Abstract
In recent decades, aquaculture has played a significant role in fulfilling the vast demand for animal protein requirements and consequently in food security. However, environmental contamination and disease prevalence are considered essential challenges for the sector. In this regard, new approaches have been paved in technology to deal effectively with such challenges. Among these, nanotechnology—as a novel and innovative tool—has a broad spectrum of uses and a tremendous potential in aquaculture and seafood preservation. It can provide new technologies for management of drugs as liberation of vaccines and therefore hold the assurance for civilized protection of farmed fish against disease-causing pathogens. This article presents a review of nanotechnology and its applications in aquaculture. Additionally, it gives a brief idea about the fish disease and classical ways of controlling pathogens. On the other hand, this review sheds the light on nanotechnology as a potential novel tool which may possibly enhance the management and the control of disease prevalence. Therefore, the importance of this technology to promote sustainable aquaculture has also been highlighted. Focusing on the role of selenium nanoparticles as an efficient element is discussed also in this article.
Keywords: Fish disease, Pathogen control, Aquaculture sustainability, Nanotechnology
Introduction
There are different protein sources from terrestrial and aquatic animals. Nevertheless, due to positive health effects and essential compositional features of food, aquatic protein options are preferable. Fish is considered an essential part of the human diet in nearly all countries in the world (Mohanty 2015). It accounts for about 17% of the global population’s intake of animal protein (Shah and Mraz 2020). Fish play an important role in nutrition, food security, and livelihoods. Fish provide the highest-quality protein sources and a wide range of other nutrients especially essential amino acids and fatty acids that our body needs, in addition to vitamins and other vital elements such as iodine and selenium which are not found in other crops or meat (Kwasek et al. 2020). The minerals existing in fish include iron, calcium, zinc, iodine (from marine fish), phosphorus, selenium, and fluorine. These minerals are extremely bioavailable once simply taken in by the body. Fish is a good source of vitamin B complex, and liver oil has a significant amount of fat-soluble vitamins A, E, K, and D, besides other vitamins like vitamin E, vitamin K, and vitamin C (Mohanty 2015). Fish oil is rich with polyunsaturated fatty acids (PUFAs) especially omega fatty acids that the human body cannot synthesize (Mohanty 2015). Omega-3 fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are the major building stones of our neural system. With a growing world population, the demand for fish and fish products is expected to increase as they are considered the cheapest and most available animal proteins compared to other animal protein sources. Fish is considered an important component for the nutrition of poor people who depend on staple food (in particular maize, rice, and other cereals), and fish help correct imbalanced calorie/protein ratio where 150 g of fish protein delivers about 50–60% of an adult’s daily protein obligation (Mohanty 2015). Strong evidence underlines how consumption of fish, and in particular oily fish, lowers the risk of coronary heart disease (CHD) mortality. However, aquaculture sector is still under uncertainty in terms of putting a question mark on its sustainability where the effect of ever-increasing waste from aquaculture has bad effects both on productivity within aquaculture systems and on the aquatic environment. In this regard, nanotechnology is quickly evolving as the new forum for science and technology for the next generation of agri-food systems’ growth and transformation (Rodrigues et al. 2017).
Fish disease
Like other animals, fish suffer from various diseases. Disease is a prime agent affecting fish mortality, especially when fish are young. There are two types of fish diseases according to their infectiousness, pathogenic diseases and non-pathogenic diseases. The last is related to poor water quality, malnutrition, etc., and not transferrable from one fish to another. Non-infectious diseases are represented by gas bubble diseases due to extensive aeration, nutritional diseases due to deficiency of certain nutrients (as vitamins and minerals), disorders caused by pollutants (agricultural and industrial), and neo-plastic and genetic anomalies referring to abnormal growth in any organ which make organ loses its function and structure (El-Sayed Ali et al. (2014a, b); El-Sayed Ali et al. (2017); and Idowu et al. (2017)). The other type of disease is the pathogenic disease which is considered a very dangerous disease because it is transferred from one fish to another and causes huge mortalities. There are three types of infectious diseases, namely bacterial (Streptococcus spp., Aeromonas hydrophila, Pseudomonas spp., and Vibrio spp. are the most common), fungal, and parasitic.
Fish defenses against disease are specific and non-specific. Specific defenses are specialized responses to particular pathogens recognized by the fish’s body that are adaptive immune responses (Chaplin 2010). In recent years, vaccines have become widely used in aquaculture and ornamental fish, for example vaccines for furunculosis in farmed salmon and koi herpes virus in koi (Sommerset et al. 2005). Non-specific defenses include skin and scales, as well as the epidermis-secreted mucus layer that captures microorganisms and inhibits growth. If pathogens break these defenses, fish can create inflammatory reactions that maximize blood flow to infected areas and deliver white blood cells that attempt to kill the pathogens.
Fish immune system
Immune system plays an important role in fish protection against any infectious agent. When any defect happens, the immune system has the ability to catch disease (Firdaus-Nawi and Saad 2016). The immune system of the fish is composed of two main components, innate and adaptive immunities (Firdaus-Nawi and Saad 2016). Innate immunity is un-specific and performs as the primary line of pathogen invasion protection, while adaptive immunity is much more specific to a particular pathogen. Innate immunity is composed of the non-specific cellular and humeral elements. The non-specific cellular portion consists of toll-like receptors (TLRs), macrophages, neutrophils, eosinophils, and non-specific cytotoxic cells, while the non-specific humoral portion includes lysozyme, the complement, interferons, C-reactive proteins, transferrins, and lectins where they are working together to prevent pathogen invasion at the preliminary stage (Firdaus-Nawi and Saad 2016). The adaptive immune system, on the other hand, comprises highly specific systemic cells and mechanisms which are divided into two major components: humoral and cellular (Firdaus-Nawi and Saad 2016). Three forms of antibodies, the IgM, IgD, and IgT, are the essential components of humoral immunity which operate on invading extracellular diseases. The cytotoxic T-lymphocyte cells are the significant component of the cellular immunity that often destroys bacterial or parasite-infected cells infected with viruses and intracellular cells (Firdaus-Nawi and Saad 2016). Both innate and adaptive immune components must work with each other efficiently to prevent body from any diseases, where innate immunity responds to invading pathogens by identifying line-encoded molecules of the germ. Toll-like receptors (TLRs) and phagocytosis are the essential components of innate immunity that shields the host from foreign invaders by identifying and ultimately killing phagocyte cells (Silva et al. 2002). In contrast, adaptive immunity recognizes pathogens through molecules created by somatic mechanisms followed by humoral and cellular reactions via B- and T-lymphocytes (Uribe et al. 2011).
Fish can protect themselves from any disease by two types of immune system that generate different defenses. The non-specific defenses/non-specific immunity includes the skin and scales as well as the layer of mucus secreted by the epidermis that traps and inhibits microorganisms development. When pathogens penetrate the skin and other physical barriers, the role of specific defense/specific immunity begins. Fish may produce an inflammatory response if pathogens breach these defenses which increases blood flow to the infected area and delivers white blood cells (WBCs) that attempt to destroy pathogens. The response of a “specific defense” to a particular pathogen recognized by the body of the fish is an immune response (Sharma et al. 2012). When pathogens go inside the fish, the two main components of specific immunity became active and antibodies may lyse bacteria and function as opsonins to facilitate phagocytosis. Neutrophils, eosinophils, and macrophages do widely against different types of infections (bacteria, intracellular infection of protozoan or bacteria, and intracellular infection of viruses, or fungus, respectively) and are considered an important cell for saving the host.
Disease treatment
As a wide range of chemicals that are currently being used in the development of the aquaculture sector, control measures have been implemented over the years. Hormones, vitamins, antibiotics, and some other chemicals were examined for various remedies in aquaculture operations. Although they do have beneficial effects, due to their residual and other side effects, they cannot be prescribed. These are divided into synthesis and neutral treatments: synthesis treatment such as disinfectants (e.g., hydrogen peroxide and green malachite), antibiotics (e.g., sulfonamides and tetracyclines), anthelmintic (e.g., pyrethroid insecticides and avermectins); natural such as probiotic, essential oil, and herbal biomedicines (Citarasu 2010; Rawn et al. 2009).
Synthetic treatment as antibiotics
Antibiotics called antimicrobial agents which can be characterized as substances capable of killing microorganisms or inhibiting their growth, following their formal Fleming discovery in 1928 (Romero et al. 2012), have become vital medicine for human and health of animal. It can originate from natural sources or artificial origins. It must be non-toxic to the host, which lets them be used as a chemo-medicine agent for bacterial disease treatment. In aquaculture, there are numerous bacterial diseases, which leads to the use of antibiotic expansion (Defoirdt et al. 2007; Romero et al. 2012), but antibiotics have numerous disadvantages in their use in aquaculture (Burridge et al. 2010) as follows:
It is not easy to estimate a specific concentration due to different dissemination and registration systems in different countries that lead to different concentrations.
Antibiotic is added to food which settles into water so fish can get it that leads to increase biological carrying in water.
Due to several numbers of bacteria, numerous antimicrobial agents are used, thus leading to increased biological carrying in water too.
