Abstract
In December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) became evident in Wuhan, China, and then spread rapidly worldwide. Numerous drugs and vaccines are under clinical trial pipeline for investigation against coronavirus disease 2019 (COVID-19) infection. The aim of this systematic review was to discuss about investigational new as well as repurposed drugs currently under trial for COVID-19 infection. An exhaustive search was carried out for this review article including scientific databases of PubMed, Embase, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, Web of Science, ScienceDirect, ProQuest, Google Scholar, and Scopus search engines using keywords of “Coronavirus,” “COVID-19,” “MERS-CoV,” “MERS,” “SARS-CoV-2,” and “SARS-CoV-1” and “Solidarity trial” and their Persian-equivalent keywords from inception until May 2020. After screening the 296 articles searched from different databases (PubMed = 97 and other search engines = 199), 52 articles were included in the final systematic review. It was found that the World Health Organization introduced a Solidarity international clinical trial to discover an effectual treatment of COVID-19. Based on established in vitro and in vivo activity against different strains of coronaviruses, four repurposed drugs – remdesivir, lopinavir/ ritonavir combination, lopinavir/ritonavir with beta-1a, chloroquine, and hydroxychloroquine – were considered for clinical trial against COVID-19. A number of other drugs and vaccines are under clinical trial pipeline for investigation against COVID-19 infection. Despite multitude of treatment options available, treatment of choice is still not well established. Moreover, optimum supportive care and monitoring of seriously ill patients is the need of the hour.
Keywords: Coronavirus, coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2, Solidarity trial
Introduction
In December 2019, the SARS-CoV-2 virus became evident in Wuhan, China, and then spread rapidly worldwide.[1] Till date, three major epidemics of respiratory distress syndrome have been caused by three strains of coronaviruses (CoVs).[2,3] In 2003, the severe acute respiratory syndrome coronavirus (SARS-CoV) was the first epidemic that was caused by coronavirus with epicenter in Guangdong, China.[4] Middle East respiratory syndrome coronavirus (MERS-CoV) was the second epidemic that emerged in 2012 in Saudi Arabia, and recently, the 2019-novel coronavirus (2019-nCoV) or coronavirus disease 2019 (COVID-19) has occurred as the third epidemic of respiratory coronavirus which was mainly centered in Wuhan province, China.[1] The novel virus was first isolated on January 7, 2020, and named as “2019-nCoV/SARS-CoV-2.” On March 11, 2020, COVID-19 was declared as coronavirus pandemic by the World Health Organization (WHO). Coronavirus-infected patients may remain asymptomatic, or they may experience mild-to-severe clinical symptoms such as pneumonia, respiratory failure, and ultimately death.[1,5] The aim of this review was to discuss about investigational new as well as repurposed drugs currently under trial for COVID-19 infection.
Materials and Methods
A systematic search of literature evaluating treatment options available for coronavirus was performed by the investigators. The databases of PubMed, Embase, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, Web of Science, ScienceDirect, ProQuest, Google Scholar, and Scopus search engines were systematically explored using the search terms “Coronavirus,” “COVID-19,” “MERS-CoV,” “SARS-CoV-2,” and “SARS-CoV-1,” “Prophylaxis,” and “Preventive” and “Solidarity trial” from inception until May 2020. Various alterations in spelling and abbreviations were applied due to the pronounced heterogeneity of this research field.
The titles and the abstracts of the relevant studies in English were procured using the search strategy, and they were independently screened by 3 authors, who eventually recaptured abstracts and the full text of articles to clinch the propriety. Dissent perseverance was executed by the fourth author. As the current pandemic is an extant public health dilemma, the systematic review protocol could not be preregistered. No presumptions or elucidation were contrived during the course of review.
