Abstract
The role of microRNA (miR)-1301-3p has been investigated in breast cancer and colorectal cancer. Dysregulation of miR-1301-3p expression in non-small cell lung cancer (NSCLC) is speculated to be associated with tumor progression, which was systemically investigated in the present study. Reverse transcription-quantitative PCR analysis was performed to detect miR-1301-3p expression in 124 paired tissue samples and cultured cell lines. The results demonstrated that miR-1301-3p expression was regulated by transfection with miR-1301-3p mimic or inhibitor, and the proliferation, migration and invasion of the transfected cells were assessed via the Cell Counting Kit-8 and Transwell assays. In addition, miR-1301-3p expression was significantly upregulated in NSCLC tissues and cells compared with normal tissues and normal cells, respectively. Notably, upregulated miR-1301-3p expression in NSCLC tissues was significantly associated with the TNM stage, lymph node metastasis and poor prognosis of patients with NSCLC. Furthermore, upregulated miR-1301-3p expression in NSCLC cells promoted cell proliferation, migration and invasion, the effects of which were reversed following miR-1301-3p knockdown. Thy-1 was identified as a direct target of miR-1301-3p, which serves as a tumor promoter in the progression of NSCLC. Taken together, the results of the present study suggest that upregulated miR-1301-3p expression in NSCLC acts as an independent prognostic factor and a tumor promoter by targeting thy-1, thus provides a potential therapeutic target for NSCLC.
Keywords: non-small cell lung cancer, microRNA-1301-3p, thy-1, progression, development
Introduction
Non-small cell lung cancer (NSCLC) is one of the most common types of lung cancer, which accounts for the majority of cancer-associated mortalities with the incidence rat of 1.3 million cases per year according to the statistical data until 2018 (1,2). Advancements in molecular biology and therapeutic strategies for NSCLC have allowed identification of novel drugs and treatments; however, the 5-year survival rate (<15%) of patients with NSCLC remains poor (3,4). Current therapies in the clinic, such as surgical resection, chemotherapy and radiotherapy, have been established with limited positive effects on the long-term survival of patients (5). Poor patients prognosis is predominantly due to advanced local invasion and distant metastasis at diagnosis (6). Recently, a number of studies have focused on investigating novel therapeutic strategies for patients with NSCLC, and identifying effective therapeutic targets (7–9).
MicroRNAs (miRNAs/miRs) are a class of short, highly conserved non-coding RNAs that regulate gene expression by binding to the 3′-untranslated region (UTR) of target mRNAs (10,11). miRNAs have the ability to predict clinical outcomes, detect cancer and monitor disease conditions (10). Previous studies have reported that miRNAs participate in the progression and development of different types of cancer, including NSCLC (12,13). A previous study demonstrated that miR-1301-3p inhibits cell proliferation, and induces cell cycle arrest and apoptosis of breast cancer (14). In prostate cancer, miR-1301-3p promotes the expansion of prostate cancer stem cells by targeting GSK3β and SFRP1, and activating the Wnt pathway (15). In addition, miR-1301-3p expression is associated with the pathological stages of colorectal cancer (16). A recent miRNA expression profile revealed that miR-1301-3p expression is significantly upregulated in NSCLC tissues compared with normal clinical samples, suggesting that miR-1301-3p participates in the development of NSCLC (17), which has not yet been fully investigated. Thy-1 is a glycosylphosphatidylinositol-linked outer membrane leaflet glycoprotein that is closely associated with idiopathic pulmonary fibrosis, which can increase the risk of lung cancer (18). Thus, it is speculated that Thy-1 may play a vital role in the development of NSCLC.
The present study aimed to investigate the role of miR-1301-3p in NSCLC to determine its association with the prognosis and progression of NSCLC. In addition, the effects of the underlying molecular mechanisms of miR-1301-3p were also investigated.