An antibiotic may be widely used in aquaculture but not for all bacteria, even bacteria which may have been affected at the beginning may have a resistance for this antibiotic later on.
The price of these chemical treatments is very high, stimulation is heavy, efficacy is low, and there are various side effects.
Moreover, it has been stated by Romero et al. (2012) that antibiotic resistance takes two forms, either inherited resistance or acquired. In the first form, an antibiotic has less power to penetrate bacterial cells and reach the mark site, or shortage attraction between antibiotic and its mark site, or absence of the target cell. In the second case, a bacterial species is normally susceptible to a specific antibiotic, but some bacterial strains gain rapid antibiotic resistance. Using antibiotics substantially leads to bacterial resistance to antibiotics.
Natural treatment such as probiotics, essential oils, and herbal biomedicines
Probiotics
Probiotics are one or more microorganisms with positive effects on the host that can survive in the digestive tract because of their acid and bile salt tolerance (Kothari et al. 2019). The intestinal Lactobacillus acidophilus and Bacillus subtilis isolated from silver carp and grass carp are considered the most potent probiotic bacteria (Irianto and Austin 2002). These probiotics have beneficial effects according to Aydın and Çek-Yalnız (2019), where they are used as:
Growth promoter: to improve growth of cultivated species in aquaculture
Pathogen inhibition: antibiotics have long been used in aquaculture for the prevention of crop diseases
Nutrient digestion enhancement
Water quality enhancement and tolerance to stress
Although probiotics have very beneficial effects, they have some disadvantages as follows:
Some strains can increase histamine levels: where histamine is a molecule that the immune system normally produces when it senses a threat. When produced in high amount, the blood flow increases, causing redness and swelling in the affected area (Branco et al. 2018).
Probiotic bacteria increase in a body may lead to toxicity of the bacteria and can cause organ failure leading to death (Kothari et al. 2019).
May pass through the intestinal mucosa and enter the bloodstream or vital organs which cause systemic or localized infections (Kothari et al. 2019).
Essential oils
Essential oils (EOs) are volatile liquid fractions that contain the substances responsible for the aromas of plants, taken from different organs, such as flowers, buds, seeds, leaves, twigs, bark, herbs, wood, fruits, and roots (Bakkali et al. 2008). The essential role of essential oils in plants is as pollinator attractors and a defense mechanism due to antibacterial, antiviral, antifungal, and insecticidal effects (Bader et al. 2010). The molecular basis of the antibacterial action is not well understood (Devi et al. 2010) as the chemical composition is different according to extract condition.
Herbal bio-medicine
Herbal bio-medicine is a part or extract from plant where plants may be delivered as whole plants or parts such as leaves, roots, seeds, or fruits and may be used as either fresh or as-formulated herbal extracts containing various solvents such as water, methanol, chloroform, and ethyl acetate (Ngo 2015). These plants have a beneficial effect and can be introduced into breeding water to enhance water quality, minimize fish stress, increase pathogen resistance, and treat fish diseases. According to Harikrishnan et al. (2011), Chakraborty and Hancz (2011), and Citarasu (2010), plants have been noted to have different effects, such as antistress, growth advancement, stimulation of appetite, enhance immunity, aphrodisiac, and ant pathogenic properties in fish and shrimp aquaculture such as alkaloids, terpenoids, tannins, saponins, and flavonoids.
Although plant drugs have beneficial effects, they have many disadvantages (Chaves et al. 2013, and Reverter et al. 2017) as follows:
There are little information about mode of action, acceptable method, and form for effective and safe management.
It is not a natural part of fish feed or of the marine environment.
May cause environmental problems due to being continuously provided to culture water
The biological activity and chemical compounds of plants and extracts can vary considerably based on the portion and type of the extract used.
Finally, the chemical composition is different according to extract condition and season.
From the previous treatment application, whether natural or synthesis, the main objectives of these treatments are improvement of water quality, reduction of stress, etc., to make fish healthy for disease prevention. Therefore, there is no direct solution to prevent bacterial disease directly, so using a vaccine with nanoparticles is more effective from previous treatment.
Vaccines
Vaccination is one of the most essential and probably the most important approach for preventing and controlling infectious fish diseases by activation of the immune system (Sahdev et al. 2014; Mohd-Aris et al. 2019). The use of vaccines has been crucial in aquaculture as a defense mechanism against pathogens to protect the host from the infections by these pathogens (Shah and Mraz 2020). It is impossible to treat many of the bacterial infections in aquatic animals only with antimicrobials (Dadar et al. 2016). There have recently been improvements in the vaccination of fish. Some of the improvements include immunization of large stocks at a time and multivalent vaccine development (Plant and LaPatra 2011). There are several types of vaccines which may be marked as killed, attenuated, DNA, synthetic peptide, recombinant vector, genetically modified, and subunit vaccines.
Vaccination decreases the use of antibiotics in aquaculture and reduces the possibility of drug resistance (Plant and LaPatra 2011). Fish vaccination began in cutthroat in 1942, by vaccinating against Aeromonas salmonicida infection (Gudding and Van Muiswinkel 2013). Vaccines available are those adjuvant to oil and injectable where adjuvants help improve immune system response as well as minimize administration frequency (Gudding and Van Muiswinkel 2013). According to Plant and LaPatra (2011) and Plaza-Diaz et al. (2019), advantages of vaccines can be summarized as follows:
Vaccination is extensively used in almost all animals in food production.
It reduces the use of antibiotics in aquaculture.
It also helps to prevent the threat of drug resistance.
Vaccines are used to prevent a specific disease outbreak from occurring and are not a therapy.
Its efficiency exists for a longer duration with one or more treatments.
No toxic side effects and healthy fish have better growth performance.
No accumulation of toxic residues.
Pathogens will not develop resistance.
No environmental impact.
Theoretically, it can control any bacterial and viral disease.
Protection at stock level can be achieved because of herd immunity and the need to license and register new vaccines is much easier than antibiotics.
Nanotechnology and aquaculture
In scientific and technological innovation, nanoscience and nanotechnology are highly promising and rapidly progressing disciplines. In both the agricultural and aquaculture fields, nanotechnology demonstrates several interdisciplinary practices.
Nanoparticle definition
A structure between 0.1 and 100 nm (1/1,000,000 mm) is typically deemed to be a nanoparticle (NP). Many industrial sectors have recognized the potential benefits of nanotechnology and their products based on nanotechnology or products containing nanoparticles are already produced in the fields of microelectronics, consumer products (e.g., personal care products, paint, automotive), and pharmaceutical industries. A variety of favorable applications are also emerging with regard to food and agriculture, such as packaging technologies, nanosensors for pathogen detection or storage conditions, nanoformulations of agrochemicals, and nano-encapsulation/nanodelivery of food ingredients (Bhattacharyya 2009).
Usage of nanotechnology
Enhancement of fish growth
In the aquaculture and seafood industries, nanotechnology has a wide potential for application. Limited information exists about the impact on marine species. Young carp and sturgeon have been shown to grow more rapidly due to the effect of iron nanoparticles. It was also noted that the diet supplemented with nano-selenium could boost fish weight, relative gain rate, and fish antioxidant status, and increase the activity of glutathione peroxidase and muscle selenium concentrations of crucian carp (Carassius auratus gibelio) (Handy et al. 2012; Bhupinder 2014)
In addition, fish growth and performance that have been tested by the abovementioned nanomaterials have shown that nanolevel delivery of nutraceuticals can boost fish growth. The direct use of silver nanoparticles in water for the treatment of fungal diseases has been found to be harmful to young trout, while a silver nanoparticle–coated water filter may prevent fungal infections of rainbow trout fish in fish farming. It can be stated here that fish health can be manipulated depending on nanotechnological applications in aquaculture systems, nano delivery of veterinary products in fish food using porous nanostructures, and nanosensors for the detection of pathogens in fish farming systems. Therefore, in a wide range of pond-ecosystem environments, nanomaterials have shown great potential (Gustavo and Dominguez 2014).
The above technology contributes to the antifouling of fishing and aquaculture nets, to antibacterial substances for aquaculture tanks and to new packaging materials for shipping seafood products, and to new equipment for detecting the shelf-life of seafood products. Naturally, the above statement clearly denotes that various nanotechnology processes can preserve food quality. In the aquaculture industry, few studies on nanoparticles have been successful, such as the rapid growth of young carps (C. auratus gibelio) with dietary iron and selenium (Fonghsu 2008; Frederick et al. 2010).
Scientists from the Russian Academy of Sciences have recorded that when fed nanoparticles of iron, young carp and sturgeon showed a faster growth rate (30% and 24%, respectively) (ETC 2003). Research has shown that different sources of selenium (nano-Se and selenomethionine) supplemented to the basal diet could boost final weight, relative gain rate, and antioxidant status (Carassius auratus gibelio). In addition, nano-Se appeared to be more successful in raising muscle selenium content than organic selenomethionine. Similarly, the growth and efficiency of the fish being tested were assessed to be higher at nanolevel delivery of these nutraceuticals (Zhou et al. 2009).
