Abstract
After training as a gastroenterologist in the UK, the author became interested in lipidology while he was a research fellow in the USA and switched careers after returning home. Together with Nick Myant, he introduced the use of plasma exchange to treat familial hypercholesterolemia (FH) homozygotes and undertook non-steady state studies of LDL kinetics, which showed that the fractional catabolic rate of LDL remained constant irrespective of pool size. Subsequent steady-state turnover studies showed that FH homozygotes had an almost complete lack of receptor-mediated LDL catabolism, providing in vivo confirmation of the Nobel Prize-winning discovery by Goldstein and Brown that LDL receptor dysfunction was the cause of FH. Further investigation of metabolic defects in FH revealed that a significant proportion of LDL in homozygotes and heterozygotes was produced directly via a VLDL-independent pathway. Management of heterozygous FH has been greatly facilitated by statins and proprotein convertase subtilisin/kexin type 9 inhibitors but remains dependent upon lipoprotein apheresis in homozygotes. In a recent analysis of a large cohort treated with a combination of lipid-lowering measures, survival was markedly enhanced in homozygotes in the lowest quartile of on-treatment serum cholesterol. Emerging therapies could further improve the prognosis of homozygous FH; whereas in heterozygotes, the current need is better detection.
Supplementary key words: atherosclerosis; cardiovascular disease; cholesterol; hyperlipidemia; hypolipidemic drugs; proprotein convertase subtilisin/kexin type 9; statins, low density lipoprotein receptor; lipoprotein (a); apheresis; familial hypercholesterolemia
Abbreviations: CHD, coronary heart disease; FCR, fractional catabolic rate; FH, familial hypercholesterolemia; Lp(a), lipoprotein (a); PCSK9, proprotein convertase subtilisin/kexin type 9
Personal perspective
The neologism in the title hints that the author was once a gastroenterologist. While training to be one at Hammersmith Hospital in London, I was awarded a Medical Research Council Traveling Fellowship to study vitamin D absorption in Kurt Isselbacher’s laboratory at the Massachusetts General Hospital in Boston. This was in 1966, and the following January saw the publication in the New England Journal of Medicine of the hugely influential five-part series of articles by Fredrickson, Levy, and Lees (1) describing the phenotypic classification of disorders of lipoprotein metabolism. As a result, my interest soon switched from vitamin D to cholesterol. I was in good company in this respect because the medical interns at the Massachusetts General Hospital during the time I was there included Mike Brown, Joe Goldstein, Tony Gotto, and Bryan Brewer, all of whom subsequently became household names in the world of lipid research. A subsequent spell of research working with Tony Gotto in Houston further increased my interest in lipids and led to my abandoning a career in gastroenterology after I returned to the UK. Instead, the late Nick Myant (2) created a clinical research position for me in his Medical Research Council Lipid Metabolism Unit at Hammersmith Hospital. His main interest at that time was studying cholesterol metabolism in patients with familial hypercholesterolemia (FH), an interest I soon came to share, and in 1975, we organized a highly successful meeting that was attended by most of those who were actively undertaking research on FH at the time (Fig. 1).
Fig. 1.
Participants in the Workshop on FH held at the Royal Postgraduate Medical School, Hammersmith Hospital, London, in September 1975.
Myant’s fascination with FH dated from 1963 when a 7-year-old girl was referred to him with extensive cutaneous xanthomas and a cholesterol level of 24 mmol/l (930 mg/dl). To investigate this further, he performed a radio-isotopic turnover study, which demonstrated that she had a markedly increased rate of cholesterol synthesis. Her hypercholesterolemia proved to be resistant to diet and drugs as well as to the ileal bypass, which she underwent subsequently, and she died from myocardial ischemia a few months later at the age of only 10 years (3). In 1970, Myant summarized the results of the various cholesterol turnover studies he and his colleagues had performed over the years and concluded that over-synthesis rather than defective removal of cholesterol was the underlying abnormality in FH (4). However, it later became apparent that the increase in cholesterol synthesis was a consequence of the disorder rather than the cause.
Phenotypic features of FH
FH is characterized by hypercholesterolemia from birth and the subsequent development of xanthomas and premature atherosclerosis within families, as was first described by Müller (5) in 1938. The dominantly inherited increase in plasma cholesterol consists largely of LDL cholesterol, which reaches 20–30 mmol/l (775–1,160 mg/dl) in homozygotes and 8–12 mmol/l (310–465 mg/dl) in heterozygotes. The frequency of FH in Europe and North America averages 1:244 for heterozygotes and 1:160,000 to 1:300,000 for homozygotes (6) but in some parts of the world is much higher. In South Africa and Quebec, the increase represents founder effects traceable to immigrant settlers from Europe, whereas in Muslim communities, it reflects the frequency of first-cousin marriages.
In homozygotes, severe hypercholesterolemia manifests itself in childhood and results in atheromatous involvement of the aortic root before puberty and sudden death from myocardial infarction or acute coronary insufficiency, usually before the age of 30. At autopsy, the aortic valve, sinuses of Valsalva, and ascending arch of the aorta are grossly infiltrated with atheroma, with the coronary ostia often narrowed to pinhole size. Atheromatous involvement of the aortic valve can cause severe stenosis, necessitating surgical reconstruction of the aortic root and valve replacement, which carries a high risk. In a recent survey in the UK, 21 of 44 homozygotes had aortic involvement, 10 of whom had undergone aortic valve replacement with a 50% operative mortality (7).
