Abstract
Purpose
To report clinical outcomes of a patient with unilateral neurotrophic keratitis following penetrating keratoplasty for lattice dystrophy treated with topical recombinant human nerve growth factor.
Observations
A 75-year-old male with lattice dystrophy and history of herpes simplex keratitis, presented with recurrent neurotrophic ulceration in the right eye two years following penetrating keratoplasty. The patient was successfully treated with topical recombinant human nerve growth factor.
Conclusion
Neurotrophic keratitis is a rare chronic disorder that affects quality of life due to the risk of vision loss. Topical recombinant human nerve growth factor is a novel and effective treatment option that may help improve optical quality and patient's satisfaction as shown in this case of recurrent neurotrophic keratitis.
Keywords: Neurotrophic keratitis, Lattice dystrophy, Growth factor
1. Introduction
Neurotrophic keratitis (NK) is a rare disease caused by impairment of corneal sensory nerves.1,2,3 Corneal nerve damage reduces the integrity of the corneal epithelium and the production of trophic factors to the nerves themselves, ultimately leading to NK.2,4 A decrease in corneal epithelium healing rate is observed, which can result in breakdown of the epithelium, development of corneal ulceration, melting, and perforation.1,3
Several surgical and non-surgical treatment options have been proposed for NK depending on the severity of the disease, including preservative-free artificial tears2, 3, 4 therapeutic contact lenses,2,3 autologous serum tears,2, 3, 4 amniotic membrane2, 3, 4 and tarsorrhaphy.2,4 More recently, Cenegermin 0.002% ophthalmic solution (Oxervate; Dompé Farmaceitici SpA, Milan, Italy), a topical recombinant nerve growth factor, has been introduced as an effective alternative for promoting corneal healing in NK.2,5, 6, 7
The purpose of this case report is to describe the clinical outcome of a patient with unilateral recurrent NK following penetrating keratoplasty (PK) for lattice corneal dystrophy (LCD) treated with topical recombinant human nerve growth factor.
2. Case report
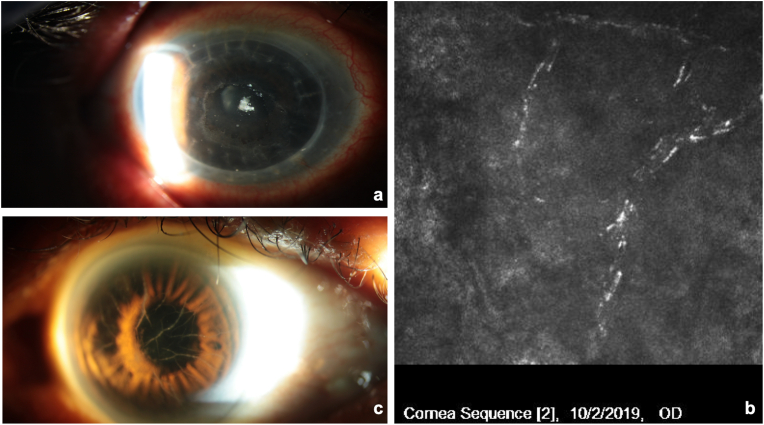
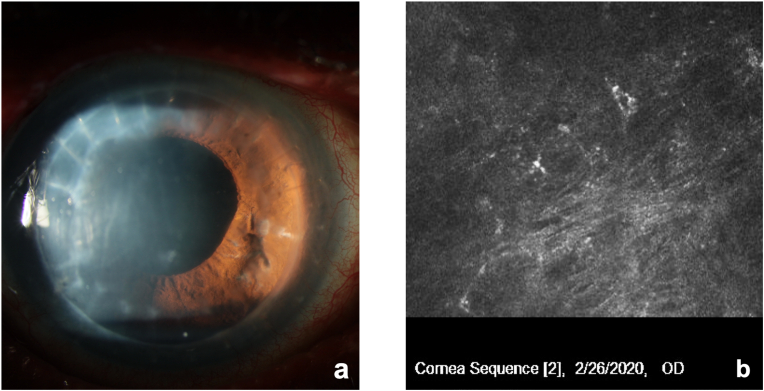
A 75-year-old patient with LCD, chronic open angle glaucoma (COAG) history of herpetic keratitis and two previous penetrating keratoplasty (PK) procedures in the right eye (OD) presented for evaluation of recurrent corneal ulcers in OD two years after the last corneal transplant. The patient had undergone phacoemulsification in that eye and had experienced one episode of herpes simplex dendritic keratitis OD 1 year following PK. At examination, distance correct visual acuity (DCVA) was 20/100 OD with a manifest refraction (MR) of +2.00–1.50 × 57, recurrence of LCD was observed at graft-host junction inferiorly, associated with reduced corneal sensation and neurotrophic ulceration OD. Treatment consisted of preservative-free artificial tears, prophylactic antibiotic (Vigamox® Ophthalmic Solution, Alcon Laboratories, Inc, Fort Worth, USA), dehydrated amniotic membrane (BioDOptix®, Labtician Ophthalmics, Canada) and therapeutic contact lens, and lateral tarsorrhaphy. After several ulceration recurrences, and failed palliative treatments, the patient was started on Cenegermin 0.002% ophthalmic solution (Oxervate; Dompé Farmaceitici SpA, Milan, Italy) 6 times a day for 8 weeks. Before treatment, DCVA was 20/100 and epithelial ulceration measured 4.3mm (V) x 4.2mm (H) (Fig. 1a). Confocal microscopy (Heidelberg Engineering