Abstract
Background
Pregnancy associated breast cancer (PABC) is a rare entity and defined as breast cancer diagnosed during pregnancy or one-year post-partum. There is sparse data especially from low and middle-income countries (LMIC) and merits exploration.
Methods
The study (2013–2020) evaluated demographics, treatment patterns and outcomes of PABC.
Results
There were 104 patients, median age of 31 years; 43 (41%) had triple-negative disease, 31(29.8%) had hormone-receptor (HR) positive and HER2 negative, 14 (13.5%) had HER2-positive and HR negative and 16(15.4%) had triple positive disease. 101(97%) had IDC grade III tumors and 74% had delayed diagnosis. 72% presented with early stage (24, EBC) or locally advanced breast cancer (53, LABC) and received either neoadjuvant (n = 49) or adjuvant (n = 26) chemotherapy and surgery. Trastuzumab, tamoxifen, and radiotherapy were administered post-delivery. At a median follow up of 27 (IQR:19–35) months, the estimated 3-year event-free survival (EFS) for EBC and LABC was 82% (95% CI: 65.2–100) and 56% (95% CI: 42–75.6%) and for metastatic 24% (95% CI: 10.1%–58.5%) respectively.
Of the 104 patients, 34 were diagnosed antepartum (AP) and 15 had termination, 2 had preterm and 16 had full-term deliveries(FTDs). Among postpartum cohort (n = 70), 2 had termination, 1 had preterm, 67 had FTDs. 83(including 17 from AP) children from both cohorts were experiencing normal milestones.
Conclusion
Data from the first Indian PABC registry showed that the majority had delayed diagnosis and aggressive features(TNBC, higher grade). Treatment was feasible in majority and stage matched outcomes were comparable to non-PABCs.
Keywords: Pregnancy associated breast cancer (PABC), Registry, Gestational, Trimester, Antepartum, Postpartum
Highlights
-
•
Pregnancy associated breast cancer (PABC) is a rare and Challenging entity with lack of data from low-middle income countries.
-
•
First Indian data showed that stage matched oncologic outcomes were comparable to non-PABC.
-
•
Obstetric outcomes were similar to non-cancer associated pregnancies with normal cognitive development.
-
•
Creating awareness and early diagnosis is of utmost importance to improve prognosis in this unique entity
1. Introduction
PABC is most commonly defined as breast cancer diagnosed during pregnancy (BCP) or one-year post-partum (BCPP) although some studies show that the breast cancer risk post-partum may extend up to 5 years after delivery and this criterion is also used for the definition [1]. Young breast cancer women have a higher predisposition to familial cancer and tend to present with advanced stage, aggressive biology disease and poor outcomes. This is despite the fact that pregnancy and breastfeeding are considered protective for the development of breast cancer [1]. Increasingly, women are delaying childbearing for personal and/or professional reasons, which may have led to an increased incidence and reporting of PABC. Breast cancer (1 in 3000) followed by cervical cancer and melanoma are among the top 3 cancers diagnosed in pregnant women [[2], [3], [4]]. Breast parenchyma undergoes proliferation and structural changes during pregnancy and have been hypothesized to be the reason for PABCs [[5], [6], [7]]. Younger women with breast cancer show distinct biological features and are known to have an aggressive biology [[8], [9], [10], [11]]. There is sparse and often conflicting data on PABC outcomes reporting worse, similar, and even favorable outcomes [6,12,13].
Inadequate distribution and access to health care facilities, increased prevalence of infectious diseases, sociocultural practices along with reduced spending on women’s health contribute to higher maternal and infant mortality in India compared to those in the developed countries [14]. Providing cancer care to a pregnant woman with equal emphasis on fetal health is a daunting task. Additionally, lack of cancer awareness, limited expertise and resources lead to delayed diagnosis. Therefore, this study aimed to analyze the PABC cases from the registry established at the Tata Memorial Centre, Mumbai, a tertiary cancer referral centre, and compare the epidemiological, diagnostic, and prognostic factors as well as maternal and fetal outcomes with the published literature.
2. Methods
We conducted a registry study from Sep 2013 to Jan 2020 of the reproductive age group women, diagnosed with BCP or BCPP defined as diagnosed within one-year post-partum. Patients with missing vital information on their pregnancy or its outcomes were excluded from the analysis. Self-reported information by the study participants was collected periodically using questionnaires and passively from the electronic medical records and case files. Pregnancy associated outcomes, the oncological outcomes and the factors affecting either of them were analyzed. Delay in diagnosis was defined as ≥ 3 months from the detection of first symptom by the patient.
2.1. Statistical analysis
Descriptive statistics were used for reporting the patient, tumor and treatment related characteristics. For the categorical variables, the difference in proportions was tested using the Chi-square test or Fisher’s exact test. We performed univariate analysis to evaluate factors affecting the outcomes and those factors that were significant on univariate analysis were used in the multivariate cox regression model. All p-values were two-sided and with an alpha of 0.05.
2.2. Survival analysis
Survival statistics were calculated by Kaplan Meier method and comparisons made using the log-rank test. The time from the date of diagnosis of PABC to the date of last follow-up or death from any cause was defined as the overall survival (OS). The time since the date of diagnosis of PABC to the date of any first event (relapse, progression or death) was defined as the event-free survival (EFS). The study was approved by Institute review board and registered to clinical trials registry India (CTRI/2017/02/007907).