In contrast, heterozygous FH often remains undiagnosed until the onset of cardiovascular symptoms in adult life. In addition to hypercholesterolemia, there may be signs of cholesterol deposition, namely, corneal arcus and tendon xanthomas. The increased frequency and premature onset of coronary heart disease (CHD) were first documented by Slack (8) and, in untreated subjects, occur about 20 years earlier than in the rest of the population. The likelihood of developing premature CHD is further increased by a family history of it and by the presence of other risk factors such as obesity, smoking, low HDL cholesterol and, especially, by a raised level of lipoprotein (a) [Lp(a)] (9).
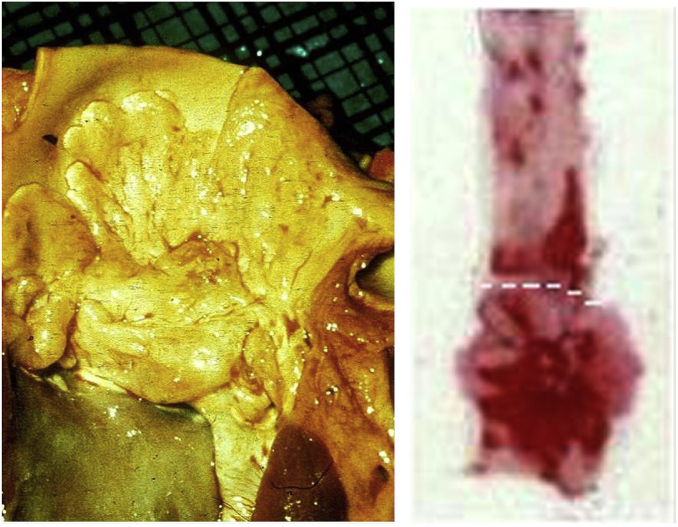
Coronary angiography shows that triple vessel disease is common in FH heterozygotes, often accompanied by disease of the left main stem, but the aortic root is affected to a much lesser extent than in homozygotes (10). It seems that atheromatous involvement of the aortic valve and root, illustrated in Fig. 2, is determined by relatively short-term exposure to very high LDL concentrations, as occurs in homozygotes and cholesterol-fed rabbits (11), rather than by long-term exposure to the less extreme concentrations of LDL seen in most heterozygotes. This difference may reflect the propensity of very high concentrations of LDL to aggregate when subjected to mechanical stress, as might occur during systolic ejection of blood from the left ventricle.
Fig. 2.
Atheroma of the aortic root at autopsy of an untreated FH homozygote who died at age 23 (left) and of a cholesterol-fed New Zealand white rabbit (right). The red areas show the intensity of staining of the rabbit aorta with Oil Red O, which is maximal below the dashed line that corresponds with the site of bisection of the human aorta. Reprinted from (11) with permission from Elsevier.
The cause of FH
Working together in Dallas in 1972, Goldstein and Brown became intrigued by a young homozygote with a serum cholesterol of 26 mmol/l (1,006 mg/dl) and severe aortic and coronary disease, reminiscent of Myant’s experience a decade earlier. Her predicament stimulated them to undertake the groundbreaking research for which they were subsequently awarded the Nobel Prize in Physiology or Medicine, as summarized below.
Because hypercholesterolemia is present in heterozygotes as well as in homozygotes, Goldstein and Brown speculated that FH was probably due to a defect in a protein involved in the feedback regulation of cholesterol synthesis. They tested this hypothesis in a series of ingenious experiments using fibroblasts cultured from the skin of FH patients and normal subjects and discovered that the activity of HMG-CoA reductase in fibroblasts cultured from their FH homozygote when incubated in lipoprotein-deficient plasma was 60–80 times greater than that of control fibroblasts. But unlike the latter, HMG-CoA reductase activity in the homozygote’s fibroblasts was not suppressed by adding LDL to the culture medium (12). Fibroblasts from FH heterozygotes exhibited a partial defect in regulation of HMG-CoA reductase. but Goldstein and Brown considered it unlikely that a defect in the gene encoding this enzyme could be the cause of FH.
Instead, they demonstrated that 125I-labeled LDL was bound to normal fibroblasts by a high-affinity saturable process, which mediated the uptake and subsequent proteolytic degradation of LDL and resulted in suppression of HMG-CoA reductase activity and, therefore, of cholesterol synthesis. They showed that fibroblasts from FH homozygotes lacked these binding sites and failed to degrade LDL and downregulate their HMG-CoA reductase activity when exposed to LDL (13). Fibroblasts from FH heterozygotes showed intermediate levels of high-affinity binding of LDL.
Goldstein and Brown concluded that high-affinity binding of LDL results in endocytosis and lysosomal degradation of LDL, the ensuing hydrolysis of cholesterol esters leading to release of free cholesterol and inhibition of HMG-CoA reductase (14). They proposed that FH was due to an abnormality of a dominantly inherited gene whose product is the LDL receptor, defects of which result in reduced catabolism of LDL and increased cholesterol synthesis. The severity of the ensuing hypercholesterolemia depended upon whether the receptor defect was partial, as in heterozygotes, or total, as in homozygotes.
Evidence that LDL catabolism was defective in vivo came from turnover studies by Langer, Strober, and Levy (15) in 1972, which showed that the fractional catabolic rate (FCR) of 125I-labeled LDL in FH heterozygotes was half that of normal subjects. However, LDL production rates were similar, which suggested the existence of an intrinsic catabolic defect rather than saturation of the normal clearance pathway by increased synthesis of LDL.
