Abstract
Enteric viruses are important human pathogens that pose a significant global health problem. These viruses infect the gastrointestinal tract, which contains a community of microbes called the “microbiota”. We and others have shown that intestinal microbiota are crucial for the replication, pathogenesis, and transmission of a variety of enteric viruses. However, the mechanisms underlying microbiota enhancement of enteric virus infection remain unclear. Interestingly, the host immune system is dependent on both the abundance and composition of the intestinal microbiota. Here we review several aspects of how microbiota influence the immune system and how this could potentially impact enteric virus infection.
Keywords: enteric viruses, microbiota, immune system, metabolites
Introduction
Within the gastrointestinal tract resides a microbial ecosystem of approximately 1014 organisms, which play a crucial role in host homeostasis (1). Importantly, microbiota are critical for the development of the host immune system (2). Specifically, microbiota influence lymphoid structure development, immune cell homeostasis and maturation, epithelial function, T cell development, induction of diverse immune cascades, production of antimicrobial peptides, inflammation, and autoimmune diseases (1, 2). Additionally, microbiota affect host biosynthesis of vitamins, hormones, and neurotransmitters, and production of bacterial byproducts such as short-chain fatty acids (SCFAs) (1, 2). Conversely, microbiota are shaped by the host environment, diet, and the use of antibiotics (3). Dysbiosis of the microbiota has been linked to a variety of diseases, including gastrointestinal, neurologic, metabolic, cardiovascular, and oncologic disease (1).
Enteric viruses are acquired through the fecal-oral route of transmission, where they infect the gastrointestinal tract, and directly contact the microbiota (4). This interaction with the microbiota has a variety of effects on enteric viruses (4). However, little is known about the interplay between microbiota, the host immune system, and enteric viruses. In this review, we discuss how the microbiota influence host homeostasis and intestinal immunity, and how these processes potentially influence enteric virus infection.
Microbiota priming of host immunity
Millions of years of coevolution between host and microbiota has forged a symbiotic relationship (2). Models of microbiota manipulation, such as germ-free or antibiotic treated mice, demonstrate altered immune response of the host (3, 5). Specifically, germ-free mice, which completely lack microorganisms, have altered immune cell homeostasis, impaired lymphoid tissue development, and altered susceptibility to pathogens, suggesting that microorganisms are crucial for proper host immunity (3). Intriguingly, little is known about how these changes impact enteric virus replication and whether the mechanisms are conserved or divergent.
Both antibiotic-treated and germ-free mice have decreased myeloid cell populations, which are known to impact a variety of viral infections (3, 6). Interestingly, macrophages are decreased in the small intestine and colon upon antibiotic treatment, and these tissues are important sites of replication for a variety of enteric viruses (7•,8•–10). Additionally, antibiotic-treated mice have impaired basal type I interferon production, and reduced populations of dendritic cells (DCs), CD4+ T cells, CD8+ T cells, T regulatory cells (FoxP3+), Th1 cells (IFNγ+), and B cells in the small intestine and colon (11). We and others have shown that several enteric viruses have decreased viral replication in intestinal tissues in antibiotic-treated mice (7•,8•,9,12). However, the mechanism for this reduction in viral replication remains unknown and it is unclear how these diverse cell populations influence enteric virus infection. To address this question, it will be important to determine how reducing specific cell populations influences enteric virus infection.
Another aspect of the immune system that is modulated by the microbiota is the production of cytokines, which have varying effects on viral infection (3, 11). Interestingly, germ-free or antibiotic-treated mice have altered cytokine levels in a variety of tissues. Specifically, several cytokines important for enteric virus infection are reduced in intestinal tissue of microbiota-depleted mice, including IL-1β, TNF-α, and IL17 (13–15). Overall, alteration of the microbiota greatly influences cytokine levels in both gastrointestinal and systemic tissues. Future studies could focus on how changes in cytokine levels in gastrointestinal tissues impact enteric virus infection.
To complicate matters, antibiotics have microbiota-independent effects on both host immunity and viral infection, meaning that antibiotics can directly modulate host cells (16•, 17•). This makes it difficult to discern whether immune changes are due to the absence of bacteria or direct effects of antibiotics on host cells. To further our understanding of the effects of antibiotics, it is crucial determine whether antibiotic effects are microbiota-dependent vs. microbiota-independent by treating germ-free mice with antibiotics and monitoring consequences.
