Abstract
Objects
To explore the specific features of cognitive function in patients with schizophrenia at different stages and its influencing factors.
Methods
The MATRICS Consensus Cognitive Battery (MCCB) and the Brief Psychiatric Rating Scale (BPRS) were administered to 208 patients with schizophrenia, including 158 clinically stable schizophrenia (CSS) and 50 first-episode patients with schizophrenia (FES), and 40 healthy controls (HC). Propensity score matching (PSM) was used to match the CSS and FES.
Results
(1) The MCCB and it,s sub-scale scores in patients with schizophrenia were lower than HC, but the score of emotion intelligence showed no significant difference between CSS and HC. (2) Before PSM, the cognitive scores of FES were significantly lower than CSS (except trail making A test, Hopkins verbal learning, category fluency). After PSM, patients with CSS still do better in performing trail making A test, emotional intelligence, continuous performances and MCCB total score. (3) BPRS total score, gender, group (FES vs CSS) and age were independent contributors to emotion intelligence, and BPRS total score had the biggest effect. (4) The effect of group (FES vs CSS) on MCCB total score and emotional intelligence was statistically significant.
Conclusions
There are significant cognitive deficits in patients with FES and CSS compared with HC. FES have greater cognitive impairments compared with CSS. Emotion intelligence of CSS may be even close to the level of HC. BPRS total score, gender, group (FES vs CSS) and age may be the independent contributors to social cognition. Group (FES vs CSS) may play an important effect on general cognition and social cognition.
Keywords: Schizophrenia, Neurocognition, Social cognition, MCCB, Influencing factors
1. Introduction
Schizophrenia is a serious mental illness, in which cognitive deficits are one of the core symptoms. It is well known that patients with schizophrenia have impairment in both neurocognitve and social cognitive functions (Evans et al., 2004; Green et al., 2000; Weinberg et al., 2016). Previous studies on specific features of cognitive deficits between clinically stable schizophrenia (CSS) and first-episode patients with schizophrenia (FES) yielded controversial results. There is clear evidence that neurocognitive performance were impaired mildly at the prodromal stage of schizophrenia and even from the childhood (Bechi et al., 2020; Bozikas and Andreou, 2011; Lewandowski et al., 2011), and then further decline lasting a long time at the clinically stable stage (McCleery et al., 2016). Other studies suggested that the neurocognitive performance of FES patients is significantly better than those of patients with schizophrenia at the CSS (Couture et al., 2006; Wu et al., 2016). However, one study also been reported that there is no significant difference in neurocognitive performance between FES patients and CSS paients (McCleery et al., 2016).
Controversial findings also have been reported in social cognitive deficits in schizophrenia (Brown et al., 2014; Fett et al., 2011; Fiszdon et al., 2013; Valaparla et al., 2017). Some studies have suggested that impairment in social cognition in patients with schizophrenia is presented both in the symptomatic and remission phases, with a higher level of deficits during the symptomatic phase (Kucharska-Pietura et al., 2005; Valaparla et al., 2017). Compared to neurocognitve deficits, social cognitve impairments are often associated with worse social and occupational functions (Couture et al., 2006; Fett et al., 2011). Although the exact relationship between social cognitive performance and disease severity, as well as current social and occupational functional status, is unclear. A few literature suggested that different domains of social cognition should be responsible for different aspects of current social and occupational functional status, as well as different psychopathological symptoms (Abdel-Hamid et al., 2009; Bell et al., 2010; Bromley and Brekke, 2010).
Cognitive impairment is considered to be a sensitive predictor of the prognosis of schizophrenia (McCleery et al., 2016; Wu et al., 2016). The importance of further research into neurocognition and social cognition in schizophrenia was highlighted by a recent NIMH-sponsored workshop report (Fiszdon et al., 2013). In this study, we examined: (1) it is to evaluated differences in social cognitive and neurocognitive performance between FES, CSS and HC. (2) Factors affecting social cognitive and neurocognitive performance in patients with schizophrenia at different stage.
2. Material and methods
2.1. Research subjects
Two hundred and eight (208) patients with schizophrenia were recruited from May 2017 to October 2017. Of those enrolled to the study, 158 patients with clinically stable schizophrenia from outpatient clinics, and 50 patients with first-episode schizophrenia who were hospitalized in the Psychiatry Department, the Third Affiliated Hospital of Sun Yat-sen University. Forty healthy controls were recruited. All participants voluntarily attended this study and gave written informed consent for participation in the study. The Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University approved this study protocol, and it conformed to the provisions of the Declaration of Helsinki.
Inclusion criteria for patients with schizophrenia included: (1) meet the diagnostic criteria for schizophrenia in the International Classification of Disease the tenth edition (ICD-10); (2) age range from 16 to 55 years old, male or female; (3) have had a period of six or more years of education.
Patients with chronically stable schizophrenia (CSS) also meet the following additional conditions: (1) the duration of the illness is at least over 2 years; (2) patients with schizophrenia who have been treated with drugs for more than 1 year were in stable condition and maintenance treatment (Conley et al., 2009); (3) patients receiving antipsychotic medications at stable dose for at least 3 months.
Patients with first-episode schizophrenia (FES) also met the following additional conditions: (1) duration of symptoms no more than 2 years; (2) in an acute episode at study enrollment; (3) all data collection should be completed within 1 week of taking the antipsychotic drugs.
Inclusion criteria for healthy controls included: (1) absence of personal or family history of psychiatric disorder assessed by mini-SCID; (2) age from 16 to 55 years old; (3) have had a period of six or more years of education.
Exclusion criteria included: (1) history of other mental illnesses (mood disorders, anxiety, eating disorders, substance abuse, mental retardation, etc.); (2) with hearing and visual disturbances who could not complete the test; (3) had a history of serious or chronic physical disease (heart failure, thyroid dysfunction, chronic hepatitis, metabolic disease history, etc.); (4) pregnant or lactating women.
