with muscle ferritin abundance (r=0.77, p=2E−13) and negatively associated with muscle transferrin receptor abundance (r=−0.76, p=1E−12). These relationships persisted when accounting for sex, age, BMI, and plasma [C-reactive protein]. In parallel with higher whole-body iron stores in our male vs. female participants, men had 2.2-fold higher muscle ferritin abundance (p=1E−4) compared with women. In accordance with lower muscle iron storage, women had 2.7-fold higher transferrin receptor abundance (p=7E−10) compared with men. We conclude that muscle iron storage and import proteins are tightly and independently related to plasma ferritin concentration in adults with obesity, suggesting that skeletal muscle may be an underappreciated iron store.
Keywords: Obesity, iron, skeletal muscle
INTRODUCTION
Iron regulation is critical to human health—iron is required for the function of numerous proteins in circulation and tissues yet toxic when it accumulates in excess (Ganz 2013). Maintaining systemic iron homeostasis requires the coordinated interplay of iron absorption from the gastrointestinal tract, transport in circulation, incorporation into iron-containing proteins, and storage/release from tissues (Ganz 2013). Unfortunately, iron homeostasis is often compromised in adults with obesity, which can lead to adverse hematological (e.g., iron-deficiency anemia) and metabolic disorders (e.g., iron overload-mediated insulin resistance; Yanoff et al. 2007, Cepeda-Lopez et al. 2015, Aigner et al. 2014). Regulation of iron homeostasis is dependent, in part, on effective storage in tissues, yet factors underlying the regulation of iron storage in different tissues in adults with obesity are not well understood.
In addition to the iron incorporated into functional proteins, iron is also stored in tissues, bound to ferritin, providing an essential pool of iron that can be released when whole-body iron status is low (Torti & Torti 2002, Walters et al. 1973). However, excess tissue iron storage is associated with adverse consequences, largely driven by iron-mediated oxidative damage and consequent cellular dysfunction (Puntarulo 2005). The liver is traditionally viewed as the key iron storage depot (Ganz 2013), and evidence indicates a strong link between elevated whole-body iron stores, high hepatic iron content, and liver dysfunction in adults with obesity (Dongiovanni et al. 2011). Although skeletal muscle is not traditionally viewed as an important iron storage site, a recent study reported elevated markers of iron storage in skeletal muscle from adults with obesity (Moreno-Navarette et al. 2016). However, the regulation of skeletal muscle iron storage in obesity has not been well described, and it is even unknown if skeletal muscle iron stores are related to whole-body iron stores.
The primary aim of this study was to examine relationships between whole-body iron stores and the abundance of the key skeletal muscle iron storage (ferritin) and import (transferrin receptor) proteins in adults with obesity. We hypothesized that plasma ferritin concentration, a marker of whole-body iron stores (Walters et al. 1973, Worwood 2007), would be positively correlated with muscle ferritin protein abundance and negatively correlated with muscle transferrin receptor abundance. Further, we hypothesized that, in concert with other known sex differences in systemic and hepatic iron homeostasis (Ganz & Nemeth 2012), men with obesity would have higher muscle ferritin protein abundance and lower muscle transferrin receptor protein abundance compared with women with obesity. Recognizing that inflammation can influence plasma ferritin concentrations, we examined potential relationships between plasma C-reactive protein concentration, plasma ferritin concentration, and skeletal muscle iron proteins.
METHODS
This study involved analyses from samples collected as part of two investigations into metabolic regulation in humans with obesity (NCT02717832 and NCT02706093). Samples collected from all participants who completed the baseline clinical study visit for one of these two protocols between June 2016 and October 2019 were analyzed for the present study, with the exception of one individual who was excluded due to a recent blood donation. In total, we studied 62 physically inactive young adults with obesity (BMI 30–40 kg/m2; age 18–44; 19 men, 43 women). All participants were non-smokers, weight stable for >6 months, and had no history of cardiometabolic disease. All women were premenopausal. We recently published a manuscript examining the effect 12-weeks of exercise training on insulin sensitivity and the baseline samples from 30 of these same participants were included in that study (Ryan et al. 2020). However, no iron-related outcomes were included in this previous publication and findings presented here are novel. The study was conducted according to the standards in the latest version of the Declaration of Helsinki. All protocols were approved by the University of Michigan Institutional Review Board (HUM00111275 and HUM00106883) and all participants provided written, informed consent.
