Abstract
Purpose:
Opioids, gabapentinoids and non-steroidal anti-inflammatory drugs (NSAIDs) may have adverse cardiovascular effects. We evaluated whether these medications were associated with incident clinically-detected atrial fibrillation (AF) or monitor-detected supraventricular ectopy (SVE), including premature atrial contractions (PACs) and supraventricular tachycardia (SVT).
Methods:
We used data from the Multi-Ethnic Study of Atherosclerosis (MESA), a cohort study that enrolled 6,814 Americans without clinically-detected cardiovascular disease in 2000–2002. At the 2016–2018 examination, 1,557 individuals received ambulatory electrocardiographic (ECG) monitoring. Longitudinal analyses investigated time-varying medication exposures at the first 5 exams (through 2011) in relation to incident clinically-detected AF through 2015 using Cox proportional hazards regression models. Cross-sectional analyses investigated medication exposures at the 2016–2018 examination and the risk of monitor-detected SVE using linear regression models.
Results:
The longitudinal cohort included 6,652 participants. During 12.4 years of mean follow-up, 982 participants (14.7%) experienced incident clinically-detected AF. Use of opioids, gabapentinoids and NSAIDs were not associated with incident AF. The cross-sectional analysis included 1,435 participants with ECG monitoring. Gabapentinoid use was associated with an 84% greater average frequency of PACs/hour (95% CI, 25–171%) and a 44% greater average number of runs of SVT/day (95% CI, 3–100%). No associations were found with use of opioids or NSAIDs in cross-sectional analyses.
Conclusions:
In this study, gabapentinoid use was associated with SVE. Given the rapid increase in gabapentinoid use, additional studies are needed to clarify whether these medications cause cardiovascular complications.
Keywords: arrhythmia, atrial fibrillation, cohort study, opioid, gabapentinoid, pharmacoepidemiology, supraventricular tachycardia
Introduction:
In the United States, millions of people suffer chronic non-cancer pain1. Between 1999–2010, opioid prescriptions for non-cancer pain quadrupled2. In more recent years of the opioid epidemic, gabapentinoid use has also increased markedly3, possibly as physicians seek treatment alternatives to opioids4. Non-steroidal anti-inflammatory drugs (NSAIDs) are also recommended as an alternative for treating chronic non-cancer pain and are among the most commonly prescribed medications for pain5. All of these medications may have unintended, adverse cardiovascular effects, but the mechanisms and the risks of specific cardiovascular outcomes are not well characterized.
Atrial fibrillation (AF) is a common arrhythmia associated with devastating consequences, including stroke, myocardial infarction, heart failure, cognitive impairment and even death6. Previous studies have found that opioids, gabapentinoids and NSAIDs may all be associated with an increased risk of AF7–12. However, our understanding of the pathophysiology of AF is incomplete. Recent studies have identified supraventricular ectopy (SVE) to be another important arrhythmia that is associated with increased risks of AF13 and stroke14,15, and investigation of the associations of opioids, gabapentinoids and NSAIDs with SVE may provide better insight into the association of AF with these drug classes.
The Multi-Ethnic Study of Atherosclerosis (MESA) is a community-based prospective cohort study that has assessed medication use and clinical AF events during up to 15 years of follow-up16. At the most recent exam, MESA also conducted extended ambulatory electrocardiographic (ECG) monitoring to identify arrhythmias17. Using MESA data, we conducted (1) longitudinal analyses to determine whether opioid, gabapentinoid and NSAID use are associated with incident clinically-detected AF, and (2) cross-sectional analyses to evaluate whether these medications are associated with the frequency of monitor-detected SVE.
Methods:
Overview and setting:
The MESA has been described in detail elsewhere16. Briefly, the study recruited 6,814 adults between 45–84 years of age who were free of clinically-recognized cardiovascular disease from 6 field centers across the U.S. to undergo baseline examination between 2000–2002 (Exam 1) with follow-up exams every 2–6 years through 2016–18 (Exam 6). The study included Asian, Hispanic, white and African-American participants. Approval for the study was obtained from the institutional review board at each participating institution, and all participants provided written informed consent.
During Exam 6, a subset of MESA participants both with and without a history of heart disease or clinically-detected AF (n=1,557) were enrolled in an ancillary study that included ambulatory ECG monitoring. Study staff applied an ECG monitoring device and asked the participant to wear it for 14 days, then to return it by mail to the manufacturer for interpretation17. The ECG monitoring device used in this study was the Zio Patch XT (iRhythm Technologies, Inc, San Francisco, CA), an FDA-approved single-channel ECG patch monitor capable of recording up to 14 days of cardiac rhythm18. Certified technicians at iRhythm processed and analyzed the ECG data and all reported arrhythmias were verified by the Epidemiological Cardiology Reading Center at Wake Forest University School of Medicine, Winston-Salem, NC. The devices were purchased for the study and the device manufacturer had no role in the study design or statistical analysis.
In longitudinal analyses, all MESA participants who had no history of clinically-detected AF at Exam 1 were included with follow up through 2015. Additional exclusions were made for those missing baseline covariates (Figure 1). In cross-sectional analyses, MESA participants who contributed to the Exam 6 ECG monitoring study and underwent at least 24 hours of continuous monitoring were included. Additional exclusions were made for those missing baseline covariates (Figure 2).