3. Results
3.1. Study population
The patient, disease stage, grade, receptor status-wise distribution and treatment related parameters are provided in Table 1. The cohort included 104 patients diagnosed with PABC; both antepartum (n = 34) and postpartum (n = 70). Majority of the women were multiparous (n = 93, 90%) and the median age was 31 years [interquartile range (IQR): 22–42 years]. After first detecting a breast lump, a median of 6 (IQR: 0.5–48) months elapsed before attaining the histological diagnosis of malignancy where 77(74%) had ≥3months of avoidable delay in diagnosis. Notably, majority (96%) of the antepartum patients had extremely dense breast parenchyma on mamamogram with irregular masses that appeared to be of equal density as the parenchyma without micro-calcifications (75%) and imposed diagnostic dilemma and add to delay in diagnosis. Only 1/25 patients with a family history of cancer, was detected with a germline pathogenic variant of BRCA1 (p.Glu23fs). There was a history of infertility treatment in three patients, and use of oral contraceptives in four patients.
Table 1.
Baseline characterstics.
| Variable | Frequency n = 104 (%) |
|---|---|
| Median age (range) in years | 31 (22–42) |
| Antepartum Cohort (BCP) | 34 (32.7) |
| Postpartum cohort (BCPP) | 70 (67.3) |
| Multiparous | 93 (92%) |
| Family history of cancer | 25 (24) |
| Median duration of symptoms [IQR] | 6 [3−12] |
| Delayed diagnosis | 77(74%) |
| Laterality | |
| Right | 53 (51) |
| Left | 51 (49) |
| Trimester | |
| 1–12weeks | 36 (61) |
| >12 to <24 | 16 (27.1) |
| >24 to <36 | 7 (11) |
| Missing | 45 |
| ECOG PS | |
| 0 | 59 (56.7) |
| 1 | 41 (39.4) |
| ≥2 | 4 (3.9) |
| Median BMI [IQR] | 24 [21–28] |
| Disease Characteristics | |
| TNBC | 43 (41.3) |
| Her2 positive and HR negative | 14 (13.5) |
| Her2 and HR positive (Triple positive) | 16 (15.4) |
| Her2 negative and HR positive | 31 (29.8) |
| Stagea | |
| EBC | 24 (23.1) |
| LABC | 51 (49) |
| MBC | 29 (27.9) |
| Grade(IDC GradeIII) | 101(97%) |
| Median week of pregnancy [IQR] | 14 [6.15–24] |
|
Treatment Characteristics | |
|
Systemic Therapy |
|
| Taxanes + Anthracyclines | 69 |
| Anthracyclines alone | 12 |
| Taxanes Alone | 10 |
| Taxanes + Carboplatin | 4 |
| Hormonal Therapy Alone | 4 |
| Trastuzumab received (n = 21 out of 30 eligible) | 15(EBC &LABC): 12 weeks (n = 9); 1year (n = 6) 6 (MBC): palliative intent trastuzumab |
| Surgery (n=65; 68 breast surgeries) | |
| Mastectomy | 27 |
| Breast Conserving Surgery | 39 |
| Breast Reconstruction | 2 |
| Locoregional Radiation Received | 77 (74) |
IQR= Interquartile Range; ECOG: Eastern Cooperative Oncology Group; PS: Performance Status.
BMI: Body Mass Index; HR= Hormone Receptors; EBC = Early Breast Cancer; LABC = Locally Advanced Breast Cancer; MBC = Metastatic Breast Cancer.
Stage is defined as per the standard AJCCC staging system eighth edition [37].
3.2. Oncological treatments
3.2.1. Systemic therapy
Among the 75 non-metastatic patients, 49 patients received neoadjuvant (NACT) while 26 patients received adjuvant chemotherapy (ACT) after surgery whereas all except 4 metastatic patients received some form of chemotherapy. Only 70% HER2-positive patients received Trastuzumab only after delivery, either as short or as long course, due to financial constraints (Table 1). In antepartum patients, chemotherapy was continued till 35–37 weeks or two weeks before a planned delivery so as to minimize chances of maternal and/or neonatal myelosuppression/septicaemia. Ondanstron, metoclopramide and histamine blockers were used for emesis as prophylaxis and/or treatment in the antepartum cohort. Growth factors were uncommonly used either as primary (n = 2) or as secondary prophylaxis (n = 4). Most of the patients tolerated the systemic therapy well with few grade III/IV complications in the form of febrile-neutropenia (n = 12), mucositis (n = 3), and 1 patient each with hypersensitivity, diarrhea and hyponatremia. One patient required short term stay in the intensive care unit for acute onset dyspnea after anthracyclines whereas one patient developed coagulopathy and HELLP syndrome after 4 cycles of NACT and had to undergo therapeutic preterm delivery. One LABC patient who received anthracyclines and trastuzumab, developed cardiomyopathy with an ejection fraction of 35% requiring interruption of trastuzumab. Dose reductions (up to 75%) were required in seven patients. There was no chemotherapy-induced death.
