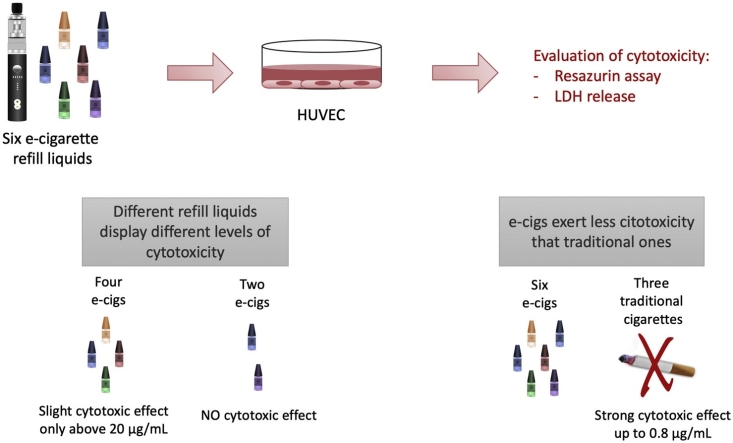
Graphical abstract
Abbreviations: e-Cig, electronic cigarette; ENDS, electronic nicotine delivery systems; EVALI, e-vaping acute lung injury; HUVEC, human umbilical vein endothelial cells; LDH, lactate dehydrogenase
Keywords: Electronic cigarettes, Refill liquids, Cytotoxicity, EVALI
Highlights
-
•
The vapors obtained from different refill liquids for e-cigarettes exert different extent of cytotoxicity.
-
•
The condensates obtained from traditional cigarettes are always highly cytotoxic in the same experimental conditions.
-
•
Accurate evaluations for refill liquids will help to correlate toxicity to chemical composition.
Abstract
The electronic cigarettes mimic combustible cigarettes through a heating technology that vaporizes a refill liquid consisting of solvents, flavors, and nicotine. E-cigarettes are sometimes still used as a support for smoking cessation, even if in 2019 an acute lung injury outbreak occurred in the USA, affecting mainly adolescents and young adults, and was correlated to eCigs. Therefore, due to the lack of a definite knowledge about the mechanism(s) of refill liquid toxicity and considering that previous investigations gave controversial results, the aim of the present study was the cytotoxicity assessment of different refill liquids on human endothelial cells, evaluated by means of two different in vitro approaches, i.e. the resazurin and the LDH release assays. Our results clearly demonstrated that different refill liquids (6 samples) display different levels of cytotoxicity in our cellular model, although their cytotoxicity was always lower than that observed for the condensate obtained from traditional cigarettes (3 samples). These results suggest that accurate evaluations should be provided for refill liquids, in particular to correlate their toxicity to their chemical composition, with the final aim of obtaining useful information for the agencies involved in the regulation of their components.
1. Introduction
The electronic cigarettes (e-cigarettes or e-Cigs), developed in the early 2000s in China, mimic combustible cigarettes by means of a heating technology able to vaporize a liquid consisting of solvents, aromas, and, eventually, nicotine [1]. This alternative to the combustible cigarettes was introduced in the United States in 2006 and its popularity increased rapidly, mostly thanks to the avoidance of the harmful tobacco combustion effects. Within few years from the first commercialization, e-cigarettes, also defined “electronic nicotine delivery systems (ENDS)” by Food and Drug Administration (FDA), quickly developed and improved, in their appearance and also in the features determining their power [1]. The liquids are generally composed of a mixture of propylene glycol, vegetable glycerin, water and various flavorings (fruit, dessert, mint, tobacco), to which nicotine can be added in different concentrations [2].
It has been estimated that nearly 500 e-cigarette brands were present by the end of 2014 and more than 7500 types of e-liquid were marketed [3,4]. A Welsh survey examining the behavior of secondary school students reported that the ever prevalence of smoking e-cigarettes was 37.3 % with respect to only 26.5 % of smoking traditional cigarettes, while in 2015 the use of e-cigarettes among US high school students was nearly three times higher than that of traditional ones (16 % vs 6%) and increased from 1.5%–20.8% (3.05 million students) in the period 2011–2018 [5,6]. An Italian study revealed that less than 3 years after the first commercialization, 3.5 millions of Italians have tried e-cigarettes at least once and more than 600,000 people use them regularly [7].