Figure 1:
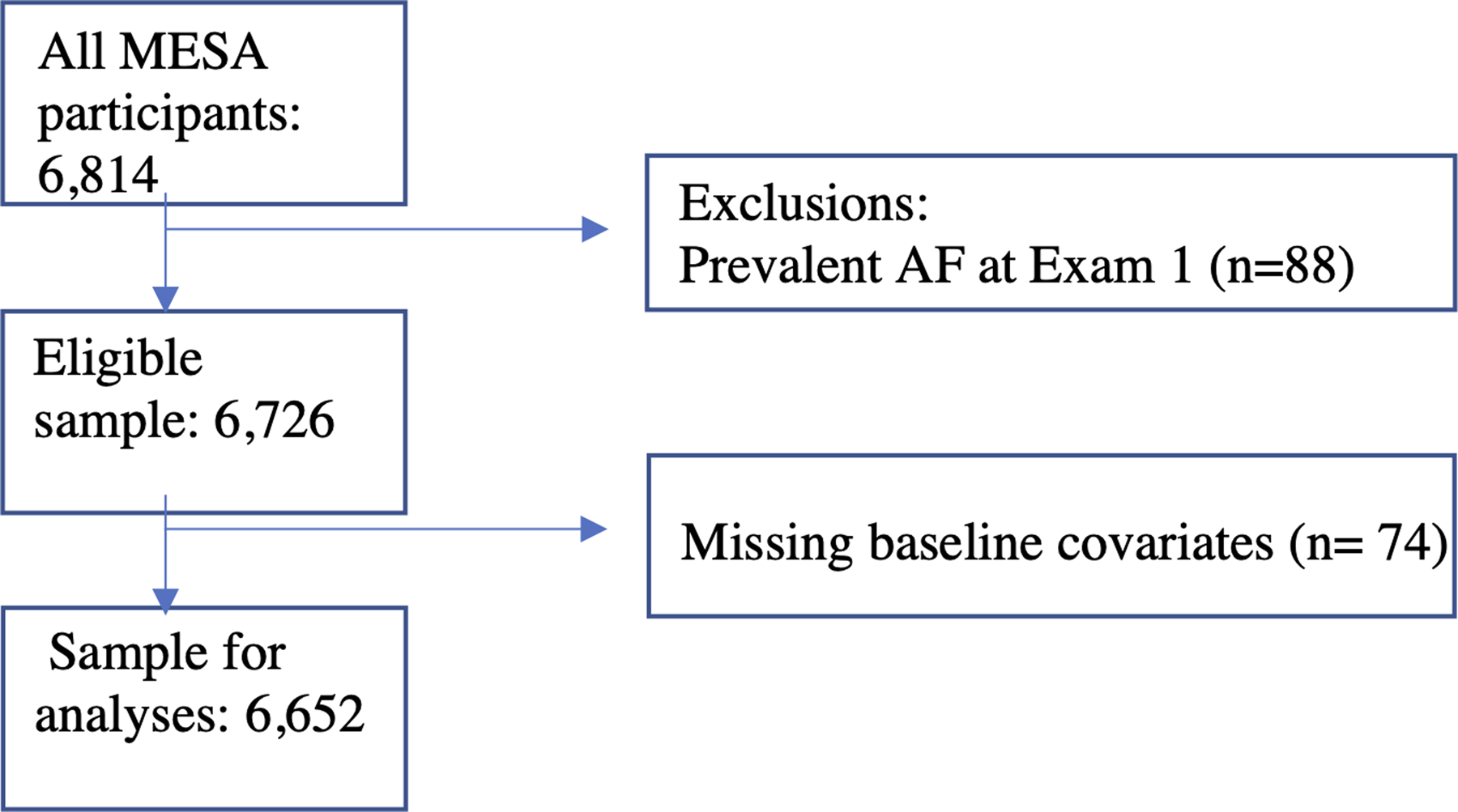
Flow chart showing inclusion criteria and exclusions for the longitudinal analysis of opioid, gabapentinoid and NSAID use and the risk of incident clinically-detected atrial fibrillation.