Inclusion criteria were the insertion of the searched keywords in the title section and keywords of the articles. Studies in English only that possessed the information of investigational drugs for the treatment of COVID-19 infection (lopinavir/ritonavir, darunavir, ribavirin, remdesivir, favipiravir, oseltamivir, interferon-alpha [IFN-α], Arbidol, chloroquine phosphate, hydroxychloroquine, camostat mesylate, tocilizumab, sarilizumab, gimsilumab, azithromycin, baricitinib, ruxolitinib, tofacitinib, convalescent plasma [CP], and anticoagulation) were included. This included randomized controlled trials (RCTs) and observational studies (including cohort and control studies) along with the case reports (lopinavir/ritonavir, darunavir, ribavirin, remdesivir, favipiravir, oseltamivir, IFN-α, Arbidol, chloroquine phosphate, hydroxychloroquine, camostat mesylate, tocilizumab, sarilizumab, gimsilumab, azithromycin, baricitinib, ruxolitinib, tofacitinib, CP, and anticoagulation).
We excluded studies in other languages other than English and when no translation was available. Duplicate studies were also excluded from the search. The literature search was in accordance with PRISMA guidelines.
Results and Discussion
After preliminary screening of the databases, we could find 296 articles (PubMed = 97 and other search engines = 199). Further screening of title and abstract resulted in the exclusion of 120 duplicate articles. Full-text screening of the remaining 176 articles was done after the removal of duplicate records. Finally, 52 articles were included in the final systematic review. A flowchart demonstrating the search is presented in Figure 1.
Figure 1.
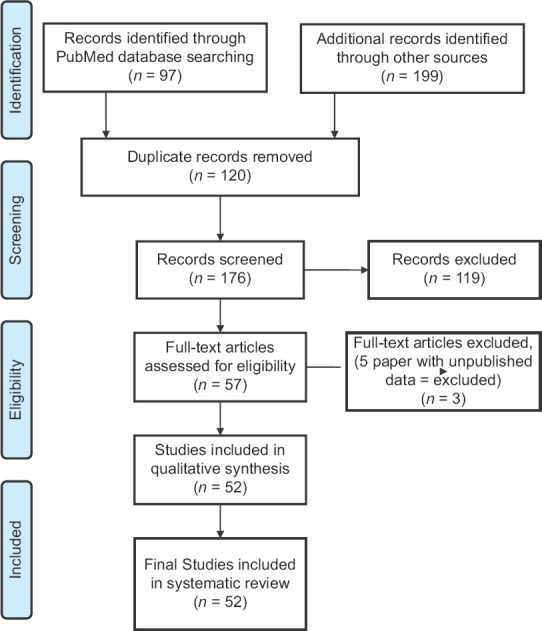
Result of systematic review search
Benefits in reducing mortality and viral load and time to clinical recovery were the primary outcome measures. The time required for negative seroconversion, length of hospital stay, overall survival, and adverse events were secondary outcome measures that were looked for while searching for investigational drugs.
It was found that the WHO introduced a Solidarity international clinical trial to discover an effectual treatment of COVID-19.
“Solidarity” clinical trial
The WHO introduced a Solidarity international clinical trial to discover an effectual treatment of COVID-19. Extensive process of clinical trial will be curbed by 80% with the help of Solidarity trial. Almost 100 countries have participated in Solidarity trial by April 21, 2020. Based on established in vitro and in vivo activity against different strains of CoVs, four repurposed drugs – remdesivir, lopinavir/ritonavir combination, lopinavir/ritonavir with beta-1a, chloroquine, and hydroxychloroquine – were considered for clinical trial against COVID-19. Anyone of the above drugs is tested against local standard care of treatment.[6]
Considerable novel and repurposed pharmacotherapeutic agents against SARS-CoV-2 are under trial, but still, the specific drug treatment is the need of the hour.[7]
Drugs that are reported to be investigated for COVID-19 infection till date are depicted in Table 1.[7,8,9]
Table 1.