Materials and methods
Patients
The present study recruited 124 patients with NSCLC at Binzhou Medical University Hospital, between January 2013 and December 2015. The inclusion criteria was as following: i) 18 years old or above; ii) histologically diagnosed with NSLC and were amenable to surgery; iii) had never undergone any kinds of anti-cancer therapy, such as chemotherapy and radiotherapy prior to surgery; iv) clinical data were completed. Patients diagnosed with other cancers were excluded. NSCLC tissues and adjacent normal tissues (about 2 cm from the lesion) were collected via surgical resection. Collected tissues were immediately frozen in liquid nitrogen and stored at −80°C until subsequent experimentation. The clinicopathological characteristics of the patients are presented in Table I. Survival analysis was performed via a 5-year follow-up survey at 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, and 60 months after surgery by telephone. The present study was approved by the Ethics Committee of Binzhou Medical University Hospital (Binzhou, China; approval no. 201212), and written informed consent was provided by all patients prior to tissue collection.
Table I.
Association between miR-1301-3p expression and the clinicopathological characteristics of patients with non-small cell lung cancer (n=124).
| Characteristic | Patient, n | Low miR-1301-3p expression (n=51) | High miR-1301-3p expression (n=73) | P-value |
|---|---|---|---|---|
| Age, years | 0.704 | |||
| <60 | 59 | 30 | 29 | |
| ≥60 | 65 | 21 | 44 | |
| Sex | 0.453 | |||
| Male | 70 | 31 | 39 | |
| Female | 54 | 20 | 34 | |
| TNM stage | 0.025a | |||
| I–II | 86 | 39 | 47 | |
| III–IV | 38 | 12 | 26 | |
| Lymph node metastasis | 0.027a | |||
| Negative | 84 | 34 | 50 | |
| Positive | 40 | 17 | 23 | |
| Differentiation | 0.136 | |||
| Well-moderate | 79 | 39 | 40 | |
| Poor | 45 | 12 | 33 | |
| Smoking | 0.270 | |||
| No | 61 | 23 | 38 | |
| Yes | 63 | 28 | 35 | |
| Tumor size, cm | 0.262 | |||
| <4 | 67 | 35 | 32 | |
| ≥4 | 57 | 16 | 41 |
P<0.05. miR, microRNA; TNM, tumor-node-metastasis.
Cell culture and transfection
The NSCLC cell lines, A549, H1299, MRC5 and SK-LU-1, and the lung epithelial cell line, BEAS-2B, were purchased from the American Typical Culture Collection. Cells were maintained in DMEM medium (Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc.), at 37°C with 5% CO2.
miR-1301-3p mimic, miR-1301-3p inhibitor or the corresponding negative controls (25 nM; Guangzhou RiboBio Co., Ltd.) were transfected into A549 and H1299 cells using Lipofectamine® 3000 transfection reagent (cat. no. L3000-015, Invitrogen; Thermo Fisher Scientific, Inc.) at room temperature for the overexpression or knockdown of miR-1301-3p. Transfected cells were available for following experiments after 24 h of the transfection. The following sequences were used: miR-1301-3p mimic, 5′-UUGCAGCUGCCUGGGAGUGACUUC-3′; and miR-1301-3p inhibitor, 5′-GAAGUCACUCCCGGCAAGCUGCAA-3′.
Reverse transcription-quantitative (RT-q)PCR
Total RNA was extracted from tissues and cultured cells using TRIzol® reagent (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Total RNA was reverse transcribed into cDNA using the TaqMan microRNA reverse transcription kit (cat. no. 4366596, Applied Biosystems; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. qPCR was subsequently performed using the SYBR Green I Master Mix kit (cat. no. 12223012, Invitrogen; Thermo Fisher Scientific, Inc.) and the 7300 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The following primer sequences were used for qPCR: miR-1301-3p forward, 5′-TTACAGCTGCCTGAGAGTGACTTA-3′ and reverse, 5′-CTCTACAGCTATATTGCCAGCCA-3′; and U6 forward 5′-CGCTTCGGCAGGCATTATATAC-3′ and reverse 5′-AAGGGGCCATGCTAATCTT-3′. The following thermocycling conditions were used for qPCR: 10 sec at 95°C, followed by 40 cycles of 5 sec at 95°C and 20 sec at 60°C. Relative expression levels were calculated using the 2−ΔΔCq method (19) and normalized to the internal reference gene U6. All experiments were performed in triplicate.