Immune modulation by bacterial derived short-chain fatty acids
A gap in the field of microbiota-virus interactions is how bacteria can indirectly influence the immune system through production of SCFAs. Bacteria within the gastrointestinal tract ferment dietary fiber to produce SCFAs such as butyrate, propionate, and acetate (18). Through fermentation of dietary fiber, bacteria produce millimolar quantities of SCFAs, making them some of the most abundant molecules in the gut (19). SCFAs are primarily produced by anaerobic bacteria, most notably Gram-positive Firmicutes in the class Clostridia, although other bacteria can produce SCFAs as well (20).
SCFAs influence host immunity by a variety of mechanisms, such as altering gene expression and intestinal metabolism, which in turn could potentially impact enteric virus infection (21–23). Additionally, SCFAs can alter immune cell development and function, such as altering regulatory T cell homeostasis in the gut (22). Interestingly, when mice were given a single dose of streptomycin, they exhibited a marked decrease in cecal Clostridia and butyrate (24). Importantly, this single dose of streptomycin also reduced shedding and pathogenesis of the enteric virus coxsackievirus B3, but not poliovirus, suggesting that two closely related enteric viruses have different requirements of the host microbiota (8).
One SCFA, butyrate, influences intestinal immunity through changes in host gene expression in the gut. Butyrate alters gene expression through inhibition of histone deacetylases, and by creating a hypoxic state through beta-oxidation of butyrate, which stabilizes the transcription factor hypoxia inducible factor 1 (25, 26). Furthermore, the intestinal inflammatory response is altered by butyrate through activation of G-protein coupled receptors (20, 27, 28). Some of the genes affected by butyrate influence viral infection. For example, butyrate upregulates expression of the coxsackievirus and adenovirus receptor (CAR), which is the receptor for coxsackievirus B3 (29). Butyrate also downregulates various innate immune genes such as CCL2, CCL7, CXCL2, IL6, IL12, NOS2, and TNF-α (21, 23, 30). A recent study demonstrated that, in cultured cells, butyrate treatment increased infection with several viruses by suppressing expression of interferon-stimulated genes (31•). Taken together, bacterial-derived butyrate regulates various innate immune genes that are important for enteric virus infection.
These findings suggest that butyrate, and perhaps other SCFAs, may influence enteric virus infection in the intestine (Figure 1). Future studies could examine the impact of specific SCFAs on a variety of enteric viruses using mouse models. For example, depletion of microbiota with antibiotics, followed by treatment with exogenous acetate, butyrate, or propionate prior to enteric virus infection could determine whether SCFAs are sufficient for enteric virus replication. Furthermore, it remains unknown if and how SCFA-mediated changes to host gene expression affects enteric virus infection. Unbiased approaches, such as analysis of SCFA-mediated transcriptomic changes by RNA sequencing in different tissues throughout the gastrointestinal tract (from conventional, antibiotic treated, or antibiotic treated plus specific SCFA mice) could reveal shared vs. unique gene expression signatures relevant to enteric virus infection.