3.2.2. Loco-regional therapy
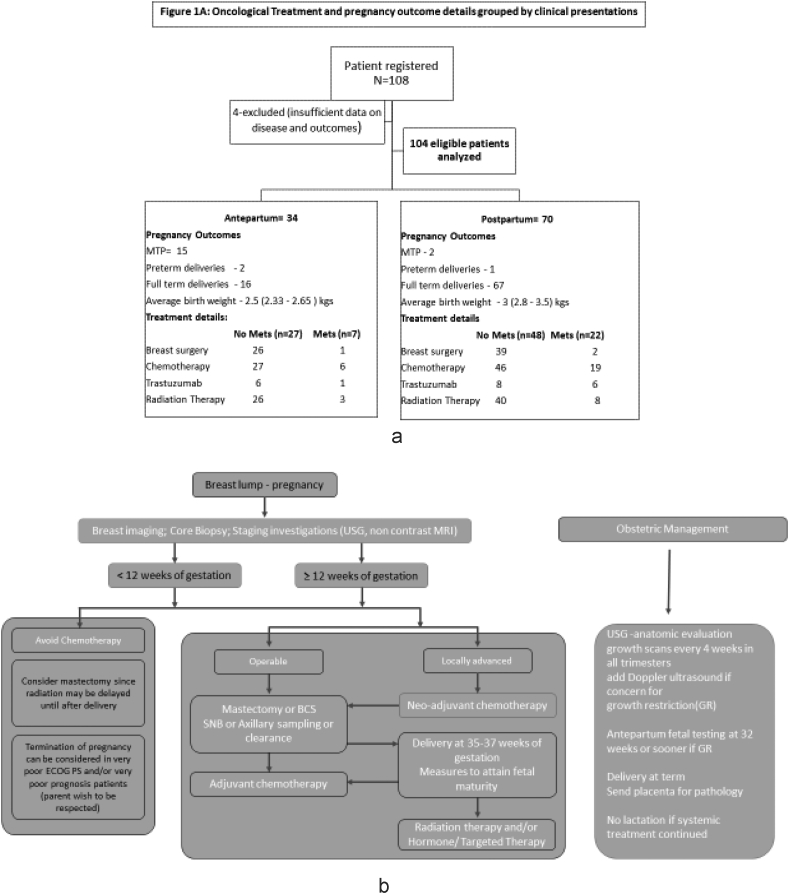
At the time of reporting only 65/75 non-metastatic patient had undergone surgery; 5 undergoing NACT whereas 5 defaulted further treatment after NACT. Two patients among those that were operated underwent cosmetic whole breast reconstructive surgery. Three metastatic patients underwent surgery where 1 was diagnosed to have metastatic disease after the surgery whereas the other 2 were oligo-metastatic and treated with radical intent. For all the women underwent surgery during pregnancy appropriate precautions were taken to avoid uterine hypoperfusion, maternal hypotension, hypoxia, hypoglycemia, pain, fever, and infection and thrombotic prophylaxis was used as appropriate. Radiation therapy (RT) was delivered to the patients whether as adjuvant therapy (n = 66) or as palliative therapy (n = 11) only post-partum. The details of the treatments grouped by the antepartum and postpartum cohort is given in Fig. 1A and treatment algorithm in Fig. 1B.
Fig. 1.
A: Oncological Treatment and pregnancy outcome details grouped by clinical presentations, B: Clinical algorithm for Management of PABC (antepartum cohort).
3.3. Survival analysis
3.3.1. Overall cohort
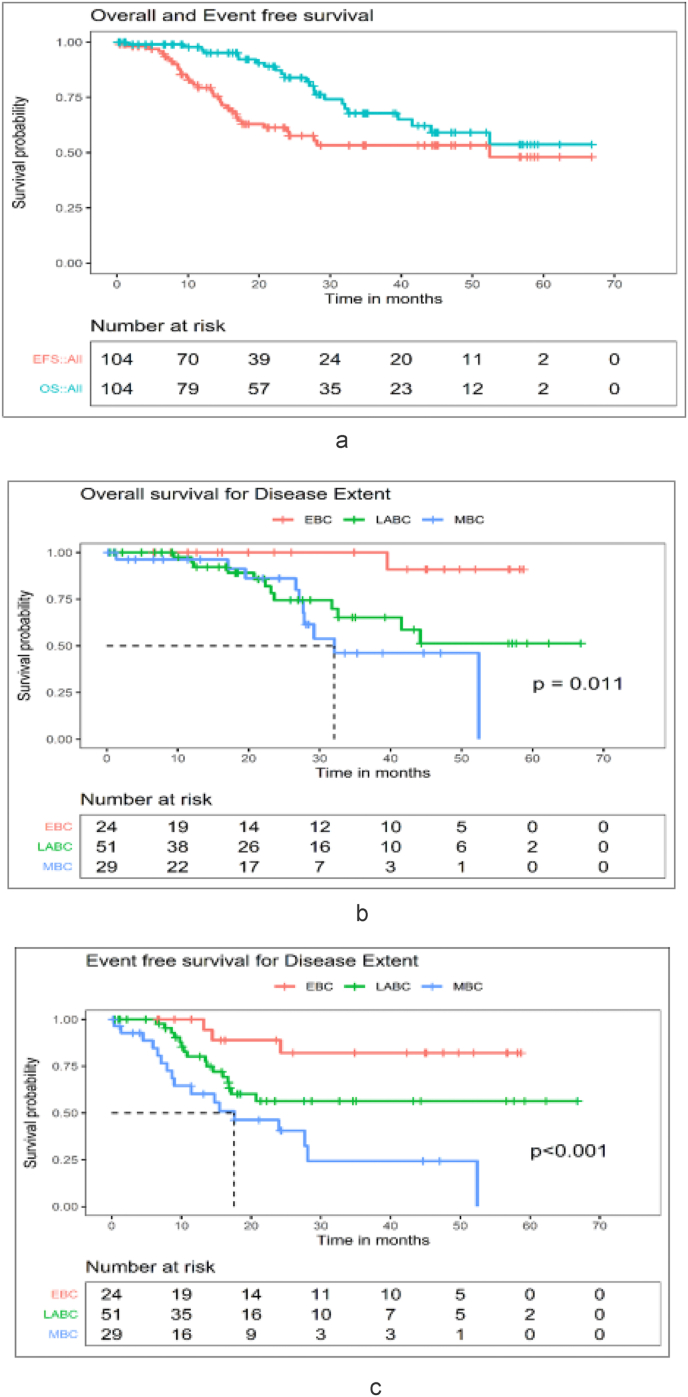
At a median follow up of 27(range 0.2–67 months), the estimated 3-years OS and EFS for the entire cohort shown in Fig. 2 was 67.8% [95% confidence intervals (CI): 56.5%–81.3%] and 53.4% (42.6%–66.8%). For EBC and LABC patients the 3-year OS (Fig. 3A) was 100 (95% CI: 100%–100%) and 65% (95% CI: 49%–86.2%) whereas the 3-year EFS (Fig. 3B) was 82% (95% CI: 65.2%–100%) and 56%(95% CI: 42%–75.6%) respectively (Fig. 3A). Metastatic breast cancer (MBC) patients had the worst outcome with a 3-year OS and EFS of 46.2% (95% CI: 26.7%–79.8%) and 24% (95% CI: 10.1%–58.5%) respectively (Fig. 2, Fig. 3). The median OS and EFS were 32 (22–42) months and 17 (5–30) months for MBC patients.