Cell proliferation assay
The proliferative ability of transfected cells was assessed via the Cell Counting Kit-8 (CCK-8) assay. Briefly, A549 and H1299 cells were seeded into 96-well plates at a density of 5×103 and incubated at 37°C with 5% CO2 for 0, 24, 48 and 72 h. Subsequently, CCK-8 reagent (Dojindo Molecular Technologies, Inc.) was added to each well and incubated for 1 h at 37°C. Cell proliferation was measured at a wavelength of 450 nm using a microplate reader (Synergy 4, BioTek Instruments, Inc.).
Migration and invasion assays
A total of 1×105 A549 and H1299 cells were plated in the upper chambers of 24-well Transwell plates in DMEM culture medium without FBS, whereas culture medium supplemented with 10% FBS was plated into the lower chambers as the chemoattractant. For the invasion assay, Transwell membranes were precoated with Matrigel (BD Biosciences) at 37°C for 1 h. Following incubation at 37°C for 48 h, the migratory/invasive cells were stained with 0.1% crystal violet at 37°C for 5 min and counted under a light microscope (magnification, ×400).
Target prediction and dual-luciferase reporter assay
The TargetScan database (http://www.targetscan.org/vert_71) was used to predict the target of miR-1301-3p and verify the 3′-UTR binding region of miR-1301-3p.
The dual-luciferase reporter assay was performed using a dual-luciferase reporter assay system (Promega Corporation), according to the manufacturer's protocol. The recombinant vectors, pGL3-thy-1-wt and pGL3-thy-1-mut were generated using the pmirGLO vector (Promega Corporation). A549 cells were seeded into 96-well plates and co-transfected with miR-1301-3p mimic or miR-1301-3p inhibitor, and pGL-thy-1-3′UTR-wt or pGL-thy-1-3′UTR-mut using Lipofectamine® 2000 transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and incubated at 37°C. Luciferase activities were detected 24 h post-transfection with reference to Renilla luciferase activity.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (IBM Corp.) and GraphPad Prism 7.0 software (GraphPad Software, Inc.). Data are presented as the mean ± standard deviation obtained from at least triplicate experiments or determination. Paired Student's t-test was used to compare differences between two groups, while one-way ANOVA followed by Tukey's post hoc test were used to compare differences between multiple groups. The average expression level of miR-1301-3p (3.63) in NSCLC tissues was used as the cut-off value to divide 124 patients with NSCLC into high (n=73) and low (n=51) miR-1301-3p expression groups. The association between miR-1301-3p expression and the clinicopathological characteristics of patients with NSCLC was estimated by the Pearson's χ2 test. The Kaplan-Meier method followed by log-rank test, and Cox regression analyses were performed to assess survival and determine the prognostic value of miR-1301-3p, respectively.
Results
miR-1301-3p expression is significantly upregulated in NSCLC tissues and cells
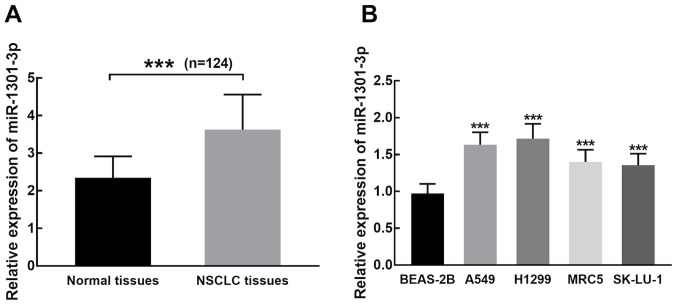
miR-1301-3p expression was significantly higher in NSCLC tissues compared with adjacent normal tissues (P<0.001; Fig. 1A). Similarly, miR-1301-3p expression was significantly upregulated in NSCLC cells compared with BEAS-2B cells (P<0.001; Fig. 1B).