Experimental Procedures
In the morning after an overnight fast, a venous blood sample was collected, and a muscle biopsy sample was obtained from the vastus lateralis. Upon collection, the muscle tissue was quickly dissected free of any adipose or connective tissue, rinsed with saline, blotted dry, flash frozen in liquid nitrogen and then stored at −80°C until further analysis. On a separate day, body composition was determined using dual-energy x-ray absorptiometry (Lunar DPX, GE, WI) and subjects completed an MRI scan for liver R2* determination (an index of liver iron content; Labranche et al. 2018).
Analytical Procedures
Circulating markers of iron status and inflammation
Plasma concentrations of ferritin (S-22, Ramco Laboratories, Inc, TX), hepcidin (hormone regulating iron homeostasis; ICE-004, Intrinsic Life Sciences, CA), high-sensitivity C-reactive protein (CR120C, Cal Biotech, CA), and IL-6 (HS600C, R&D Systems, MN) were assessed using enzyme-linked immunosorbent assays. Hemoglobin concentration and hematocrit were assessed by the University of Michigan Pathology Laboratory (Sysmex XN9000).
Muscle ferritin and transferrin receptor abundance
Skeletal muscle ferritin and transferrin receptor protein abundance were assessed using immunoblotting. A small piece (~25mg) of muscle tissue was homogenized with 2×5mm steel beads (TissueLyser II, Qiagen, CA, USA) in ice cold radioimmunoprecipitation assay buffer (#9806, Cell Signaling, MA, USA) supplemented with protease and phosphatase inhibitors, rotated at 50 rpm for 1 hour at 4°C, and then centrifuged at 15,000g for 15 minutes at 4°C. The supernatant was collected, and protein concentration was determined using a bicinchoncic acid assay. Fifteen μg of protein were separated onto handcast 10% gels via SDS-PAGE. Proteins were then transferred onto nitrocellulose membranes and stained with Memcode total protein stain (#24580, ThermoFisher, MA)—the total protein stain signal intensity in each lane was quantified to normalize protein expression (Moritz 2017). Membranes were blocked in 5% BSA in TBS-T for 2 hours and incubated with appropriate primary antibodies overnight at 4°C. Primary antibodies for ferritin (ab75973, RRID:AB_1310222) and transferrin receptor (ab84036, RRID:AB_10673794) were obtained from Abcam (MA). This primary antibody for ferritin recognizes both the heavy and light chain of ferritin. Membranes were then incubated with appropriate secondary antibody for 90 minutes, developed using enhanced chemiluminescence, and imaged. To reduce gel-to-gel variability, an internal standard sample (composite skeletal muscle lysate from 8 obese individuals) was loaded on each gel and protein expression was also normalized to this lane.
Liver MRI
Liver R2* is an MRI-derived parameter that is directly proportional to liver iron content (Wood et al. 2005, Labranche et al. 2018). MRI scans were conducted using a 3 Tesla clinical MRI system (Philips Healthcare, the Netherlands). A multi-echo Dixon product sequence was performed with a breath-hold lasting ≥ 16 s. Nominal acquisition conditions of this fast-field-echo sequence were: 77 axial 3D slices; 6 echos (TE=0.96ms + n*0.7ms, n=0,1,5); TR=6.2ms; flip angle =3°; acquisition matrix = 160(R/L) by 160(A/P); parallel imaging SENSE = 2; and FOV = 400mm. Images were converted to 3D Meta-Image format files using in-house MatLab routines and the T2* meta-image file was examined and quantified using 3D Slicer ver4.6.2. All analyses were completed by a single trained investigator who was blinded with respect to all participant characteristics. Large regions of interest (ROIs) were manually defined on three, 5mm axial slices (each separated by 10mm) through the right lobe of the liver; large vessels were avoided. Hepatic T2* was quantified as the average T2* value within the 3 slices. The coefficient of variation for T2* for the 3 slices within each scan averaged 5.1%. Hepatic R2* was calculated as 1/T2* and expressed in Hz.