Water filtration and remediation
For the removal of contaminants from water, nano-enabled technologies are available today. In aquaculture applications for holding aerobic and anaerobic biofilm for the removal of ammonia, nitrites, and nitrate contaminants, nanomaterials in the form of activated materials such as carbon or alumina, with additives such as zeolite and iron-containing compounds, can be used. Ultrafine nanoscale iron powder can also be used as an important method for the cleaning of less toxic, simpler carbon compounds such as trichloroethane, carbon tetrachloride, dioxins, and polychlorinated biphenyls, thereby paving the way for nano-aquaculture (Rather et al. 2011).
Fish harvesting
Fishing lures are painted to represent light to draw the attention of fish in order to capture them. However, only in one direction do these traditional lures reflect light. Compared to the case where a lure without a polyimide coating is used, the surface of the lure is colored and then nanocoated with a polyimide film to solve this problem, which increases the probability of catching fish 2 to 3 times (Rather et al. 2011).
Biofouling control
By improving disease control, feeding formulation, and biofouling control, nanotechnology can improve aquaculture production and shrimp culture. Biofouling is unwanted bacteria (as biofilm), and it is possible to monitor invertebrates (mussels and barnacles) and algae (seaweeds and diatoms) by coating or painting nanostructures through the incorporation of metal oxide nanoparticles such as ZnO, CuO, and SiO2. This can be accomplished by creating an effective antifouling surface and enhancing the efficiency of antifouling control (Rajeshkumar et al. 2008; Handy 2012). This antifouling could be used in fishing and aquaculture networks, antibacterial substances for aquaculture tanks, and new packaging materials for marine products.
Removal of heavy metals
For successful heavy metal removal, which is related to high absorption propensity and cost-effectiveness, ligand-based nanocoating can be used as it can be regenerated by treating the bifunctional self-assembling ligand in situ with the previously used nanocoating media. In this method, crystal clear technology is used for water purification; several metal layers are bonded to one substrate (Farmen 2009). Today, because of their high reactivity and large surface area, nanomaterials have been commonly used for heavy metal removal from water/wastewater.
Nanoparticles of metal oxides, such as nanosized ferric oxides, aluminum oxides, manganese oxides, cerium oxides, magnesium oxides, and titanium oxides, have a high surface area and specific aqueous system affinity for heavy metal adsorption. To date, the development of new technologies for the synthesis of metal oxide nanoparticles as well as better practical applications, i.e., composite materials or granular oxides, has become a hot topic for evaluating their heavy metal removal under various experimental conditions or revealing the underlying mechanism of metal removal based on mathematical models or analytical techniques such as XAS and NMM (Hua et al. 2012; Kumar and Chawla 2014).
A detailed research on the effects of humic acid and fulvic acid on heavy metal removal by various nanomaterials from aqueous solutions, primarily including iron-based nanomaterials, carbon-based nanomaterials, and photocatalytic nanomaterials, has been reported by Tang et al. (2014). In addition, this study addressed interaction mechanisms and examined the possible environmental mechanisms of humic acid and fulvic acid.
Chitosan nanoparticles are used as adsorbents in the removal of heavy metals. Recent studies have focused on the removal of heavy metal from clays such as kaolinite, bentonite, and montmorillonite by chitosan nanoparticles due to the intrinsic ability of clays, including chitosan and chitin, to remove heavy metal. Studies on nano chitosan-clay composite for metal ion removal have been reported in recent years (Futalan et al. 2011; Pandey and Mishra 2011). For heavy metal removal from aqueous solution, chitosan-magnetite nanocomposites were also indicated for (Namdeo and Bajpai 2008; Fang et al. 2017).
Nanotechnology devices for aquatic environment management
The application of nanotechnology in seawater shrimp aquaculture showed that the nanodevice was able to reduce the rate of water exchange and increase both the quality of water and the survival rate of shrimp and thus the yield (Wen et al. 2003). Among several nanodevices, nanonet treatment was the best device; the results showed a 100% increase in fish survival rate, and a decrease in both water nitrite and nitrate; and nitrite decreased to as low as 1/4 of the control group. Nanotechnology has also increased the pH of water and greatly improved the efficiency of water (Liu et al. 2008).
In China at present, nano-863 is a high-tech agricultural product that is commonly used. This product is developed by applying high-temperature sintering nanomaterials and good light-absorbing properties to a ceramic material carrier. Nano-863 has been commonly used in livestock raising, crop growing, and aquaculture
Nanotechnology as a new tool in fish diseases
Nanotechnology is capable of observing, measuring, manipulating, and producing things on a nanometer scale. A nanometer (nm) is an SI (Syst’eme international d’Unit’es) unit of 10−9 duration or one-billionth of a meter distance (Mongillo 2007; Can et al. 2011). These new materials are developed with specific physical or chemical properties that are derived from their small size, form, surface area, conductivity, or surface chemistry, and have found numerous applications in textiles, electronics, engineering, and medicine (Abhilash 2010; Hernando 2007). The name “nanomaterial” is built on the word “nano,” which comes from the Greek word meaning “dwarf.” Usually, the term nanomaterial is used for materials ranging from 1 to 100 nm in size (Rai and Ingle 2012).
Thus, nanotechnology is considered a solution which can effectively prevent and monitor diseases and pathogens, and multiply the benefits of aquaculture. Some of the fish health applications of nanotechnology are antibacterial or antifungal surfaces developed using porous nanostructures and nanosensors in aquaculture systems for the detection of pathogens in water and the nanodelivery of veterinary products and fish medicines through fish foods (Muruganandam et al. 2019). The usage level of nano-trace elements is up to 100% higher than that of conventional inorganic trace elements, which is normally very limited as the former reaches the animal body by direct penetration (Muruganandam et al. 2019; Luis et al. 2019; Shah and Mraz 2020).
Controlling virus, bacteria, and fungi would involve early detection and the elimination of pathogens could be through nanomaterials, as they operate on the same scale as a particle-infecting virus or disease (Muruganandam et al. 2019; Luis et al. 2019; Shah and Mraz 2020). Chitosan-based wrapping around vaccines as a nano-encapsulation carrier for effective therapy of bacteria and viruses causing fish disease has been documented as nano-encapsulated materials are durable and with high temperature or acidity. These nanomaterials are becoming very useful for effective agriculture in the development of pathogen-free fish seedlings and shrimp or prawn post-larvae (Muruganandam et al. 2019).
Other applications in aquaculture and marine industries
In the fishery sector, nanotechnologies are widely used for many purposes such as water purification, fish pond sterilization, nanofeed for fish nourishment, and aquatic disease management. Nanotechnologies have been commonly used to treat water and breed fish. The application of nanotechnologies in aquaculture of seawater shrimp showed that the nanosystem was capable of improving water quality, decreasing water exchange rates, and increasing shrimp survival and yield (Wen et al. 2003).
Fishing and aquaculture industries can be pioneered by using nanotechnology with new technologies such as rapid disease detection, increasing fish’s ability to absorb drugs such as hormones, medicines, and nutrients (Dar et al. 2019; Muruganandam et al. 2019). According to Mozafari et al. (2008), Kutlay (2009), and Muruganandam et al. (2019), nanotechnology has high ability to be used in the aquaculture and marine industries especially in:
Color and taste;
Bio-availability of chemical compounds;
Prevention of decay;
Encapsulation and regulated release of food materials;
Improved bio-availability, durability, and shelf-life of critical ingredients;
Defense of food products against spoilage;
Carriers of nutrients, nutraceuticals, enzymes, food additives, and anti-microbial products;
Water nanofiltration: development of more effective aquaculture fish feed. Improving the physical, chemical, and nutritional consistency of feeds and their respective ingredients by applying nanotechnology in the various stages of their manufacturing
Researchers believe that nanotechnology may have the potential to provide disease- and pollution-safe fish pools. Another possible application of nanotechnology in seafood is the use of various protection and packaging techniques to provide product safety by preventing enzymatic and microbial spoilage (Dursun et al. 2010; Can et al. 2011).
Recently, it was observed that there is increasing application of nanoparticles in aquaculture (Khosravi-Katuli et al. 2017). Nanoparticles of elements such as selenium, iron, and chitosan added to diet sources may improve fish production. Researchers believe that nanotechnologies may have the ability to provide disease- and pollution-safe fish ponds. Another possible application of nanotechnologies is the use of various protection and packaging techniques to ensure the safety of seafood by avoiding mildew and microbial spoilage (Can et al. 2011). Scientists from the Russian Academy of Sciences recorded that when fed iron nanoparticles, young carp and sturgeon showed a faster growth rate (30% and 24% respectively) (Ma et al. 2011). Several other studies have indicated that smaller particles produce stronger immune responses than their larger equivalents (Minigo et al. 2007; Mottram et al. 2007; Manolova et al. 2008). According to Abdel-Tawwab et al. (2019), using nano chitosan increased growth performance of Nile tilapia, and antioxidant-stimulated activity was also observed. Activities of catalase, superoxide dismutase, lysozyme, and respiratory burst also increased which lead to improve immunity. According to Udo et al. (2018), using nano chitosan on African catfish improved water quality, daily weight gain, feed utilization, and survival as well as body composition. Another study of using chitosan in Nile tilapia culture (Wang and Li 2011) improved final weight, daily weight gain, and feed conversion ratio of the fish.