Fig. 2.
A: Overall cohort - OS and EFS (N = 104), B: Overall cohort - OS - Disease extent (N = 104), C: Overall cohort - EFS - Disease extent (N = 104).
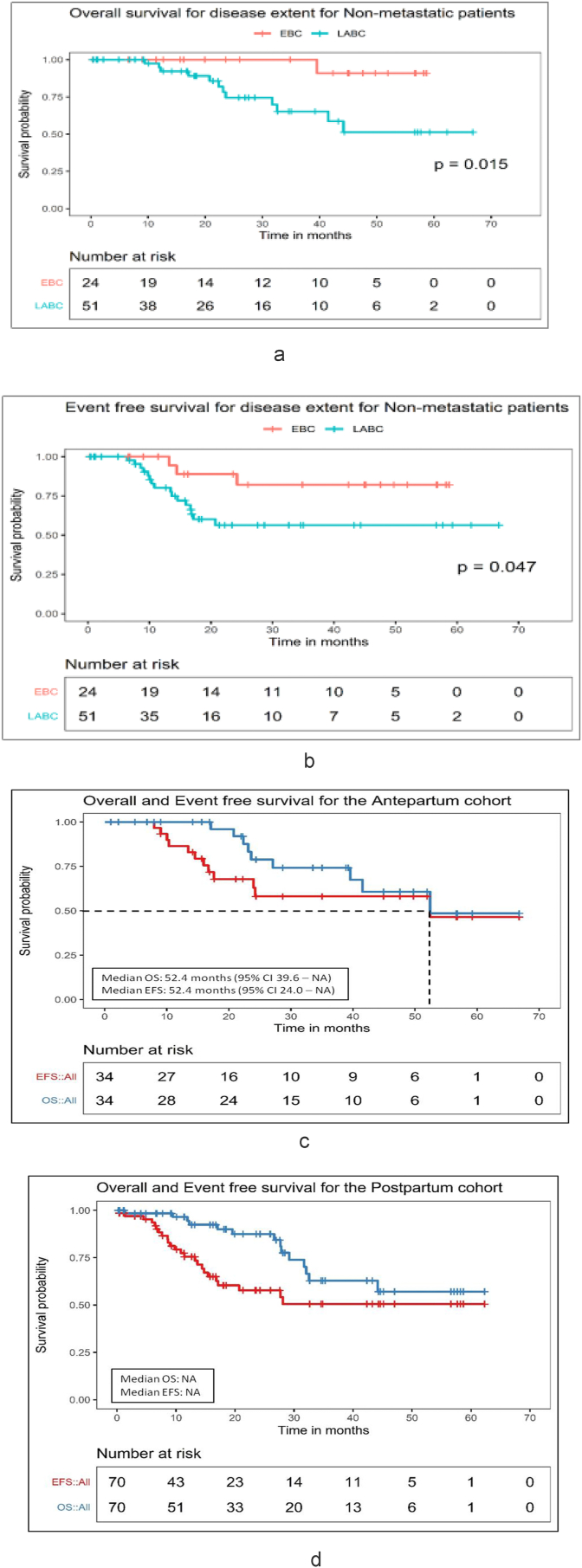
Fig. 3.
A: Non- Metastatic cohort - OS - Disease extent (N = 75), B: Non- Metastatic cohort - EFS - Disease extent (N = 75), C: Antepartum Cohort - OS and EFS (N = 34), D: Postpartum Cohort - OS and EFS (N = 70).
3.3.2. BCP Patients(diagnosed antepartum)
At the median follow-up of 38 (range 1–67) months, the median OS was 52.4 (95% CI 39.6 – NA) months and median EFS was 52.4 (95% CI 24.0 – NA) months. The estimated 3 years OS and EFS for this cohort as shown in the figure is 74.2% (95% CI 58.3%–94.4%) and 58.1% (95% CI 41.6%–81.2%) respectively (Fig. 3 D).
3.3.3. BCPP Patients(diagnosed postpartum)
At the median follow up of 24 (range 0.5–62) months, the median OS and EFS was not achieved. The estimated 3 years OS and EFS for the entire cohort shown in the figure is 62.8% (95% CI 47.9%–82.3%) and 50.5% (95% CI 37.3%–68.4%) respectively (Fig. 3 D).