Figure 2:
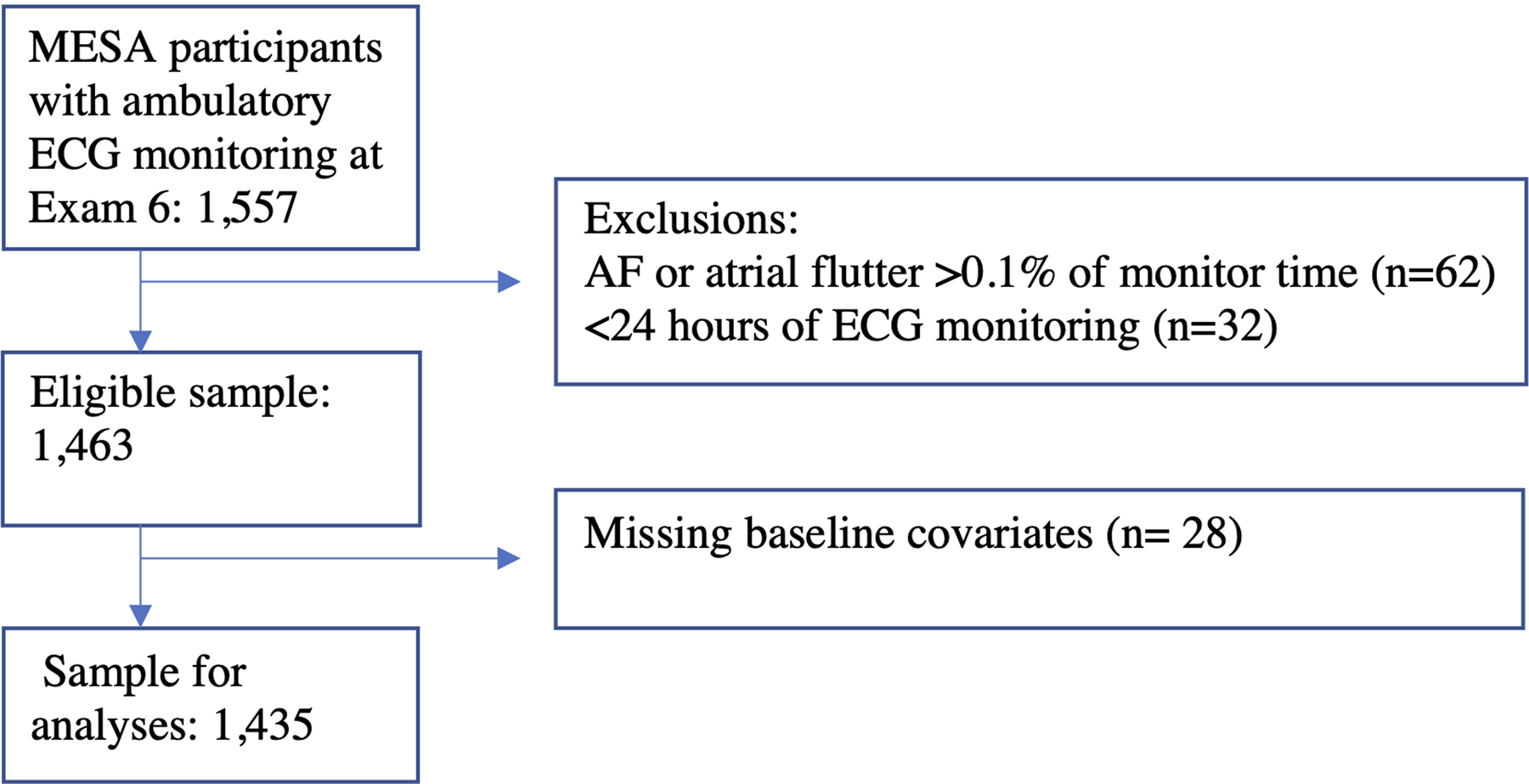
Flow chart showing inclusion criteria and exclusions for the cross-sectional analysis of opioid, gabapentinoid and NSAID use and the risk of monitor-detected supraventricular ectopy.
Exposure:
The longitudinal analyses evaluated time-varying exposure to a) opioid, b) gabapentinoid or c) NSAID medications at the first five exams (Exams 1–5) compared with nonusers. At each study visit, MESA participants were asked to bring all prescription and over-the-counter medications they were currently using, and a technician recorded the medication information19. These medication inventory data were used to assess opiate, gabapentinoid and NSAID use, which were reevaluated at each subsequent exam. The cross-sectional analysis evaluated use of opioids, gabapentinoids or NSAIDs at Exam 6 compared with nonusers as the reference.
Outcome:
The outcome of interest in longitudinal analyses was incident clinically-detected AF, which was ascertained through December 2015 from (1) ICD-9 and ICD-10 (International Classification of Diseases, Ninth and Tenth Revisions) discharge diagnosis codes from hospitalizations during regular MESA events follow-up, and (2) for participants enrolled in fee-for-service Medicare, from ICD-9 and ICD-10 inpatient discharge diagnosis codes or outpatient codes from Medicare claims data20.
The outcome of interest in cross-sectional analyses was monitor-detected SVE. This included (1) the frequency of premature atrial contractions (PACs), defined as the mean count of PACs per hour during the monitoring period for each patch and (2) the mean frequency of runs of SVT, defined as 4 or more consecutive PACs. We also examined the incidence of runs of supraventricular tachycardia (SVT) as a binary outcome.
Covariates:
The following potential confounders, assessed at baseline (Exam 1), were adjusted for in the longitudinal analysis of time-varying medication use and incident AF: site (Baltimore, MD; Chicago, IL; Los Angeles County, CA; New York, NY; St. Paul, MN; and Winston Salem, NC), age (linear), height (cm, linear), weight (lb, linear), glucose status (normal, impaired fasting glucose [IFG], diabetes), treated hypertension (yes-no, combining information on self-reported hypertension and self-reported antihypertensive medication use), systolic blood pressure (mmHg, linear), smoking (never, former, current) and current alcohol use (yes-no).
The cross-sectional analysis of medication use at Exam 6 and monitor-detected SVE was adjusted for potential confounders assessed during Exam 6. These confounders included the same variables that were considered in the longitudinal analyses, as well as physical activity (metabolic equivalent [MET]/min, linear), self-perceived health (poor-fair, good-excellent), self-reported pain interfering with work (moderate-extreme, little-not at all) and history of myocardial infarction, stroke, or heart failure.
Statistical analysis:
In longitudinal analyses, time-varying medication use and AF incidence were modeled using Cox proportional hazards regression models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). The time scale was time since baseline exam. Participants were censored at the earliest of date of death, an AF event, loss to follow up, or end of follow up.