2.2. Assessment tools
2.2.1. Survey of demographic and general clinical data
The demographic and general clinical data of study subjects were collected by an questionnaire developed in-house, including: age, sex, ethnicity, disease course, previous treatment history with antipsychotics (type, duration and dose of medications), years of education, lifestyle (smoking, drinking, weekly exercise), alcohol drinker mean drinking alcohol regularly at least once a week.
2.2.2. MATRICS consensus cognitive battery (MCCB)
MCCB is a standard cognitive assessment tool for schizophrenia (Kern et al., 2008). It includes 10 subscales consisting of 7 cognitive dimensions, namely attention/alertness, information processing speed, verbal learning, visual learning, working memory, reasoning and problem solving, and social cognitive. Raw scores are converted to T scores. An MCCB total T score is the sum of subscale T scores. The higher T scores, the better cognitive performance. The standardized Chinese version was used in this study (Shi et al., 2015).
2.2.3. The Brief Psychiatric Rating Scale (BPRS)
BPRS is a scale for assessing the severity of psychotic symptoms. It is suitable for the majority of patients with severe psychotic symptoms, especially for patients with schizophrenia. There are 18 items in total and it consists of five sub-factors, namely anxiety and depression, lack of vitality, thinking disorder, activation, and hostile suspicion. The total score reflects the severity of the psychotic symptoms. The higher the total score, the worse the condition.
2.2.4. Mini-SCID patients/non-patients version
The MINI-International Neuropsychiatric Interview (M.I.N.I.) is a simple, effective and reliable interview tool developed by Sheehan and Lecrubier (Lecrubier et al., 1997). It was mainly used to screen and diagnose patients with schizophrenia or healthy controls.
2.3. Statistical methods
IBM SPSS 23.0 software package was used to analyze data. The distribution of the variables was assessed using the Shapiro-Wilk test. Normally distributed continuous variables were presented as mean ± standard deviation (SD), demographic and general clinical data were tested by one-way analysis of variance (ANOVA). Skewed distributed continuous variables were tested by Wilcoxon rank-sum test or Kruskal-Wallis H test. The Chi-square test was used for category variables. Fisher's least significant difference (LSD) test was used to perform post-hoc pair-wise comparison between groups of CSS, FES and HC. Covariate analysis were used to adjust confounding factors. Propensity score matching (PSM) was used to analyze the differences between groups of patients with schizophrenia, and the 1:1 nearest neighbor matching method was used to set the caliper value to 0.02. Taking the MCCB T score and emotional management T score as dependent variables, correlation analysis and stepwise multiple regression analysis were performed to screen independent affecting factors of cognitive function. The polyline was created by Graphpad prism7.0 software. All P-values were two tailed with significance level set at 0.05.
3. Results
Table 1 shows that first-episode patients with schizophrenia (FES), clinically stable patients with schizophrenia (CSS), and healthy controls (HC) were similar in gender distribution and smoking (P > 0.05), but with a significant difference in age, education, alcohol drinking, exercise, disease course, and BPRS scores (P < 0.05).
Table 1.
The demographic and general clinical characteristics of participants.
| CSS |
FES |
HC |
F/χ2 | P | Corrected P⁎ | CSS vs FES | CSS vs HC | FES vs HC | |
|---|---|---|---|---|---|---|---|---|---|
| (n = 158) | (n = 50) | (n = 40) | |||||||
| Age (yrs) | 28.30 ± 7.70 | 22.30 ± 6.74 | 25.25 ± 6.79 | 30.646 | <0.001 | <0.001 | <0.001 | 0.020 | 0.061 |
| Education (yrs) | 13.15 ± 2.98 | 12.24 ± 2.44 | 14.68 ± 2.94 | 17.033 | <0.001 | <0.001 | 0.053 | 0.003 | <0.001 |
| Gender | 0.610 | 1.830 | 0.568 | 0.358 | 0.740 | ||||
| Male (%) | 78 (49.37%) | 27 (54%) | 23 (57.5%) | 0.988 | |||||
| Female (%) | 80 (50.63%) | 23 (46%) | 17 (42.5%) | ||||||
| Smoker (%) | 23 (14.56%) | 8 (16.0%) | 11 (27.5%) | 3.840 | 0.147 | 0.441 | 0.803 | 0.053 | 0.184 |
| Alcohol drinker (%) | 8 (5.06%) | 2 (4.0%) | 11 (27.5%) | 23.344 | <0.001 | <0.001 | 0.759 | <0.001 | 0.002 |
| Exercise (%) | 50 (31.65%) | 12 (24.0%) | 5 (12.5%) | 6.223 | 0.045 | 0.135 | 0.303 | 0.016 | 0.166 |
| Drug dose (DDD) | 396.45 ± 227.82 | 365.71 ± 257.74 | −0.839 | 0.402 | 1.206 | ||||
| Disease course (m) | 80.08 ± 61.10 | 6.79 ± 8.38 | −10.10 | <0.001 | <0.001 | ||||
| BPRS total score | 29.75 ± 9.08 | 41.02 ± 8.69 | 22.48 ± 3.01 | 85.705 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
DDD: drug dose is the average daily chlorpromazine equivalent mg/d; CSS:clinically stable patients with schizophrenia; FES: first-episode patients with schizophrenia; HC: healthy controls.
All indicated p values were after Bonferroni's correction.
3.1. Comparison of cognitive performance between FES, CSS and HC
After using covariance analysis to control the confounding factors (age, education, and BPRS scores), there was still a significant difference in cognitive performance (MCCB and its subscales T scores) between FES, CSS and HC (Table 2). The MCCB and subscales T scores of patients with CSS and FES were significantly lower compared with the HC group (Table 2), but the score of emotion intelligence had no significant difference between CSS and HC.
Table 2.