Depicting investigational pharmacotherapeutic agents for treatment of COVID-19 infection
| Pharmacological class of drug | Name of drug | Mechanism of action |
|---|---|---|
| Antiviral drugs | Lopinavir/ritonavir | Inhibits 3-chymotrypsin-like protease |
| Darunavir | Inhibits 3-chymotrypsin-like protease | |
| Ribavirin | RNA dependent | |
| RNA polymerase | ||
| Remdesivir | RNA dependent | |
| RNA polymerase | ||
| Favipiravir | RNA dependent | |
| RNA polymerase | ||
| Neuraminidase inhibitor | Oseltamivir | Inhibits influenza A and B virus neuraminidase |
| Interferons | IFN-α | Interferes with viral replication |
| Broad-spectrum antiviral | Arbidol | Targets S protein/ACE2 interaction |
| Inhibits membrane fusion of the viral envelope | ||
| Antimalarial drug | Chloroquine phosphate | Inhibition of viral release into intracellular space, upregulate cell surface ACE-2, reduce glycosylation of ACE-2 |
| Hydroxychloroquine | ||
| Serine protease inhibitor | Camostat mesylate | Type 2 transmembrane serine protease |
| IL-6 receptor antagonist | Tocilizumab | Binds IL-6 receptor |
| Prevents IL-6 receptor activation | ||
| Inhibits IL-6 signaling | ||
| Sarilizumab | Binds IL-6 receptor | |
| Prevents IL-6 receptor activation | ||
| Inhibits IL-6 signaling | ||
| GM- CSF inhibitor | Gimsilumab | Acts against GM-CSF |
RNA=Ribonucleic acid, ACE=Angiotensin-converting enzyme 2, GM-CSF=Granulocyte-macrophage colony-stimulating factor
Repurposed drugs investigated under Solidarity trial
Remdesivir
Remdesivir is a broad-spectrum antiviral investigational drug.[10] Remdesivir is an adenosine analog and has been emerged as a promising antiviral drug against SARS/MERS-CoV. It gets incorporated into nascent viral RNA chains and causes its premature termination. Remdesivir was found to inhibit virus infection efficiently in a human cell line (human liver cancer Huh-7 cells), which is sensitive to 2019-nCoV.[11]
In in vitro studies, it has shown inhibition of replication of both SARS-CoV and MERS-CoV.[12,13]
However, when remdesivir was combined with IFN-β, the results shown were superior to lopinavir, ritonavir, and IFN-β both in vitro and in a MERS-CoV mouse model.[14]
Remdesivir is a nucleotide analog that acts by inhibiting RNA polymerase enzyme. In a study on compassionate use of remdesivir among patients with COVID-19, the drug was administered in a dose of 200 mg intravenously on day 1, followed by 100 mg daily for the remaining 9 days of treatment. The study reported clinical improvement in 36 of 53 patients (68%).[15]
Recently, remdesivir has been approved for emergency use in COVID-19 infection.[16]
Lopinavir with ritonavir booster
Lopinavir is an antiretroviral drug which acts by inhibiting protease enzyme. It is used with ritonavir which is also a cytochrome enzyme inhibitor that increases the half-life of ritonavir.[17]
It was documented in a randomized open-labeled trial that a combination of lopinavir with ritonavir booster treatment added to standard supportive care among the SARS CoV-2-infected patients, but it was not associated with clinical improvement or mortality in seriously ill patients with COVID-19 different from that associated with standard care alone.[18,19]
Interferons
It has been noted in the previously published literature that IFN-I treatment has activity against MERS-CoV and SARS-CoV.[20] It has been investigated in numerous experiments both in vitro and in vivo.[14]
The IFNβ subtype appears to be the most suited for early stages of COVID-19 treatment.[21]
Chloroquine and hydroxychloroquine
Chloroquine, which is used as an antimalarial drug, has recently been documented as a potential broad-spectrum antiviral drug. It increases viral endosomal pH and also interferes with the glycosylation of cellular receptors of SARS-CoV.
In Vero E6 cells, chloroquine was found to have action at both entry and at postentry stages of the 2019-nCoV infection.[22,23]
The multimodal action of chloroquine in inhibition of coronavirus is illustrated in Figure 2.
Figure 2.