How do the nanoparticles work?
Nanosafety-related concerns still exist and must be tackled before their full-scale implementation. Toxicological effects of nanoparticles depend on various factors including complex interplay between particle characteristics such as diameter, form, surface charge, concentration, time of exposure, nature of the nanoparticles, medium composition, route of particle administration, and target species immune system. Despite the available information, several points of criticism are hindering the exact understanding of safety of nanoparticles in aquaculture. Firstly, the way nanoparticles are administrated in aquaculture can be very different: addition to food, to water media, or in aquaculture facilities. Nevertheless, the existing studies in aquatic toxicology are inadequate to fulfill the request for nanoparticle safety in aquaculture like their route of administration, their concentration, and exposure time. Concentrations are sometimes lower or higher than that applied or expected to be applied in aquaculture leading to unrealistic results. Thus, it is not possible to infer about the potential adverse effects on the final consumer. It is necessary to explore the safety of nano-based aquaculture considering not only relatively short-term treatment periods (less than 40 days) but also the whole cultured products along their life cycle from the egg/larva to the table, including water quality. Secondly, due to the fact that aquatic organisms are cultured in different environments, nano-based products can behave very differently like the derived effects; thus, it might be interesting to explore how nano-safety could be influenced by environmental factors mainly pH, salinity, and temperature (Khosravi-Katuli et al. 2017).
Nano-selenium
Selenium is an essential trace element which is required for ordinary body functions and animal metabolism (Prashanth et al. 2015). It plays a significant role in the physiology of fish by enhancing the physiological status of the animal and immune systems. Selenium supplementation avoids cell damage and plays a significant role in the growth, fertility, and immune functions of fish. It also provides organism protection against oxygen-free radicals which are produced during stressful conditions or when an animal is exposed to certain kinds of toxicity (Khurana et al. 2019). According to Le et al. (2013), selenium supplementation increases the immunity of fish by enhancing lysozyme activity and fish red blood cell (RBC) count, where the RBC and the proportion of hematocrit can be enhanced with an appropriate diet in tilapia (El-Hammady et al. 2007).
The most beneficial effect of selenium is in the nano form, which is more effective than the bulky one. Due to its high bioavailability and lower toxicity, the nano form of selenium is a novel type that receives more interest than inorganic and organic forms (Shi et al. 2011; Khurana et al. 2019), where inorganic compounds are more toxic than organic ones. The biological properties of selenium nanoparticles (SeNPs) depend on their size: there is greater activity in smaller particles (Torres et al. 2012).
Nano-selenium (Nano-Se) benefits from the ability to use selenium at zero oxidation (Se0), which has low toxicity and excellent bioavailability relative to other organic and inorganic oxidation states (Torres et al. 2012). It is very unstable and is easily turned into a dormant form. However, its stabilization can be achieved by encapsulation with chitosan (Zhai et al. 2017).
Forms of dietary selenium
Nanotechnology holds promise for medicine and nutrition because nanometer-dimensional materials exhibit novel properties other than those of both isolated atoms and bulk materials. Albrecht et al. (2006) and Zhou et al. (2009) had shown that specific Se (selenium) sources (nano-Se and selenomethionine) supplemented to the basal diet could boost final weight, relative gain rate, GSH-Px activity, and muscle Se concentrations of Gibelium. In addition, nano-Se increasing muscle selenium content tended to be more effective than organic selenomethionine. Nano-Se in crucian carps could have a specific metabolism pathway and mechanism of deposition. According to Deng and Chen (2003), a high dose of selenium nanoparticles and sodium selenite in feed (2.5 mg/kg) of Nile tilapia (Oreochromis niloticus) used to estimate the growth performance revealed that high doses of sodium selenite did not cause a significant gain in fish weight compared to the control group. In the case of selenium nanoparticles, an increase in growth rate of 51.9% than the control was observed, which indicates that a high concentration of selenium nanoparticles has low or zero toxicity compared with sodium selenite.
Advantages of selenium nanoparticles
Nano-scale selenium has a wide range of uses in biomedicine.
It has an effect on oxidative stress reduction (Kojouri and Sharifi 2013).
In addition to its use as antioxidant with a decreased toxicity risk, nano-Se also has the potential to be a chemical preventive agent which acts as anticancer (Wang and Webster 2012).
Numerous studies have shown nano-Se has antimicrobial effect and antifungal activity (Cremonini et al. 2016).
It has protective effects against metal intoxication (Ansar et al. 2017).
It has an immune stimulatory effect (Azdi et al. 2013).
Last but not least, nano-Se has beneficial effects on a number of physiological functions (Shi et al. 2010).
Nano-selenium in aquaculture
Various types of Se nanoparticle supplementation have been researched in fish species including crucian carp (Wang et al. 2007), artemia (Juhász et al. 2017), Carassius auratus gibelio (Zhou et al. 2009), rainbow trout (Naderi et al. 2017), and common barbell (Kouba et al. 2014). Some of these research indicate that more digestible, better preserved, and more biologically active than inorganic forms are organic forms of Se. According to Zhou et al. (2009), Ashouri et al. (2015), and Saffari et al. (2017), it has been shown that selenium nanoparticles can enhance growth efficiency, boost muscle content, and enhance carp antioxidant protection mechanism compared to organic Se.
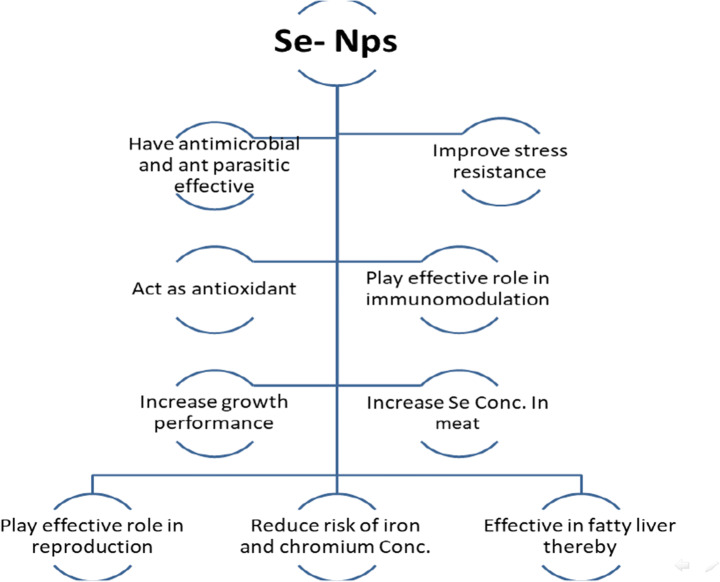
Studies on application of Se nanoparticles in fisheries and livestock indicate that nanoparticles improve reproduction, digestion, development, immunomodulation, and toxicity concentration relative to other forms of Se (Khosravi-Katuli et al. 2017). Nanosupplementation can also decrease oxidative stress and boost fish and livestock productivity (Wang et al. 2013) (Fig. 1).
Fig. 1.
Beneficial role of selenium nanoparticles
Qin et al. (2016) showed that a diet with 0.2 mg/kg SeNP increased weight gain rate and reduced feed coefficient compared to those diets with 0, 0.1, 0.4, 0.8, and 1.6 mg/kg SeNP. Dietary supplementation with SeNP offers resistance to hypoxia stress and improves immunity and disease resistance.
Kumar et al. (2017) demonstrated fish reared under lead (Pb) and high temperature (34 °C) for 72 days. Thermal tolerance and cellular metabolic stress were evaluated. Thermal tolerance increased with SeNP feeding and also improved the oxidative and metabolic enzymes. Incorporation of SeNPs @ 1 mg/kg in diet could protect Pangasius hypophthalmus against Pb and thermal stress.
Zhou et al. (2008) found the highest value of Se content in muscle was observed in SeNP. Survival rate and feed conversion ratio were not affected by the dietary treatments compared with selenomethionine. Moreover, Saffari et al. (2017) showed that SeNP acts more efficiently on growth performance and antioxidant defense system of common carp than organic and inorganic sources of Se. Additionally, Juhász et al. (2017) showed that the moderate level of selenium enrichment (~ 4 mg/kg dry matter) influences the rearing efficiency of fish larvae, but higher dosages could cause adverse effects.
Nanovaccinology as a new technology in fish treatment
Nanotechnology converging with biotechnology has made great progress in biomedicine (Zhao et al. 2014) and has expanded its application in the field of vaccinology giving rise to a new science field called nanovaccinology (Mamo and Poland 2012; Zhao et al. 2014). Nanovaccines are vaccines designed with an antigen or a group of antigens containing an appropriate nanoparticle. They are emerging as a new class of vaccines that specifically target the body’s infection site through the use of the body’s immune system and inhibit spread of infections and diseases (Vinay et al. 2018). The production of the vaccine has undergone a shift from this conventional method of using whole pathogen to using only the protein and peptide antigens required to reduce the unwanted side effects, but the immunogenicity of these antigens has declined dramatically (Smith et al. 2015). To enhance the immunogenicity of vaccines, nanoparticles are used as carriers and/or adjuvants. The immune system can be well activated due to the similar scale (size) between the NPs and the pathogens, resulting in triggered cellular and humoral immunity responses (Vinay et al. 2018; and Gheibi Hayat and Darroudi 2019). Other advantages of nanovaccine include enhanced blood flow stability to increase blood shelf-life, increased activation of the immune system, no need for booster doses, no need to maintain the cold chain, and the ability to actively target (Gheibi Hayat and Darroudi 2019). Nanovaccine is more beneficial and suitable for disease prevention compared with other types of medicines and vaccines.