Figure 1.
miR-1301-3p expression is upregulated in NSCLC tissues and cells. (A) miR-1301-3p expression was significantly higher in NSCLC tissues compared with adjacent normal tissues. (B) miR-1301-3p expression was significantly upregulated in the NSCLC cell lines, A549, H1299, MRC5 and SK-LU-1, compared with normal BEAS-2B epithelial cells. ***P<0.001 vs. normal tissues and BEAS-2B cells. miR, microRNA; NSCLC, non-small cell lung cancer.
miR-1301-3p expression is significantly associated with TNM stage and lymph node metastasis
The average expression level of miR-1301-3p (3.63) in NSCLC tissues was used as the cut-off value to divide 124 patients with NSCLC into high (n=73) and low (n=51) miR-1301-3p expression groups. As presented in Table I, patients with high miR-1301-3p expression were significantly associated with positive lymph node metastasis (P=0.027) and an advanced clinical stage (P=0.025).
Overexpression of miR-1301-3p is associated with a poor prognosis of patients with NSCLC
Survival information was obtained via a 5-year follow-up survey and plotted using the Kaplan-Meier method. As presented in Fig. 2, patients with high miR-1301-3p expression had a significantly shorter overall survival time than those with low miR-1301-3p expression (Log-rank P=0.022).
Figure 2.
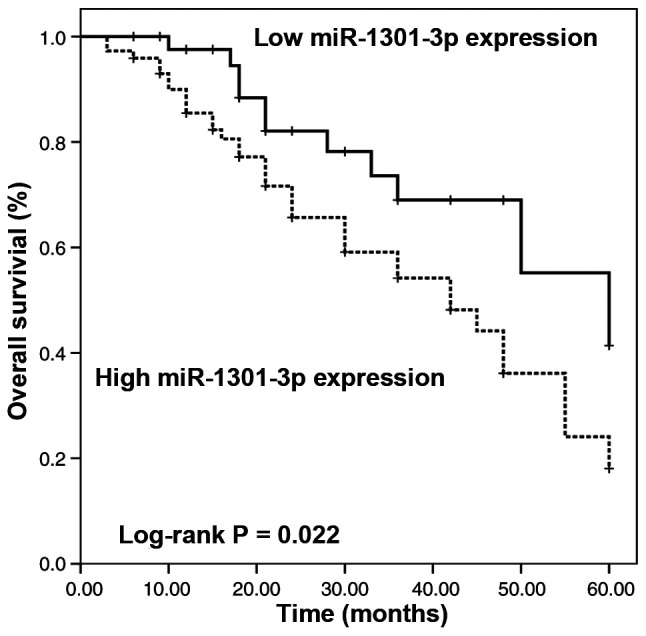
Kaplan-Meier curve summarizing the survival information of patients based on miR-1301-3p expression. Patients with high miR-1301-3p expression had a significantly shorter overall survival time than those with low miR-1301-3p expression. Log-rank P=0.022. miR, microRNA.
Cox regression analysis was performed to determine the prognostic value of miR-1301-3p. The results demonstrated that miR-1301-3p expression [hazard ration (HR), 2.450; 95% confidence interval (CI), 1.166–5.150; P=0.018], TNM stage (HR, 2.162; 95% CI, 1.093–4.276; P=0.027) and lymph node metastasis (HR, 2.181; 95% CI, 1.087–4.374; P=0.028) were independent factors for the clinical prognosis of patients with NSCLC (Table II).
Table II.
Cox regression analysis of miR-1301-3p and survival of patients with NSCLC.
| Characteristic | HR factor | 95% CI | P-value |
|---|---|---|---|
| miR-1301-3p | 2.450 | 1.166–5.150 | 0.018 |
| Age | 1.223 | 0.657–2.276 | 0.525 |
| Sex | 1.290 | 0.671–2.481 | 0.445 |
| TNM stage | 2.162 | 1.093–4.276 | 0.027 |
| Lymph node metastasis | 2.181 | 1.087–4.374 | 0.028 |
| Differentiation | 1.548 | 0.824–2.907 | 0.175 |
| Smoking | 1.502 | 1.785–2.873 | 0.219 |
| Tumor size | 1.445 | 0.758–2.756 | 0.263 |
miR, microRNA; TNM, tumor-node-metastasis; HR, hazard ratio; CI, confidence interval.