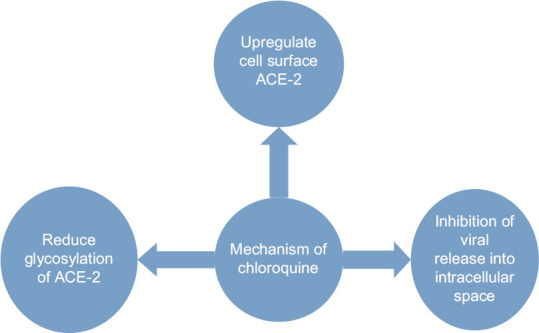
Depicting mechanism of action of chloroquine in coronavirus disease 2019 infection
It has been documented in a study that hydroxychloroquine treatment was significantly associated with reduction/disappearance of viral load in COVID-19 patients. This effect was augmented by the addition of azithromycin. In this study, patients were randomized to receive 600 mg of hydroxychloroquine daily, and their viral load in nasopharyngeal swabs was tested daily in a hospital setting.[24]
Another Chinese study recommended treatment of patients diagnosed as mild, moderate, and severe cases of COVID-19 pneumonia and without contraindications to chloroquine, with 500 mg chloroquine twice a day for 10 days.[25]
Hydroxychloroquine has a better clinical safety profile than that of chloroquine (during long-term use). Thus, it can be tolerated in higher daily dose with less concerns of drug–drug interactions.[26]
Data from in vitro and in vivo studies are limited, with mixed results and confounded with bias. Chloroquine is known to cause prolonged QT syndrome and torsade de pointes. Other drugs causing cardiovascular side effects are shown in Table 2.
Table 2.
Established cardiovascular effects of investigational drugs for COVID-19
| Pharmacologic class of drug | Name of drug | Cardiovascular side effect |
|---|---|---|
| Antimalarial[27,28] | Chloroquine | Prolongation of QT interval, arrhythmias in seriously ill patients, torsade de pointes |
| Antimalarial[27,28] | Hydroxychloroquine | Prolongation of QT interval, arrhythmias in seriously ill patients, torsade de pointes |
| Protease inhibitor[27,28] | Lopinavir; ritonavir | QT prolongation, torsade de pointes |
| Macrolide[27,28] | Azithromycin | QT prolongation, torsade de pointes |
| Janus kinase inhibitors[29,30] | Baricitinib, ruxolitinib, tofacitinib | DVT and PE |
DVT=Deep vein thrombosis, PE=Pulmonary embolism
Other drugs under investigation for COVID-19 infection
Ribavirin
Ribavirin is a guanosine analog and acts against both RNA and DNA viruses. It acts by multiple mechanisms. It interferes with the functioning of polymerase enzyme. It also causes the destabilization of viral RNA and also inhibits inosine monophosphate dehydrogenase and thereby inhibits the formation of guanosine.[31]
It has shown its efficacy in SARS-CoV and MERS-CoV epidemic. Initially, it was given in a dose of 4-g oral loading dose followed by a 1.2-g oral dose every 8 h.[32,33,34] The dose was then modified to 500 mg IV BID or TID.
Low cost of ribavirin and its efficacy to treat COVID infection justify its use in clinical trials.[35]
Favipiravir
Favipiravir has been shown its efficacy in the treatment of influenza, and Ebola virus is basically a prodrug and acts by inhibiting RNA-dependent RNA polymerase inhibitor.[36,37]
Favipiravir has shown its antiviral efficacy against SARS infection in in vitro studies.[1,36]
In an open-labeled trial, favipiravir was given orally in a dose of 1600mg twice daily on day 1 and 600mg twice daily on days 2–14. It was compared against lopinavir/ritonavir, while IFN-α was administered to all participants. A shorter viral clearance time was found with significant improvement in chest imaging which was observed. Favipiravir showed better therapeutic responses on COVID-19 in terms of disease progression and viral clearance.[38]
Monoclonal antibody Tocilizumab
Tocilizumab is a humanized monoclonal antibody which acts against the interleukin-6 receptor. It was suggested in recent literature that IL-6 is one of the most crucial cytokines which was documented to be involved in COVID-19-induced cytokine storms. Thus, tocilizumab emerged as one of the treatment strategies among COVID-19-infected patients. Luo P et al. implied the effectiveness of tocilizumab treatment in COVID-19 patients with a risk of cytokine storms and emphasized that the dose of tocilizumab can be repeated among critically ill patients with elevated IL-6.[39,40]
Gimsilumab
Gimsilumab is a fully human monoclonal antibody. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is documented to be involved in hyperinflammation of the lung and increase in pro-inflammatory cytokines and chemokines. Gimsilumab is thought to act on these GM-CSFs.[41]
Recombinant human angiotensin-converting enzyme 2
It was confirmed in an in vitro study that angiotensin-converting enzyme 2 (ACE2) expression corroborates with susceptibility to SARS-CoV infection. Hence, it was assumed that higher ACE2 expression might also lead to a higher risk of SARS-CoV-2 infection.[42]
In COVID-19 patients, coronary artery disease and hypertension are considered as remarkable mortality.[43]
Angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) in addition to their main pharmacological effect to inhibit ACE1 or block angiotensin II type 1 receptor, they are able to upregulate ACE2 expression and render the patient susceptible to the infection of SARS-CoV-2.