In cross-sectional analyses, linear regression with log-transformed outcomes was used to estimate ratios of geometric means of the per-unit time rates of PACs and runs of SVT. If participants had zero PACs per hour or zero runs of SVT per day, we imputed a value of 0.170 for PACs per hour (the smallest value for those with PACs recorded) and imputed a value of 0.071 runs of SVT per day (the smallest value for those with runs of SVT recorded) before log-transforming. To examine the association between medication use and the incidence of any runs of SVT, relative rate regression using a Poisson likelihood and an offset equal to the log of the monitoring time until the first run of SVT was used.
Sensitivity analyses:
We conducted sensitivity analyses that considered participants to be users of opioid, gabapentinoid or NSAID medications only after reported use at 2 or more consecutive exams for the AF incidence analysis. Once participants met this criterion, they were considered always exposed. This approach was used to identify participants with a greater likelihood of current medication use and avoided misclassifying participants as users who may have used a drug for only a brief period of time before discontinuing use. We also conducted separate analyses for the use of the most common opioid medications (hydrocodone, tramadol) and the most common gabapentinoid medication (gabapentin).
Results:
From Exam 1 (2000–2002) to Exam 6 (2016–2018), the use of opioids increased gradually from 2.8% to 5.3%, while the use of gabapentinoids increased markedly from 0.7% to 6.1% (Figure 3).
Figure 3:
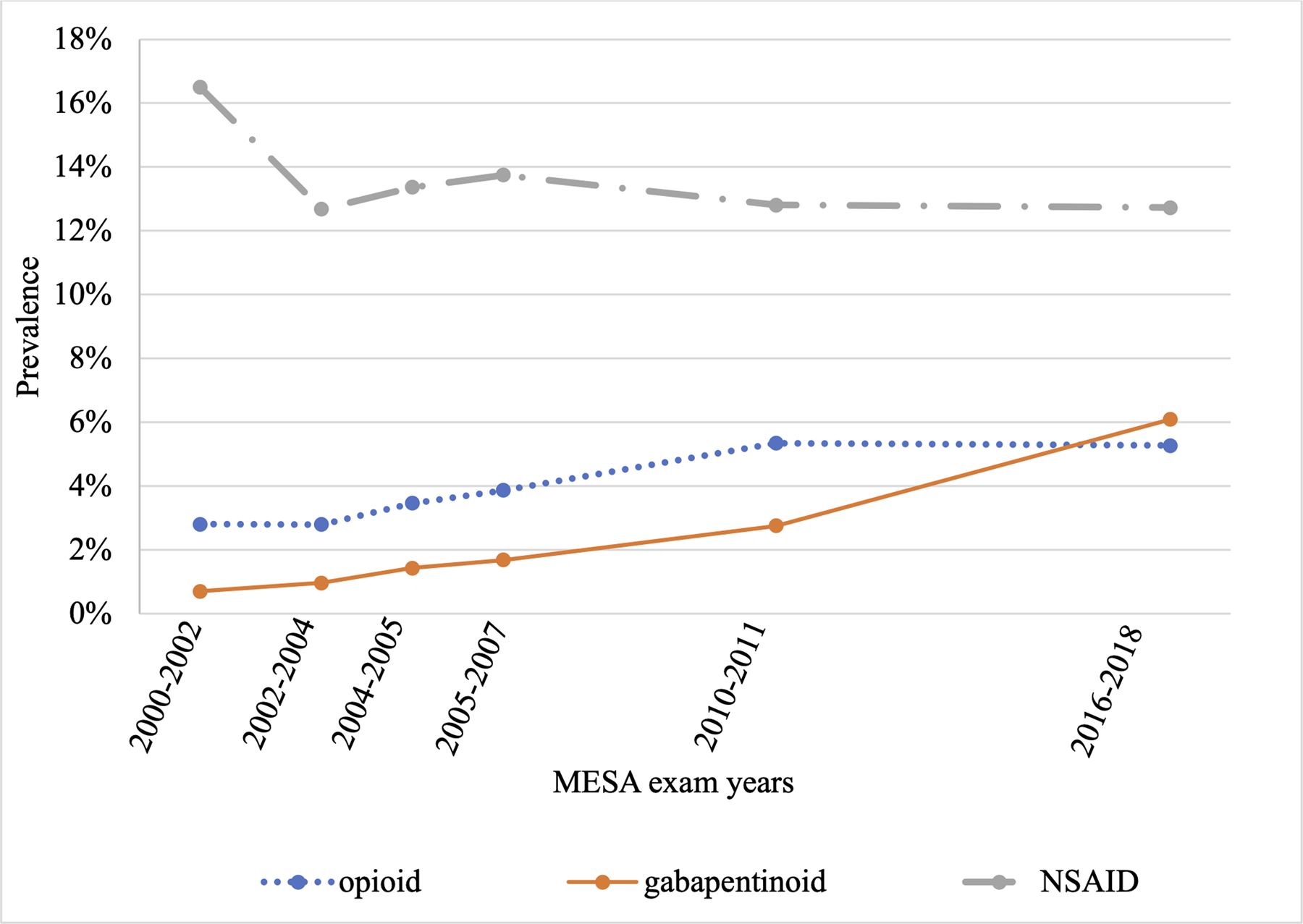
The prevalence of opioid, gabapentinoid and NSAID use from Exam 1 (2000–2002) to Exam 6 (2016–2018) in the Multi-Ethnic Study of Atherosclerosis.