Overexpression of miR-1301-3p promotes proliferation, migration and invasion of NSCLC cells
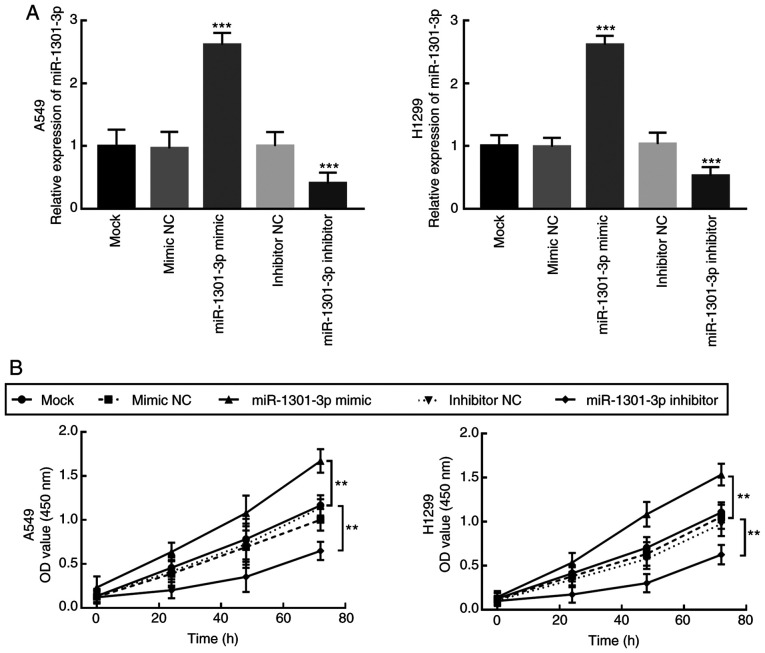
To determine the biological function of miR-1301-3p in NSCLC, miR-1301-3p expression was regulated by transfection with miR-1301-3p mimic or miR-1301-3p inhibitor. RT-qPCR analysis demonstrated that transfection with miR-1301-3p mimic significantly increased miR-1301-3p expression in A549 and H1299 cells, while miR-1301-3p inhibitor significantly decreased miR-1301-3p expression compared with the mock and negative controls (P<0.001; Fig. 3A).
Figure 3.
Overexpression of miR-1301-3p enhances the proliferation of non-small cell lung cancer cells in vitro. (A) miR-1301-3p expression was overexpressed in A549 and H1299 cells following transfection with miR-1301-3p mimic, and downregulated following transfection with miR-1301-3p inhibitor. (B) A549 and H1299 cell proliferation was enhanced following overexpression of miR-1301-3p and inhibited following miR-1301-3p knockdown. **P<0.01, ***P<0.001 vs. mock groups. miR, microRNA; NC, negative control; OD, optical density.