Therefore, it was suggested that ACEIs and ARBs should be avoided in COVID infection.[44] Moreover, it has been documented in recent findings that serum level of angiotensin II is significantly elevated in COVID-19 patients and it shows a linear positive correlation to viral load and lung injury.[45]
Thus, there are mixed recommendations for ACEI and ARB, whether to continue or discontinue. Despite these, it has been suggested not to discontinue these drugs to prevent potential lung and heart injury, although exogenous supplement of rhACE2 may be a potential way to prevent and treat COVID-19.
Potential nCoV-RBD-hACE2 interaction-blocking peptides were designed that may restrict viral attachment and entry. Experimental validation is needed for these peptides need to test their therapeutic efficacy.[46]
Azithromycin
Viral load reduction in COVID-19 patients was found to be significantly associated with hydroxychloroquine treatment which was further reinforced by the addition of azithromycin. However, its use along with chloroquine might result in prolonged QT syndrome.[24]
Convalescent plasma
CP or immune plasma has materialized as one-fourth buoyant treatments for COVID-19 infection. Plasma that is collected from an infected individual which is then transfused into infected patients as a postexposure prophylaxis is termed as CP.[47,48]
Antibodies that are derived from CP are able to neutralize a virus by inhibition of its replication.[49]
The use of CP therapy among patients infected with COVID-19 was approved by FDA on March 24, 2020.[50]
Corticosteroids and nonsteroidal anti-inflammatory drugs
Regarding the use of corticosteroids and nonsteroidal anti-inflammatory drugs, mixed results have been documented as per the paramount knowledge of researchers. There is a dire need to exercise caution regarding the use of corticosteroids in COVID-19 patients until emergence of further evidence.[51]
Anticoagulation
It is becoming evident from the recent studies that disseminated intravascular coagulation can complicate severe coronavirus disease and these patients are at high risk of venous thromboembolism. D-dimer might prove to significantly important in predicting outcome among these patients.[52]
Expert consensus has recommended the application of heparin in seriously ill COVID-19 patients because they are at a high risk of disseminated intravascular coagulation and venous thromboembolism. Tang N et al. emphasized the association of low-molecular-weight heparin with better prognosis in seriously ill COVID-19 patients meeting sepsis-induced coagulopathy criteria or with strikingly upraised D-dimer.[53,54]
The measurement of D-dimer and fibrinogen levels in screening of coagulation tests is suggested.[55]
Other drugs under clinical trial pipeline for investigation against COVID-19 infection are galidesivir, leronlimab, brilacidin, neuraminidase inhibitors, oseltamivir, umifenovir, sarilumab, Vitamin C, darunavir, IL-1 receptor antagonist (anakinra and emapalumab), Arbidol, and camostat mesylate.[56] Various vaccines including BCG vaccination are under pipeline.[57]
According to statistics by the Ministry of Health and Family Welfare, SARS-CoV-2 virus-induced COVID-19 has, to date (April 30, 2020), 23651 active cases, 8324 cured/discharged patients, and 1074 deaths.[58]
To standstill this expansion in the number of cases of this unprecedented even of coronavirus infection in India, a national lockdown was implemented on March 22, 2020. However, till date, there is no cure for this unrivaled incidence.