Statistical Analysis
Data were checked for normality and non-normally distributed variables were log-transformed prior to statistical analysis. The primary statistical analyses involved simple and multiple linear regression analyses to determine the relationship between plasma ferritin concentration and muscle protein abundances. We compared men and women using independent samples t-tests. P-values <0.05 were considered statistically significant.
RESULTS
Across all participants, we found a strong positive correlation between plasma ferritin concentration and muscle ferritin abundance (r=0.77, p=2E−13; Figure 1A). Using multiple regression analysis, plasma ferritin concentration was independently related to muscle ferritin abundance (rpartial=0.70, p=8E−10), whereas sex, age, BMI, and plasma CRP concentration were not (Table 1). Although inflammation can increase plasma ferritin concentrations, there was no positive correlation between plasma ferritin concentration and either plasma CRP or IL-6 concentration (Figure 1B,D). As expected, there was no significant correlation between plasma ferritin concentration and BMI (Figure 1F).
Figure 1. Relationship between plasma ferritin concentration and skeletal muscle iron protein abundance in adults with obesity.
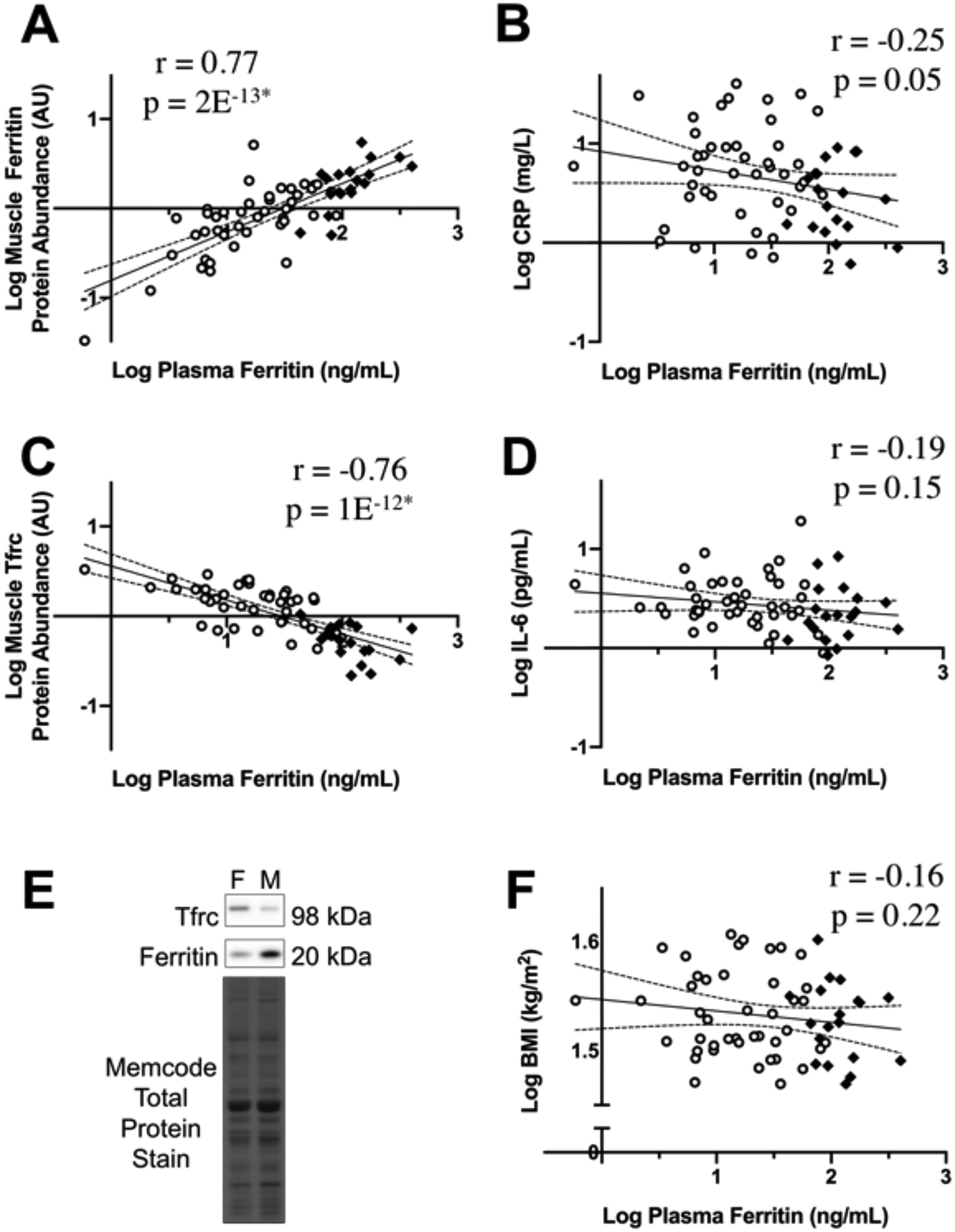
The relationship between plasma ferritin concentration and A) skeletal muscle ferritin abundance, B) plasma C-reactive protein concentration (CRP), C) skeletal muscle transferrin receptor (Tfrc) abundance, D) plasma IL-6 concentration, and F) body mass index. Panel E shows