E-cigarettes have been proposed as an aid for people who want to stop smoking and as a useful tool to reduce the damages derived from active and passive traditional smoking, since they emit a steam flux, converting a liquid into an inhalable aerosol, without releasing the toxic substances whose formation is due to tobacco combustion. However, the utility of e-cigarette as an effective product to stop smoking traditional cigarettes has not yet been proven and the American Heart Association (AHA) recommended caution in their use [8]. A FDA warning against the uncontrolled use of e-cigarettes as a "healthy" alternative to the traditional cigarette or as a tool for smoking cessation from tobacco also indicates that it is necessary to check the presence and the possible release of toxic contaminants and the impact of e-cigarettes to public health, in particular among youngest users [9]. Furthermore, while in some countries e-cigarettes are still considered a help for smoking cessation, an acute lung injury outbreak occurred in USA in 2019, affecting mainly adolescents and young adults. This syndrome has been named E-Vaping Acute Lung Injury (EVALI) [10], and the criteria for its diagnosis have been described in detail [11], although further information about the specific components responsible for EVALI development are needed.
The increasing efforts in evaluating the safety of e-cigarettes and controlling their market raised from the fact that the information on the actual chemical composition of the refill liquids and the emissions produced by their vaporization are not yet complete. Although research indicates that e-cigarettes release lower levels of known toxicants, e.g. carbon monoxide and acrolein, with respect to traditional cigarettes [12], the health risk assessment associated with the use of e-cigarette is the focus of many ongoing preclinical and clinical investigations, as summarized by Eltorai and refs. therein [1].
The objective of this research project is to evaluate the effects on human health ascribable to the exposure to hazardous substances released by e-cigarettes, using the immortalized human endothelial umbilical vein cells HUVEC/tert2 as an in vitro model. With the aim of evaluating the possible toxicity related to the use of e-cigarettes, in this study we examined the effect of the condensation vapors of refill liquids and compared them with the smoke condensation vapors produced by traditional cigarette combustion.
2. Materials and methods
2.1. Materials
This study was performed on HUVEC/Tert2 cells obtained from Evercyte (Vienna, Austria). l-Glutamine and the Endothelial Cell Growth Medium EGM-2 were purchased from Lonza (Basel, Switzerland), Dulbecco’s Phosphate Buffered Saline, the enzyme TrypLE and Defined Trypsin Inhibitor DTI were purchased from Gibco (Thermo Fischer Scientific, Waltham, MA USA). The other reagents used in this study were obtained from Sigma-Aldrich (Vienna, Austria).
2.2. Smoke production and condensate collection
A modified Borgwaldt RM 1/G-R58.02 smoking machine was used to produce 70 mL aerosol in 3,0 s with a 30 s-interval between two consecutive puffs. For generating the e-cigarette vapor a Joyetech vTwo mini with Eleaf coil 0,15 O (Ni) e-cigarette was used, set at 15 W for PG/VG 50/50 samples, and it was triggered directly by the smoking machine. After 5 puffs, the aerosol generated in the next 100 puffs (or more, to reach 0200 g at least of condensate) was collected in a 20 mL glass vial with 2 mL of PBS. In this study 6 condensates of e-Liquid and 3 condensates of traditional cigarette were obtained and examined (Table 1). e-liquid brands and names are not reported in order to avoid commercial use of this publication.
Table 1.
Sample names and description.
| Sample Name | Type | Ingredients |
|---|---|---|
| Sample A | e-liquid | Propylene glycol, glycerol, Nicotine (20 mg/mL), Flavors |
| Sample B | e-liquid | Propylene glycol, glycerol, Nicotine (20 mg/mL), Flavors |
| Sample C | e-liquid | Propylene glycol, glycerol, Nicotine (20 mg/mL), Flavors |
| Sample D | e-liquid | Propylene glycol, glycerol, Nicotine (20 mg/mL), Vanillin (1.3 %), Flavors |
| Sample E | e-liquid | Propylene glycol, glycerol, Nicotine (20 mg/mL), Flavors |
| Sample F | e-liquid | Propylene glycol, glycerol, Nicotine (20 mg/mL), Flavors (Mandarin Oil) |
| Sample G | Traditional cigarette | |
| Sample H | Traditional cigarette | |
| Sample I | Traditional cigarette |
2.3. Sample preparation
Condensates were taken up in PBS and diluted with culture medium (final 20 % of PBS) to obtain a final concentration of 20 μg/mL, used as the highest concentration to test. Such concentration was chosen according to Putzhammer et al. [4]. Diluted condensates were extensively mixed and filtered with a 0.2 μm syringe filter before use. For each sample, 4 more solutions at different concentration were prepared by serial dilution in culture medium, as described by Putzhammer et al. [4].