For asymptomatic corona warriors treating patients with suspected or confirmed COVID-19, and for asymptomatic household contacts of confirmed cases, chemoprophylaxis has been recommended with hydroxychloroquine (400 mg twice on day 1 and then 400 mg once a week thereafter) by the Indian Council of Medical Research under the Ministry of Health and Family Welfare. However, the evaluation of individual risk–benefit ratio should be warranted.[59]
Strength of the study
A comprehensive and reproducible search strategy was applied to review the literature, and the available incidence regarding investigational drugs for COVID-19 infection was compiled in this article. In spite of all the limitations, our study is the first study to describe about Solidarity trial in detail.
To conclude, it is very demanding to establish the safety and efficacy of these investigational pharmacotherapeutic agents in the management of COVID-19 due to the dearth of evidence. Nonetheless, the gold standard drug for its treatment should be assured after proper understanding of risk–benefit ratio.
However, many potential frontiers in the treatment of corona disease have been elaborated in our study, and the establishment of their efficacy by large-scale RCTs is the need of the hour.
Limitations
This review article is an overview of various relevant published data in this area of practice. Despite thorough review of drugs, May 2020, many continuously evolving newer drugs under trial may be missed as their data might not be published online.
Conclusion
Corona pandemic has brought a huge challenge to health-care system. Testing a new drug is itself a giant challenge in such a state of dire emergency. In spite of multitude treatment options available, there is still a dire need to search an ideal drug molecule with lesser side effects, which may improve the patient compliance. Moreover, optimum supportive care and monitoring of seriously ill patients is the need of the hour. The safe and effective management skills of health-care professionals in this unprecedented time of coronavirus infection are highly appreciated. Nonetheless, long-term data of RCTs of their investigational drugs should validate their efficacy and safety in the management of COVID-19 infection. The development of impressive vaccines to discontinue the spread of SARS-CoV-2 is the need of the hour.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 2.Sarma P, Prajapat M, Avti P, Kaur H, Kumar S, Medhi B. Therapeutic options for the treatment of 2019-novel coronavirus: An evidence-based approach. Indian J Pharmacol. 2020;52:1–5. doi: 10.4103/ijp.IJP_119_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham RL, Donaldson EF, Baric RS. A decade after SARS: Strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11:836–48. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. SARS (Severe Acute Respiratory Syndrome) World Health Organization; [Last accessed on 2020 Apr 27]. Available from: https://www.who.int/ith/diseases/sars/en/ [Google Scholar]
- 5.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization.Org. Solidarity Clinical Trial for COVID-19 Treatments. [Last accessed on 2020 Apr 30]. Available from: http://www.who.int.diseases.novel-coronavirus-2019.sol .
- 7.Borges do Nascimento IJ, Cacic N, Abdulazeem HM, von Groote TC, Jayarajah U, Weerasekara I, et al. Novel coronavirus infection (COVID-19) in humans: A scoping review and meta-analysis. J Clin Med. 2020;9:941. doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: The mystery and the miracle. J Med Virol. 2020;92:401–2. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prajapat M, Sarma P, Shekhar N, Avti P, Sinha S, Kaur H, et al. Drug targets for corona virus: A systematic review. Indian J Pharmacol. 2020;52:56–65. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis. 2020;34:101615. doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agostini ML, Andres EL, Sims AC, et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018;9(2):e00221–18. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396):eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382:2327–36. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDA. Remdesivir EUA Letter of Authorization FDA. [Last accessed on 2020 May 04]. Available from: http://www.fda.gov.media.download .
- 17.Vie AB. Kaletra Prescribing Information. 2020. [Last accessed on 2020 Apr 28]. Available from: www.rxabbvie.com/pdf/kaletratabpi.pdf .