representative images of the ferritin protein band, Tfrc protein band, and the Memcode total protein stain from a female (F) and male (M) participant. Men are indicated by black diamonds and women by open circles. The regression line is indicated in black, with the 95% confidence intervals represented by dotted lines. *p≤1E−12.
Table 1.
Multiple linear regression analysis of factors influencing muscle iron proteins in adults with obesity.
| Model 1: Muscle Ferritin Protein Abundance | |||
|---|---|---|---|
| Adjusted R2 = 0.59; p=9E−11 | |||
| Variable | Partial r | p-value | |
| Plasma [Ferritin] | 0.70 | 8E−10* | |
| Sex | −0.19 | 0.16 | |
| Plasma [CRP] | −0.10 | 0.44 | |
| Age | 0.16 | 0.24 | |
| BMI | 0.05 | 0.73 | |
| Model 2: Muscle Transferrin Receptor Protein Abundance | |||
| Adjusted R2 = 0.61; p=3E−11 | |||
| Variable | Partial r | p-value | |
| Plasma [Ferritin] | −0.54 | 1E−5* | |
| Sex | −0.33 | 0.01* | |
| Plasma [CRP] | 0.001 | 0.99 | |
| Age | −0.12 | 0.38 | |
| BMI | −0.22 | 0.10 | |
CRP, C-Reactive Protein; BMI, body mass index.
p<0.05.
Consistent with its positive association with muscle ferritin abundance, plasma ferritin concentration was negatively correlated with muscle transferrin receptor abundance (r=−0.76, p=1E−12, Figure 1C). Multiple regression analysis revealed that plasma ferritin concentration was independently related to muscle transferrin receptor abundance (rpartial=−0.54, p=1E−5). Muscle transferrin receptor abundance was also independently related to sex (rpartial=−0.33, p=0.01) but not to age, BMI, or plasma CRP concentration (Table 1).