The comparison of MCCB and sub-scales scores between schizophrenia subjects with clinically stable, first-episode and healthy controls.
| CSS |
FES |
HC |
F | P | Corrected P⁎ | CSS vs FES | CSS vs HC | FES vs HC | |
|---|---|---|---|---|---|---|---|---|---|
| (n = 158) | (n = 50) | (n = 40) | |||||||
| TMT | 46.75 ± 0.96 | 46.15 ± 1.94 | 58.45 ± 2.04 | 13.822 | <0.001 | <0.001 | 0.787 | <0.001 | <0.001 |
| SC | 34.22 ± 0.94 | 28.31 ± 1.90 | 47.79 ± 2.01 | 20.064 | <0.001 | <0.001 | 0.007 | <0.001 | <0.001 |
| HVLT | 32.36 ± 1.11 | 31.54 ± 2.24 | 45.86 ± 2.36 | 13.806 | <0.001 | <0.001 | 0.750 | <0.001 | <0.001 |
| SS | 38.34 ± 1.15 | 31.85 ± 2.32 | 46.39 ± 2.45 | 8.068 | <0.001 | <0.001 | 0.016 | 0.003 | <0.001 |
| DS | 39.85 ± 1.07 | 33.43 ± 2.16 | 48.70 ± 2.27 | 10.429 | <0.001 | <0.001 | 0.010 | <0.001 | <0.001 |
| NAB | 40.54 ± 1.27 | 31.14 ± 2.56 | 52.11 ± 2.70 | 13.801 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 |
| BVMT | 38.00 ± 1.11 | 31.80 ± 2.25 | 47.70 ± 2.37 | 10.601 | <0.001 | <0.001 | 0.017 | <0.001 | <0.001 |
| CF | 38.84 ± 0.70 | 37.21 ± 1.43 | 44.44 ± 1.50 | 6.584 | 0.002 | 0.006 | 0.319 | 0.001 | 0.001 |
| EIT | 50.84 ± 1.15 | 44.51 ± 2.34 | 54.26 ± 2.46 | 3.847 | 0.023 | 0.069 | 0.019 | 0.206 | 0.008 |
| CPT | 37.51 ± 0.95 | 29.42 ± 1.93 | 48.45 ± 2.02 | 20.488 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| MCCB | 32.04 ± 1.16 | 23.25 ± 2.35 | 50.68 ± 2.47 | 30.919 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
1. The data was presented as Mean ± SE; 2. the confounding factors of age, years of education, BPRS scores were controlled by Covariance analysis; 3. CSS: clinically stable patients with schizophrenia; FES: first-episode patients with schizophrenia; HC: healthy controls; 4. TMT: trail making A test; SC: symbol coding; HVLT: Hopkins verbal learning test; SS: spatial span; DS: digital sequence; NAB: maze; BVMT: brief visuospatial memory test; CF: category fluency; EIT: emotion intelligence test; CPT: Continuous performance test; MCCB: MCCB total T score.
All indicated p values were after covariates controlling (age, education, and BPRS scores) and Bonferroni's correction.
3.2. Comparison of cognitive performance between FES, CSS
Using schizophrenia groups as the dependent variable, age, gender, and years of education were selected as covariates, using a 1:1 nearest neighbor matching method with a caliper value of 0.02. After performing PSM, a total of 40 subjects with first episode schizophrenia and 40 patients with clinically stable schizophrenia were matched. There was no statistical difference between the two groups in terms of age, gender, years of education, and drug dosage, smoking, alcohol drinking, exercise, but there was a statistical difference between the two groups in the course of disease and the total score of BPRS, as shown in Table 3.
Table 3.
Comparison of general demographic data after PSM in subjects with first-episode schizophrenia and clinically stable schizophrenia.
| After PSM |
z/χ2 | P | ||
|---|---|---|---|---|
| FES |
CSS |
|||
| n = 40 | n = 40 | |||
| Age (yrs) | 22 (18.25, 28) | 22 (19, 29.50) | −0.150 | 0.881 |
| Gender | ||||
| Male | 20 (50.0%) | 23 (57.5%) | 0.453 | 0.501 |
| Female | 20 (50.0%) | 17 (42.5%) | ||
| Education (yrs) | 12 (12, 15) | 12 (9.75, 15.75) | −0.050 | 0.960 |
| Smoker (%) | 7 (17.5%) | 7 (17.5%) | 0.000 | 1.000 |
| Alcohol drinker (%) | 2 (5.0%) | 4 (10.0%) | 0.180 | 0.671 |
| Exercise (%) | 10 (25.0%) | 18 (45.0%) | 3.516 | 0.061 |
| Drug dose (DDD) | 346.93 (221.72, 590.63) | 304.19 (150.00, 547.50) | −0.664 | 0.507 |
| Disease course (m) | 2 (1, 6) | 57 (36, 93) | −7.369 | <0.001 |
| BPRS total score | 40 (36.5, 46) | 29.50 (24, 34.50) | −5.005 | <0.001 |
DDD is the average daily chlorpromazine equivalent mg/d.
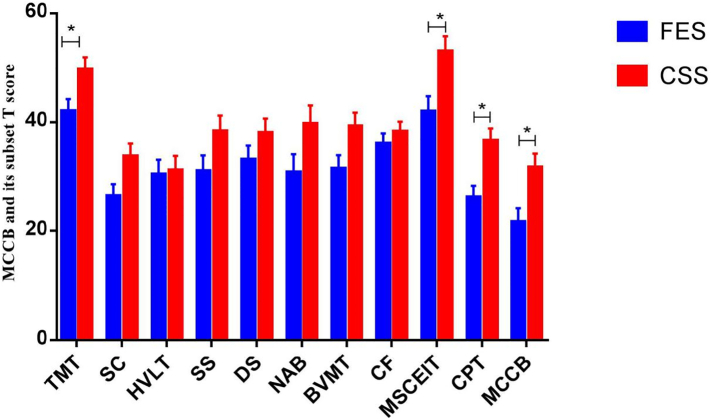
In terms of cognitive function, clinically stable schizophrenia group are superior to first-episode schizophrenia group in performing symbol coding, verbal learning, spatial span, digital sequence, maze, visuospatial memory, emotional intelligence, continuous performance, MCCB total score still have significant statistical differences between the two groups (P < 0.05), see Table 4.