The use of nanoparticles to vaccinate farmed fish is a unique technique. In drug administration, nanoparticles have multi-faceted advantages such as vaccine delivery and thus carry promises to enhance the safety of farmed fish against diseases caused by pathogens. There is concern however that the benefits of the delivery of nanoparticles may also be followed by environmental and health risks (Walker 2004).
In aquaculture, vaccination has had a significant impact on the management and prevention of infectious diseases despite the fact that there are many infectious diseases for which the production of an effective vaccine was difficult to achieve (Brudeseth et al. 2013; Carmen and Forlenza 2016; Luis et al. 2019; Shah and Mraz 2020). Research into fish health has played a crucial role in the enormous growth of salmon aquaculture, which resulted from the shift from freshwater ponds to the use of sea pens in the 1970s. Salmon, which is farmed in open sea pens, is generally vulnerable to disease. Well-transmitted fish pathogens in water and high stocking densities and short distances between farms allow for high transmission to date; salmon farming and fish health are concerned that epidemic bacterial diseases can be prevented by excessive use of antibiotics which once again have not been environmentally sustainable (Bhattacharyya et al. 2015).
Why using nanoparticles instead of bulk material in vaccine production is recommended?
Nano-immune stimulators are particles of the nanoscale (20–100 nm) that can actually improve in vivo vaccine effectiveness better than bulk molecules (Smith et al. 2013; Irvine et al. 2013). Some NPs are able to boost the host immunity by stimulating different immune cells. NP size, shape, and surface chemistry are essential factors that determine their ability for immune response activation. In general, NPs can induce immune system reactions by significantly increasing the defense gene synthesis and inflammatory reactions (Smith et al. 2013).
Nanoparticles may be absorbed by cellular endocytosis mechanism due to their nano size (Zaman et al. 2013; Zhao et al. 2014; Luis et al. 2019; Shah and Mraz 2020) that promotes the cellular uptake of antigens and enhances antigen presentation capability (Shaalan et al. 2016). Reports have shown that nanotechnology application improves vaccine solubility, stability, targeting, biocompatibility, and permeability (Doll et al. 2013; Lai et al. 2013; Shah and Mraz 2020).
Nanoparticles also have adjuvant properties that can improve antigen efficacy. Nanoparticles enter living cells by cellular endocytosis because of their very small size (Treuel et al. 2013; Zhao et al. 2014). Adjuvants help improve immune response and also reduce administration frequency (Evensen 2009). Researchers have shown that nanotechnology application improves the solubility, stability, targeting, biocompatibility, and permeability of vaccines (Doll et al. 2013; Lai et al. 2013; Luis et al. 2019).
Immune system and nanovaccine
Innate immunity
Macrophages and monocytes are cells that are strongly heterologous that spread throughout the body. Macrophages process and display antigens to produce an adaptive immune response. Macrophages can be easily targeted by surface-engineered NPs due to their intrinsic phagocytic nature, in which cognate ligands agonist to macrophage receptors and can be conjugated on the NP surface. In order to facilitate interactions between NPs and macrophage receptors, many physicochemical parameters of NPs such as thickness, surface load, hydrophobicity, surface topography, and material structure can be optimized (Nel et al. 2009).
Adaptive immunity
T- and B-cells of the adaptive immune system display a receptor set for the identification of a variety of antigens. T-cell immune activation or suppression may determine the fate of a disease. A variety of therapeutic strategies based on NP have been developed to control activity of T-cells against viral, bacterial, or fungal infections (Fang et al. 2018). Through B-cell receptors, B-cells can recognize and react to microbial surface antigens (Kim et al. 2006; Kim et al. 2014). Antigen-specific B-cells were activated and clonally expanded using engineered NPs for the production of vaccines against various diseases.
The immunity of Nile tilapia and Streptococcus pyogenes resistance by creation of antibodies based on the use of selenium nanoparticles, adjuvants, and nanovaccine (dead bacteria) lead to lower mortality rates and attain the greatest survival rate to maximize economic profit (Wang and Li 2011).
Conclusion
Fish is considered a very important source of high-quality protein, fatty acids, vitamins, and other vital elements such as iodine and selenium which do not exist in other crops or meat. Thus, fish is a valuable food for most people. Focusing on the cultured fish species all over the world, they suffer from various infectious and non-infectious diseases like most aquatic organisms due to water mixed with agricultural and industrial pollutants and due to intensive aquaculture. Moreover, the stress on fish leads to increased spread of diseases, and the capacity to capture illnesses (bacteria-fungal-viral) is increasing owing to low immunity of fish in captivity. Thus, fish diseases have attracted attention significantly in recent years.
Moreover, despite the wide expansion of fish culture and economic loss due to infections in intensive culture systems, reliable information on the side effects of antibiotic and indirect treatment by synthesis and natural product have been proved. Classical methods of treatment and remedy have demonstrated significant disadvantages. Nowadays, nanovaccination is a new attempt to enhance the immunogenicity of vaccines using nanoparticles as carriers and/or adjuvants. The immune system can be well activated due to the similar scale (size) between the nanoparticles and the pathogens, resulting in triggered cellular and humoral immunity responses. Thus, using nanotechnology in aquaculture has become a comprehensive tool for solving a lot of problems, not only disease diagnosis and treatment but also water quality control, fish nutrition, environmental management, etc. This review also sheds light on the role of selenium nanoparticles as a novel efficient element in the field of aquaculture. It has great bioavailability and comparatively low toxicity compared to other selenium types, thus being preferable for many aspects.
Finally, we emphasized that the use of nanomaterials and nanotechnology in aquaculture sector will be a slow process. However, as demonstrated throughout this review, many possibilities indicate the existence of a promising path to solving the major problems in aquaculture including disease control and may extend to diet formulation and other marine industries in the near future.
Availability of data and materials
Not applicable
Authors’ contributions
The authors had equal role in the work.
Funding
Not applicable
Declarations
Ethical approval
Ethics required is approved. The Ethical Committee of the Alexandria University and Kafr Elsheikh University approved the fish handling procedures.
Consent to participate
The authors all participated in the work.
Consent to publish
The authors certify that the publisher is permitted to publish this work.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Tawwab M, Razek NA, Abdel-Rahman AM. Immunostimulatory effect of dietary chitosan nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.) Fish Shellfish Immunol. 2019;88:254–258. doi: 10.1016/j.fsi.2019.02.063. [DOI] [PubMed] [Google Scholar]
- Abhilash M. Potential applications of nanoparticles. Int J Pharm Bio Sci. 2010;V1(1):1–12. [Google Scholar]
- Albrecht MA, Evans CW, Raston CL. Green chemistry and the health implications of nanoparticles. Green Chem. 2006;8:417–432. doi: 10.1039/b517131h. [DOI] [Google Scholar]
- Ansar S, Alshehri SM, Abudawood M, Hamed SS, Ahamad T. Antioxidant and hepatoprotective role of selenium against silver nanoparticles. Int J Nanomedicine. 2017;12:7789–7797. doi: 10.2147/IJN.S136748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H. Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio) Aquaculture. 2015;446:25–29. doi: 10.1016/j.aquaculture.2015.04.021. [DOI] [Google Scholar]
- Aydın F, Çek-Yalnız Ş. Effect of probiotics on reproductive performance of fish. Nat Eng Sci. 2019;4(2):153–162. [Google Scholar]
- Azdi MH, Mahdavi M, Setayesh N, Esfandyar M, Shahverdi AR. Selenium nanoparticle-enriched Lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer. Daru. 2013;21(1):33. doi: 10.1186/2008-2231-21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader A, Cioni PL, Flamini G. GC-MS analysis of the essential oils of ripe fruits, roots and flowering aerial parts of Elaeoselinum asclepium subsp. meoides growing in Sicily. In: Natural product communications. 2010;5(7):1111–1114. [PubMed] [Google Scholar]
- Bakkali F, Averbeck S, Averbeck D. Idaomar M. Biological effects of essential oils--a review. Food Chem Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A (2009) Nanoparticles-from drug delivery to insect pest control 1(1), 1–7.