Inc, Heidelberg, Germany) showed reduced density of the subepithelial nerve plexus and hyperreflective deposits consistent with amyloid deposition (Fig. 1b). In the left eye we observed only lattice dystrophy without any associated persistent epithelial defects or reduced corneal sensitivity (Fig. 1c). At 4 weeks, DCVA was stable at 20/100, but epithelial ulceration decreased to 3.6 mm (V) x 3.0mm (H) (Fig. 2). At 8 weeks, DCVA improved to 20/50 with a MR of +0.75–0.50 × 81, and corneal epithelium was completely healed (Fig. 3a). Confocal microscopy showed improved density of the subepithelial nerve plexus (Fig. 3b). The patient remains stable 6 months after treatment.
Fig. 1.
Patient post-penetrating keratoplasty for lattice corneal dystrophy at baseline. a) central neurotrophic ulceration measuring 4.4mm (V) x 4.2mm (H) in the right eye. b) Confocal microscopy of the right eye shows reduced density of the subepithelial nerve plexus and hyperreflective deposits consistent with amyloid deposition. c) slit lamp photo of the left eye illustrates lattice dystrophy without associated corneal erosion.
Fig. 2.
Patient at 4-week treatment with Cenegermin 0.002% We note increased epithelial healing, and neurotrophic ulceration measuring 3.6mm (V) x 3.2mm (H).
Fig. 3.
Patient at 8-week treatment with Cenegermin 0.002%. a) Complete epithelial healing and absence of ulceration. b) Confocal microscopy shows improved density of the subepithelial nerve plexus.
3. Discussion
Neurotrophic keratitis is a rare degenerative disease that affects approximately 5/10,000 patients worldwide.2 Herpetic keratitis, herpes varicella-zoster keratitis and surgical procedures for trigeminal neuralgia are the most common causes of NK.4 We report a case of a patient with LCD and herpetic keratitis who developed NK 2 years following PK.
Several ocular or systemic conditions can lead to NK, including herpetic keratitis, chronic severe blepharitis, Sjogren's syndrome chemical burns, prior eye surgeries, chronic use of topical medications, diabetes mellitus or central nervous system diseases.1,2,4 Our patients had LCD, an entity that affects the corneal stroma and may be associated with recurrent corneal erosions, eventually leading to corneal scarring.8 Penetrating keratoplasty or deep anterior lamellar keratoplasty (DALK) are effective treatment options, but recurrence of the disease has been demonstrated to be around 5% on the first year and as high as 26% at 8 years.8 One year following PK, our patient experienced recurrence of LCD, and, two years after corneal transplantation, stromal deposits, decreased corneal sensation, and recurrent epithelial erosions were observed.
In vivo confocal microscopy (IVCM) is a non-invasive method to assess the sub-basal corneal nerve plexus.9,10 It may also be used to evaluate the response of ocular treatments (eg. Nerve growth factor) on corneal nerves.10 Studies using laser-scanning confocal microscopy (LSCM) have reported normal nerve density varying from 19.1 ± 4.5mm/mm2 to 25.9 ± 6.7mm/mm2.9 In NK, a decrease in the total number and density of sub-basal corneal nerves have been described.9 Interestingly, treatment with autologous plasma therapy have been associated with an increase in corneal sensitivity and corneal nerve regeneration. In LCD, it is possible to observe reflective linear branching filaments in the stroma, and long nerve fiber bundles in the subepithelial nerve plexus.9 In our patient, we observed improvement of corneal nerve density via IVCM after 8 weeks of treatment with recombinant nerve growth factor.