Table 4.
Comparison of cognitive function before and after PSM in subjects with first-episode schizophrenia and clinically stable schizophrenia.
| Before PSM |
t | P | After PSM |
t/z | P | |||
|---|---|---|---|---|---|---|---|---|
| FES |
CSS |
FES |
CSS |
|||||
| n = 50 | n = 158 | n = 40 | n = 40 | |||||
| Neurocognition | ||||||||
| TMT | 44.80 ± 12.85 | 46.61 ± 11.69 | −0.930 | 0.353 | 44.03 ± 13.54 | 48.18 ± 10.70 | −1.521 | 0.132 |
| SC | 26.76 ± 12.20 | 34.34 ± 11.82 | −3.920 | <0.001 | 24 (13.50, 35.50) | 36 (24.25, 46) | −3.273 | 0.001 |
| HVLT | 29.24 ± 14.01 | 32.31 ± 14.49 | −1.316 | 0.190 | 26 (14.75, 36.75) | 36.50 (25.50, 44.75) | −2.101 | 0.036 |
| SS | 29.76 ± 13.60 | 38.39 ± 14.77 | −3.667 | <0.001 | 13 (10, 29.50) | 42 (30, 51.75) | −3.499 | <0.001 |
| DS | 33.20 ± 14.49 | 39.56 ± 13.70 | −2.824 | 0.005 | 32.50 (20, 43) | 41 (28.50, 49.50) | −2.395 | 0.017 |
| NAB | 30.62 ± 17.37 | 40.42 ± 16.25 | −3.654 | <0.001 | 29.50 (10, 43.75) | 46 (31.75, 53.75) | −2.899 | 0.004 |
| BVMT | 31.72 ± 13.58 | 37.77 ± 14.00 | −2.683 | 0.008 | 31.30 ± 13.92 | 40.05 ± 12.49 | −2.959 | 0.004 |
| CF | 36.55 ± 8.51 | 38.75 ± 9.06 | −1.507 | 0.133 | 35.46 ± 9.05 | 39.20 ± 9.29 | −1.811 | 0.074 |
| CPT | 27.61 ± 11.05 | 37.78 ± 12.04 | −5.265 | <0.001 | 26.21 ± 11.46 | 36.20 ± 10.70 | −4.008 | <0.001 |
| Social cognition | ||||||||
| MSCEIT | 42.88 ± 13.75 | 50.64 ± 14.85 | −3.252 | <0.001 | 41.10 ± 12.42 | 54.20 ± 13.30 | −4.522 | <0.001 |
| Overall cognition | ||||||||
| MCCB | 22.14 ± 12.62 | 31.97 ± 15.04 | −4.141 | <0.001 | 15 (10, 30) | 36 (21.50, 45.25) | −4.025 | <0.001 |
Further using covariance analysis to control the impact of disease course and disease severity on cognitive function, we found that subjects with clinically stable schizophrenia still have better cognitive functions than first-episode schizophrenia in performing trail making A test, emotional intelligence, continuous performance test and MCCB total T score (P < 0.05), see Fig. 1.
Fig. 1.
Comparison of cognitive function between patients with first-episode schizophrenia and clinically stable schizophrenia.
CSS: clinically stable patients with schizophrenia; FES: first-episode patients with schizophrenia.
TMT: trail making A test; SC: symbol coding; HVLT: Hopkins verbal learning test; SS: spatial span; DS: digital sequence; NAB: maze; BVMT: brief visuospatial memory test; CF: category fluency; EIT: emotion intelligence test; CPT: continuous performance test; MCCB: MCCB total T score.
3.3. Correlation between cognitive performance and clinical variables in patients with schizophrenia
Two-level (FES vs CSS) diagnostic variable as group in a single model to evaluate the possibly different effect of the predictor variables. Taking MCCB total score and emotion intelligence test score as the dependent variables, the analysis of factors affecting the cognitive function and found that: MCCB total score and emotion intelligence score of schizophrenia subjects was positively associated with disease course, negatively correlated with BPRS total score (Table 5).
Table 5.
Linear correlation analysis of general clinical data and cognitive function of subjects with schizophrenia.
| MCCB total score |
Emotion intelligence |
|||
|---|---|---|---|---|
| r | P | r | P | |
| Age | −0.036 | 0.605 | −0.050 | 0.472 |
| Education (yrs) | 0.002 | 0.980 | 0.071 | 0.307 |
| Drug dose (DDD) | −0.089 | 0.200 | −0.014 | 0.846 |
| Disease course (m) | 0.230 | 0.001 | 0.167 | 0.016 |
| BPRS total scscore | −0.242 | <0.001 | −0.263 | <0.001 |
Taking MCCB total score and emotion intelligence score as dependent variables, gender, age, years of education, drug dosage, disease course, alcohol drinking, exercise and BPRS total score as independent variables, stepwise regression analysis was performed for subjects with schizophrenia: BPRS total score, gender, group, age have found to be independent contributors to emotion intelligence (P < 0.05), the exploratory variable BPRS total score had the biggest effect on emotion intelligence score according to the values of standardized coefficients. The influence of group on MCCB total score and emotion intelligence was statistically significant (P < 0.05), and CSS was better than the FES according to the values of standardized coefficients (Table 6).
Table 6.