2.4. Cell culture
To evaluate the cytotoxic effect of condensates, HUVEC/tert2 immortalized human umbilical vein endothelial cells were used following a related method described by Putzhammer et al. [4]. Briefly, cells were cultured in Endothelial Cell Growth Medium – BBE without substitution of FBS at 37 °C, 5 % CO2 and 20 % O2. 25,000 HUVEC/Tert2 cells were seeded per well in 24-well plates. After 24 h of incubation, HUVEC/Tert2 cells were treated with 20 μg/mL e-liquid sample in Endothelial Cell Growth Medium – BBE. The PBS concentration in the culture medium was adjusted to 20 % in all treated wells. Cells treated with medium and 20 % PBS were used as controls. Cell Lysis was determined 24 h after treatment by measuring the release of lactate dehydrogenase (LDH). The metabolic activity of the cells was determined 48 h after treatment by Resazurin assay. Briefly, cells were maintained in a humidified atmosphere with 5% CO2, in endothelial growth medium (EGM, Lonza) and routinely passaged in 0.2 % gelatine-coated (Sigma, Steinheim, Germany) polysterene culture flasks (TPP, Switzerland).
2.5. Resazurin assay
Resazurin assay was used to determine the metabolic activity of live HUVEC/Tert2 cells by means of a colorimetric assay. Briefly, treatment medium was discarded from cells and culture medium with 30 μg/mL Resazurin was added. The turnover of Resazurin to Resorufin was analyzed after 2 h of incubation fluorescent measurements (excitation 540 nm / emission 590 nm). All experiments were measured in technical as well as biological triplicates.
2.6. Lactate dehydrogenase (LDH) release assay
The LDH Assay quantitatively measures the amount of lactate dehydrogenase (LDH), a stable cytosolic enzyme released after membrane lysis as consequence of cell death. The release of LDH in culture supernatants was measured using the CytoTox 96® non-radioactive cytotoxicity assay kit purchased from Promega (Milan, Italy). Briefly, 50 μL of supernatants of cells treated as described above were collected and analyzed according to manufacturer`s instructions. LDH release in supernatants was measured with a 30-minute coupled enzymatic assay. In detail, the LDH released from dead cells, in which cell membrane is disrupted, induced the conversion of a tetrazolium salt (iodonitro-tetrazolium violet) into a red formazan product. Therefore, the amount of red color formation is proportional to LDH concentration in cell supernatants, and thus to the number of dead cells [13]. The absorbance was measured at 490 nm and results were expressed as percentage of cytotoxicity with respect to the “maximum LDH release” obtained with the complete lysis of the cells.
2.7. Statistical analysis
Statistical analyses were performed by means of the GraphPad Prism software ver. 8.0 (GraphPad Software Inc., San Diego, CA, USA). Data are expressed as mean ± standard error of the mean (SEM). The experimental results were compared by one-way analysis of variance (ANOVA) or Student’s t-test, when appropriate. In the case of statistically significant differences (α = 0.05), ANOVA was followed by the Tukey’s post-hoc test [14]. A P value < 0.05 was considered statistically significant.