Fig 1. Influence of Intestinal Microbiota on Host Physiology.
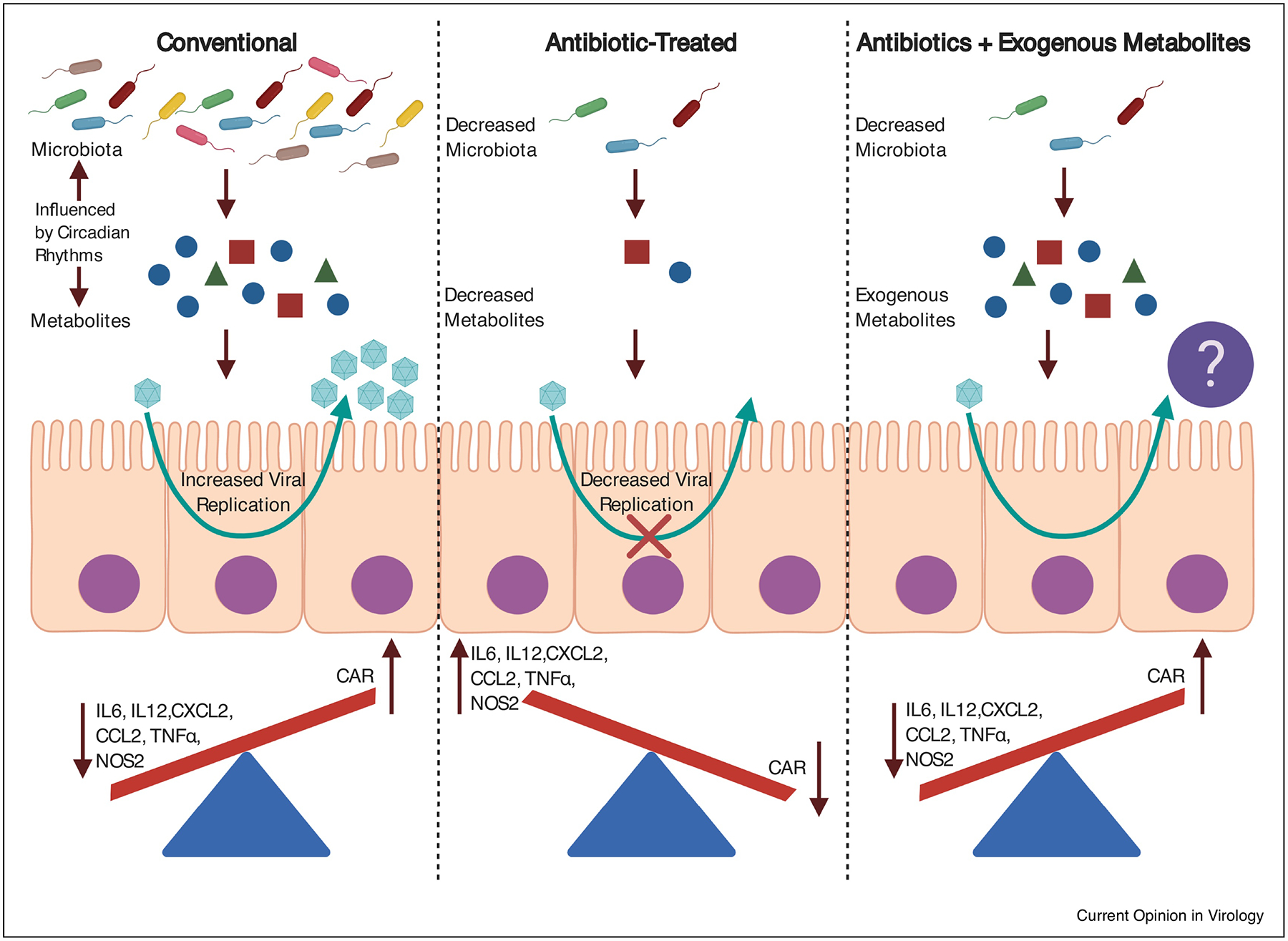
Microbially-derived short-chain fatty acids alter innate immune genes relevant to enteric virus infection. Both microbiota and microbial-derived metabolites are influenced by circadian rhythms. Upon antibiotic treatment, short-chain fatty acids are significantly reduced. Antibiotic treatment also reduces enteric virus infection. It remains unknown if exogenously added metabolites are sufficient to rescue enteric virus infection. Figure was created with BioRender.
The microbiota also modifies host metabolites such as bile acids, which can have both pro- and anti-viral effects on certain enteric viruses. Specifically, these bile acids bind to the human norovirus capsid protein and are important cofactors for efficient binding to CD300lf, the cell surface receptor for murine norovirus (32, 33). Interestingly, bacterial-modified bile acids prime type-III interferon induction to suppress murine norovirus infection in the proximal intestine (34). Furthermore, bile acids suppress rotavirus replication by downregulating rotavirus-induced lipid synthesis, suggesting that bile acids influence the replication of enteric viruses through various mechanisms (35). Taken together, bacterial-modified bile acids modulate infection of various enteric viruses.
Circadian effects on microbiota, immunity, and enteric virus infection
There has been growing evidence of a link between circadian rhythms and oscillations in the microbiota and host response pathways. Circadian rhythms are roughly 24-hour oscillations in a variety of processes that are entrained by the diurnal cycle of the planet (36). This rhythmicity is perpetuated by a set of transcription factors, so called “clock proteins”, which control expression of ~3–20% of genes in any mammalian cell or tissue. Thus, major physiological processes are under circadian control, including metabolism and immunity (37, 38). Additionally, microbiota undergo diurnal oscillations in both function and composition along the different tissues of the gastrointestinal tract (35•, 36•). These fluctuations in the microbiota are driven by host feeding rhythms (41). Furthermore, fluctuation in the amount of bacterial attachment to the colonic epithelium is also driven by diurnal oscillations (35•, 36•). However, little is known about how these fluctuations impact immune cell populations throughout the gastrointestinal tract. Moreover, it remains unclear if cytokine levels fluctuate throughout the gastrointestinal tract over a 24-hour circadian cycle. To date, it remains unknown how circadian rhythms impact enteric virus infection. Since enteric viruses require the host microbiota to establish infection in the host, it will be illuminating to determine the impact of microbial diurnal oscillations on intestinal immunity and how this influences enteric virus infection.