The longitudinal cohort included 6,652 participants with mean age of 62, of whom 636 (10%) used opioids, 240 (4%) used gabapentinoids and 2,282 (34%) used NSAIDs at one or more exams (Table 1). The most commonly used opioid medications were hydrocodone and tramadol (each used by 37% of those with opioid use), and the most commonly used gabapentinoid medication was gabapentin (used by 91% of those with gabapentinoid use). During 12.4 years of mean follow up, 982 participants (14.7%) experienced incident AF. Use of opioids, gabapentinoids, and NSAIDs were not significantly associated with the risk of incident AF compared to no use (Table 2). Findings from the sensitivity analysis requiring medication use at 2 or more exams resulted in similar null findings for all classes of medications (Supplemental Table 1) and findings from the sensitivity analysis investigating the most commonly used opioid and gabapentinoid medication types resulted in similar findings (Supplemental Table 2).
Table 1:
Baseline characteristics of Multi-Ethnic Study of Atherosclerosis participants in longitudinal analytic sample (N=6,652) based on medication use
| No opioid use (n=6,016) | Opioid use (n=636) | No gabapentinoid use (n=6,412) | Gabapentinoid use (n=240) | No NSAID use (n=4,370) | NSAID use (n=2,282) | |
|---|---|---|---|---|---|---|
| Characteristic | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Age, mean (sd), years | 62.0 (10.2) | 62.6 (10.2) | 62.0 (10.2) | 62.9 (9.4) | 62.9 (10.3) | 60.4 (9.9) |
| Female | 2910 (48) | 234 (37) | 3062 (48) | 82 (34) | 2245 (51) | 899 (39) |
| Race/ethnicity | ||||||
| White | 2275 (38) | 271 (43) | 2442 (38) | 104 (43) | 1450 (33) | 1096 (48) |
| Chinese | 771 (13) | 21 (3) | 774 (12) | 18 (8) | 706 (16) | 86 (4) |
| African American | 1636 (27) | 200 (31) | 1771 (28) | 65 (27) | 1235 (28) | 601 (26) |
| Hispanic | 1334 (22) | 144 (23) | 1425 (22) | 53 (22) | 979 (22) | 499 (22) |
| Glucose status | ||||||
| Normal | 4449 (74) | 448 (70) | 4748 (74) | 149 (62) | 3144 (72) | 1753 (77) |
| Impaired fasting glucose | 830 (14) | 93 (15) | 887 (14) | 36 (15) | 634 (15) | 289 (13) |
| Diabetes | 737 (12) | 95 (15) | 777 (12) | 55 (23) | 592 (14) | 240 (11) |
| Treated hypertension | 1943 (32) | 276 (43) | 2105 (33) | 114 (48) | 1452 (33) | 767 (34) |
| Smoking | ||||||
| Current | 758 (13) | 113 (18) | 838 (13) | 33 (14) | 557 (13) | 314 (14) |
| Former | 2178 (36) | 259 (41) | 2350 (37) | 87 (36) | 1560 (36) | 877 (38) |
| Never | 3080 (51) | 264 (41) | 3224 (50) | 120 (50) | 2253 (51) | 1091 (48) |
| Current alcohol use | 3344 (56) | 349 (55) | 3586 (56) | 107 (45) | 2269 (52) | 1424 (62) |
| BMI (kg/m2) | ||||||
| Normal weight (BMI<25) | 1797 (30) | 110 (17) | 1859 (29) | 48 (20) | 1433 (33) | 474 (21) |
| Overweight (BMI 25–30) | 2377 (39) | 226 (36) | 2517 (39) | 86 (36) | 1716 (39) | 887 (39) |
| Obese (BMI>30) | 1842 (31) | 300 (47) | 2036 (32) | 106 (44) | 1211 (28) | 921 (40) |
| Systolic blood pressure, mean(sd), mmHg | 126.2 (21.3) | 129.2 (23.0) | 126.3 (21.4) | 129.2 (23.1) | 126.8 (21.4) | 125.8 (21.5) |
| History of sleep apneaa | 169 (3) | 38 (6) | 194 (3) | 13 (6) | 129 (3) | 78 (4) |
History of physician-diagnosed sleep apnea assessed was assessed from questionnaires at exam 2 (2002–2004) and was available on 6,082/6652 study participants. Percentages are calculated for those with non-missing data.
Abbreviations: NSAID, non-steroidal anti-inflammatory drug; SD, standard deviation.
Table 2:
Medication use and risk of clinically-detected atrial fibrillation in longitudinal analyses
| Opioid use | Gabapentinoid use | NSAID use | |||
|---|---|---|---|---|---|
| Unadjusted HR (95%CI) | Adjusted HRa (95%CI) | Unadjusted HR (95%CI) | Adjusted HRa (95%CI) | Unadjusted HR (95%CI) | Adjusted HRa (95%CI) |
| 1.33 (1.00, 1.76) | 1.17 (0.88, 1.56) | 1.28 (0.83, 1.97) | 1.06 (0.69, 1.64) | 0.90 (0.75–1.09) | 1.07 (0.89–1.30) |
Adjusted for: age, sex, site, race, height, weight, diabetes, treated hypertension, systolic blood pressure, smoking, alcohol use.