Multiple linear regression analysis of the total MCCB score and emotional intelligence score of subjects with schizophrenia.
| Unstandardized coefficients |
Standardized coefficients |
P | 95.0%CI |
R2 | Adjusted R2 | |||
|---|---|---|---|---|---|---|---|---|
| B | Beta | Lower bound | Upper bound | |||||
| Emotion intelligence | (Constant) | 64.732 | <0.001 | 52.724 | 76.741 | |||
| BPRS total score | −0.322 | −0.217 | 0.004 | −0.540 | −0.105 | 0.068 | 0.063 | |
| Gender | −5.539 | −0.183 | 0.005 | −9.418 | −1.660 | 0.102 | 0.093 | |
| Group | 6.875 | 0.195 | 0.011 | 1.571 | 12.180 | 0.120 | 0.107 | |
| Age | −0.302 | −0.158 | 0.024 | −0.564 | −0.041 | 0.142 | 0.125 | |
| MCCB total score | (Constant) | 21.900 | <0.001 | 25.943 | ||||
| Group | 10.068 | 0.286 | <0.001 | 5.430 | 14.707 | 0.082 | 0.077 | |
4. Discussion
In this study, after controlling for the confounding factors, we found that the MCCB T score and its subscale T scores of FES and CSS were significantly lower than healthy controls, indicating that cognitive performance in patients with schizophrenia is impaired. Harvey et al. (Harvey and Rosenthal, 2017) reported that the cognitive performance of patients with schizophrenia was lower than that of healthy people of the same age, and similar to healthy people three decades older. Our findings have again confirmed that cognitive impairment is the core symptom of schizophrenia and extends through the entire course of schizophrenia.
This study found that the CSS neurocognition (including trail making A test and continuous performance) and social cognition (emotional intelligence test/EIT) were significantly better than FES, while the BPRS score was significantly lower than FES. This result reflected that the psychopathological symptoms of FES were significantly worse than that of CSS, and cognitive performance of patients with schizophrenia decreased sharply during the first episode. However, the results are not consistent with one previous studies (Bozikas and Andreou, 2011) that the cognitive performance of CSS was much worse than that of FES, mainly reflected in category fluency, trail marking A, digital sequencing, verbal learning, maze, and emotional intelligence. There were also studies (Horan et al., 2012; McCleery et al., 2016) which reported no significant difference in cognitive deficits between FES and CSS. Other studies documented that cognitive performance was maintained at a relatively stable level for a long time in CSS (Aas et al., 2014; Guo et al., 2011; Olivier et al., 2015). It is well known that acute psychopathological symtoms have negative impact on neurocognitive function. In our study, we used covariate analysis and PSM method to control the influence of psychotic symptoms on cognitive function, and found that the cognitive function of FES was still lower than that of CSS, which supports the present viewpoint that cognitive impairment in patients with schizophrenia is one of the five-dimensional symptoms independent of positive and negative symptoms. One possible explaination for the difference in neurocognitive function between FES and CCS in our study is that the marked decline in cognitive function in FES may be due to the short burst of pro-inflammatory cytokines and the reduction of anti-inflammatory cytokines which cause the damage of nerve synapses and the damage of the blood-brain barrier, suggesting that cognitive impairment is significantly related to elevated CRP and reduced BDNF levels in schizophrenia (Bora, 2019; Hori et al., 2017; Jacomb et al., 2018). Some studies believe that schizophrenia is a severe mental illness with chronic low-grade inflammation, are correlated with elevated levels of CRP, pro-inflammatory cytokines (IL-6, IL-1beta and TNFalpha) and anti-inflammatory factors (TGF beta, IL-10, sIL-2, IL-1RA)(Dubois et al., 2018). Prompt and effective treatment can partially ameliorate the inflammatory process and improve the cognitive function. We also noted that the average age of CSS in our study was much younger than that of McCleery's study (McCleery et al., 2014). Therefore, other factors such as duration of the illness (Drake et al., 2020), consistency of treatment, life style including nutrition, exercise, comorbid physical illness and substance abuse may play a significant role in neurocognitive function decline. In view of the possible instability of the FES diagnosis, the possibility that some samples reporting more preserved cognition in FEP compared to chronic schizophrenia may include patients who will later receive another diagnosis in the psychosis spectrum maybe another explanation.
Social cognition includes the various domains of theory of mind (ToM), emotional processing (EP), social perception (SP) and attributional styles (AS) (Mehta et al., 2014; Penn et al., 1997; Penn et al., 2008). The Mayer-Salovey-Caruso Emotional Intelligence test (MSCEIT) in MCCB reflects the individual EP domain of social cognition. Previous studies (Kohler et al., 2010; Penn et al., 1997; Penn et al., 2008) showed that patients with schizophrenia, both during the symptomatic phase and in remission, exhibited higher deficits in EP, suggesting that EP deficits may be trait markers. However, there are still doubts about the social cognitive performance of patients with schizophrenia at different stages. One study (Kucharska-Pietura et al., 2005) reported higher level of impairment in chronic patients with schizophrenia than patients in the early stage of schizophrenia and healthy controls. Other studies (Green et al., 2012; Horan et al., 2012) reported comparable levels of social cognitive impairment for both early stage and chronic patients, and revealed social cognitive deficits were stable across the phases of illness. Using covariate analysis to control the influence of psychotic symptoms, education, gender and age on emotional intelligence performance, we still found that the emotional intelligence score of FES was significantly lower than that of CSS and HC, but no significant difference was found between CSS and HC. After PSM, patients with CSS still do better in performing emotional intelligence test compare with FES. This suggests that patients with schizophrenia during the symptomatic phase may have more severe impairment of social cognitive performance, while the social cognitive function of chronic stable patients with schizophrenia was significantly improved and even close to the level of social cognitive performance of healthy controls. Therefore, the divergent results between our study and previous studies may be partly explained by the differences in race, social culture, study design and illness duration. Our subjects are young and middle-aged, all of whom are Han ethnicity, living in the south of China. A recent longitudinal study (Valaparla et al., 2017) reported similar findings to ours that patients with schizophrenia in India during the symptomatic phase showed higher levels of social cognitive deficits, but showed lower levels of social cognitive deficits during the clinical remission phase. Our study suggests that the social cognitive performance of patients with FES is expected to close to the level of healthy people after active treatment. Therefore, the first episode is the critical period for the treatment of schizophrenia.