To date, approximately 3,000 variants in the LDL receptor gene have been described, 1,300 of which can cause FH (16). An identical clinical syndrome occurs as a result of inheritance of mutations at the apoB locus, which result in a functionally defective form of LDL. This disorder, familial defective apoB-100, has a frequency of about 0.1% in people of European descent. Rarely, FH is caused by dominantly inherited gain-of-function mutations of a gene encoding proprotein convertase subtilisin/kexin type 9 (PCSK9), which results in increased degradation of LDL receptors and an unusually severe clinical phenotype. FH can also be caused by recessively inherited loss-of-function mutations of a gene encoding an adaptor protein involved in the clathrin-mediated internalization of the LDL receptor, which results in a milder phenotype than dominantly inherited forms of the condition and is termed autosomal recessive hypercholesterolemia. A recent survey detected mutations of the LDL receptor, apoB, and PCSK9 genes in only 60% of patients with clinically defined FH in North America (17), the likelihood being that many mutation-negative subjects have a polygenic basis for their hypercholesterolemia.
ApoB metabolism in FH
Non-steady-state turnover studies of the kinetics of LDL in FH patients showed that the FCR of LDL remained subnormal even after a marked reduction in pool size following plasma exchange (18). This finding supported the concept that defective catabolism of LDL was an intrinsic feature of FH and not secondary to the increased production of apoB-containing lipoproteins that we demonstrated in simultaneous steady-state studies of VLDL and LDL turnover in homozygotes (19). In collaboration with Canadian colleagues, we later confirmed that increased production of LDL also occurs in FH heterozygotes and showed that this reflected VLDL-independent synthesis of LDL (20). The increased production of directly secreted LDL apoB particles (Table 1) and, hence, increased availability of apoB for intrahepatic conjunction with apo(a), might explain why, for any given apo(a) genotype, Lp(a) levels are 3-fold higher in FH patients than in normal subjects, with a clear dose effect according to the number of defective LDL receptor alleles (21). This explanation gains credence from evidence that, in contrast with LDL, decreased catabolism does not explain raised Lp(a) levels in FH (22).
TABLE 1.
Simultaneous studies of turnover of 125I-VLDL apoB and 131I-LDL apoB in control subjects and FH patients
| Subjects | VLDL |
LDL |
Direct LDL |
||
|---|---|---|---|---|---|
| FCR (per hour) | Synthesis (mg/kg/day) | FCR (per day) | Synthesis (mg/kg/day) | Synthesis (%) | |
| Control (n = 6) | 0.37 | 21.5 | 0.37 | 13.0 | 0 |
| HeFH (n = 3) | 0.30 | 23.2 | 0.24 | 24.6 | 12 |
| HoFH (n = 3) | 0.28 | 10.8 | 0.12 | 18.5 | 41 |
Shepherd et al. (23) had earlier performed turnover studies in Glasgow that involved simultaneous administration of native 125I- and 131I-labeled LDL coupled with 1,2-cyclohexanedione, a chemical modification known to inhibit binding of LDL to fibroblasts. They showed that the FCR of 125I-labeled LDL but not that of 131I-LDL-cyclohexanedione was reduced in FH heterozygotes compared with normal subjects, implying decreased receptor-mediated catabolism of LDL in FH (23). Using their technique, we subsequently demonstrated an almost complete absence of receptor-mediated LDL catabolism in two homozygotes (24).
The likelihood that the abnormalities of receptor-mediated catabolism revealed by these turnover studies reflected impaired uptake of LDL by hepatic receptors was supported by in vitro binding studies using cell membranes prepared from liver biopsies obtained during routine abdominal surgery. Our results showed that saturable binding of 125I-LDL was reduced by approximately 50% in FH heterozygotes compared with normal subjects; when the data were pooled, they showed an inverse correlation between the saturable binding of LDL by liver membranes and the concentration of cholesterol in plasma (25). This finding underlined the importance of hepatic LDL receptors in regulating plasma cholesterol, a premise that was later confirmed when Starzl and colleagues transplanted a healthy liver into an FH homozygote and reduced her plasma cholesterol to normal (26).
Pharmacological treatment of FH
The management of homozygous FH remains a major therapeutic challenge. Diet has little impact on the hypercholesterolemia, and the same applies to drug therapy except for maximum doses of the most potent statins. Partial ileal bypass is ineffective, and although portacaval shunt occasionally has a dramatic effect, its outcome is unpredictable. Liver transplantation remedies the hepatic deficiency of LDL receptors but has the disadvantage of requiring long-term immunosuppression. Gene therapy offers a possible means of treating this disorder but so far has proved disappointing. Currently the safest and most reliable way of reducing cholesterol levels in homozygotes is to undertake lipoprotein apheresis at weekly or fortnightly intervals, as discussed below. In contrast, the vast majority of heterozygotes respond to drug therapy and relatively few require apheresis.
Pre-statin and statin era compounds
Bile acid sequestrants (colestyramine and colestipol) were for many years the drugs of choice for heterozygous FH, achieving reductions in LDL cholesterol of up to 30% when given in doses of 24–30 g/day. However, they are poorly tolerated because of gastrointestinal side-effects and, except where safety is an overriding concern as in pregnancy, they have largely been replaced by HMG-CoA reductase inhibitors (statins). The latter class of drug, first used to treat FH by Endo and colleagues (27), has revolutionized the outlook for most FH patients and provides a safe and effective means of lowering LDL cholesterol, although not Lp(a). Rosuvastatin and atorvastatin are the most potent statins and can lower LDL cholesterol by over 50% when given in doses of 40 and 80 mg/day, respectively. Ezetimibe is a useful adjuvant in FH patients failing to achieve target levels of LDL or as monotherapy for those intolerant of statins. Combining a high dose of statin with 10 mg of ezetimibe daily has been shown to decrease LDL cholesterol by >60%.