3.3.4. Variables affecting OS and EFS
The details of the univariate analysis of factors and their respective correlation with either the OS or the EFS for the overall cohort and subgroups are provided in Table 1 A and B(supplements).The factors analyzed in univariate analysis were age, body mass index, ECOG-PS, duration of symptoms, parity, diagnosis during pregnancy or postpartum, family History of cancer, delay in diagnosis, receptor status; treatment specifications-regimen type, multimodality care received or not, pregnancy termination done or not, birth weight and lactating status. Multivariate cox regression analysis for EFS in the overall cohort could be not be conducted since disease extent was the only statistically significant factor in the univariate analysis. Similarly, due to lower event rates in the non-metastatic, antepartum and postpartum patient cohorts, multivariate analysis could not be conducted for OS. The cox proportional hazards model for the OS (entire cohort) and the EFS (postpartum cohort) showed that the patients with greater disease extent were significantly more likely to have an event and poor outcome as compared to patients with EBC (Table 2). These models were validated with Harrell’s concordance statistic of 0.69 and the proportionality assumption was not violated.
Table 2.
Multivariate analysis for factors affecting OS and EFS.
| Overall Survival overall cohort |
Event Free survival in postpartum cohort |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Exp(B) | 95.0% CI for Exp(B) |
Sig. | Exp(B) | 95.0% CI for Exp(B) |
Sig. | ||||
| Lower | Upper | Lower | Upper | ||||||
| Disease extent | EBC (Ref.) | ||||||||
| LABC | 7.793 | 1.007 | 60.310 | 0.049 | 2.104 | 0.454 | 9.767 | 0.342 | |
| MBC | 8.066 | 0.912 | 71.310 | 0.06 | 5.191 | 1.031 | 26.136 | 0.046 | |
| Radiation therapy |
Yes (Ref.) | ||||||||
| No |
0.4159 |
0.146 |
1.184 |
0.1 |
0.588 |
0.2078 |
1.668 |
0.319 |
|
|
Overall Survival overall cohort |
Event Free survival in postpartum cohort |
||||||||
| Disease extent | EBC (Ref.) | ||||||||
| LABC | 7.793 | 1.007 | 60.310 | 0.049 | 2.104 | 0.454 | 9.767 | 0.342 | |
| MBC | 8.066 | 0.912 | 71.310 | 0.06 | 5.191 | 1.031 | 26.136 | 0.046 | |
| Radiation therapy | Yes (Ref.) | ||||||||
| No | 0.4159 | 0.146 | 1.184 | 0.1 | 0.588 | 0.2078 | 1.668 | 0.319 | |
EBC = Early Breast Cancer, LABC = Locally advanced Breast Cancer, MBC = Metastatic Breast Cancer.
3.4. Pregnancy related details
Among the 34 antepartum patients 14 were diagnosed during the first, 13 during the second and 7 during the third trimester. Fifteen patients opted for medical termination of pregnancies (MTP); among them 5 patients had metastatic disease. There were 18 live births including 16 full term, and 2 preterm deliveries with average birth -weight of 2.55 Kgs (SD 0.484), one patient was yet to deliver at the time of analysis. One preterm baby required ventilatory support immediately postpartum while the other developed strabismus and seizures at the age of 6 months with delay in attaining cognitive milestones. All the remaining 17 babies were alive and attaining normal milestones.One infant who had hydroureteronephrosis antenatally was diagnosed with posterior urethral valve after birth and was surgically corrected at the age of 11 months. All 18 neonates were started on top-feeds to allow uninterrupted systemic therapy delivery post-partum. Two of these mothers experienced moderate anxiety whereas one failed to bond with the child.
Among the 70 patients diagnosed with PABC postpartum, 2 underwent MTPs (1 had metastatic disease), 1 had a preterm delivery and, the rest had full term deliveries with an average birthweight of 3.03 kgs (SD 0.43). The difference between the birth weights of antepartum and postpartum cohort was statistically significant (p = 0.002). Within the post-partum cohort also a neonate had developed hydroureteronephrosis and expired at 6 months from severe diarrhea; another neonate died on day 18 (of unknown cause). All the other 66 babies were alive and attaining normal milestones.
4. Discussion
Ours is the first PABC registry created in India providing insight on the uncommon presentation of breast cancer in pregnant women or those that have delivered within a year of the cancer diagnosis. This registry was thought to be necessary to systematically study the incidence, patterns of presentation, demographic, tumor and treatment related details and survival outcomes of both the patients and their babies in India. The exact incidence of PABC in India is not known due to under reporting and absence of national PABC registry till now. The median age at diagnosis in our study was 31 years which is similar to the published literature [3]. For majority of these women, this was not their first pregnancy (excluding delayed childbearing as the likely etiology) which is also in alignment with the published data suggesting that the average age at first childbirth in India is still around 20 years of age [[15], [16], [17]].
There was a delay in establishing the cancer diagnosis (median 6 months) since first noticing a lump and is similar to the observations made by other PABC studies reporting delays between 1 and 13 months [18,19]. The higher proportion of postpartum patients compared to antepartum patients, similar to other series, could be a reflection of the delay in diagnosis [[19], [20], [21], [22], [23], [24]]. Even though multiple pregnancies and breast feeding are considered protective for breast cancer, the increased levels of pregnancy-associated hormones as well as process of involution may predispose women to develop breast cancer. This is corroborated by a study reporting the importance of oxytocic molecular signatures in mammary and extramammary regions and its effect on neoplastic process [22,25]. Lack of awareness about breast cancer in young, reluctance to seek medical attention, inexperienced obstetrician or midwife, compounded by reduced sensitivity of clinical or self-breast examination and difficulty in detecting a breast lump due to pregnancy and lactation related changes in breast on mammography and denial are among the few reasons for the delay [[26], [27], [28]]. Our data reinforces the importance of educating pregnant women and care providers on the risks of PABC as well as the impact of early detection of cancer on the outcomes.