3. Results
3.1. Cytotoxicity of e-cigarette condensates
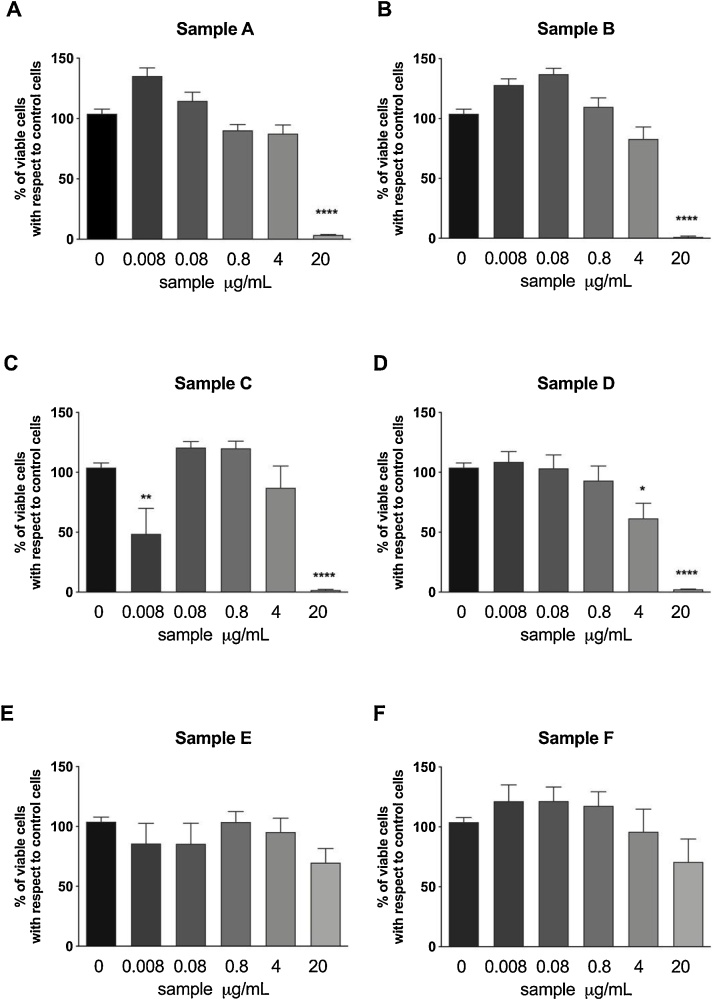
The four e-liquid condensates A, B, C and D induced a significant reduction in HUVEC/Tert2 cell metabolism at the concentration of 20 μg/mL (Fig. 1). Thus, cell vitality at this concentration was below 3.5 % for the first four e-cigarette condensates, whereas at lower concentrations (4 μg/mL or lower) they showed hardly any cytotoxic effect in sample A, B, C and D. The metabolic activity remained above 60 %.
Fig. 1.
Cytotoxic effect of e-cigarette condensates evaluated by means of the resazurin assay. Data are reported as mean ± SEM of two independent experiments, each run in triplicates. *p < 0.05, ** p < 0.01, ****p < 0.0001 vs. control cells treated with medium.
The two e-liquid condensates E and F hardly showed any cytotoxic effect on HUVEC/Tert2 cells and showed at the highest concentrations only minor, not significant effects on metabolic activity, which remained above 69 % even at the concentration of 20 μg/mL.
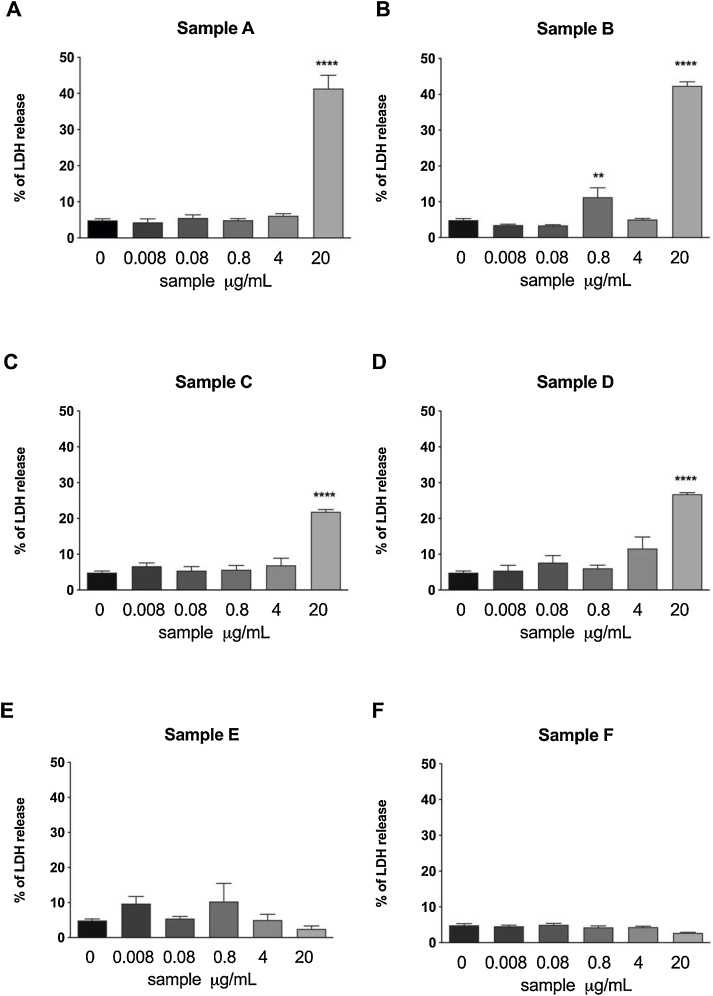
Accordingly, the e-liquid condensates A, B, C and D caused a significant increase in LDH release within the first 24 h, arguing for a cell lytic effect of these samples, at the highest concentration of 20 μg/mL (Fig. 2). Sample A and B exerted a stronger lytic effect compared to sample C and D, with LDH levels of about 40 % and 25 % of the lysis control, respectively.