A survey of more than 1,000 patients with FH in the UK in 1999 showed that their mortality from CHD had halved since 1992, arguably reflecting the universal use of statins to treat such patients from 1989 onwards, when simvastatin was first licensed in Britain (28). This conclusion is supported by data from The Netherlands, which showed that statins increased CHD-free survival among patients with heterozygous FH by 76% and that the risk of myocardial infarction in those over the age of 55 on statins was no greater than that of the Dutch population at large (29).
PCSK9 inhibitors
For statin-intolerant individuals and for patients with refractory FH, a new class of lipid-lowering agent has recently become available. These are the PCSK9 inhibitors, monoclonal antibodies that reduce the rate of degradation of LDL receptors by PCSK9 and thereby promote receptor-mediated LDL uptake by the liver. When given by injection every 2–4 weeks, their LDL-lowering effect is equivalent to that of a high-dose potent statin and is additive to the effect of the latter.
Like the discovery of the LDL receptor, the discovery of PCSK9 inhibitors resulted from studies of patients with rare genetic mutations. PCSK9 belongs to a family of proteolytic enzymes, one of its functions being to induce the lysosomal degradation of the LDL receptor. In 2003, hypercholesterolemia due to an increase in LDL levels was reported in members of two French families with a gain-of-function mutation of PCSK9, and, two years later, it was reported that loss-of-function mutations of the gene were associated with subnormal levels of LDL and a markedly reduced risk of CHD (30). This pointed to the potential of PCSK9 inhibition as a means of treating hypercholesterolemia, either as a substitute for statins in statin-intolerant subjects or as an adjunct in statin-refractory subjects, given that the ability of statins to upregulate the LDL receptor and thereby lower LDL is limited by the increase in PCSK9 levels that they induce (31)
Current pharmacological approaches to inhibiting PCSK9 are humanized mAbs, two of which, evolocumab and alirocumab, are approved for use in patients with or at high risk of developing atherosclerotic CVD. A recent meta-analysis of 24 randomized trials in over 10,000 subjects given various doses of evolocumab or alirocumab by subcutaneous injection once or twice monthly showed a mean reduction in LDL cholesterol of 47% (32). Data from individual trials of evolocumab showed reductions in LDL cholesterol of 60% at maximum dosage that were similar irrespective of whether patients did or did not have heterozygous FH (33, 34) or of whether they were or were not receiving concomitant statin therapy (35). Open label trials of evolocumab showed that doses of 140 mg every 2 weeks or 420 mg monthly for 11 months halved the risk of cardiovascular events (36).
In the meta-analysis referred to above, Lp(a) levels were reduced by 25%, which is roughly half the reduction achieved in LDL cholesterol. This illustrates one of the limitations of PCSK9 mAbs and probably reflects the fact that Lp(a) is not catabolized by the LDL receptor pathway. Nevertheless, a similarly modest 27% reduction in Lp(a) by evolocumab in patients with high Lp(a) levels in FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) was associated with a decrease in cardiovascular events, so should not be sneezed at (37).
From the practical point of view, PCSK9 mAbs clearly have enormous potential as an adjunct to statins and ezetimibe in the treatment of patients with heterozygous FH and as an alternative in statin-refractory or -intolerant patients with CVD. However, their efficacy in homozygous FH is much less, especially in those who are receptor negative, with reductions in LDL cholesterol and Lp(a) on evolocumab averaging only 23% and 12%, respectively (38), and often they will complement rather than replace lipoprotein apheresis.
The most recent development in the field of PCSK9 inhibition is Inclisiran, a siRNA nucleotide molecule that prevents the translation of PCSK9 in the liver (39). Given twice yearly, it achieved a 50% reduction in LDL cholesterol over and above the effect of background statin/ezetimibe therapy. Currently, this compound is undergoing long-term phase 3 trials, which, if successful, might eventually lead to it supplanting the anti-PCSK9 mAbs in current use. The availability of the twin LDL receptor-upregulating options of statins and PCSK9 inhibitors means that raised LDL cholesterol levels can be lowered very effectively in the majority of eligible patients providing they exhibit some degree of residual LDL receptor activity.
Microsomal triglyceride transfer protein inhibitor, lomitapide
Lomitapide is licensed to treat the extreme hypercholesterolemia seen in many patients with homozygous FH (40). Unlike all the other lipid-lowering drugs described above, it inhibits the secretion of apoB-containing lipoproteins and so does not depend upon LDL receptor activity. In essence it induces an iatrogenic form of abetalipoproteinemia [recessively inherited microsomal triglyceride transfer protein (MTP) deficiency] and has the latter’s drawback of inducing diarrhea and a fatty liver. It remains to be seen whether the latter will eventually lead to hepatic fibrosis, but in patients with this extreme form of hyperlipidemia, which predisposes to premature atherosclerosis and death, this risk seems worth taking. Existing (40) and unpublished data show that lomitapide decreases LDL cholesterol by 50% in FH homozygotes, and in some patients, it either eliminates the need for apheresis or reduces its frequency when given as an adjunct.
Lipoprotein apheresis for FH
Lipoprotein apheresis provides an extracorporeal means of reducing raised plasma levels not only of LDL but also Lp(a) and triglyceride-rich lipoproteins. The term includes all the various procedures used to achieve this objective, including plasma exchange and the selective removal of apoB-containing lipoproteins from whole blood or plasma by adsorption, precipitation, or differential filtration.