Unlike our study and that by Johansson et al., Hou et al. found a correlation between a positive family history and PABC which could be due to the differences in the PABC definition and geo-ethnic and racial differences of the cohorts [27,28]. Since younger reproductive age-group women are more commonly associated with both the PABC and the hereditary breast cancer age at presentation may act as a confounder and therefore such associations need to be evaluated carefully. Nevertheless, PABC patients that meet the criteria should be offered genetic counselling and if needed testing. Furthermore, BRCA mutation carriers are at a high risk of contralateral breast and ovarian cancer and require counselling regarding different prophylactic treatment strategies and follow-up and even preimplantation genetic diagnosis as appropriate [16,24,27].
The tumor-related characteristics like the grade and the receptor-status distribution (higher proportion of TNBCs) are higher compared to the other series indicating association of PABC with aggressive biology [6,27,29,30]. This could also be explained by the fact that in India breast cancer occurs a decade earlier and the TNBC and HER2-neu positivity rates are higher even in the non-pregnant women than in the west [31]. Still, the proportion of TNBCs are higher in PABC cohort in comparison with the non-PABC young breast cancer patients that present to our institute (34%, unpublished data) and suggest aggressive biology disease. The chemotherapy indications and agents used for PABC patients are the same as in non-pregnant breast cancer patient. However, special consideration is given to the physiologic changes of pregnancy which effect the pharmacokinetics of systemic treatment including progressive increase in the maternal body weight, plasma volume, hepatorenal perfusion, cytochrome P450 activity (third trimester), and serum albumin concentrations. The fetus and amniotic fluid act as a third space, sequestering the medications from the mother.
The systemic anti-cancer drugs are teratogenic and are contraindicated for use during the 1st trimester [16,17,19,27]. In second and third trimester, anthracycline-based regimen have been shown to be safe without increase in perinatal deaths, still births or long term cognitive outcomes and were preferred in our PABC patients (Table 1) [19,27,32]. There is limited data about the safety of taxanes with some older series showed increased birth complications but not hampering with infant growth [20]. Even though the recent data may be more reassuring one should exercise caution [33]. Chemotherapy-induced toxicities were similar to the other reported study [21]. Trastuzumab, tamoxifen and radiotherapy all agents known to cause teratogenic effects antepartum were used in post-partum setting only wherever indicated [6]. The stage of the disease and the trimester guided the timing and type of surgery during the pregnancy where equal emphasis was given to maternal and fetal wellbeing. Radiation therapy like other studies was not offered during pregnancy and hence, if women was keen for conservation surgeries, it was performed after the delivery or in the last trimester of pregnancy to prevent delay in adjuvant RT [16,19,27].
The OS and the EFS were comparable to other PABC studies as well as stage and age -matched non-pregnant cohort especially in EBC(unpublished Institutional data from other large study of young breast cancer patients) and worsened with increased disease extent which was the only independent predictor found for survival. This again emphasizes the fact that increasing cancer awareness among young women may lead to early diagnosis and better outcome and is in sync with few other studies [20,21].
In our study approximately one in two underwent MTP, mostly in the 1st trimester. A third of them were diagnosed with metastatic disease which may have influenced their decision. For most aggressive biology, poor prognostic cancers diagnosed in the first or early second trimester, various international guidelines suggest opting for MTP, so that standard treatment can be delivered without delay to optimize oncological outcomes [16,34]. Nevertheless, treatment decisions in such uncommon and complex situations should be taken in a multidisciplinary setting involving the parents in the decision-making process. Luminal-A subtype of disease, early stage and precious pregnancy are few clinical situations where pregnancy should be continued if the patient and the father desire. Conception and pregnancy following successful treatment of breast cancer is possible despite the use of alkylating agents and breast radiotherapy and should be informed to all women diagnosed with advanced cancer prior to completing 20 weeks of gestation.
Our study showed fewer incidences of birth complications and impaired cognitive development in children born in antepartum cohort (1/18 child with preterm delivery); this is similar to other studies wherein morbidities and cognitive development were found to be related with each added month to delivery rather than chemotherapy cycles [18,21,23,27]. However, some studies have reported transient dyspnea (10%) and requirement of special attention in 11% school-aged children. This discrepancy could be due to sparse data because of the uncommon presentation [18,29]. The birth weight was significantly lower in antepartum cohort which might be related to the fetus’ exposure to chemotherapy. While there are studies with similar finding there are other studies also that suggest no impact on birthweight [7,32,35,36]. This could be also be attributed to the over-cautious attitude of obstetricians and tendency to deliver the fetus preterm in woman with PABC without any pressing medical indication. Morbidity and mortality in newborn babies are directly related to gestational age at delivery, hence, utmost care should be exercised while taking these decisions [[33], [34], [35]] [[33], [34], [35]] [[33], [34], [35]]. Longitudinal studies focusing on the maternal and child related outcomes including behavioral and emotional development would be necessary to guide regarding selection of appropriate treatment strategies.
5. Limitations
The nonrandomized, nature of the study with small patient numbers introduce some bias. However, due to its uncommon presentation, conducting randomized studies may not be possible. Since some information was based on self-reporting, the education levels can have impact on the integrity of that data. However, we confirmed the patient history with medical records for this data set which greatly reduced the chances of such interpretational errors.