- Bhattacharyya A, Reddy SJ, Hasan MM, Adeyemi MM, Marye RR. Nanotechnology-a unique future technology in aquaculture for the food security. International Journal of Bioassays. 2015;4(7):4115–4126. [Google Scholar]
- Bhupinder SS. Nanotechnology in agri-food production: an overview. Nanotechnol Sci Appl. 2014;7:31–53. doi: 10.2147/NSA.S39406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco ACCC, Yoshikawa FSY, Pietrobon AJ, Sato MN (2018) Role of histamine in modulating the immune response and inflammation. Mediators of Inflammation 2018 |Article ID 9524075 | 10 pages | 10.1155/2018/9524075. [DOI] [PMC free article] [PubMed]
- Brudeseth BE, Wiulsrod BN, Fredriksen K, Lindmo KE, Lokling M, Bordevik N, Steine A, Klevan GK. Status and future prospects of vaccines for industrialized fin-fish farming. Fish Shellfish Immunol. 2013;35:1759–1768. doi: 10.1016/j.fsi.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Burridge L, Weis JS, Cabello F, Pizarro J, Bostick K. Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture. 2010;306(1- 4):7–23. doi: 10.1016/j.aquaculture.2010.05.020. [DOI] [Google Scholar]
- Can E, Kizak V, Kayim M, Can SS, Kutlu B, Ates M, Kocabasl M, Demirtas N. Nanotechnological applications in aquaculture seafood industries and adverse effects of nanoparticles on environment. J Mater Sci Eng. 2011;5:605–609. [Google Scholar]
- Carmen WEE, Forlenza L. Oral vaccination of fish: lessons from humans and veterinary species. Dev Comp Immunol. 2016;64:118–137. doi: 10.1016/j.dci.2016.03.024. [DOI] [PubMed] [Google Scholar]
- Chakraborty SB, Hancz C. Application of phytochemicals as immunostimulant, antipathogenic and antistress agents in finfish culture. Rev Aquac. 2011;3:103–119. doi: 10.1111/j.1753-5131.2011.01048.x. [DOI] [Google Scholar]
- Chaplin DD. Overview of the immune response. Journal of Allergy and Clinical Immunology. 2010;125(2 Suppl 2):S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves TP, Santana CP, Véras G, Brandão DO, Felismino DC, Medeiros ACD, Trovão DMBM. Seasonal variation in the production of secondary metabolites and antimicrobial activity of two plant species used in Brazilian traditional medicine. Afr J Biotechnol. 2013;12:847–853. [Google Scholar]
- Citarasu T. Herbal biomedicines: a new opportunity for aquaculture industry. Aquac Int. 2010;18(3):403–414. doi: 10.1007/s10499-009-9253-7. [DOI] [Google Scholar]
- Cremonini E, Zonaro E, Donini M, Lampis S, Boaretti M, Dusi S, Melotti P, Lleo MM, Vallini G. Biogenic selenium nanoparticles: characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb Biotechnol. 2016;9(6):758–771. doi: 10.1111/1751-7915.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M, Dhama K, Vakharia VN, Hoseinifar SH, Karthik K, Tiwari R, Khandia R, Munjal A, Salgado-Miranda C, Joshi SK. Advances in aquaculture vaccines against fish pathogens: global status and current trends. Reviews in Fisheries Science & Aquaculture. 2016;25(3):184–217. doi: 10.1080/23308249.2016.1261277. [DOI] [Google Scholar]
- Dar AH, Rashid N, Majid I, Hussain S, Dar MA (2019) Nanotechnology interventions in aquaculture and seafood preservation. Crit Rev Food Sci Nutr:1–10 [DOI] [PubMed]
- Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P. Alternatives to antibiotics to control bacterial infections: luminescent vibriosis in aquaculture as an example. Trends Biotechnol. 2007;25(10):472–479. doi: 10.1016/j.tibtech.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Deng YS, Chen QJ. Affects of nano-selenium on the growth of Nile tilapia (Oreochromis niloticus) Inland Aquatic Production Journal. 2003;6:28–30. [Google Scholar]
- Devi KP, Nisha SA, Sakthivel R, Pandian SK. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J Ethnopharmacol. 2010;130(1):107–115. doi: 10.1016/j.jep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Doll TA, Raman S, Dey R, Burkhard P. Nano scale assemblies and their biomedical applications. J R Soc Interface. 2013;10(80):20120740. doi: 10.1098/rsif.2012.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun S, Erkan N, Yesiltas M. Application of natural biopolymer based nanocomposite films in seafood. J Fish Sci. 2010;4(1):50–77. [Google Scholar]
- El-Hammady AKI, Ibrahim SA, El-Kasheif MA. Synergistic reactions between vitamin E and selenium in diets of hybrid tilapia (Oreochromis niloticus × Oreochromis aureus) and their effect on the growth and liver histological structure. Egyptian Journal of Aquatic Biology and Fisheries. 2007;11:53–58. doi: 10.21608/ejabf.2007.1914. [DOI] [Google Scholar]
- El-Sayed Ali T, Abdel-Aziz SH, El-Sayed AM, Zeid S. Structural and functional effects of early exposure to 4-nonylphenol on gonadal development of Nile tilapia (Oreochromis niloticus): a-histological alterations in ovaries. Fish Physiol Biochem. 2014;40:1509–1519. doi: 10.1007/s10695-014-9943-6. [DOI] [PubMed] [Google Scholar]
- El-Sayed Ali T, Abdel-Aziz SH, El-Sayed AM, Zeid S. Structural and functional effects of early exposure to 4-nonylphenol on gonadal development of Nile tilapia (Oreochromis niloticus): a-histological alterations in testes. Fish Physiol Biochem. 2014;40:1495–1507. doi: 10.1007/s10695-014-9944-5. [DOI] [PubMed] [Google Scholar]
- El-Sayed Ali T, El-Sayed AM, Abdel-Razek Eissa M, Hanafi H. Effects of dietary biogen and sodium butyrate on hematological parameters, immune response and histological characteristics of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac Int. 2017;26:139–150. doi: 10.1007/s10499-017-0205-3. [DOI] [Google Scholar]
- ETC (Action Group on Erosion, Technology and Concentration) 2003 Down on the farm: the impact of nanoscale technologies on food and agriculture, http://www.etcgroup.org/en/materials/publications.html?pub_id=80)
- Evensen Ø. Development in fish vaccinology with focus on delivery methodologies, adjuvants and formulations. Use Veterinary Drugs Vaccines Mediterranean Aquaculture. 2009;86:177–186. [Google Scholar]
- Fang Z, Zhao Y, Warner RD, Johnson SK. Active and intelligent packaging in meat industry. Trends Food Sci Technol. 2017;61:60–71. doi: 10.1016/j.tifs.2017.01.002. [DOI] [Google Scholar]
- Fang P, Li X, Dai J, Cole L, Camacho JA, Zhang Y, Ji Y, Wang J, Yang X-F, Wang H. Immune cell subset differentiation and tissue inflammation. J Hematol Oncol. 2018;11:97. doi: 10.1186/s13045-018-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmen L. Commercialization of nanotechnology for removal of heavy metals in drinking water. In: Savage N, Diallo M, Duncan J, Street A, Sustich R, editors. Nanotechnology applications for clean water. Norwich: William Andrew Inc; 2009. pp. 115–130. [Google Scholar]
- Firdaus-Nawi M, Saad M. Major components of fish immunity: a review. Pertanika J Tropical Agric Sci. 2016;39(4):393–420. [Google Scholar]
- Fonghsu K (2008) Nanoemulsions for pharmaceutical and nutraceutical delivery in cancer and inflammation. Ph.D. thesis University of Massachusetts Lowell.