As expected, men had significantly higher plasma ferritin and hepcidin concentrations, as well as higher liver R2* (index of liver iron content; Table 2). Interestingly, in parallel with higher whole-body iron stores, men also had 2.2-fold higher muscle ferritin abundance (p=1E−4) compared with women (Table 2). As expected based on their lower muscle ferritin abundance, women had 2.7-fold higher muscle transferrin receptor abundance (p=7E−10) compared with men.
Table 2.
Subject characteristics and iron-related parameters in men and women with obesity.
| Men (n=19) | Women (n=43) | p-value | |
|---|---|---|---|
| Age (years) | 31 ± 6 | 30 ± 7 | 0.87 |
| Body mass index (kg/m2) | 34 ± 3 | 34 ± 3 | 0.46 |
| Body Fat (%) | 37 ± 3 | 46 ± 4 | 2E−12* |
| Plasma [Ferritin] (ng/mL) | 135 ± 88 | 24 ± 21 | 3E−14* |
| Plasma [Hepcidin] (ng/mL) | 45 ± 25 | 23 ± 15 | 7E−5* |
| Hemoglobin concentration (g/dL)a | 14.6 ± 0.7 | 12.4 ± 1.3 | 1E−6* |
| Hematocrit (%)a | 43 ± 3 | 38 ± 3 | 1E−5* |
| Plasma [CRP] (mg/dL) | 0.4 ± 0.3 | 1.0 ± 1.0 | 0.01* |
| Plasma [IL-6] (pg/ml) | 2.7 ± 2.0 | 3.6 ± 3 | 0.09 |
| Liver R2* (Hz)b | 58 ± 10 | 43 ± 9 | 7E−6* |
| Muscle Ferritin Protein Abundance (AU) | 2.2 ± 1.2 | 1.0 ± 0.8 | 1E−4* |
| Muscle Tfrc Protein Abundance (AU) | 0.6 ± 0.2 | 1.5 ± 0.7 | 7E−10* |
Data are presented as mean ± SD.
p≤0.01.
Hemoglobin concentration and hematocrit were not obtained from 6 men and 8 women so n=13 men and n=32 women for these parameters.
MRI scans were not obtained on 2 men and 8 women so liver R2* sample sizes were n=17 men and n= 35 women. BMI, body mass index; CRP: C-reactive protein; Tfrc, transferrin receptor.
DISCUSSION
Our novel findings indicate that plasma ferritin concentration, a marker of whole-body iron stores, is tightly and independently associated with skeletal muscle ferritin and transferrin receptor abundance in adults with obesity. We interpret this to suggest coordinated regulation of muscle and whole-body iron stores, such that whole-body iron stores play a very important role in determining the magnitude of iron storage in skeletal muscle. Additionally, our data reveal large sex differences in these muscle iron proteins, which appear to be driven by sex differences in whole-body iron stores. These findings have important implications for the understanding of tissue and systemic iron homeostasis in adults with obesity.
A mobilizable pool of iron stored in tissues is critical for providing iron for erythropoiesis—dietary iron absorption is relatively inefficient, and inadequate iron storage can result in iron-deficiency anemia (Worwood 2007, Zimmermann & Hurrell 2007). On the other hand, excessive tissue iron storage is associated with organ toxicity due to iron-mediated oxidative damage (Puntarulo 2007). The liver is traditionally viewed as the primary iron storage site (Ganz 2013), and a role for muscle as an iron store is not widely recognized. Although iron concentration in skeletal muscle is much lower than that found in the liver, the large muscle mass in humans results in comparable total iron content among these tissues (Torrance et al. 1968). The strong correlations we observed between plasma ferritin concentration and muscle ferritin and transferrin receptor abundance suggest that alterations in whole-body iron status are accompanied by changes in muscle iron storage, supporting a previously underappreciated role for skeletal muscle in the regulation of iron homeostasis.