Fig. 2.
Cytotoxic effect of e-cigarettes evaluated by means of the LDH release assay. Data are reported as mean ± SEM of two independent experiments, each run in triplicates. ****p < 0.0001 vs. control cells treated with medium.
The last two samples of e-liquid condensate samples E and F didn’t induce LDH release after a 24h-treatment, suggesting the absence of cytotoxicity, as also demonstrated by the Resazurin assay.
3.2. Cytotoxicity of traditional cigarette condensates
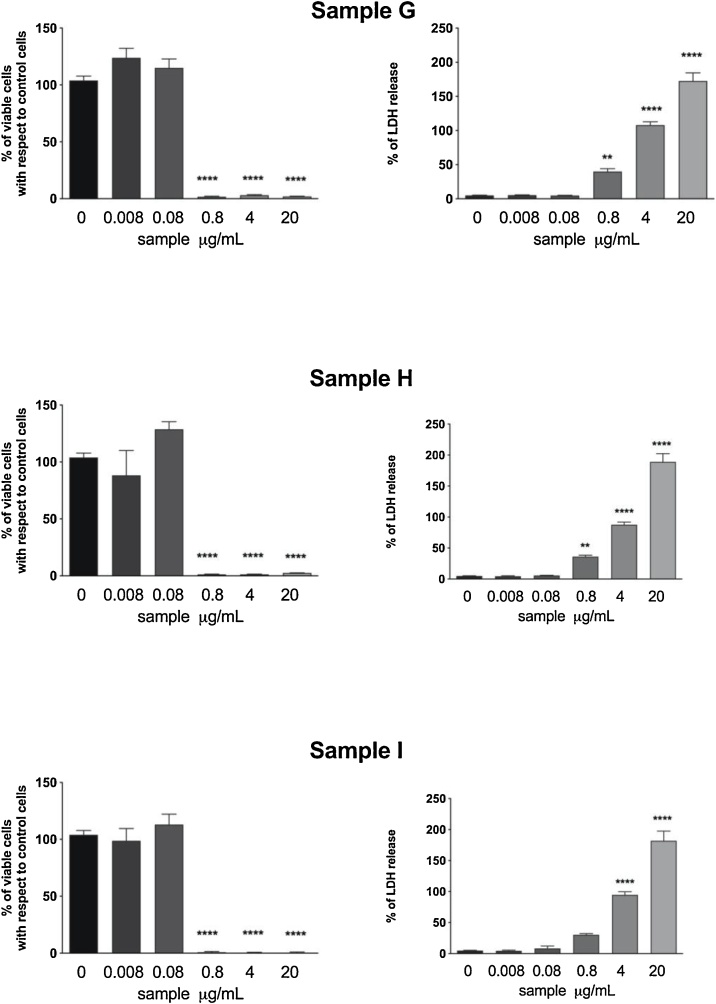
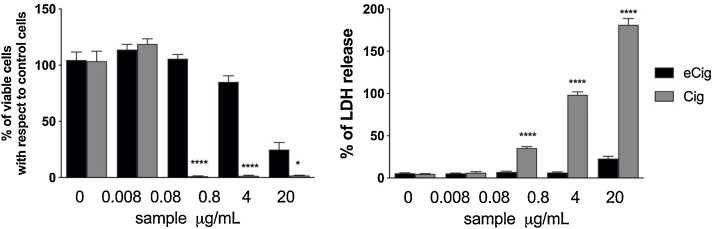
By contrast, condensate samples deriving from three different traditional cigarette brands (G, H, and I) exerted a much stronger cytotoxic effect, since the metabolic activity was under 3% up to the concentration of 0.8 μg/mL and, accordingly, an elevated LDH release starting from 0.8 and increased at 4 and 20 μg/mL in a dose dependent manner could be observed (Fig. 3).