Both linear correlation and multiple stepwise regression analysis showed that the severity of psychopathological symptoms (BPRS score) had significant effects on the emotion intelligence score of patients with schizophrenia, and showed that clinical psychopathological symptoms were closely inversely related to patients' social cognitive performances. Our result is consistent with most of reports (Kanchanatawan et al., 2018; Man et al., 2018; Trampush et al., 2015). Disorganization is one of the main clinical psychopathological features of schizophrenia, and it seems to have the most significant impact on social cognitive performance. Previous studies found a link between the dimension “disorganization” and failure in various neuropsychological tests (Johnson et al., 2009; Klingberg et al., 2006). Minor and Lysaker (Minor and Lysaker, 2014) reported that disorganization seems to show a greater inverse association with cognitive processes. Social cognition has repeatedly been shown to be compromised in most patients with schizophrenia (Lee et al., 2004), is crucially linked to social behavioral competence, and is the most significant predictor of severe social behavioral abnormalities regardless of illness phase (Brune, 2005). The association between disorganization in patients with schizophrenia and social cognitive impairment has been highlighted in numerous studies (Abdel-Hamid et al., 2009; Urbach et al., 2013).
Our study showed that gender had significant, independent effect on emotion intelligence scores. Cabello et al. (Cabello et al., 2016) found that gender significantly affected the emotional management ability of healthy adults. Evidence from cross sectional studies suggests that, compared to females, males have poorer emotional processing abilities (Navarra-Ventura et al., 2018). Megias et al. (Megias et al., 2018) reported that women with schizophrenia had better emotional management and less aggressive behavior than men. However, Zheng et al. (Zheng et al., 2015) reported that the emotion management ability of stable male patients with schizophrenia was significantly better than that of female patients with schizophrenia. Whatever the controversy, both our study and existing researches support that gender has an impact on social cognitive function. In addition, Our study found that age and patients with schizophrenia' cognitive function are correlated, which is consistent with the majorities of studies reports (Cabello et al., 2016; Husa et al., 2017). With the increase of age, people's social cognition show upward trend.
5. Limitations
There are several limitations in this study: Current study is cross sectional, so the findings could not provide a causal association. Because of in acute episode at the stage of enrollment, the fact that possible problems in reliability of the assessment in FES may weak the strength of our conclusion should be considered. Our study has a relatively small sample size in a population with relatively homogenous cultural background. Therefore, a large sample size and perspective study conducted in various cultural population will be necessary to verify our findings.
6. Conclusion
Although this is a cross-sectional study, we firstly used MCCB to systematically assess and compare the difference of neurocognitive and social cognitive performance between east Asia patients with schizophrenia at different stage and healthy controls. What we found may provide some clinical implications. (1) The result suggests that the first episode should be the critical period for the treatment of schizophrenia. So, in clinical practice, we need to pay attention to the impact of pychopathological symptoms on social cognitive and general cognitive performance in patients with schizophrenia. The rapid control of psychotic symptoms is beneficial to the improvement of general cognitive and social cognitive functions. (2) To the first-episode patients with schizophrenia, improving social cognitive function is that we need to pay attention to gender and age.
CRediT authorship contribution statement
Shengyun Chen: Methodology, Formal analysis, Data curation,Writing - original draft. Yaxi Liu: Methodology, Formal analysis. Dennis Liu: Data curation, Writing - review & editing. Guican Zhang:Formal analysis, Data curation. Xiaoli Wu:Methodology, Formal analysis, Data curation, Writing - review & editing.
Declaration of competing interest
There is no conflict of interest in relation to this paper.
Acknowledgments
Acknowledgement
This study was funded by the National Key Research and Development Program of China (2016YFC1306900) and the Science and Technology Program of Guangdong Province (2016A020215075).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scog.2021.100195.
Appendix A. Supplementary data
Stepwise multiple regression analysis were performed to screen independent affecting factors of cognitive function.
References
- Aas M., Dazzan P., Mondelli V., Melle I., Murray R.M., Pariante C.M. A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Frontiers in Psychiatry. 2014;4(182) doi: 10.3389/fpsyt.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Hamid M., Lehmkamper C., Sonntag C., Juckel G., Daum I., Brune M. Theory of mind in schizophrenia: the role of clinical symptomatology and neurocognition in understanding other people’s thoughts and intentions. Psychiatry Res. 2009;165(1–2):19–26. doi: 10.1016/j.psychres.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Bechi M., Agostoni G., Buonocore M., Bosinelli F., Spangaro M., Bianchi L. The influence of premorbid adjustment and autistic traits on social cognitive dysfunction in schizophrenia. J. Int. Neuropsychol. Soc. 2020;26(3):276–285. doi: 10.1017/S1355617719000961. [DOI] [PubMed] [Google Scholar]