- Frederick K, Kuhn ME, Tarver T. Newinsights into food and health. Food Technology -Champaign Then Chicago. 2010;64(5):44–49. [Google Scholar]
- Futalan CM, Kan CC, Dalida ML, Pascua C, Wan MW. Fixed-bed column studies on the removal of copper using chitosan immobilized on bentonite. Carbohydr Polym. 2011;83:697–704. doi: 10.1016/j.carbpol.2010.08.043. [DOI] [Google Scholar]
- Gheibi Hayat SM, Darroudi M. Nanovaccine: a novel approach in immunization. J Cell Physiol. 2019;234(8):12530–12536. doi: 10.1002/jcp.28120. [DOI] [PubMed] [Google Scholar]
- Gudding R, Van Muiswinkel WB. A history of fish vaccination: science-based disease prevention in aquaculture. Fish and Shellfish Immunology. 2013;35(6):1683–1688. doi: 10.1016/j.fsi.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Gustavo A, Dominguez (2014) Nanotechnology in the aquaculture industry. Sustainable Nano 1
- Handy RD (2012) FSBI briefing paper: nanotechnology in fisheries and aquaculture. Fisheries Society of the British Isles (www.fsbi.org.uk/assets/brief-nanotechnology-fisheriesaquaculture.pdf)
- Handy RD, Cornelis G, Fernandes T, Tsyusko O, Decho A, Sabo-Attwood T, Metcalfe C, Steevens JA, Klaine SJ, Koelmans AA, Horne N (2012) Ecotoxicity test methods for engineered nanomaterials: practical experiences and recommendations from the bench. Environ Toxicol Chem:1–29 [DOI] [PubMed]
- Harikrishnan R, Heo J, Balasundaram C, Kim M-C, Kim J-U, Han Y-J, Heo M-S. Effect of traditional Korean medicinal (TKM) triherbal extract on the innate immune system and disease resistance in Paralichthys olivaceus against Uronema marinum. Vet Parasitol. 2011;170:1–7. doi: 10.1016/j.vetpar.2010.01.046. [DOI] [PubMed] [Google Scholar]
- Hernando GA. Nanotecnología y nanopartículas magnéticas: la Física actual en lucha contra la enfermedad. Rev. Real Academia de Ciencias Exactas, Físicas y Naturales (Esp) 2007;101(2):321–327. [Google Scholar]
- Hua M, Zhang S, Pan B, et al. Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater. 2012;212:317–331. doi: 10.1016/j.jhazmat.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Idowu TA, Adedeji HA, Sogbesan OA. Fish disease and health management in aquaculture production. International Journal Environmental & Agricultural Science. 2017;1:2–6. [Google Scholar]
- Irianto A, Austin B (2002) Review Probiotics in aquaculture. Journal of Fish Diseases (1997):633–642
- Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhász P, Lengyel S, Udvari Z, Sándor AN, Stündl L. Optimised selenium enrichment of Artemia sp. feed to improve red drum (Sciaenops ocellatus) larvae rearing. Acta Biol Hung. 2017;68:255–266. doi: 10.1556/018.68.2017.3.3. [DOI] [PubMed] [Google Scholar]
- Khosravi-Katuli K, Prato E, Lofrano G, Guida M, Vale G, Libralato G. Effects of nanoparticles in species of aquaculture interest. Environ Sci Pollut Res. 2017;24(21):17326–17346. doi: 10.1007/s11356-017-9360-3. [DOI] [PubMed] [Google Scholar]
- Khurana A, Tekula S, Saifi MA, Venkatesh P, Godugu C. Therapeutic applications of selenium nanoparticles. Biomed Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- Kim YM, Pan JYJ, Korbel GA, Peperzak V, Boes M, Ploegh HL. Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation. Proceedings of the National Academy of Sciences USA. 2006;103:3327–3332. doi: 10.1073/pnas.0511315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MG, Park JY, Shon Y, Kim G, Shim G, Oh Y-K. Nanotechnology and vaccine development. Asian Journal of Pharmacological Science. 2014;9(5):227–235. doi: 10.1016/j.ajps.2014.06.002. [DOI] [Google Scholar]
- Kojouri GA, Sharifi S. Preventing effects of nano-selenium particles on serum concentration of blood urea nitrogen, creatinine, and total protein during intense exercise in donkey. Journal of Equine Veterinary Science (JEVS) 2013;33(8):597–600. doi: 10.1016/j.jevs.2012.09.008. [DOI] [Google Scholar]
- Kothari D, Patel S, Kim SK. Probiotic supplements might not be universally-effective and safe: a review. Biomed Pharmacother. 2019;111:537–547. doi: 10.1016/j.biopha.2018.12.104. [DOI] [PubMed] [Google Scholar]
- Kouba A, Velíšek J, Stará A, Masojídek J, Kozák P. Supplementation with sodium selenite and selenium-enriched microalgae biomass show varying effects on blood enzymes activities, antioxidant response, and accumulation in common barbel (Barbus barbus) Biomed Res Int. 2014;2014(2014):408270. doi: 10.1155/2014/408270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Chawla J. Removal of cadmium ion from water/wastewater by nano-metal oxides: a review. Water Qual Expo Health. 2014;5:215–226. doi: 10.1007/s12403-013-0100-8. [DOI] [Google Scholar]
- Kumar N, Krishnani KK, Gupta SK, Singh NP. Selenium nanoparticles enhanced thermal tolerance and maintain cellular stress protection of Pangasius hypophthalmus reared under lead and high temperature. Respir Physiol Neurobiol. 2017;246:107–116. doi: 10.1016/j.resp.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Kutlay M (2009) The factors that effect adoption of nanotechnological foods by society, Workshop of Nanotechnological Risks, Yeditepe University, Istanbul.
- Kwasek K, Thorne-Lyman AL, Phillips M. Can human nutrition be improved through better fish feeding practices? A review paper. Crit Rev Food Sci Nutr. 2020;60:3822–3835. doi: 10.1080/10408398.2019.1708698. [DOI] [PubMed] [Google Scholar]
- Lai W, Hu Z, Fang Q. The concerns on biosafety of nanomaterials. JSM Nanotechnol Nanomed. 2013;1(2):1009. [Google Scholar]
- Le KT, Fotedar R, Partridge G. Selenium and vitamin E interaction in the nutrition of yellowtail kingfish (): physiological and immune responses. Aquac Nutr. 2013;20:303–313. doi: 10.1111/anu.12079. [DOI] [Google Scholar]
- Liu J, Zhang YD, Zhang ZM (2008) Application study on nanobiotechnology in increasing yield benefit of rice, maize and soybean. J Anhui Agric Sci 36:15814–15816. (In Chinese with English abstract)
- Luis AIS, Campos EVR, de OliveiraJL FLF. Trends in aquaculture sciences: from now to use of nanotechnology for disease control. Rev Aquac. 2019;11:119–132. doi: 10.1111/raq.12229. [DOI] [Google Scholar]
- Ma R, Rupam S, Md A, Ahmad S, Neeraj K, Mohammad K, Ramya VL (2011) Nanotechnology: a novel tool for aquaculture and fisheries development. A prospective mini-review. Fisheries and Aquaculture Journal 2011. 10.4172/2150-3508.1000016
- Mamo T, Poland GA. Nanovaccinology: the next generation of vaccines meets 21st century materials science and engineering. Vaccine. 2012;30:6609–6611. doi: 10.1016/j.vaccine.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. E J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- Minigo G, Scholzen A, Tang CK, Hanley JC, Kalkanidis M, Pietersz GA, Apostolopoulos V, Plebanski M. Poly-L-lysine-coated nanoparticles: a potent delivery system to enhance DNA vaccine efficacy. Vaccine. 2007;25:1316–1327. doi: 10.1016/j.vaccine.2006.09.086. [DOI] [PubMed] [Google Scholar]
- Mohanty BP. book: Conspectus on inland fisheries management, Chapter: # 2, Publisher: ICAR - Central Inland Fisheries Research Institute, Editors: A. K. Das and D. Panda. 2015. Nutritional value of food fish; pp. 15–21. [Google Scholar]
- Mohd-Aris A, Muhamad-Sofie MHN, Zamri-Saad M, Daud HM, Ina-Salwany MY. Live vaccines against bacterial fish diseases: a review. Veterinary World. 2019;12(11):1806–1815. doi: 10.14202/vetworld.2019.1806-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongillo FJ. Nanotechnology 101. Westport, Connecticut/London: Greenwood Press; 2007. [Google Scholar]
- Mottram P, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, Ghildyal R, Vardaxis N, Plebanski M. Type 1 and type 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm. 2007;4:73–84. doi: 10.1021/mp060096p. [DOI] [PubMed] [Google Scholar]
- Mozafari MR, Johnson C, Hatziantoniou S, Demetzos S. Nanoliposomes and their applications in food nanotechnolog. Journal of Liposome Research. 2008;18(4):309–327. doi: 10.1080/08982100802465941. [DOI] [PubMed] [Google Scholar]
- Muruganandam M, Chipps SR, Ojasvi PR. On the advanced technologies to enhance fisheries production and management. Acta Scientific Agriculture. 2019;3(8):216–222. doi: 10.31080/ASAG.2019.03.0589. [DOI] [Google Scholar]
- Naderi M, Keyvanshokooh S, Salati AP, Ghaedi A. Combined or individual effects of dietary vitamin E and selenium nanoparticles on humoral immune status and serum parameters of rainbow trout (Oncorhynchus mykiss) under high stocking density. Aquaculture. 2017;474:40–47. doi: 10.1016/j.aquaculture.2017.03.036. [DOI] [Google Scholar]
- Namdeo M, Bajpai SK. Chitosan-magnetite nanocomposites (CMNs) as magnetic carrier particles for removal of Fe(III) from aqueous solutions. Colloids Surf A Physicochem Eng Aspects. 2008;320:161–168. doi: 10.1016/j.colsurfa.2008.01.053. [DOI] [Google Scholar]
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- Ngo H. The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture. 2015;446:88–96. doi: 10.1016/j.aquaculture.2015.03.014. [DOI] [Google Scholar]
- Pandey S, Mishra SB. Organic-inorganic hybrid of chitosan/organoclay bionanocomposites for hexavalent chromium uptake. J Colloid Interface Sci. 2011;361:509–520. doi: 10.1016/j.jcis.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Plant KP, LaPatra SE. Advances in fish vaccine delivery. Dev Comp Immunol. 2011;35(12):1256–1262. doi: 10.1016/j.dci.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Advances in Nutition. 2019;10(suppl_1):S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashanth L, Kattapagari KK, Chitturi RT, Baddam VRR, Prasad LK. A review on role of essential trace elements in health and disease. Journal of dr. ntr university of health sciences. 2015;4(2):75. doi: 10.4103/2277-8632.158577. [DOI] [Google Scholar]
- Qin F, Shi M, Yuan H, Yuan L, Lu W, Zhang J, Tong J, Song X. Dietary nanoselenium relieves hypoxia stress and, improves immunity and disease resistance in the Chinese mitten crab (Eriocheir sinensis) Fish shellfish immunol. 2016;54:481–488. doi: 10.1016/j.fsi.2016.04.131. [DOI] [PubMed] [Google Scholar]
- Rai M, Ingle A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl Microbiol Biotechnol. 2012;94:287–293. doi: 10.1007/s00253-012-3969-4. [DOI] [PubMed] [Google Scholar]
- Rajeshkumar S, Ahmed VPI, Parameswaran V, et al. Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in Asian sea bass (Lates calcarifer) to protect from Vibrio manguillarum. Fish Shellfish Immunol. 2008;25:47–56. doi: 10.1016/j.fsi.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Rather MA, Sharma R, Aklakur M, Ahmad S, Kumar N, Khan M, Ramya VL (2011) Nanotechnology: a novel tool for aquaculture and fisheries development. A prospective mini-review. Fish Aquacult J 16:1–15
- Rawn DFK, Krakalovich T, Forsyth D, Roscoe V. Analysis of fin and non-fin fish products for azamethiphos and dichlorvos residues from the Canadian retail market. International Journal of Food Science & Technology. 2009;44:1510–1516. doi: 10.1111/j.1365-2621.2007.01678.x. [DOI] [Google Scholar]
- Reverter M, Tapissier-Bontemps N, Sasal P, Saulnier D. Use of medicinal plants in aquaculture. Diagnosis and control of diseases of fish and shellfish. 2017;9:223–261. doi: 10.1002/9781119152125.ch9. [DOI] [Google Scholar]
- Rodrigues SM, Demokritou P, Dokoozlian N, Hendren CO, Karn B, Mauter MS, et al. Nanotechnology for sustainable food production: promising opportunities and scientifific challenges. Environmental Science: Nano. 2017;4:767–781. [Google Scholar]
- Romero J, Feijoo CG, Navarrete P (2012) Antibiotics in aquaculture–use, abuse and alternatives, health and environment in aquaculture, Dr. Edmir Carvalho (Ed.), ISBN; 978-953-51-0497-1. Tech, Available from: http://www. intechopen. com/books/healthandenvironment-in-aquaculture/antibioticsin-aquacultureuse-abuse-and-alternatives.