Our report in 1975 of the first use of plasma exchange to treat two FH homozygotes (41) heralded a step change in the management of this disorder and was the forerunner of the currently recommended first-line treatment, selective lipoprotein apheresis. We subsequently described the beneficial effects of 3 years of bi-weekly plasma exchange in reducing the serum cholesterol and size of xanthomas, and stabilizing aorto-coronary lesions on angiography (42), and later reported a statistically significant increase in the longevity of five homozygotes treated with bi-weekly plasma exchange in the UK and USA for 6–9 years, as compared with their untreated deceased homozygous siblings (43). This was the first time that lowering LDL cholesterol had been shown to reduce mortality in this hitherto fatal disorder. The history and scientific basis of lipoprotein apheresis are discussed in detail elsewhere (44) and UK and international guidelines on its use in homozygous FH have been published recently (45, 46).
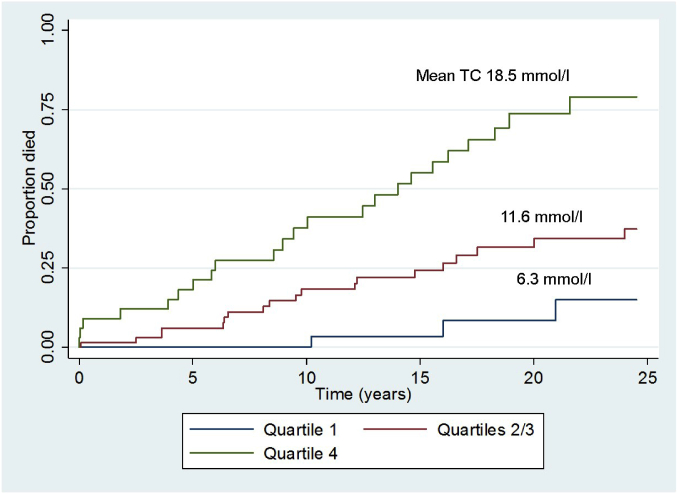
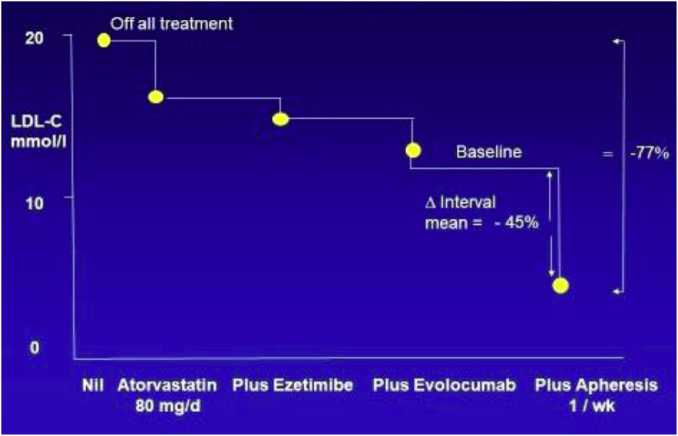
In view of the fact that the number of patients in those earlier studies was small, my collaborators and I recently undertook a retrospective survey of lipid levels and clinical outcomes of 133 FH homozygotes treated with a combination of lipid-lowering measures between 1990 and 2014 in South Africa and the UK (47). We divided these previously statin-naive homozygotes into quartiles according to their on-treatment levels of serum cholesterol and compared the occurrence of any death, cardiovascular death, and major adverse cardiovascular events between the quartiles during 25 years of follow-up, using Cox and competing risks regression analysis. As shown in Fig. 3, homozygotes in quartile 4, with the highest on-treatment serum cholesterol, had a hazard ratio of 11.5 for all-cause mortality compared with those in quartile 1 with the lowest on-treatment cholesterol. Those in quartiles 2 and 3 combined, with an intermediate level of on-treatment cholesterol, had a hazard ratio of 3.6 compared with quartile 1. These differences were statistically significant (P < 0.001) and remained so after adjustments for confounding factors. Significant differences between quartiles were also evident for cardiovascular deaths and major adverse cardiovascular events. These findings provided unequivocal evidence that the extent of reduction of serum cholesterol achieved by a combination of therapeutic measures, including statins, ezetimibe, lipoprotein apheresis, and evolocumab, is the main determinant of survival in homozygous FH. A quantitative assessment of the contribution of each of these measures to LDL lowering in a hypothetical homozygote is illustrated in Fig. 4. In this example, apheresis is the dominant contributor to LDL lowering, based on two plasma or blood volumes being treated weekly in conjunction with combination drug therapy. The importance of apheresis in improving survival in FH homozygotes was recently confirmed by Stefanutti et al. (48).
Fig. 3.
Time to death from any cause after start of treatment in FH homozygotes according to quartile of on-treatment total cholesterol (TC). Quartile 1, <8.1 mmol/l (<313 mg/dl); Quartiles 2/3, 8.1–15.1 mmol/l (313–584 mg/dl); Quartile 4 >15.1 mmol/l (>584 mg/dl). Reproduced from (47) with permission of Oxford University Press.
Fig. 4.
Sequential contributions of a high dose of a statin, ezetimibe, evolocumab, and lipoprotein apheresis to LDL lowering in a hypothetical FH homozygote. To convert millimoles per liter to milligrams per deciliter, multiply by 38.7.
Future prospects
The advent of statins in the 20th century and of PCSK9 inhibitors in the 21st century revolutionized the management of heterozygous FH. The most pressing issue now is the detection of affected individuals before they develop cardiovascular adverse events and here, cascade screening allied to genetic diagnosis provides a cost effective approach (49, 50).