This is the data from first registry on PABC from India from a large tertiary cancer centre. Majority of the patients had aggressive biology (TNBC and higher grades) and delayed diagnosis. The treatments for breast cancer in women pregnant or otherwise are similar, with a few differences governed by the balance of oncologic versus obstetric outcome and decisions are generally trimester-dependent. Treatment was feasible in the majority of patients and stage, matched outcomes are comparable to non-PABC, hence, creating awareness and early diagnosis is of utmost importance to improve prognosis in this unique entity. Long-term effects of chemotherapy on cognitive and other milestone development needs exploration in larger prospective cohorts and collaborations.
Sources of support
Roche Products (India) Pvt Limited provided external funding, however had no role in design of the study, data acquisition, analysis, manuscript writing or decision to submit.
Funding source
Roche Products (India) Pvt limited (only external funding was provided; sponsors was not involved in design, data collection, analysis, drafting manuscript or submission decision).
Authors contribution statement
Jyoti Bajpai (JB): Conception, design, patient recruitment, management, follow-up, analysis, first and final manuscript [First and Corresponding author).
Vijay Simha (VS): patient recruitment, treatment, follow-up, analysis, first and final manuscript.
Sudeep Gupta (SG): design, management follow-up, statistical analysis, final manuscript.
Rajeev Sarin (RS): design, management, follow-up, lab reports, analysis, final manuscript.
Shylasree TS (ST) and Reema Pathak(RP): patient recruitment, treatment, follow-up, first and final manuscript.
Smruti Mokal(SM): Statistical analysis.
Tanuja Sheth(TS) and Palak Popat(PP): lab reports, final manuscript.
Sonal Dandekar(SD): recruitment, follow-up, final manuscript.
Declaration of competing interest
This manuscript has been read and approved by all the authors, the requirements for authorship have been met. We believe that the manuscript represents honest work and this information is not provided in another form.
Acknowledgments
We deeply appreciate the contribution of Dr. Hope Rugo (Professor, University of California, San Francisco Comprehensive Cancer Centre), entire breast disease management group at Tata Memorial Centre, patients and families for their trust and support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.02.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Azim H.A., Brohée S., Peccatori F.A. Biology of breast cancer during pregnancy using genomic profiling. Endocr Relat Canc. 2014;21(4):545–554. doi: 10.1530/ERC-14-0111. [DOI] [PubMed] [Google Scholar]
- 2.Eibye S., Kjær S.K., Mellemkjær L. Incidence of pregnancy-associated cancer in Denmark, 1977-2006. Obstet Gynecol. 2013;122(3):608–617. doi: 10.1097/AOG.0b013e3182a057a2. [DOI] [PubMed] [Google Scholar]
- 3.Peccatori F.A., Azim J.A., Orecchia R. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(SUPPL.6) doi: 10.1093/annonc/mdt199. [DOI] [PubMed] [Google Scholar]
- 4.Haas J.F. Pregnancy in association with a newly diagnosed cancer: a population-based epidemiologic assessment. Int J Canc. 1984;34(2):229–235. doi: 10.1002/ijc.2910340214. [DOI] [PubMed] [Google Scholar]
- 5.Cardonick E., Dougherty R., Grana G., Gilmandyar D., Ghaffar S., Usmani A. Breast cancer during pregnancy. Canc J. 2010;16(1):76–82. doi: 10.1097/PPO.0b013e3181ce46f9. [DOI] [PubMed] [Google Scholar]
- 6.Amant F., von Minckwitz G., Han S.N. Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J Clin Oncol. 2013;31(20):2532–2539. doi: 10.1200/JCO.2012.45.6335. [DOI] [PubMed] [Google Scholar]
- 7.Loibl S., Han S.N., von Minckwitz G. Treatment of breast cancer during pregnancy: an observational study. Lancet Oncol. 2012;13(9):887–896. doi: 10.1016/S1470-2045(12)70261-9. [DOI] [PubMed] [Google Scholar]
- 8.Cancello G., Maisonneuve P., Rotmensz N. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann Oncol. 2010;21(10):1974–1981. doi: 10.1093/annonc/mdq072. [DOI] [PubMed] [Google Scholar]
- 9.Azim H.A., Michiels S., Bedard P.L. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Canc Res. 2012;18(5):1341–1351. doi: 10.1158/1078-0432.CCR-11-2599. [DOI] [PubMed] [Google Scholar]
- 10.Collins L.C., Marotti J.D., Gelber S. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Canc Res Treat. 2012;131(3):1061–1066. doi: 10.1007/s10549-011-1872-9. [DOI] [PubMed] [Google Scholar]
- 11.Narod S.A. Breast cancer in young women. Nat Rev Clin Oncol. 2012;9(8):460–470. doi: 10.1038/nrclinonc.2012.102. [DOI] [PubMed] [Google Scholar]
- 12.Azim H.A., Jr., Botteri E., Renne G. The biological features and prognosis of breast cancer diagnosed during pregnancy: a case-control study. Acta Oncol (Madr) 2012;51(5):653–661. doi: 10.3109/0284186X.2011.636069. [DOI] [PubMed] [Google Scholar]