Interestingly, circadian disorders can cause microbial dysbiosis, which impacts host metabolism (35•). Feces isolated from mice displayed significant rhythmicity in the levels of SCFAs such as acetate, propionate, and butyrate, which are important molecules for driving host metabolism and immunity (42). Furthermore, intestinal microbiota are required for rhythmic recruitment of histone deacetylase 3 to target gene promoters, which rhythmically regulates host metabolism and drives expression of hundreds of downstream gene targets (36). Given the importance of SCFAs in modulating host immunity and that SCFAs are known to fluctuate throughout the day, it would be interesting to determine whether enteric virus infection is dependent on the time of day that the host is inoculated.
Conclusions
Considering all these findings, there is a clear need to further understand how the intestinal microbiota influences enteric virus infection and whether microbiota derived effects on host immunity is playing a role in enteric virus infection. Many questions remain about mechanisms of both direct and indirect effects on the microbiota on enteric virus infection. Which immune cells influence enteric virus infection and are these populations altered by the microbiota? Do changes in host immunity through abundance of microbial-derived SCFAs impact enteric virus enteric virus infection and if so, how? What is the impact of circadian rhythms on enteric virus infection and do microbiota fluctuations play a role? Elucidating the effects of the microbiota on the immune system and host homeostasis and how this influences enteric virus infection is a promising field of study. Future studies on the host microbiota, and how they modulate host immunity, may inspire new therapeutic approaches targeting enteric viruses.
Funding information and acknowledgements
We thank Carolyn Sturge for critical review of the manuscript. Work in J.K.P.’s lab is funded through NIH NIAID grant R01 AI74668, a Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Diseases Award, and a Faculty Scholar grant from the Howard Hughes Medical Institute. MWA was supported in part by NIH NIAID grant T32 AI007520.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared
References and recommended reading
Papers of particular interest, published with the period of review, have been highlighted as:
• of special interest
- 1.Lynch SV, Pedersen O. 2016. The Human Intestinal Microbiome in Health and Disease. N Engl J Med 375:2369–2379. [DOI] [PubMed] [Google Scholar]
- 2.Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science 336:1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy EA, King KY, Baldridge MT. 2018. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front Physiol 9:1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson CM. 2019. Enteric viruses exploit the microbiota to promote infection. Curr Opin Virol 37:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, Wherry EJ, Artis D. 2012. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37:158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stegelmeier AA, van Vloten JP, Mould RC, Klafuric EM, Minott JA, Wootton SK, Bridle BW, Karimi K. 2019. Myeloid Cells during Viral Infections and Inflammation. Viruses 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. 2011. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334:249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First paper to demonstrate that enteric viruses replication and pathogenesis is promoted by bacteria.
- 8.Robinson CM, Woods Acevedo MA, McCune BT, Pfeiffer JK. 2019. Related Enteric Viruses Have Different Requirements for Host Microbiota in Mice. J Virol 93. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper highlights that two related enteric viruses have different requirements for the host microbiota.
- 9.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, Virgin HW. 2015. Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science 347:266–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. 2009. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol 183:6041–50. [DOI] [PubMed] [Google Scholar]
- 11.Ekmekciu I, von Klitzing E, Fiebiger U, Escher U, Neumann C, Bacher P, Scheffold A, Kuhl AA, Bereswill S, Heimesaat MM. 2017. Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice. Front Immunol 8:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, Golovkina TV. 2011. Successful transmission of a retrovirus depends on the commensal microbiota. Science 334:245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw MH, Kamada N, Kim YG, Nunez G. 2012. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med 209:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira MR, Tafuri WL, Afonso LC, Oliveira MA, Nicoli JR, Vieira EC, Scott P, Melo MN, Vieira LQ. 2005. Germ-free mice produce high levels of interferon-gamma in response to infection with Leishmania major but fail to heal lesions. Parasitology 131:477–88. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4:337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopinath S, Kim MV, Rakib T, Wong PW, van Zandt M, Barry NA, Kaisho T, Goodman AL, Iwasaki A. 2018. Topical application of aminoglycoside antibiotics enhances host resistance to viral infections in a microbiota-independent manner. Nat Microbiol 3:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A seminal paper that demonstrates that antibiotics confer broad antiviral resistance.
- 17.Woods Acevedo MA, Pfeiffer JK. 2020. Microbiota-independent antiviral effects of antibiotics on poliovirus and coxsackievirus. Virology 546:20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An important finding that antibiotics alter enteric virus replication in a microbiota-independent manner.