- Bell M.D., Fiszdon J.M., Greig T.C., Wexler B.E. Social attribution test–multiple choice (SAT-MC) in schizophrenia: comparison with community sample and relationship to neurocognitive, social cognitive and symptom measures. Schizophr. Res. 2010;122(1–3):164–171. doi: 10.1016/j.schres.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E. Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: a meta-analysis. Psychol. Med. 2019;49(12):1971–1979. doi: 10.1017/S0033291719001685. [DOI] [PubMed] [Google Scholar]
- Bozikas V.P., Andreou C. Longitudinal studies of cognition in first episode psychosis: a systematic review of the literature. Aust N Z J Psychiatry. 2011;45(2):93–108. doi: 10.3109/00048674.2010.541418. [DOI] [PubMed] [Google Scholar]
- Bromley, E., Brekke, J.S. 2010. Assessing function and functional outcome in schizophrenia. Curr Top Behav Neurosci. 4(3–21. [DOI] [PubMed]
- Brown E.C., Tas C., Can H., Esen-Danaci A., Brune M. A closer look at the relationship between the subdomains of social functioning, social cognition and symptomatology in clinically stable patients with schizophrenia. Compr. Psychiatry. 2014;55(1):25–32. doi: 10.1016/j.comppsych.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Brune, M. 2005. Emotion recognition, ‘theory of mind’, and social behavior in schizophrenia. Psychiatry Res. 133(2–3), 135–147. [DOI] [PubMed]
- Cabello R., Sorrel M.A., Fernandez-Pinto I., Extremera N., Fernandez-Berrocal P. Age and gender differences in ability emotional intelligence in adults: a cross-sectional study. Dev. Psychol. 2016;52(9):1486–1492. doi: 10.1037/dev0000191. [DOI] [PubMed] [Google Scholar]
- Conley R.R., Boggs D.L., Kelly D.L., McMahon R.P., Dickinson D., Feldman S. The effects of galantamine on psychopathology in chronic stable schizophrenia. Clin. Neuropharmacol. 2009;32(2):69–74. doi: 10.1097/WNF.0B013E31816F2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture, S.M., Penn, D.L., Roberts, D.L. 2006. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 32 Suppl 1(S44–S63. [DOI] [PMC free article] [PubMed]
- Drake R.J., Husain N., Marshall M., Lewis S.W., Tomenson B., Chaudhry I.B. Effect of delaying treatment of first-episode psychosis on symptoms and social outcomes: a longitudinal analysis and modelling study. Lancet Psychiatry. 2020;7(7):602–610. doi: 10.1016/S2215-0366(20)30147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois T., Reynaert C., Jacques D., Lepiece B., Patigny P., Zdanowicz N. Immunity and psychiatric disorders: variabilities of immunity biomarkers are they specific? Psychiatr. Danub. 2018;30(Suppl. 7):447–451. [PubMed] [Google Scholar]
- Evans J.D., Bond G.R., Meyer P.S., Kim H.W., Lysaker P.H., Gibson P.J. Cognitive and clinical predictors of success in vocational rehabilitation in schizophrenia. Schizophr. Res. 2004;70(2–3):331–342. doi: 10.1016/j.schres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Fett A.K., Viechtbauer W., Dominguez M.D., Penn D.L., van Os J., Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci. Biobehav. Rev. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fiszdon J.M., Fanning J.R., Johannesen J.K., Bell M.D. Social cognitive deficits in schizophrenia and their relationship to clinical and functional status. Psychiatry Res. 2013;205(1–2):25–29. doi: 10.1016/j.psychres.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F., Kern R.S., Braff D.L., Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr. Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green M.F., Bearden C.E., Cannon T.D., Fiske A.P., Hellemann G.S., Horan W.P. Social cognition in schizophrenia, part 1: performance across phase of illness. Schizophr. Bull. 2012;38(4):854–864. doi: 10.1093/schbul/sbq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Zhai J., Wei Q., Twamley E.W., Jin H., Fang M. Neurocognitive effects of first- and second-generation antipsychotic drugs in early-stage schizophrenia: a naturalistic 12-month follow-up study. Neurosci. Lett. 2011;503(2):141–146. doi: 10.1016/j.neulet.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Harvey P.D., Rosenthal J.B. Cognitive and functional deficits in people with schizophrenia: evidence for accelerated or exaggerated aging? Schizophr. Res. 2017;196:14–21. doi: 10.1016/j.schres.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Horan W.P., Green M.F., DeGroot M., Fiske A., Hellemann G., Kee K. Social cognition in schizophrenia, part 2: 12-month stability and prediction of functional outcome in first-episode patients. Schizophr. Bull. 2012;38(4):865–872. doi: 10.1093/schbul/sbr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Yoshimura R., Katsuki A., Atake K., Igata R., Konishi Y. Relationships between serum brain-derived neurotrophic factor, plasma catecholamine metabolites, cytokines, cognitive function and clinical symptoms in Japanese patients with chronic schizophrenia treated with atypical antipsychotic monotherapy. World J Biol Psychiatry. 2017;18(5):401–408. doi: 10.1080/15622975.2016.1212172. [DOI] [PubMed] [Google Scholar]
- Husa, A.P., Moilanen, J., Murray, G.K., Marttila, R., Haapea, M., Rannikko, I., et al. 2017. Lifetime antipsychotic medication and cognitive performance in schizophrenia at age 43 years in a general population birth cohort. Psychiatry Res. 247(130–138. [DOI] [PMC free article] [PubMed]
- Jacomb, I., Stanton, C., Vasudevan, R., Powell, H., O'Donnell, M., Lenroot, R., et al. 2018. C-reactive protein: higher during acute psychotic episodes and related to cortical thickness in schizophrenia and healthy controls. Frontiers in Immunology. 9(2230. [DOI] [PMC free article] [PubMed]
- Johnson I., Ben A.O., Kebir O., Dellagi L., Amado I., Tabbane K. Evaluation of correlations between cognitive performances and clinical dimensions of schizophrenia. Tunis Med. 2009;87(10):664–669. [PubMed] [Google Scholar]
- Kanchanatawan, B., Thika, S., Anderson, G., Galecki, P., Maes, M. 2018. Affective symptoms in schizophrenia are strongly associated with neurocognitive deficits indicating disorders in executive functions, visual memory, attention and social cognition. Prog Neuropsychopharmacol Biol Psychiatry. 80(Pt C), 168–176. [DOI] [PubMed]