Abbreviations: NSAID, non-steroidal anti-inflammatory drug; HR, hazard ratio; CI, confidence interval.
Among 1,435 participants included in the cross-sectional analysis, the median (interquartile range [IQR]) duration of cardiac monitoring) was 13.8 (12.9–14.0) days; 1,433 (99%) participants experienced PACs and 1,186 (83%) experienced at least one run of SVT. Among those experiencing PACs, the median frequency of PACs/hour was 4.1 (IQR1.3–18.4) while among those experiencing SVT, the median frequency of runs of SVT/day was 0.5 (0.08–1.2). There were 78 (5%) opioid users, 86 (6%) gabapentinoid users and 198 (14%) NSAID users. Opioid users and gabapentinoid users had a greater comorbidity burden than nonusers, while NSAID users and nonusers were similar, with the exception of greater weight among NSAID users (Table 3). Users of any of the medications of interest self-reported more moderate-to-extreme pain than nonusers.
Table 3:
Baseline characteristics of Multi-Ethnic Study of Atherosclerosis participants in cross-sectional analytic sample (N=1,453) based on medication use
| No opioid use (n=1357) | Opioid use (n=78) | No gabapentinoid use (n=1349) | Gabapentinoid use (n=86) | No NSAISD use (n=1237) | NSAID use (n=198) | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age, mean (sd), years | 73.3 (8.3) | 73.4 (8.8) | 72.2 (8.3) | 75.4 (8.5) | 73.6 (8.4) | 71.8 (7.7) |
| Female | 691 (51) | 48 (62) | 693 (51) | 46 (53) | 622 (50) | 117 (59) |
| Race/ethnicity | ||||||
| White | 553 (41) | 32 (41) | 554 (41) | 31 (36) | 481 (39) | 104 (53) |
| Chinese | 197 (15) | 1 (1) | 193 (14) | 5 (6) | 190 (15) | 8 (4) |
| African American | 330 (24) | 23 (29) | 323 (24) | 30 (35) | 302 (24) | 51 (26) |
| Hispanic | 277 (20) | 22 (28) | 279 (21) | 20 (23) | 264 (21) | 35 (18) |
| Glucose status | ||||||
| Normal | 729 (54) | 39 (50) | 725 (54) | 43 (50) | 658 (53) | 110 (56) |
| Impaired fasting glucose | 329 (24) | 10 (13) | 327 (24) | 12 (14) | 302 (24) | 37 (19) |
| Diabetes | 299 (22) | 29 (37) | 297 (22) | 31 (36) | 277 (22) | 51 (26) |
| Treated hypertension | 809 (60) | 59 (76) | 806 (60) | 62 (72) | 748 (60) | 120 (61) |
| Smoking | ||||||
| Current | 78 (6) | 9 (12) | 82 (6) | 5 (6) | 73 (6) | 14 (7) |
| Former | 641 (47) | 45 (58) | 646 (48) | 40 (47) | 590 (48) | 96 (48) |
| Never | 638 (47) | 24 (31) | 621 (46) | 41 (48) | 574 (46) | 88 (44) |
| Current alcohol use | 600 (44) | 34 (44) | 596 (44) | 38 (44) | 515 (42) | 119 (60) |
| BMI (kg/m2) | ||||||
| Normal weight (BMI<25) | 394 (29) | 19 (24) | 398 (29) | 15 (17) | 371 (30) | 42 (21) |
| Overweight (BMI 25–30) | 535 (39) | 32 (41) | 535 (40) | 32 (37) | 493 (40) | 74 (37) |
| Obese (BMI>30) | 428 (32) | 27 (35) | 416 (31) | 39 (46) | 373 (30) | 82 (42) |
| Systolic blood pressure, mean(sd), mmHg | 127.3 (20.3) | 125.8 (18.1) | 127.4 (20.2) | 124.4 (18.8) | 127.1 (20.2) | 128.1 (19.8) |
| Moderate/vigorous PA, median (IQR), met-min/wk | 3,645(1,635–7,035) | 3,086 (1,260–5,775) | 3683 (1680–7020) | 2100 (855–5035) | 3600 (1583–6720) | 3701 (1755–7770) |
| Fair/poor general health | 181 (13) | 20 (26) | 170 (13) | 31 (36) | 176 (14) | 25 (13) |
| Moderate/extreme pain | 250 (18) | 45 (58) | 257 (19) | 38 (44) | 232 (19) | 62 (31) |
| History of MI | 36 (3) | 1 (1) | 33 (2) | 4 (5) | 33 (3) | 4 (2) |
| History of stroke | 30 (2) | 2 (3) | 31 (2) | 1 (1) | 28 (2) | 4 (2) |
| History of HF | 16 (1) | 4 (5) | 17 (1) | 3 (3) | 18 (1) | 2 (1) |
| History of obstructive sleep apneaa | 326 (43) | 21 (40) | 318 (43) | 29 (58) | 297 (44) | 50 (41) |
Obstructive sleep apnea data came from MESA sleep polysomnography data collected at exam 5 (2010–2012) and was available on a sub-sample of participants included in these cross-sectional analyses (n=798/1,453). Percentages are calculated for those with non-missing data.