- 18.Cao B, Wang Y, Wen D, Liu W, et al. A trial of Lopinavir-ritonavir in adults hospitalized with severe Covid-19. New Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stower H. Lopinavir-ritonavir in severe COVID-19. Nat Med. 2020;26:465. doi: 10.1038/s41591-020-0849-9. [DOI] [PubMed] [Google Scholar]
- 20.Stockman LJ, Bellamy R, Garner P. SARS: Systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallarda E, Lescureb FX, Yazdanpanahb Y. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178:1047912. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–9. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Multicenter collaboration group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:185–8. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Credible Meds. COVID-19 Experimental Therapies and TdP Risk. [Last retrieved on 2020 Mar 24]. Available from: https://crediblemeds.org/blog/covid-19-experimental-therapies-and-tdprisk/
- 28.Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for Drug Interactions on QTc in Exploratory COVID-19 Treatment. Circulation. 2020;141(24):e906–e907. doi: 10.1161/CIRCULATIONAHA.120.047521. [DOI] [PubMed] [Google Scholar]
- 29.Olumiant (baricitinib) Tablets Package Insert. Indianapolis, IN: Lilly USA, LLC; 2019. [Google Scholar]
- 30.Xeljanz (tofacitinib) Package Insert. New York, NY: Pfizer, Inc; 2019. [Google Scholar]
- 31.Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falzarano D, de Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel beta coronavirus replication by a combination of interferon-alpha2b and ribavirin. Sci Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peiris J, Chu C, Cheng V, Chan K, Hung I, Poon L, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet. 2003;361:1767–72. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee N, Hui D, Wu A, Chan P, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–94. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 35.Khalili JS, Zhu H, Mak NSA, Yan Y, Zhu Y. Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. J Med Virol. 2020;92:740–6. doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela CS. Günther successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res. 2014;105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Madelain V, Oestereich L, Graw F, Nguyen TH, De Lamballerie X, Mentré F, et al. Ebola virus dynamics in mice treated with favipiravir. Antiviral Res. 2015;123:70–7. doi: 10.1016/j.antiviral.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Cai Q, Yang M, Liu D, et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study [published online ahead of print, 2020 Mar 18] Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.03.007. 10.1016/j.eng. 2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roivant starts gimsilumab dosing in Covid-19 trial. [Last accessed on 2020 May 02]. Available from http://www.clinicaltrialsarena.com.news.roivantgimsilum .
- 42.Hofmann H, Geier M, Marzi A, Krumbiegel M, Peipp M, Fey GH, et al. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem Biophys Res Commun. 2004;319:1216–21. doi: 10.1016/j.bbrc.2004.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lia Y, Cheng X, Zeng Q, Chen Z, Wang Z, Yuan J, et al. Expert recommendations for management and treatment of cardiovascular diseases under the epidemic situation of novel coronavirus pneumonia in Hubei province. J Clin Cardiol. 2020;36:201–3. [Google Scholar]
- 45.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–74. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barh D, Tiwari S, Silva Andrade B, et al. Potential chimeric peptides to block the SARS-CoV-2 spike receptor-binding domain.F1000Res. 2020;9:576. doi: 10.12688/f1000research.24074.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teixeira da Silva JA. Convalescent plasma: A possible treatment of COVID-19 in India. Med J Armed Forces India. 2020;76(2):236–237. doi: 10.1016/j.mjafi.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bloch E.M, Shoham S, Casadevall A. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020 doi: 10.1172/JCI138745. doi: 10.1172/JCI138745. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Erp EA, Luytjes W, Ferwerda G, van Kasteren PB. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol. 2019;10:548. doi: 10.3389/fimmu.2019.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanne JH. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020;368:m1256. doi: 10.1136/bmj.m1256. [DOI] [PubMed] [Google Scholar]
- 51.Russell B, Moss C, Rigg A, Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: Should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14:1023. doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189(5):846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–9. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2 [published online ahead of print, 2020 Apr 3] J Thromb Thrombolysis. 2020:1–4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 57.WHO. Bacille Calmette-Guérin. Vaccination and COVID-19. [Last assessed on 2020 May 02]. Available from: https://www.who.int/news-room/commentaries/detail/bacilli-calmette-gu%C3%A9rin-(bcg)-vaccination-and-covid-19 .
- 58.Ministry of Health and Family Welfare. Government of India. COVID-19 INDIA. [Last assessed 2020 May 03; Last accessed on 2020 Apr 30]. Available from: https://www.mohfw.gov.in/
- 59.National Taskforce for COVID-19. Advisory on the use of Hydroxy-Chloroquine as Prophylaxis for SARS-CoV-2 Infection. [Last accessed on 2020 May 02]. Available from: https://www.mohfw.gov.in/pdf/Advisoryontheuseof HydroxychloroquinasprophylaxisforSARSCoV2infection.pdf .