The opposite applies to homozygous FH where the diagnosis is seldom in doubt but where effective treatment remains problematic. The latest potential advance in therapy is the angiopoietin-like protein 3 (ANGPTL3) inhibitor, evinacumab, which has been shown to lower LDL cholesterol by 49% in homozygotes (51) by a mechanism that is independent of the LDL receptor. When used in combination with a statin and a PCSK9 inhibitor, evinacumab achieved an 80% reduction in plasma cholesterol and marked regression of atherosclerosis in genetically modified hyperlipidemic mice (52), but the clinical usefulness of this approach remains to be assessed.
The results of over half a century of clinical and basic research by numerous individuals, notably Fredrickson and Levy, Goldstein and Brown, Endo, and many others, including the author and his colleagues as recalled in this review, have greatly advanced our understanding of the causes, cardiovascular consequences, and treatment of FH. The need for and benefits of early detection and treatment of FH in children to prevent CVD in adulthood was underlined recently (53, 54). Public health policies aimed at improving its detection and management have been proposed in a Global Call to Action by representatives from 40 countries (55), and it is hoped that this initiative will promote the attainment of those objectives as well as increase awareness of FH throughout the world.
Conflict of interest
The author declares no conflicts of interest with the contents of this article.
References
- 1.Fredrickson D.S., Levy R.I., Lees R.S. Fat transport in lipoproteind–an integrated approach to mechanisms and disorders. N. Engl. J. Med. 1967;276 doi: 10.1056/NEJM196701052760107. 34–42, 94–103, 148–156, 215–225, 273–281. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons G., Soutar A., Thompson G. In Memoriam: Nick Myant (1917–2015) J. Lipid Res. 2015;56:1081–1084. doi: 10.1194/jlr.E060129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston I.D., Davis J.A., Moutafis C.D., Myant N.B. Ileal bypass in the management of familial hypercholesterolaemia. Proc. R. Soc. Med. 1967;60:746–748. [PMC free article] [PubMed] [Google Scholar]
- 4.Myant N.B. The regulation of cholesterol metabolism as related to familial hypercholesterolaemia. Sci. Basis Med. Annu. Rev. 1970;1970:230–259. [PubMed] [Google Scholar]
- 5.Müller C. Angina pectoris in hereditary xanthomatosis. Arch. Intern. Med. (Chic.). 1939;64:675–700. [Google Scholar]
- 6.Cuchel M., Bruckert E., Ginsberg H.N., Raal F.J., Santos R.D., Hegele R.A., Kuivenhoven J.A., Nordestgaard B.G., Descamps O.S., Steinhagen-Thiessen E. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur. Heart J. 2014;35:2146–2157. doi: 10.1093/eurheartj/ehu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson G.R., Seed M., Naoumova R.P., Neuwirth C., Walji S., Aitman T.J., Scott J., Myant N.B., Soutar A.K. Improved cardiovascular outcomes following temporal advances in lipid-lowering therapy in a genetically-characterised cohort of familial hypercholesterolaemia homozygotes. Atherosclerosis. 2015;243:328–333. doi: 10.1016/j.atherosclerosis.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Slack J. Risks of ischaemic heart-disease in familial hyperlipoproteinaemic states. Lancet. 1969;2:1380–1382. doi: 10.1016/s0140-6736(69)90930-1. [DOI] [PubMed] [Google Scholar]
- 9.Seed M., Hoppichler F., Reaveley D., McCarthy S., Thompson G.R., Boerwinkle E., Utermann G. Relation of serum lipoprotein (a) concentration and apolipoprotein (a) phenotype to coronary heart disease in patients with familial hypercholesterolemia. N. Engl. J. Med. 1990;322:1494–1499. doi: 10.1056/NEJM199005243222104. [DOI] [PubMed] [Google Scholar]
- 10.Rallidis L., Naoumova R.P., Thompson G.R., Nihoyannopoulos P. Extent and severity of atherosclerotic involvement of the aortic root in familial hypercholesterolaemia. Heart. 1998;80:583–590. doi: 10.1136/hrt.80.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson G.R. Atherosclerosis in cholesterol-fed rabbits and in homozygous and heterozygous LDL receptor-deficient humans. Atherosclerosis. 2018;276:148–154. doi: 10.1016/j.atherosclerosis.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein J.L., Brown M.S. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc. Natl. Acad. Sci. USA. 1973;70:2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown M.S., Goldstein J.L. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3- methylglutaryl coenzyme A reductase activity. Proc. Natl. Acad. Sci. USA. 1974;71:788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown M.S., Goldstein J.L. Receptor-mediated endocytosis: Insights from the lipoprotein receptor system. Proc. Natl. Acad. Sci. USA. 1979;76:3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langer T., Strober W., Levy R.I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J. Clin. Invest. 1972;51:1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leigh S., Futema M., Whittall R., Taylor-Beadling A., Williams M., den Dunnen J.T., Humphries S.E. The UCL low-density lipoprotein receptor gene variant database: pathogenicity update. J. Med. Genet. 2017;54:217–223. doi: 10.1136/jmedgenet-2016-104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg A., Fazio S., Duell P.B., Baass A., Udata C., Joh T., Riel T., Sirota M., Dettling D., Liang H. Molecular characterization of familial hypercholesterolemia in a North American cohort. J. Endocr. Soc. 2019;4:bvz015. doi: 10.1210/jendso/bvz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson G.R., Spinks T., Ranicar A., Myant N.B. Non-steady-state studies of low density lipoprotein turnover in familial hypercholesterolaemia. Clin. Sci. Mol. Med. 1977;52:361–369. doi: 10.1042/cs0520361. [DOI] [PubMed] [Google Scholar]