Abbreviations: NSAID, non-steroidal anti-inflammatory drug; SD, standard deviation; PA, physical activity; IQR, interquartile range; MI, myocardial infarction; HF, heart failure
The use of opioids and NSAIDs at Exam 6 was not significantly associated with the frequency of PACs, or frequency of runs of SVT. Gabapentinoid use was associated with an 84% greater frequency of PACs (95% CI, 25% to 171%) and with a 44% greater frequency of runs of SVT (95% CI, 3% to 100%) (Table 4). Gabapentinoid use was associated with a greater incidence rate of runs of SVT, but no association was found for use of opioids or NSAIDS (Supplemental Table 3).
Table 4:
Cross-sectional associations between medication use and monitor-detected supraventricular ectopy, Multi-Ethnic Study of Atherosclerosis Exam 6
| Opioid | Gabapentinoid | NSAID | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| PACs/hour, geometric mean ratio (95% CI) | 1.04 (0.68, 1.56) | 1.03 (0.68, 1.55) | 2.11 (1.41–3.17) | 1.84 (1.25–2.71) | 1.20 (0.91–1.58) | 1.28 (0.98–1.67) |
| SVT/day, geometric mean ratio (95% CI) | 1.16 (0.82, 1.65) | 1.19 (0.84–1.69) | 1.51 (1.08–2.10) | 1.44 (1.03–2.00) | 1.10 (0.87–1.39) | 1.09 (0.87–1.37) |
Adjusted for: age, sex, site, race, height, weight, diabetes, treated hypertension, systolic blood pressure, smoking, alcohol use, history of myocardial infarction, stroke and heart failure, physical activity, self-reported health, and self-reported pain interfering with work.
Abbreviations: NSAID, non-steroidal anti-inflammatory drug; PACs, premature atrial contractions; SVT, supraventricular tachycardia; CI, confidence interval.
Discussion:
Using extended ambulatory ECG monitoring, a sensitive and unbiased method for detecting arrhythmias, we found that gabapentinoid use was associated with measures of SVE, which may reflect pathologic changes in the atrial myocardium and represent an important biomarker for AF and stroke. Several studies have reported increased risks of cardiovascular events among participants with a greater burden of SVE13,15,21,22. The use of opioids or NSAIDs was not associated with SVE, and we did not find evidence of associations between longitudinal opioid, gabapentinoid or NSAID use and incident clinically-detected AF, although the wide confidence intervals did not exclude clinically-meaningful increases in risk.
Our null findings for clinical AF events contrast with previously published reports. A cross-sectional analysis in the Reasons for Geographic and Racial Differences in Stroke study found that opioid use was associated with a 1.35 greater odds (95%CI 1.16–1.57) of AF identified from a single resting 12-lead ECG or self-report11. In addition, several studies that used diagnosis codes from electronic health databases to identify AF events have found that NSAID use was associated with greater AF risk, with relative risks ranging from 1.18–1.447–10. The confidence intervals for the drug associations with incident AF in our study were wide due to the limited sample size, and they overlap with estimates from previous studies, which may explain discordant findings. Alternatively, residual confounding, different methods for outcome ascertainment, and differences in the underlying study populations may explain differences. It is notable that neither opiates nor NSAIDs were associated with cross-sectional measures of SVE, which may be sensitive biomarkers of AF risk.
One prior study found the rate of prescriptions for AF-related medications increased following the start of gabapentin or pregabalin12. We did not find an increase in incidence of AF based on gabapentinoid use, but did detect a greater frequency of PACs and of runs of SVT among users of gabapentinoids. Mechanistically, the therapeutic target of gabapentinoids, α2δ subunits of voltage-gated calcium channels, can accentuate calcium influx in the heart when activated12,23, which may explain an increased risk of arrhythmias. During the course of the present study, there was an eight-fold increase in gabapentinoid use, which mirrors national trends3. With the rapid increase in gabapentinoid medication use, it is important to identify unintended adverse effects of these drugs, since they are considered safe alternatives to opioids24,25.
There are several limitations of the present study. First, while medication inventory can provide a valid assessment of medication use26, it was possible to assess medication use only five times during approximately 15 years of follow-up. This may result in misclassification of exposure between the study visits. Second, our power to detect small but clinically-important increases in the risk of clinically-detected AF was limited due to the relatively small number who developed incident AF and the low prevalence of medication use. Third, residual confounding due to unmeasured or misclassified variables is possible because of the observational study design. Strengths of our study include the unbiased methods for assessing arrhythmias, the rich data on potential confounding factors, the community-based study design, and the multi-ethnic study population. Furthermore, we did not rely on prescription records of medication use, which may misclassify patients who did not take their prescribed medications and fail to capture most use of NSAIDs, which are often purchased over the counter.
In conclusion, given the rapid increase in gabapentinoid use, our finding of a greater burden of SVE associated with these medications may be of public health importance. Additional studies are needed to clarify whether this class of medications can cause clinically-relevant arrhythmias and other cardiovascular complications.
Supplementary Material
Key points:
Opioids, gabapentinoids and NSAIDs may have adverse cardiovascular effects, including arrhythmias.
We conducted analyses to evaluate whether longitudinal medication use was associated with incident clinically-detected atrial fibrillation.
We also conducted analyses to evaluate whether cross-sectional medication use was associated with monitor-detected supraventricular ectopy.