- 18.von Engelhardt W, Bartels J, Kirschberger S, Meyer zu Duttingdorf HD, Busche R. 1998. Role of short-chain fatty acids in the hind gut. Vet Q 20 Suppl 3:S52–9. [PubMed] [Google Scholar]
- 19.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. 2008. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27:104–19. [DOI] [PubMed] [Google Scholar]
- 20.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. 2016. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 165:1332–1345. [DOI] [PubMed] [Google Scholar]
- 21.Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS. 2016. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 167:1137. [DOI] [PubMed] [Google Scholar]
- 22.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–50. [DOI] [PubMed] [Google Scholar]
- 23.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillis CC, Hughes ER, Spiga L, Winter MG, Zhu W, Furtado de Carvalho T, Chanin RB, Behrendt CL, Hooper LV, Santos RL, Winter SE. 2018. Dysbiosis-Associated Change in Host Metabolism Generates Lactate to Support Salmonella Growth. Cell Host Microbe 23:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. 2011. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol 32:335–43. [DOI] [PubMed] [Google Scholar]
- 26.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. 2015. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 17:662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. 2009. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. 2013. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145:396–406 e1–10. [DOI] [PubMed] [Google Scholar]
- 29.Kuster K, Grotzinger C, Koschel A, Fischer A, Wiedenmann B, Anders M. 2010. Sodium butyrate increases expression of the coxsackie and adenovirus receptor in colon cancer cells. Cancer Invest 28:268–74. [DOI] [PubMed] [Google Scholar]
- 30.Chang PV, Hao L, Offermanns S, Medzhitov R. 2014. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A 111:2247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chemudupati M, Kenney AD, Smith AC, Fillinger RJ, Zhang L, Zani A, Liu SL, Anderson MZ, Sharma A, Yount JS. 2020. Butyrate reprograms expression of specific interferon stimulated genes. J Virol doi: 10.1128/JVI.00326-20. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper demonstrates that a microbially derived metabolite, butyrate, alters expression of antiviral genes induced by type I interferon response.
- 32.Kilic T, Koromyslova A, Hansman GS. 2019. Structural Basis for Human Norovirus Capsid Binding to Bile Acids. J Virol 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson CA, Wilen CB, Dai YN, Orchard RC, Kim AS, Stegeman RA, Hsieh LL, Smith TJ, Virgin HW, Fremont DH. 2018. Structural basis for murine norovirus engagement of bile acids and the CD300lf receptor. Proc Natl Acad Sci U S A 115:E9201–E9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grau KR, Zhu S, Peterson ST, Helm EW, Philip D, Phillips M, Hernandez A, Turula H, Frasse P, Graziano VR, Wilen CB, Wobus CE, Baldridge MT, Karst SM. 2020. The intestinal regionalization of acute norovirus infection is regulated by the microbiota via bile acid-mediated priming of type III interferon. Nat Microbiol 5:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y, Chang KO. 2011. Inhibitory effects of bile acids and synthetic farnesoid X receptor agonists on rotavirus replication. J Virol 85:12570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuang Z, Wang Y, Li Y, Ye C, Ruhn KA, Behrendt CL, Olson EN, Hooper LV. 2019. The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science 365:1428–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohawk JA, Green CB, Takahashi JS. 2012. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35:445–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi JS. 2017. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18:164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z, Segal E, Elinav E. 2014. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159:514–29. [DOI] [PubMed] [Google Scholar]; • Seminal paper demonstrating that the gut microbiota exhibits diurnal osscilations in both function and composition.
- 40.Thaiss CA, Levy M, Korem T, Dohnalova L, Shapiro H, Jaitin DA, David E, Winter DR, Gury-BenAri M, Tatirovsky E, Tuganbaev T, Federici S, Zmora N, Zeevi D, Dori-Bachash M, Pevsner-Fischer M, Kartvelishvily E, Brandis A, Harmelin A, Shibolet O, Halpern Z, Honda K, Amit I, Segal E, Elinav E. 2016. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 167:1495–1510 e12. [DOI] [PubMed] [Google Scholar]; • This study highlights that diurnal rhymicity in the microbiota programs transcriptomic changes in the intestine.
- 41.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, Chang EB. 2015. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17:681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segers A, Desmet L, Thijs T, Verbeke K, Tack J, Depoortere I. 2019. The circadian clock regulates the diurnal levels of microbial short-chain fatty acids and their rhythmic effects on colon contractility in mice. Acta Physiol (Oxf) 225:e13193. [DOI] [PubMed] [Google Scholar]