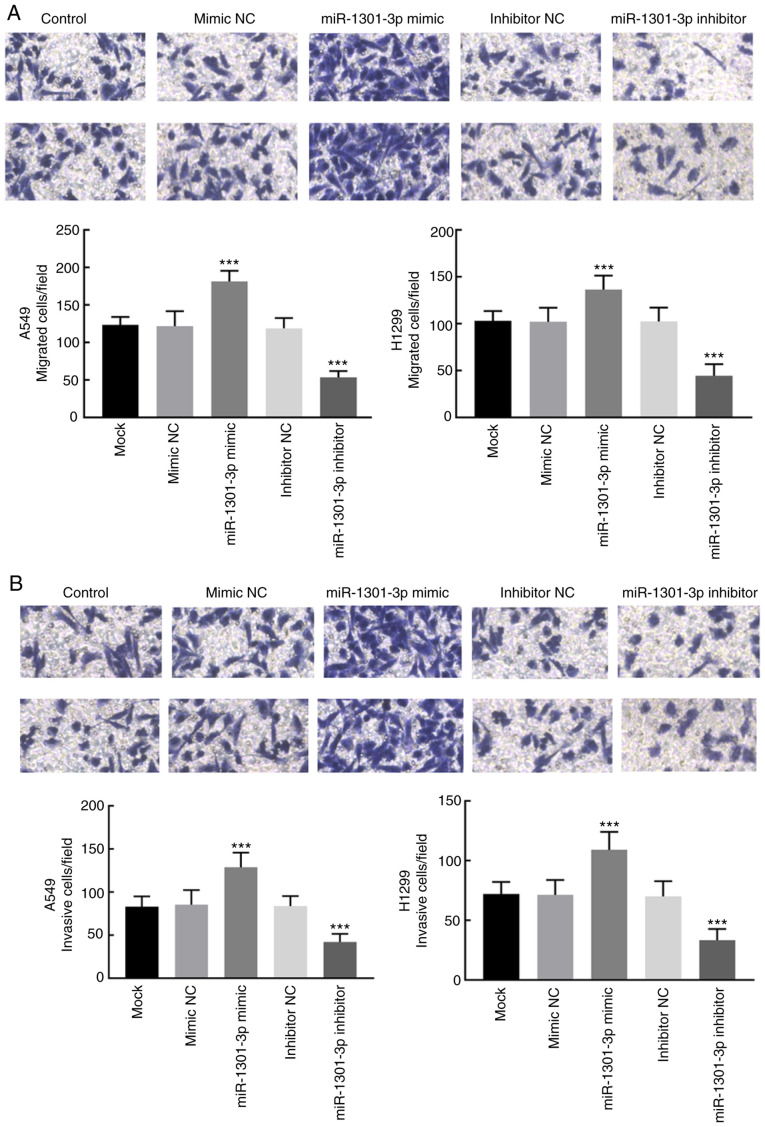
Proliferation of the transfected cells was detected via the CCK-8 assay. Overexpression of miR-1301-3p promoted A549 and H1299 cell proliferation, whereas miR-1301-3p knockdown significantly suppressed the proliferation of A549 and H1299 cells (P<0.01; Fig. 3B). Similarly, the migratory and invasive abilities of NSCLC cells significantly enhanced following overexpression of miR-1301-3-3p, the effects of which were reversed following miR-1301-3-3p knockdown (P<0.001; Fig. 4A and B).
Figure 4.
Overexpression of miR-1301-3p promotes the migratory and invasive abilities of non-small cell lung cancer cells in vitro. (A) The migration of A549 and H1299 cells was promoted following overexpression of miR-1301-3p and inhibited following miR-1301-3p knockdown. (B) The invasion of A549 and H1299 cells was promoted following overexpression of miR-1301-3p and inhibited following miR-1301-3p knockdown. ***P<0.001 vs. mock groups. miR, microRNA; NC, negative control.
Thy-1 is a direct target of miR-1301-3p
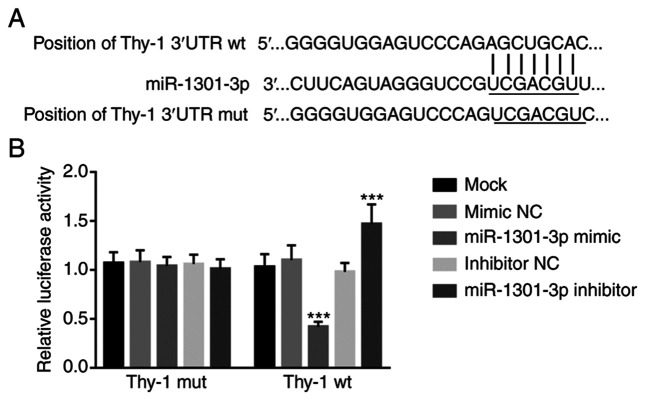
To determine the molecular mechanism underlying the biological function of miR-1301-3p in NSCLC, the target of miR-1301-3p was investigated. The target of miR-1301-3p was first predicted using the TargetScan database, and the potential binding sites are presented in Fig. 5A, which was further confirmed via the dual-luciferase reporter assay.
Figure 5.