The regulation of iron homeostasis in obesity is complex, as evidenced by iron deficiency in many obese adults and iron overload in others (Aigner et al. 2014). These opposing iron-related disorders are associated with different health complications in obesity. Whereas iron deficiency leads to anemia (Zimmermann & Hurrell 2007), high body iron stores have been associated with metabolic dysfunction and the development of type 2 diabetes (Jiang et al. 2004, Jehn et al. 2004). The mechanisms linking iron and metabolic dysfunction are incompletely understood (Fernandez-Real et al. 2015), but some pre-clinical evidence indicates that excess muscle iron impairs peripheral insulin signaling (Cui et al. 2019). Although whole-body iron stores vary markedly among adults with obesity (Aigner et al. 2014), it was reported that markers of iron content in skeletal muscle correlated with BMI, suggestive of increased muscle iron storage in obesity (Moreno-Navarette et al. 2016). Our findings suggest that whole-body iron stores are a key determinant of muscle iron storage among adults with obesity. Because we did not study normal-weight individuals in this study, we cannot conclude whether this phenomenon contributes to differences in iron homeostasis often reported between normal-weight and obese groups (Aigner et al. 2014). While we do not discount the possibility that other obesity-related factors may also affect muscle iron storage, the strong and independent correlation we found between plasma ferritin concentration and muscle ferritin abundance suggests that the impact of other obesity-related factors may be secondary to whole-body iron stores. Based on our findings, we contend that studies comparing muscle iron storage between groups should consider the influence of whole-body iron status. Further studies are warranted to determine if the tight relationship we observed between plasma ferritin concentration and skeletal muscle ferritin abundance in individuals with BMI 30–40 kg/m2 extends across a larger range of BMI.
Our data are the first to our knowledge to reveal sex differences in the abundance of the key iron storage and import proteins in human skeletal muscle. Sex differences in whole-body iron stores are well-established, and the large sex difference in muscle iron protein abundance we observed fits well with the notion of coordinated regulation of whole-body and muscle iron storage. There was no difference in BMI between the men and women in our study but, as expected, women had significantly higher body fat percentage. It is possible that elevated adiposity may partially contribute to lower iron storage in women. However, menstrual blood loss has been identified as a primary factor driving sex differences in whole-body iron storage (Worwood 2007).
Experimental considerations
The aim of this study was to assess the relationship between whole-body and skeletal muscle iron stores. We did not assess factors responsible for differences in whole-body iron stores between individuals (e.g., dietary iron intake, dietary iron absorption, menstrual blood losses, etc.). Additionally, we did not control the timing of testing relative to the menstrual phase, but plasma ferritin concentration and other circulating iron biomarkers remain relatively stable across the menstrual cycle (Puolakka 1980, Belza et al. 2005). Therefore, it is unlikely that standardizing testing relative to the menstrual phase would have altered our findings. It is important to recognize that inflammation can influence plasma ferritin concentration, which can impact the interpretation of plasma ferritin concentration as a biomarker of total body iron stores (Worwood 2007). There is strong evidence that acute infection and chronic inflammatory diseases result in increases in plasma ferritin concentration independent of iron stores (Worwood 2007). Obesity is often accompanied by low-grade inflammation, and the plasma CRP and IL-6 concentrations in our participants with obesity reflect the expected elevated levels of inflammation (i.e., compared to plasma CRP and IL-6 values reported in normal-weight populations; Aronson et al. 2004, Kern et al. 2001). While we cannot discount a potential small influence of inflammation on plasma ferritin concentrations in our study, multiple pieces of evidence suggest that our key findings are not driven by a confounding influence of inflammation on the interpretation of plasma ferritin concentration. First, there were no positive associations between plasma ferritin and either plasma CRP or IL-6 concentrations in our participants, whether we looked across all participants or within men/women. Despite more than 2-fold higher mean plasma CRP concentrations in the women, mean plasma ferritin concentration was more than 5-fold higher in men. Finally, our novel finding that plasma ferritin concentration is tightly correlated with muscle ferritin abundance—independent of plasma CRP concentration—supports a link between plasma ferritin concentration and tissue iron stores.