- 13.Litton J.K., Warneke C.L., Hahn K.M. Case control study of women treated with chemotherapy for breast cancer during pregnancy as compared with nonpregnant patients with breast cancer. Oncol. 2013;18(4):369–376. doi: 10.1634/theoncologist.2012-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moller A.B., Patten J.H., Hanson C. Monitoring maternal and newborn health outcomes globally: a brief history of key events and initiatives. Trop Med Int Health. 2019;24(12):1342–1368. doi: 10.1111/tmi.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bongaarts J., Blanc A.K. Estimating the current mean age of mothers at the birth of their first child from household surveys. Popul Health Metrics. 2015;13(1):25. doi: 10.1186/s12963-015-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardoso F., Kyriakides S., Ohno S. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 17.Bajpai J., Shylasree T. Pregnancy-associated breast cancer: controversies and consensus! Oncobiology and Targets. 2016;3(1):6. doi: 10.4103/2395-4469.192739. [DOI] [Google Scholar]
- 18.Amant F., Van Calsteren K., Halaska M.J. Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: an observational study. Lancet Oncol. 2012;13(3):256–264. doi: 10.1016/S1470-2045(11)70363-1. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz R., Herrero C., Strasser-Weippl K. Epidemiology and pathophysiology of pregnancy-associated breast cancer: a review. Breast. 2017;35:136–141. doi: 10.1016/j.breast.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Lambe M., Hsieh C.C., Trichopoulos D., Ekbom A., Pavia M., Adami H.O. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331(1):5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- 21.Chie W.C., Hsieh C.C., Newcomb P.A. Age at any full-term pregnancy and breast cancer risk. Am J Epidemiol. 2000;151(7):715–722. doi: 10.1093/oxfordjournals.aje.a010266. [DOI] [PubMed] [Google Scholar]
- 22.Albrektsen G., Heuch I., Hansen S., Kvåle G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Canc. 2005;92(1):167–175. doi: 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo J.C., Yu T., Hurd T.C. Breast cancer in pregnancy: a literature review. Arch Surg. 2003;138(1):91–98. doi: 10.1001/archsurg.138.1.91. [DOI] [PubMed] [Google Scholar]
- 24.Hou N., Ogundiran T., Ojengbede O. Risk factors for pregnancy-associated breast cancer: a report from the Nigerian Breast Cancer Study. Ann Epidemiol. 2013;23(9):551–557. doi: 10.1016/j.annepidem.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassoni P., Sapino A., Marrocco T., Chini B., Bussolati G. Oxytocin and oxytocin receptors in cancer cells and proliferation. J Neuroendocrinol. 2004;16(4):362–364. doi: 10.1111/j.0953-8194.2004.01165.x. [DOI] [PubMed] [Google Scholar]
- 26.Ahn B.Y., Kim H.H., Moon W.K. Pregnancy- and lactation-associated breast cancer: mammographic and sonographic findings. J Ultrasound Med. 2003;22(5):491–497. doi: 10.7863/jum.2003.22.5.491. [DOI] [PubMed] [Google Scholar]
- 27.Johansson A.L.V., Andersson T.M.L., Hsieh C.C., Cnattingius S., Dickman P.W., Lambe M. Family history and risk of pregnancy-associated breast cancer (PABC) Breast Canc Res Treat. 2015;151(1):209–217. doi: 10.1007/s10549-015-3369-4. [DOI] [PubMed] [Google Scholar]
- 28.Taylor D., Lazberger J., Ives A., Wylie E., Saunders C. Reducing delay in the diagnosis of pregnancy-associated breast cancer: how imaging can help us. J Med Imaging Radiat Oncol. 2011;55(1):33–42. doi: 10.1111/j.1754-9485.2010.02227.x. [DOI] [PubMed] [Google Scholar]
- 29.Bae S.Y., Jung S.P., Jung E.S. Clinical characteristics and prognosis of pregnancy-associated breast cancer: poor survival of luminal B subtype. Oncol. 2018;95(3):163–169. doi: 10.1159/000488944. [DOI] [PubMed] [Google Scholar]
- 30.Gooch J.C., Chun J., Kaplowitz E. Pregnancy-associated breast cancer in a contemporary cohort of newly diagnosed women. Breast J. 2019 doi: 10.1111/tbj.13510. [DOI] [PubMed] [Google Scholar]
- 31.Nair N., Shet T., Parmar V. Breast cancer in a tertiary cancer center in India - an audit, with outcome analysis. Indian J Canc. 2018;55(1):16. doi: 10.4103/ijc.IJC_484_17. [DOI] [PubMed] [Google Scholar]
- 32.Amant F., Loibl S., Neven P., Van Calsteren K. Breast cancer in pregnancy. Lancet. 2012;379(9815):570–579. doi: 10.1016/S0140-6736(11)61092-1. [DOI] [PubMed] [Google Scholar]
- 33.OʼLaughlin A., So S., Fleischer L., Akoto S., Cardonick E. Safety of taxane chemotherapy in breast cancer during pregnancy [28O] Obstet Gynecol. 2019;133:169–170. doi: 10.1097/01.aog.0000558888.89911.f0. [DOI] [Google Scholar]
- 34.Daly M.B., Pilarski R., Yurgelun M.B. NCCN guidelines insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 1.2020. J Natl Compr Canc Netw. 2020;18(4):380–391. doi: 10.6004/jnccn.2020.0017. [DOI] [PubMed] [Google Scholar]
- 35.Van Calsteren K., Heyns L., De Smet F. Cancer during pregnancy: an analysis of 215 patients emphasizing the obstetrical and the Neonatal outcomes. J Clin Oncol. 2010;28(4):683–689. doi: 10.1200/JCO.2009.23.2801. [DOI] [PubMed] [Google Scholar]
- 36.Melamed N., Klinger G., Tenenbaum-Gavish K. Short-term neonatal outcome in low-risk, spontaneous, singleton, late preterm deliveries. Obstet Gynecol. 2009;114(2):253–260. doi: 10.1097/AOG.0b013e3181af6931. [DOI] [PubMed] [Google Scholar]
- 37.Giuliano A.E., Edge S.B., Hortobagyi G.N. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25:1783–1785. doi: 10.1245/s10434-018-6486-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.