- 19.Soutar A.K., Myant N.B., Thompson G.R. Simultaneous measurement of apolipoprotein B turnover in very low and low density lipoproteins in familial hypercholesterolaemia. Atherosclerosis. 1977;28:247–256. doi: 10.1016/0021-9150(77)90174-5. [DOI] [PubMed] [Google Scholar]
- 20.Teng B., Sniderman A.D., Soutar A.K., Thompson G.R. Metabolic basis of hyperapobetalipoproteinemia. Turnover of apolipoprotein B in low density lipoprotein and its precursors and subfractions compared with normal and familial hypercholesterolemia. J. Clin. Invest. 1986;77:663–672. doi: 10.1172/JCI112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraft H.G., Lingenhel A., Raal F.J., Hohenegger M., Utermann G. Lipoprotein(a) in homozygous familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2000;20:522–528. doi: 10.1161/01.atv.20.2.522. [DOI] [PubMed] [Google Scholar]
- 22.Ma L., Waldmann E., Ooi E.M., Chan D.C., Barrett H.P., Watts G.F., Parhofer K.G. Lipoprotein (a) and low-density lipoprotein apolipoprotein B metabolism following apheresis in patients with elevated lipoprotein (a) and coronary artery disease. Eur. J. Clin. Invest. 2019;49:e13053. doi: 10.1111/eci.13053. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd J., Bicker S., Lorimer A.R., Packard C.J. Receptor-mediated low density lipoprotein catabolism in man. J. Lipid Res. 1979;20:999–1006. [PubMed] [Google Scholar]
- 24.Thompson G.R., Soutar A.K., Spengel F.A., Jadhav A., Gavigan S.J., Myant N.B. Defects of receptor-mediated low density lipoprotein catabolism in homozygous familial hypercholesterolemia and hypothyroidism in vivo. Proc. Natl. Acad. Sci. USA. 1981;78:2591–2595. doi: 10.1073/pnas.78.4.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harders-Spengel K., Wood C.B., Thompson G.R., Myant N.B., Soutar A.K. Difference in saturable binding of low density lipoprotein to liver membranes from normocholesterolemic subjects and patients with heterozygous familial hypercholesterolemia. Proc. Natl. Acad. Sci. USA. 1982;79:6355–6359. doi: 10.1073/pnas.79.20.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilheimer D.W., Goldstein J.L., Grundy S.M., Starzl T.E., Brown M.S. Liver transplantation to provide low-density-lipoprotein receptors and lower plasma cholesterol in a child with homozygous familial hypercholesterolemia. N. Engl. J. Med. 1984;311:1658–1664. doi: 10.1056/NEJM198412273112603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto A., Sudo H., Endo A. Therapeutic effects of ML-236B in primary hypercholesterolemia. Atherosclerosis. 1980;35:259–266. doi: 10.1016/0021-9150(80)90124-0. [DOI] [PubMed] [Google Scholar]
- 28.Mortality in treated heterozygous familial hypercholesterolaemia: implications for clinical management. Scientific Steering Committee on behalf of the Simon Broome Register Group. Atherosclerosis. 1999;142:105–112. [PubMed] [Google Scholar]
- 29.Versmissen J., Oosterveer D.M., Yazdanpanah M., Defesche J.C., Basart D.C., Liem A.H., Heeringa J., Witteman J.C., Lansberg P.J., Kastelein J.J. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337:a2423. doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert G. Unravelling the functional significance of PCSK9. Curr. Opin. Lipidol. 2007;18:304–309. doi: 10.1097/MOL.0b013e3281338531. [DOI] [PubMed] [Google Scholar]
- 31.Seidah N.G., Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 32.Navarese E.P., Kolodzieiczak M., Schulze V., Gurbel P.A., Tantry U., Lin Y., Brockmeyer M., Kandzari D.E., Kubica J.M., D’Agostino R.B., Sr. Effects of proprotein convertase subtilisin/ kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann. Intern. Med. 2015;163:40–51. doi: 10.7326/M14-2957. [DOI] [PubMed] [Google Scholar]
- 33.Raal F.J., Stein E.A., Dufour R., Turner T., Civeira F., Burgess L., Langslet G., Scott R., Olsson A.G., Sullivan D. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:331–340. doi: 10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- 34.Koren M.J., Scott R., Kim J.B., Knusel B., Liu T., Lei L., Bolognese M., Wasserman S.M. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380:1995–2006. doi: 10.1016/S0140-6736(12)61771-1. [DOI] [PubMed] [Google Scholar]
- 35.Blom D.J., Hala T., Bolognese M., Lillestol M.J., Toth P.D., Burgess L., Ceska R., Roth E., Koren M.J., Ballantyne C.M., DESCARTES Investigators A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N. Engl. J. Med. 2014;370:1809–1819. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 36.Sabatine M.S., Giugliano R.P., Wiviott S.D., Raal F.J., Blom D.J., Robinson J., Ballantyne C.M., Somaratne R., Legg J., Wasserman S.M. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015;372:1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 37.O’Donoghue M.L., Fazio S., Giugliano R.P., Stroes E.S., Kanevsky E., Gouni-Berthold I., Im K., Pineda A.L., Wassermann S.M., Ceska R. Lipoprotein (a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139:1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184. [DOI] [PubMed] [Google Scholar]