We observed that gabapentinoid use was associated with an increase in supraventricular ectopy, which may be a sensitive biomarker of atrial fibrillation risk.
Given the rapid increase in gabapentinoid use in our study population and across the nation, additional studies are needed to clarify whether these medications cause cardiovascular complications.
Acknowledgements:
The authors thank the other investigators, the staff and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding: This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). This work was additionally supported by a National Heart, Lung and Blood Institute grant, R01HL127659. JSF was supported by National Heart, Lung and Blood Institute grants K08HL116640 and R01HL142599. BNH is supported by a National Heart, Lung and Blood Institute grant T32HL007828.
Footnotes
Conflict of interest: Dr. Psaty serves on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. Dr. Floyd has consulted for Shionogi Inc. Other authors have no conflicts of interest to disclose.
Publisher's Disclaimer: Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
References:
- 1.Tompkins DA, Hobelmann JG, Compton P. Providing chronic pain management in the “Fifth Vital Sign” Era: Historical and treatment perspectives on a modern-day medical dilemma. Drug Alcohol Depend. 2017;173 Suppl 1:S11–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease C, Prevention. Vital signs: overdoses of prescription opioid pain relievers---United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- 3.Johansen ME. Gabapentinoid Use in the United States 2002 Through 2015. JAMA Intern Med. 2018;178(2):292–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman CW, Brett AS. Gabapentin and Pregabalin for Pain - Is Increased Prescribing a Cause for Concern? N Engl J Med. 2017;377(5):411–414. [DOI] [PubMed] [Google Scholar]
- 5.Abdulla A, Adams N, Bone M, et al. Guidance on the management of pain in older people. Age Ageing. 2013;42 Suppl 1:i1–57. [DOI] [PubMed] [Google Scholar]
- 6.Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuang SY, Hsu PF, Lin FJ, et al. Association between nonsteroidal anti-inflammatory drugs and atrial fibrillation among a middle-aged population: a nationwide population-based cohort. Br J Clin Pharmacol. 2018;84(6):1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt M, Christiansen CF, Mehnert F, Rothman KJ, Sorensen HT. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study. BMJ. 2011;343:d3450. [DOI] [PubMed] [Google Scholar]
- 9.De Caterina R, Ruigomez A, Rodriguez LA. Long-term use of anti-inflammatory drugs and risk of atrial fibrillation. Arch Intern Med. 2010;170(16):1450–1455. [DOI] [PubMed] [Google Scholar]
- 10.Chao TL C; Chen S; Wang K; et al. The association between the use of non-steroidal anti-inflammatory drugs and atrial fibrillation: A nationwide case–control study. International Journal of Cardiology. 2013;168:312–316. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi WT, O’Neal WT, Khodneva Y, et al. Association Between Opioid Use and Atrial Fibrillation: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. JAMA Intern Med. 2015;175(6):1058–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortiz de Landaluce L, Carbonell P, Asensio C, Escoda N, Lopez P, Laporte JR. Gabapentin and Pregabalin and Risk of Atrial Fibrillation in the Elderly: A Population-Based Cohort Study in an Electronic Prescription Database. Drug Saf. 2018;41(12):1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewland TA, Vittinghoff E, Mandyam MC, et al. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013;159(11):721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engstrom G, Hedblad B, Juul-Moller S, Tyden P, Janzon L. Cardiac arrhythmias and stroke: increased risk in men with high frequency of atrial ectopic beats. Stroke. 2000;31(12):2925–2929. [DOI] [PubMed] [Google Scholar]
- 15.Binici Z, Intzilakis T, Nielsen OW, Kober L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121(17):1904–1911. [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 17.Heckbert SR, Austin TR, Jensen PN, et al. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: The Multi-Ethnic Study of Atherosclerosis. J Electrocardiol. 2018;51(6):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang MJ, Roetker NS, Folsom AR, Alonso A, Heckbert SR, Chen LY. Feasibility of using a leadless patch monitor in community cohort studies: the multi-ethnic study of atherosclerosis. Pacing Clin Electrophysiol. 2018;41:1389–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45(6):683–692. [DOI] [PubMed] [Google Scholar]
- 20.Heckbert SR, Wiggins KL, Blackshear C, et al. Pericardial fat volume and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis and Jackson Heart Study. Obesity (Silver Spring). 2017;25(6):1115–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen BS, Kumarathurai P, Falkenberg J, Nielsen OW, Sajadieh A. Excessive Atrial Ectopy and Short Atrial Runs Increase the Risk of Stroke Beyond Incident Atrial Fibrillation. J Am Coll Cardiol. 2015;66(3):232–241. [DOI] [PubMed] [Google Scholar]
- 22.Chong BH, Pong V, Lam KF, et al. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace. 2012;14(7):942–947. [DOI] [PubMed] [Google Scholar]
- 23.Chilkoti G, Wadhwa R, Saxena A, Khurana P. Could pregabalin premedication predispose to perioperative atrial fibrillation in patients with sepsis? Saudi J Anaesth. 2014;8(Suppl 1):S115–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy. 2018;11:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanthanna H, Gilron I, Rajarathinam M, et al. Benefits and safety of gabapentinoids in chronic low back pain: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2017;14(8):e1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith NL, Psaty BM, Heckbert SR, Tracy RP, Cornell ES. The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol. 1999;52(2):143–146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