CONCLUSION
In conclusion, our findings indicate that muscle iron storage and import proteins are tightly and independently correlated with plasma ferritin concentration in adults with obesity. We contend that this relationship suggests coordinated regulation of muscle and whole-body iron stores, such that the magnitude of muscle iron storage may be determined, at least in part, by whole-body iron stores. We also reveal large sex differences in these iron-related proteins in skeletal muscle, which appear to be driven by the sex differences in whole-body iron stores. Together, our findings suggest that skeletal muscle may be an important iron store and highlight the need for further investigation into the functional implications of muscle iron storage in humans.
New Findings.
What is the central question of this study?
Obesity is associated with complex perturbations to iron homeostasis. We examined if plasma ferritin concentration (a biomarker of whole-body iron stores) is related to the abundance of ferritin (the key tissue iron storage protein) in skeletal muscle in adults with obesity.
What is the main finding and its importance?
Plasma ferritin concentration was tightly correlated with the abundance of ferritin in skeletal muscle, and this relationship persisted when accounting for sex, age, body mass index, and plasma C-reactive protein concentration. Our findings suggest that skeletal muscle may be an important iron store.
ACKNOWLEDGEMENTS
We thank the participants for their dedicated efforts and Suzette Howton for her contributions as study coordinator.
FUNDING
This study was supported by the United States National Institutes of Health (R01DK077966, P30DK089503, T32DK007245, F32DK117522), the Canadian Institutes of Health Research (338735 and 146190) and the American Diabetes Association (1-16-ICTS-048).
Footnotes
ADDITIONAL INFORMATION
The data that support the findings of this study are available from the corresponding author upon reasonable request.
COMPETING INTERESTS: The authors have no conflicts of interest to declare.
REFERENCES
- Aigner E, Feldman A, & Datz C (2014). Obesity as an emerging risk factor for iron deficiency. Nutrients, 6(9), 3587–3600. 10.3390/nu6093587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson D, Bartha P, Zinder O, Kerner A, Markiewicz W, Avizohar O, … & Levy Y (2004). Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int J Obesity, 28(5), 674–679. 10.1038/sj.ijo.0802609 [DOI] [PubMed] [Google Scholar]
- Belza A, Henriksen M, Ersbøll AK, Thilsted SH, & Tetens I (2005). Day-to-day variation in iron-status measures in young iron-deplete women. Br J Nutr, 94(4), 551–556. 10.1079/BJN20051461 [DOI] [PubMed] [Google Scholar]
- Cepeda-Lopez AC, Melse-Boonstra A, Zimmermann MB, & Herter-Aeberli I (2015). In overweight and obese women, dietary iron absorption is reduced and the enhancement of iron absorption by ascorbic acid is one-half that in normal-weight women. Am J Clin Nutr, 102(6), 1389–1397. 10.3945/ajcn.114.099218 [DOI] [PubMed] [Google Scholar]
- Cui R, Choi SE, Kim TH, Lee HJ, Lee SJ, Kang Y, … & Lee KW (2019). Iron overload by transferrin receptor protein 1 regulation plays an important role in palmitate‐induced insulin resistance in human skeletal muscle cells. FASEB J, 33(2), 1771–1786. 10.1096/fj.201800448R [DOI] [PubMed] [Google Scholar]
- Dongiovanni P, Fracanzani AL, Fargion S, & Valenti L (2011). Iron in fatty liver and in the metabolic syndrome: a promising therapeutic target. J Hepatol, 55(4), 920–932. 10.1016/j.jhep.2011.05.008 [DOI] [PubMed] [Google Scholar]
- Fernández-Real JM, McClain D, & Manco M (2015). Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care, 38(11), 2169–2176. 10.2337/dc14-3082 [DOI] [PubMed] [Google Scholar]