Fig. 3.
Cytotoxic effect of traditional cigarettes evaluated by means of resazurin assay (left column) and LDH release (right column). Data are reported as mean ± SEM of two independent experiments, each run in triplicates. ** p < 0.01, ****p < 0.0001 vs. control cells treated only with medium.
Finally, we compared the cytotoxic effect of the e-cigarette samples taken together with that of traditional cigarettes, demonstrating that mean results indicate the formers as much safer compared to the latter, according to both tests (Fig. 4). In particular, we observed that the mean values of toxicity relative to traditional cigarette condensates started to be significantly higher than those of e-Cigs at 0.8 μg/mL, and this increase showed dose dependency, as far as the LDH release is concerned.
Fig. 4.
Comparison of cytotoxic effect of e-cigarettes and traditional cigarettes evaluated by means of resazurin and LDH assays. Data are reported as mean ± SEM of two independent experiments, each run in triplicates. *p < 0.5 and ****p < 0.0001 vs. the corresponding e-cig concentration treatment.
4. Discussion
The specific aims of this study were the evaluation of the toxicological effects resulting from the vapors of e-cigarettes and the comparison between the toxicological effects of e-cigarettes and traditional cigarettes. In particular, we analyzed 6 different refill liquids for e-cigarettes, in order to ascertain whether they have different cytotoxic effects in a 2D cellular model, i.e. the HUVEC/Tert2 cells. We also compared their toxicity with that induced by normal cigarettes of three different popular brands. In agreement with previous studies (see e.g., [12] and refs therein), we demonstrated that traditional cigarettes are significantly more toxic with respect to e-cigarettes, and this difference is evident starting from a condensate concentration of 0.8 μg/mL. Besides, we demonstrated for the first time that different refill liquids display significantly different toxicity on human cells, since two out of the six samples analyzed display no cytotoxicity at all, whereas the other four caused a significant decrease of cell viability when used at high concentration (20 μg/mL).
Although it has been suggested that e-cigarettes release lower levels of classic toxicants, such as carbon monoxide and acrolein, with respect to traditional cigarettes [12], many ongoing preclinical and clinical investigations are assessing the possible risk for health of this novel device [1], and the need for accurate toxicological evaluation was recently confirmed by the EVALI outbreak. The risk assessment for human health associated with exposure to the refill liquids for e-cigarettes relies on the identification of the risks related to these potentially hazardous substances and their mixtures. According to their composition, the toxic effects of the refill liquids can include the effect of nicotine, aldehydes (acetaldehyde, formaldehyde, acrolein), the BTEX mixture (benzene, toluene, ethylbenzene, styrene, xylenes); polycyclic aromatic hydrocarbons (naphthalene, fluorine, phenanthrene, anthracene, fluoranthene, pyrene, crisene) or metals (As, Cd, Cr, Mn, Ni, Pb, Sn), all substances that can be found in both refill liquids and condensation vapors.
Recent studies have raised the issue of the possible implication of flavorings for the production of toxic derivatives, e.g. carbonyls, although conflicting results have been reported [15]. Moreover, a recent study from Kerasioti et al. demonstrated that some flavorings added to e-cig liquids induce oxidative stress-related toxicity, thus suggesting that they could affect, in combination with nicotine, the safety profile of e-cig refills [16]. A systematic review on this issue has observed that these conflicting results could be ascribable to the great variability the methodologies that have been used, e.g. different analytical methods, puffing patterns and aerosol collection, even thought it could be reasonable that in the normal conditions of use, the carbonyl emissions from e-cigs are substantially lower than that from tobacco cigarettes [17]. Another study demonstrated that acute e-cig smoking didn’t affect complete blood count of both smokers and first-time smokers, at variance with tobacco cigarettes that induced an increase of white blood cell count, particularly of lymphocytes and granulocytes. Nevertheless, the authors concluded that more studies are needed to understand the toxicological profile of e-cig refill liquids and correlate toxicity with their components, especially in terms of nicotine content, which can be extremely different in the marketed refill liquids [18].