Patients with NK have compromised ocular surgery integrity and reduced corneal sensation caused by damage to the trigeminal sensory fibers. Our patient presented recurrent ocular surface disease despite prior treatment with preservative-free artificial tears, cyclosporine 0.05%, amniotic membrane, therapeutic contact lens and lateral tarsorrhaphy. These type of palliative treatment present higher risks of disease recurrence.1 Cenegermin 0.002% is a recombinant human nerve growth factor (rhNGF) that focus on restoring corneal integrity by targeting the underlying pathophysiology.1,5,6 Clinical trials in the United States and Europe have proven rhNGF to be safe and effective in improving lesion healing in 74% of patients after one 8 week course treatment.1,7 Improvement of quality of vision and NK signs and symptoms has also been reported.1,7 Our patient who had severe neurotrophic keratitis and was put on Cenegermin 0.002% 2 years after initial diagnosis achieved partial corneal healing at 4 weeks and complete corneal healing after 8 weeks.
In previous studies, pain was the most common adverse event due to nociceptor sensitization caused by rhNGF.1 Our patient reported moderate pain during the treatment course, but it did not prevent the conclusion of the 8-week treatment. Furthermore, similar to previous studies,1,6,7 we did not observe an improvement in subjective corneal sensitivity, as measured by cotton swab. Nevertheless, contrary to prior studies, we described an improvement in visual acuity from 20/100 pre-treatment to 20/50 8 week post treatment.
In this case report we observed complete corneal healing and improved visual acuity in a patient with NK associated with history of herpes simplex epithelial keratitis, and recurrent LCD after PK. The patient was treated with rhNGF following failed treatment with standard and palliative options.
4. Conclusion
Topical recombinant human nerve growth factor is a novel therapeutic option proven to be safe and effective for refractory NK. It reduces the disease's recurrence rate, restores ocular surface integrity and has the potential to improve one's quality of life.
Patient consent
Written patient consent to publish the described case was obtained.
Funding
No funding or grant support.
Authorship
All author attests that they meet the current ICMJE criteria for authorship.
Intellectual property
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Disclosures
The authors have no financial disclosures.
Declaration of competing interest
No conflict of interest exist.
References
- 1.Pfludfelder S.C., Massaro-Giordano M., Perez V.L., Hamrah P. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlle pivotal trial. Ophthalmology. 2020;127:14–26. doi: 10.1016/j.ophtha.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Mastropasqua L., MAssarro-Giodano G., Nubile M., Sacchetti M. Understanding the pathogenesis of neurotrophic keratitis: the role of corneal nerves. J Cell Physiol. 2017;232:717–724. doi: 10.1002/jcp.25623. [DOI] [PubMed] [Google Scholar]
- 3.Sacchetti M., Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–579. doi: 10.2147/OPTH.S45921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saad S, Abdelmassih Y, Saad R, Guindolet D, El Khoury S et al. Neurotrophic keratitis: frequency, etiologies, clinical management and outcomes. Ocul Surf. [DOI] [PubMed]
- 5.Sheha H., Tighe S., Hashem O., Hayashida Y. Update on Cenegermin eye drops in the treatment of neurotrophic keratitis. Clin Ophthalmol. 2019;13:1973–1980. doi: 10.2147/OPTH.S185184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bremond-Gignac D, Daruich A, Robert MP, Chiambaretta F. Recent innovations with drugs in clinical trials for neurotrophic keratitis and refractory corneal ulcers. Expet Opin Invest Drugs, 28:11, 1013-1020. [DOI] [PubMed]
- 7.Bonini S., Lambiase A., Rama P., Sinigali F., for the REPARO Study Group Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125:1332–1343. doi: 10.1016/j.ophtha.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Mohamed A., Chaurasia S., Ramappa M., Murthy S., Garg P. Outcomes of keratoplasty in lattice corneal dystrophy in a large cohort of Indian eyes. Indian J Ophthalmol. 2018;66(5):666–672. doi: 10.4103/ijo.IJO_1150_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruzat A., Qazi Y., Hamrah P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf. 2017;15(1):15–47. doi: 10.1016/j.jtos.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J., Markoulli M. Automatic analysis of corneal nerves imaged using in vivo confocal microscopy. Clin Exp Optom. 2018;101:147–161. doi: 10.1111/cxo.12640. [DOI] [PubMed] [Google Scholar]