- Ganz T (2013). Systemic iron homeostasis. Physiol Rev, 93(4), 1721–1741. 10.1152/physrev.00008.2013 [DOI] [PubMed] [Google Scholar]
- Ganz T, & Nemeth E (2012). Hepcidin and iron homeostasis. Biochim Biophys Acta, 1823(9), 1434–1443. 10.1016/j.bbamcr.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehn M, Clark JM, & Guallar E (2004). Serum ferritin and risk of the metabolic syndrome in US adults. Diabetes Care, 27(10), 2422–2428. 10.2337/diacare.27.10.2422 [DOI] [PubMed] [Google Scholar]
- Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, & Hu FB (2004). Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA, 291(6), 711–717. 10.1001/jama.291.6.711 [DOI] [PubMed] [Google Scholar]
- Kern PA, Ranganathan S, Li C, Wood L, & Ranganathan G (2001). Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. American Journal of Physiology-Endocrinology And Metabolism, 280(5), E745–E751. 10.1152/ajpendo.2001.280.5.E745 [DOI] [PubMed] [Google Scholar]
- Labranche R, Gilbert G, Cerny M, Vu KN, Soulières D, Olivié D, … & Tang A (2018). Liver iron quantification with MR imaging: a primer for radiologists. Radiographics, 38(2), 392–412. 10.1148/rg.2018170079 [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Blasco G, Xifra G, Karczewska-Kupczewska M, Stefanowicz M, Matulewicz N, … & Fernández-Real JM (2016). Obesity is associated with gene expression and imaging markers of iron accumulation in skeletal muscle. J Clin Endocrinol Metab, 101(3), 1282–1289. 10.1210/jc.2015-3303 [DOI] [PubMed] [Google Scholar]
- Moritz CP (2017). Tubulin or not tubulin: heading toward total protein staining as loading control in western blots. Proteomics, 17(20), 1600189. 10.1002/pmic.201600189 [DOI] [PubMed] [Google Scholar]
- Puntarulo S (2005). Iron, oxidative stress and human health. Mol Aspects Med, 26(4–5), 299–312. 10.1016/j.mam.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Puolakka J (1980). Serum ferritin in the evaluation of iron status in young healthy women. Acta Obstet Gynecol Scand, 59(sup95), 35–41. 10.3109/00016348009156378 [DOI] [PubMed] [Google Scholar]
- Ryan BJ, Schleh MW, Ahn C, Ludzki AC, Gillen JB, Varshney P, … & Horowitz JF (2020). Moderate-intensity exercise and high-intensity interval training affect insulin sensitivity similarly in obese adults. J Clin Endocrinol Metab, 10.1210/clinem/dgaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance JD, Charlton RW, Schmaman A, Lynch SR, & Bothwell TH (1968). Storage iron in ‘muscle’. J Clin Path, 21(4), 495–500. 10.1136/jcp.21.4.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti FM, & Torti SV (2002). Regulation of ferritin genes and protein. Blood, 99(10), 3505–3516. 10.1182/blood.V99.10.3505 [DOI] [PubMed] [Google Scholar]
- Walters GO, Miller FM, & Worwood M (1973). Serum ferritin concentration and iron stores in normal subjects. J Clin Path, 26(10), 770–772. 10.1136/jcp.26.10.770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, & Coates TD (2005). MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood, 106(4), 1460–1465. 10.1182/blood-2004-10-3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worwood M (2007). Indicators of iron status of populations: ferritin. In: Assessing the Iron Status of Populations. 2nd ed. World Health Organization: Geneva, Switzerland, pp 35–73. [Google Scholar]
- Yanoff LB, Menzie CM, Denkinger B, Sebring NG, McHugh T, Remaley AT, & Yanovski JA (2007). Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obesity, 31(9), 1412–1419. 10.1038/sj.ijo.0803625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann MB, & Hurrell RF (2007). Nutritional iron deficiency. Lancet, 370(9586), 511–520. 10.1016/S0140-6736(07)61235-5 [DOI] [PubMed] [Google Scholar]