As far as nicotine is concerned, a clinical study by Antoniewicz and collaborators demonstrated that the aerosol of nicotine-containing e-cigarette caused a significant increase in heart rate and arterial stiffness of healthy volunteers, together with an obstruction of the conducting airways, displaying an acute impact on vascular and pulmonary function (Antoniewicz et al., 2019). However, in our experimental model, the difference between the toxicity induced by standard and e-cigarettes likely cannot be ascribed to nicotine only. In fact, the average nicotine amount released in the 100 puffs condensed in our experiments was about 20 mg. The corresponding amount for cigarettes was 70−140 mg, given that the nicotine content declared by the producers was between 7 and 14 mg/cigarette and on average 10 cigarette were needed to produce 100 puffs. The nicotine concentration in the cigarette smoke condensates was a factor of 3.5–7 times higher than the nicotine concentration in the e-cigarette vapour condensates. This difference seems unlikely to explain the large difference in cytotoxicity found between cigarettes and e-cigarette (Fig. 4). Indeed, Fig. 4 shows that a dilution of ten times of the cigarette smoke condensate is not sufficient to reduce their cytotoxic effect to be comparable to the one of e-cigarette vapor condensates.
As far as the other toxic components are concerned, further studies are ongoing in our laboratory with the aim of assessing their specific toxicity. In conclusion, this study gives the first demonstration that different refill e-liquids can have a peculiar toxicity spectrum. Further studies are needed to correlate this finding to their chemical composition, in order to obtain useful information for the agencies involved in the regulation of refill liquid components.
CRediT authorship contribution statement
Sara De Martin: Formal analysis, Data curation, Writing - original draft, Writing - review & editing. Daniela Gabbia: Data curation, Writing - original draft. Sara Bogialli: Supervision, Resources. Franco Biasioli: Investigation, Methodology. Andrea Boschetti: Investigation, Methodology. Ronald Gstir: Methodology, Investigation, Validation, Writing - review & editing. Daniela Rainer: Methodology, Investigation, Validation, Writing - review & editing. Luca Cappellin: Conceptualization, Validation, Formal analysis, Project administration, Funding acquisition, Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Edited by: Dr. A.M Tsatsaka
References
- 1.Eltorai A.E., Choi A.R., Eltorai A.S. Impact of electronic cigarettes on various organ systems. Respir. Care. 2019;64:328–336. doi: 10.4187/respcare.06300. [DOI] [PubMed] [Google Scholar]
- 2.Manzoli L., Flacco M.E., Ferrante M., La Vecchia C., Siliquini R., Ricciardi W., Marzuillo C., Villari P., Fiore M., ISLESE Working Group Cohort study of electronic cigarette use: effectiveness and safety at 24 months. Tob. Control. 2017;26:284–292. doi: 10.1136/tobaccocontrol-2015-052822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besaratinia A., Tommasi S. An opportune and unique research to evaluate the public health impact of electronic cigarettes. Cancer Causes Control CCC. 2017;28:1167–1171. doi: 10.1007/s10552-017-0952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putzhammer R., Doppler C., Jakschitz T., Heinz K., Förste J., Danzl K., Messner B., Bernhard D. Vapours of US and EU market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen K.A. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students — United States, 2011–2018. MMWR Morb. Mortal. Wkly. Rep. 2018;67 doi: 10.15585/mmwr.mm6745a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lacy E., Fletcher A., Hewitt G., Murphy S., Moore G. Cross-sectional study examining the prevalence, correlates and sequencing of electronic cigarette and tobacco use among 11–16-year olds in schools in Wales. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-012784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallus S., Lugo A., Pacifici R., Pichini S., Colombo P., Garattini S., La Vecchia C. e-cigarette awareness, use, and harm perceptions in Italy: a national representative survey. Nicotine Tob. Res. 2014;16:1541–1548. doi: 10.1093/ntr/ntu124. [DOI] [PubMed] [Google Scholar]