- Kern R.S., Nuechterlein K.H., Green M.F., Baade L.E., Fenton W.S., Gold J.M. The MATRICS consensus cognitive battery, part 2: co-norming and standardization. Am. J. Psychiatry. 2008;165(2):214–220. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- Klingberg S., Wittorf A., Wiedemann G. Disorganization and cognitive impairment in schizophrenia: independent symptom dimensions? Eur. Arch. Psychiatry Clin. Neurosci. 2006;256(8):532–540. doi: 10.1007/s00406-006-0704-0. [DOI] [PubMed] [Google Scholar]
- Kohler C.G., Walker J.B., Martin E.A., Healey K.M., Moberg P.J. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr. Bull. 2010;36(5):1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharska-Pietura, K., David, A.S., Masiak, M., Phillips, M.L. 2005. Perception of facial and vocal affect by people with schizophrenia in early and late stages of illness. Br J Psychiatry. 187(523–528. [DOI] [PubMed]
- Lecrubier Y., Sheehan D.V., Weiller E., Amorim P., Bonora I., Harnett Sheehan K. The MINI international neuropsychiatric interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. European Psychiatry. 1997;12(5):224–231. [Google Scholar]
- Lee K.H., Farrow T.F., Spence S.A., Woodruff P.W. Social cognition, brain networks and schizophrenia. Psychol. Med. 2004;34(3):391–400. doi: 10.1017/s0033291703001284. [DOI] [PubMed] [Google Scholar]
- Lewandowski K.E., Cohen B.M., Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol. Med. 2011;41(2):225–241. doi: 10.1017/S0033291710001042. [DOI] [PubMed] [Google Scholar]
- Man, L., Lv, X., Du XD, Yin, G., Zhu, X., Zhang, Y., et al. 2018. Cognitive impairments and low BDNF serum levels in first-episode drug-naive patients with schizophrenia. Psychiatry Res. 263(1–6. [DOI] [PubMed]
- McCleery A., Ventura J., Kern R.S., Subotnik K.L., Gretchen-Doorly D., Green M.F. Cognitive functioning in first-episode schizophrenia: MATRICS consensus cognitive battery (MCCB) profile of impairment. Schizophr. Res. 2014;157(1–3):33–39. doi: 10.1016/j.schres.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery A., Lee J., Fiske A.P., Ghermezi L., Hayata J.N., Hellemann G.S. Longitudinal stability of social cognition in schizophrenia: a 5-year follow-up of social perception and emotion processing. Schizophr. Res. 2016;176(2–3):467–472. doi: 10.1016/j.schres.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megias A., Gomez-Leal R., Gutierrez-Cobo M.J., Cabello R., Fernandez-Berrocal P. The relationship between aggression and ability emotional intelligence: the role of negative affect. Psychiatry Res. 2018:270.1074–270.1081. doi: 10.1016/j.psychres.2018.05.027. [DOI] [PubMed] [Google Scholar]
- Mehta U.M., Thirthalli J., Bhagyavathi H.D., Keshav K.J., Subbakrishna D.K., Gangadhar B.N. Similar and contrasting dimensions of social cognition in schizophrenia and healthy subjects. Schizophr. Res. 2014;157(1–3):70–77. doi: 10.1016/j.schres.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Minor K.S., Lysaker P.H. Necessary, but not sufficient: links between neurocognition, social cognition, and metacognition in schizophrenia are moderated by disorganized symptoms. Schizophr. Res. 2014;159(1):198–204. doi: 10.1016/j.schres.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Navarra-Ventura G., Fernandez-Gonzalo S., Turon M., Pousa E., Palao D., Cardoner N. Gender differences in social cognition: a cross-sectional pilot study of recently diagnosed patients with schizophrenia and healthy subjects. Can. J. Psychiatr. 2018;63(8):538–546. doi: 10.1177/0706743717746661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier M.R., Killian S., Chiliza B., Asmal L., Schoeman R., Oosthuizen P.P. Cognitive performance during the first year of treatment in first-episode schizophrenia: a case-control study. Psychol. Med. 2015;45(13):2873–2883. doi: 10.1017/S0033291715000860. [DOI] [PubMed] [Google Scholar]
- Penn D.L., Corrigan P.W., Bentall R.P., Racenstein J.M., Newman L. Social cognition in schizophrenia. Psychol. Bull. 1997;121(1):114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- Penn D.L., Sanna L.J., Roberts D.L. Social cognition in schizophrenia: an overview. Schizophr. Bull. 2008;34(3):408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Kang L., Yao S., Ma Y., Li T., Liang Y. The MATRICS consensus cognitive battery (MCCB): co-norming and standardization in China. Schizophr. Res. 2015;169(1–3):109–115. doi: 10.1016/j.schres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trampush J.W., Lencz T., DeRosse P., John M., Gallego J.A., Petrides G. Relationship of cognition to clinical response in first-episode schizophrenia spectrum disorders. Schizophr. Bull. 2015;41(6):1237–1247. doi: 10.1093/schbul/sbv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach, M., Brunet-Gouet, E., Bazin, N., Hardy-Bayle, M.C., Passerieux, C. 2013. Correlations of theory of mind deficits with clinical patterns and quality of life in schizophrenia. Frontiers in Psychiatry. 4(30. [DOI] [PMC free article] [PubMed]
- Valaparla, V.L., Nehra, R., Mehta, U.M., Thirthalli, J., Grover, S. 2017. Social cognition of patients with schizophrenia across the phases of illness - a longitudinal study. Schizophrenia Research. 190(150–159. [DOI] [PubMed]
- Weinberg D., Lenroot R., Jacomb I., Allen K., Bruggemann J., Wells R. Cognitive subtypes of schizophrenia characterized by differential brain volumetric reductions and cognitive decline. JAMA Psychiatry. 2016;73(12):1251–1259. doi: 10.1001/jamapsychiatry.2016.2925. [DOI] [PubMed] [Google Scholar]
- Wu, J.Q., Chen, D.C., Tan, Y.L., Xiu, M.H., De Yang, F., Soares, J.C., et al. 2016. Cognitive impairments in first-episode drug-naive and chronic medicated schizophrenia: MATRICS consensus cognitive battery in a Chinese Han population. Psychiatry Res. 238(196–202. [DOI] [PubMed]
- Zheng M.J., C S., L K. Emotional management in stable patient with schizophrenia. Chinese Journal of Psychiatry. 2015;48(4):227–231. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stepwise multiple regression analysis were performed to screen independent affecting factors of cognitive function.