- Saffari S, Keyvanshokooh S, Zakeri M, Johari SA, Pasha-Zanoosi H. Effects of different dietary selenium sources (sodium selenite, selenomethionine and nanoselenium) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio) Aquac Nutr. 2017;23:611–617. doi: 10.1111/anu.12428. [DOI] [Google Scholar]
- Sahdev P, Ochyl PL, Moon JJ. Biomaterials for nanoparticle vaccine delivery systems. Pharm Res. 2014;31:2563–2582. doi: 10.1007/s11095-014-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaalan M, Saleh M, El-Mahdy M, El-Matbouli M. Recent progress in application of nanoparticles in fish medicine: a review. Nanomed Nanotechnology. 2016;12(3):701–710. doi: 10.1016/j.nano.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Shah BR, Mraz J. Advances in nanotechnology for sustainable aquaculture and fisheries. Rev Aquac. 2020;12:925–942. doi: 10.1111/raq.12356. [DOI] [Google Scholar]
- Sharma M, Shrivastav AB, Sahni YP, Pandey G. Overviews of the treatment and control of common fish diseases. International Research Journal of Pharmacy. 2012;3(7):123–127. [Google Scholar]
- Shi L-G, Yang R-J, Yue W-B, Xun W-J, Zhang C-X, Ren Y-S, Shi L, Lei F-U. Effect of elemental nano-selenium on semen quality, glutathione peroxidase activity, and testis ultrastructure in male Boer goats. Anim Reprod Sci. 2010;118(2-4):248–254. doi: 10.1016/j.anireprosci.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Shi L, Xun W, Yue W, Zhang C, Ren Y, Shi L, Wang Q, Yang R, Lei F. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Ruminant Research- SMALL RUMINANT RES. 2011;96:49–52. doi: 10.1016/j.smallrumres.2010.11.005. [DOI] [Google Scholar]
- Silva JRMC, Staines NA, Hernandez-Blazquez FJ, Porto-Neto LR, Borges JCS. Phagocytosis and giant cell formation at 0° C by macrophage (MO) of Notothenia coriiceps. J Fish Biol. 2002;60(2):466–478. [Google Scholar]
- Smith DM, Simon JK, Baker JR. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Morton LD, Ulery BD. Nanoparticles as synthetic vaccines. Curr Opin Biotechnol. 2015;34:217–224. doi: 10.1016/j.copbio.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Sommerset I, Krossøy B, Biering E, Frost P. Vaccines for fish aquaculture. Expert review of vaccine. 2005;4:89–101. doi: 10.1586/14760584.4.1.89. [DOI] [PubMed] [Google Scholar]
- Tang WW, Zeng GM, Gong JL, Liang J, Xu P, Zhang C, Huang BB (2014) Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: a review. Sci Total Environ 468– 469:1014–1027. 10.1016/j.scitotenv.2013.09.044, 468-469 [DOI] [PubMed]
- Torres SK, Campos VI, León CG, Rodríguez-Llamazares BSM, Rojas BSM, González BM, Smith BC, Mondaca MA, Campos ÁVL, Leon CG, AMA M, Rodríguez-Llamazares S, Rojas SM, González M, Smith C. Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J Nanopart Res. 2012;14(11):1236. doi: 10.1007/s11051-012-1236-3. [DOI] [Google Scholar]
- Treuel L, Jiang X, Nienhaus GU. New views on cellular uptake and trafficking of manufactured nanoparticles. Journal of Royal Society Interface. 2013;10:20120939. doi: 10.1098/rsif.2012.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo IU, Etukudo U, Anwana UIU. Effects of chitosan and chitosan nanoparticles on water quality, growth performance, survival rate and meat quality of the African catfish, Clarias gariepinus. Nanoscience. 2018;1(1):12–25. doi: 10.31058/j.nano.2018.11002. [DOI] [Google Scholar]
- Uribe C, Folch H, Enríquez R, Moran G. Innate and adaptive immunity in teleost fish: a review. Vet Med. 2011;56(10):486–503. doi: 10.17221/3294-VETMED. [DOI] [Google Scholar]
- Vinay TN, Bhat S, Gon Choudhury T, Paria A, Jung MH, Shivani Kallappa G, Jung SJ. Recent advances in application of nanoparticles in fish vaccine delivery. Reviews in Fisheries Science & Aquaculture. 2018;26(1):29–41. doi: 10.1080/23308249.2017.1334625. [DOI] [Google Scholar]
- Walker PJ (2004) Disease emergence and food security: global impact of pathogens on sustainable aquaculture production. In: Fish, aquaculture and food security, sustaining fish as a food supply. A conference conducted by the ATSE Crawford Fund Parliament House; pp.45-52.
- Wang Y, Li J. Effects of chitosan nanoparticles on survival, growth and meat quality of tilapia, Oreochromis niloticus. Nanotoxicology. 2011;5(3):425–431. doi: 10.3109/17435390.2010.530354. [DOI] [PubMed] [Google Scholar]
- Wang Q, Webster TJ. Nanostructured selenium for preventing biofilm formation on polycarbonate medical devices. J Biomed Mater Res A. 2012;100(12):3205–3210. doi: 10.1002/jbm.a.34262. [DOI] [PubMed] [Google Scholar]
- Wang Y, Han J, Li W, Xu Z. Effect of different selenium source on growth performances, glutathione peroxidase activities, muscle composition and selenium concentration of allogynogenetic crucian carp (Carassius auratus gibelio) Anim Feed Sci Technol. 2007;134:243–251. doi: 10.1016/j.anifeedsci.2006.12.007. [DOI] [Google Scholar]
- Wang Y, Yan X, Fu L. Effect of selenium nanoparticles with different sizes in primary cultured intestinal epithelial cells of crucian carp, Carassius auratus gibelio. Int J Nanomedicine. 2013;8:4007–4013. doi: 10.2147/IJN.S43691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JQ, Cai DW, Ding YL, Yu LS, Huang JW. Summary report on experiment of Qiangdi nanometer 863 biological assistant growth unit in sea shrimp farming. J Mod Fish Inf. 2003;10:12–15. [Google Scholar]
- Zaman M, Good MF, Toth I. Nano vaccines and their mode of action. Methods. 2013;60:226–231. doi: 10.1016/j.ymeth.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Zhai X, Zhang C, Zhao G, Stoll S, Ren F, Leng X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. Journal of Nanobiotechnology. 2017;15(1):4. doi: 10.1186/s12951-016-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Seth A, Wibowo N, Zhao C-X, Mitter N, Yu C, Anton PJ, Middelberg APJ. Nanoparticle vaccines. Vaccine. 2014;32(3):327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- Zhou B-C, Liu Z-Y, Wang G-C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol. 2008;99:4717–4722. doi: 10.1016/j.biortech.2007.09.073. [DOI] [PubMed] [Google Scholar]
- Zhou X, Wang Y, Gu Q, Li W. Effects of different dietary selenium sources (Selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio) Aquac. 2009;291:78–81. doi: 10.1016/j.aquaculture.2009.03.007. [DOI] [Google Scholar]