- 8.Bhatnagar A., Whitsel L.P., Ribisl K.M., Bullen C., Chaloupka F., Piano M.R., Robertson R.M., McAuley T., Goff D., Benowitz N., American Heart Association Advocacy Coordinating Committee, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Quality of Care and Outcomes Research Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130:1418–1436. doi: 10.1161/CIR.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottlieb M.A. Regulation of e-cigarettes in the United States and its role in a youth epidemic. Children. 2019;6:40. doi: 10.3390/children6030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzortzi A., Kapetanstrataki M., Evangelopoulou V., Behrakis P. A systematic literature review of e-cigarette-related illness and injury: not just for the respirologist. Int. J. Environ. Res. Public Health. 2020;17:2248. doi: 10.3390/ijerph17072248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel D.A., Jatlaoui T.C., Koumans E.H., Kiernan E.A., Layer M., Cates J.E., Kimball A., Weissman D.N., Petersen E.E., Reagan-Steiner S., Godfred-Cato S., Moulia D., Moritz E., Lehnert J.D., Mitchko J., London J., Zaki S.R., King B.A., Jones C.M., Patel A., Delman D.M., Koppaka R., Griffiths A., Esper A., Calfee C.S., Hayes D., Rao D.R., Harris D., Smith L.S., Aberegg S., Callahan S.J., Njai R., Adjemian J., Garcia M., Hartnett K., Marshall K., Powell A.K., Adebayo A., Amin M., Banks M., Cates J., Al-Shawaf M., Boyle-Estheimer L., Briss P., Chandra G., Chang K., Chevinsky J., Chiang K., Cho P., DeSisto C.L., Duca L., Jiva S., Kaboré C., Kenemer J., Lekiachvili A., Miller M., Mohamoud Y., Perrine C., Shamout M., Zapata L., Annor F., Barry V., Board A., Evans M.E., Gately A., Hoots B., Pickens C., Rogers T., Vivolo-Kantor A., Cyrus A., Boehmer T., Glidden E., Hanchey A., Werner A., Zadeh S.E., Pickett D., Fields V., Hughes M., Neelam V., Chatham-Stephens K., O’Laughlin K., Pomeroy M., Atti S.K., Freed J., Johnson J., McLanahan E., Varela K., Layden J., Meiman J., Roth N.M., Browning D., Delaney A., Olson S., Hodges D.F., Smalley R. Update: interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury — United States, October 2019. Morb. Mortal. Wkly. Rep. 2019;68:919–927. doi: 10.15585/mmwr.mm6841e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McRobbie H., Phillips A., Goniewicz M.L., Smith K.M., Knight-West O., Przulj D., Hajek P. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, Carbon Monoxide, and Acrolein. Cancer Prev. Res. Phila. Pa. 2015;8:873–878. doi: 10.1158/1940-6207.CAPR-15-0058. [DOI] [PubMed] [Google Scholar]
- 13.Frión-Herrera Y., Gabbia D., Díaz-García A., Cuesta-Rubio O., Carrara M. Chemosensitizing activity of Cuban propolis and nemorosone in doxorubicin resistant human colon carcinoma cells. Fitoterapia. 2019;136 doi: 10.1016/j.fitote.2019.104173. [DOI] [PubMed] [Google Scholar]
- 14.Gabbia D., Pozza A.D., Albertoni L., Lazzari R., Zigiotto G., Carrara M., Baldo V., Baldovin T., Floreani A., Martin S.D. Pregnane X receptor and constitutive androstane receptor modulate differently CYP3A-mediated metabolism in early- and late-stage cholestasis. World J. Gastroenterol. 2017;23:7519–7530. doi: 10.3748/wjg.v23.i42.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kouretas D., Tsatsakis A., Poulas K. Editorial: alternative tobacco products: toxicology and health issues. Food Chem. Toxicol. 2018;118:523–525. doi: 10.1016/j.fct.2018.05.056. [DOI] [PubMed] [Google Scholar]
- 16.Kerasioti E., Veskoukis A.S., Skaperda Z., Zacharias A., Poulas K., Lazopoulos G., Kouretas D. The flavoring and not the nicotine content is a decisive factor for the effects of refill liquids of electronic cigarette on the redox status of endothelial cells. Toxicol. Rep. 2020;7:1095–1102. doi: 10.1016/j.toxrep.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farsalinos K.E., Gillman G. Carbonyl emissions in e-cigarette aerosol: a systematic review and methodological considerations. Front. Physiol. 2017;8:1119. doi: 10.3389/fphys.2017.01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flouris A.D., Poulianiti K.P., Chorti M.S., Jamurtas A.Z., Kouretas D., Owolabi E.O., Tzatzarakis M.N., Tsatsakis A.M., Koutedakis Y. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012;50:3600–3603. doi: 10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]