- 38.Raal F.J., Hovingh G.K., Blom D.J., Santos R.D., Harada-Shiba M., Bruckert E., Couture P., Soran H., Watts G.F., Kurtz C. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in 106 homozygous familial hypercholesterolaemia patients: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol. 2017;5:280–290. doi: 10.1016/S2213-8587(17)30044-X. [DOI] [PubMed] [Google Scholar]
- 39.Ray K.K., Stoekenbroek R.M., Kallend D., Leiter L.A., Landmesser U., Wright R.S., Wijngaard P., Kastelein J.J.P. Effect of an siRNA therapeutic targeting PCSK9 on atherogenic lipoproteins. Circulation. 2018;138:1304–1316. doi: 10.1161/CIRCULATIONAHA.118.034710. [DOI] [PubMed] [Google Scholar]
- 40.Cuchel M., Meagher E.A., du Toit Theron H., Blom D.J., Marais A.D., Hegele R.A., Averna M.R., Sirtori C.R., Shah P.K., Gaudet D. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–46. doi: 10.1016/S0140-6736(12)61731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson G.R., Lowenthal R., Myant N.B. Plasma exchange in the management of homozygous familial hypercholesterolaemia. Lancet. 1975;1:1208–1211. doi: 10.1016/s0140-6736(75)92193-5. [DOI] [PubMed] [Google Scholar]
- 42.Thompson G.R., Myant N.B., Kilpatrick D., Oakley C.M., Raphael M.J., Steiner R.E. Assessment of long-term plasma exchange for familial hypercholesterolaemia. Br. Heart J. 1980;43:680–688. doi: 10.1136/hrt.43.6.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson G.R., Miller J.P., Breslow J.L. Improved survival of patients with homozygous familial hypercholesterolaemia treated by plasma exchange. Br. Med. J. 1985;291:167l–1673. doi: 10.1136/bmj.291.6510.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson G. World Scientific Publishing Europe Ltd.; London: 2020. Medicine My Vocation Fishing My Recreation: Memoirs of a Physician and Flyfisherman. [Google Scholar]
- 45.France M., Rees A., Datta D., Thompson G., Capps N., Ferns G., Ramaswami U., Seed M., Neely D., Cramb R. HEART UK statement on the management of homozygous familial hypercholesterolaemia in the United Kingdom. Atherosclerosis. 2016;255:128–139. doi: 10.1016/j.atherosclerosis.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Stefanutti C., Julius U., Watts G.F., Harada-Shiba M., Cossu M., Schettler V.J., De Silvestro G., Soran H., Roeters van Lennep J., Pisciotta L., MIGHTY MEDIC Multinational Society Towards an international consensus-integrating lipoprotein apheresis and new lipid-lowering drugs. J. Clin. Lipidol. 2017;11:858–871.e3. doi: 10.1016/j.jacl.2017.04.114. [DOI] [PubMed] [Google Scholar]
- 47.Thompson G.R., Blom D.J., Marais A.D., Seed M., Pilcher G.J., Raal F.J. Survival in homozygous familial hypercholesterolaemia is determined by the on-treatment level of serum cholesterol. Eur. Heart J. 2018;39:1162–1168. doi: 10.1093/eurheartj/ehx317. [DOI] [PubMed] [Google Scholar]
- 48.Stefanutti C., Pang J., Di Giacomo S., Wu X., Wang X., Morozzi C., Watts G.F., Lin J. A cross-national investigation of cardiovascular survival in homozygous familial hypercholesterolemia: the Sino-Roman Study. J. Clin. Lipidol. 2019;13:608–617. doi: 10.1016/j.jacl.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Crosland P., Maconachie R., Buckner S., McGuire H., Humphries S.E., Qureshi N. Cost-utility analysis of searching electronic health records and cascade testing to identify and diagnose familial hypercholesterolaemia in England and Wales. Atherosclerosis. 2018;275:80–87. doi: 10.1016/j.atherosclerosis.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Umans-Eckenhausen M.A., Sijbrands E.J., Kastelein J.J., Defesche J.C. Low-density lipoprotein receptor gene mutations and cardiovascular risk in a large genetic cascade screening population. Circulation. 2002;106:3031–3036. doi: 10.1161/01.cir.0000041253.61683.08. [DOI] [PubMed] [Google Scholar]
- 51.Gaudet D., Gipe D.A., Pordy R., Ahmad Z., Cuchel M., Shah P.K., Chyu K.Y., Sasiela W.J., Chan K.C., Brisson D. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N. Engl. J. Med. 2017;377:296–297. doi: 10.1056/NEJMc1705994. [DOI] [PubMed] [Google Scholar]
- 52.Pouwer M.G., Pieterman E.J., Worms N., Keijzer N., Jukema J.W., Gromada J., Gusarova V., Princen H.M.G. Alirocumab, evinacumab, and atorvastatin triple therapy regresses plaque lesions and improves lesion composition in mice. J. Lipid Res. 2020;61:365–375. doi: 10.1194/jlr.RA119000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramaswami U., Humphries S., Priestley-Barnham L., Green P., Wald D., Capps N., Anderson M., Dale P., Morris A.A. Current management of children and young people with heterozygous familial hypercholesterolaemia – HEART UK statement of care. Atherosclerosis. 2019;290:1–8. doi: 10.1016/j.atherosclerosis.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Luirink I.K., Wiegman A., Kusters D.M., Hof M.H., Groothoff J.W., de Groot E., Kastelein J.J.P., Hutten B. 20-Year follow-up of statins in children with familial hypercholesterolemia. N. Engl. J. Med. 2019;381:1547–1556. doi: 10.1056/NEJMoa1816454. [DOI] [PubMed] [Google Scholar]
- 55.Representatives of the Global Familial Hypercholesterolemia Community Reducing the clinical and public health burden of familial hypercholesterolemia: a global call to action. JAMA Cardiol. 2020 January 2 doi: 10.1001/jamacardio.2019.5173. Epub ahead of print. [DOI] [PubMed] [Google Scholar]