Thy-1 is a direct target of miR-1301-3p. (A) The predicting binding sites of miR-1301-3p in the 3′-UTR of Thy-1-wt and Thy-1-mut. (B) Overexpression of miR-1301-3p inhibited the luciferase activity of Thy-1, while miR-1301-3p knockdown enhanced the luciferase activity of Thy-1. ***P<0.001 vs. mock groups. miR, microRNA; UTR, untranslated region; wt, wild-type; mut, mutant; NC, negative control.
The luciferase activity of Thy-1 was inhibited following overexpression of miR-1301-3p and increased following miR-1301-3p knockdown in the Thy-1 wild-type group (P<0.001; Fig. 5B). Conversely, neither miR-1301-3p overexpression or knockdown affected the luciferase activity of Thy-1 in the Thy-1 mutant group (Fig. 5B), suggesting that Thy-1 is a direct target of miR-1301-3p.
Discussion
NSCLC is a common malignant tumor that accounts for the majority of lung cancer cases, including adenocarcinoma, squamous cell carcinoma, large cell carcinoma and several other subtypes (20). The clinical outcome of NSCLC remains unsatisfactory due to the high metastasis and recurrence rates (4). It is well-known that miRNAs play important roles in the progression and development of several diseases, including cancers (21–23). For example, miR4766-5p, miR-1915-3p and miR-615-3p are associated with tumor development in gastric cancer (24–26). Several miRNAs have been identified as effective biomarkers for the progression of NSCLC. For example, miR-330-3p has been identified as an upregulated miRNA in NSCLC, in which upregulation remarkably promotes the proliferation, invasion and migration of NSCLC cells by activating the MAPK/ERK signaling pathway (27). The prognostic value of several miRNAs has also been reported in NSCLC and different types of cancer (28,29). For example, downregulated miR-5702 expression is associated with the clinical progression and poor prognosis of patients with NSCLC (30). Furthermore, miR-552 has been identified as a prognostic predictor for patients with colorectal cancer (31).
In the present study, miR-1301-3p expression was significantly upregulated in NSCLC tissues and cell lines, which is consistent with the miRNA expression profile of NSCLC (17). Dysregulation of miR-1301-3p expression was significantly associated with the TNM stage and lymph node metastasis of patients, suggesting that miR-1301-3p may be involved in the development of NSCLC. In addition, overexpression of miR-1301-3p was associated with the poor prognosis of patients and was identified as an independent indicator for the clinical prognosis of NSCLC, along with advanced TNM stage and lymph node metastasis, which is consistent with a previous study on colorectal cancer (32,33).
The levels of miR-1301 and its biological functions vary in different types of cancer. For example, downregulated miR-1301-3p expression in breast cancer acts as a tumor suppressor that inhibits cell proliferation and induces cell apoptosis (14). Similarly, in colorectal cancer, overexpression of miR-1301-3p suppresses cell proliferation and invasion, induces cell apoptosis, and decreases the volume and weight of colorectal tumors (33). Conversely, miR-1301-3p promotes cell expansion of prostate cancer stem cells by inhibiting GSK3β and SFRP1 and activating the Wnt pathway (15).
In the present study, overexpression of miR-1301-3p promoted the proliferation, migration and invasion of NSCLC cells, suggesting that miR-1301-3p may exert a tumor promoting role in the progression of NSCLC. In addition, thy-1 was identified as a direct target of miR-1301-3p. Thy-1 is a glycosylphosphatidylinositol-linked outer membrane leaflet glycoprotein (34). In a previous study, thy-1 has been reported to suppress myofibroblastic differentiation of lung fibroblasts, which is closely associated with the occurrence of lung cancer (35). In addition, thy-1 has also been reported to exert adverse impacts on the prognosis of lung cancer (36). In melanoma, thy-1 contributes to cell metastasis by mediating the adhesion of melanoma cells (37). Thus, it was concluded that the promoting effects of miR-1301-3p were mediated by regulating thy-1.
Among the clinicopathological characteristics of patients with NSCLC, tumor size, differentiation and smoking status are important factors to assess the clinical outcome of patients (38–40). However, the small sample size limited the results of the present study, which failed to exhibit a significant association between these factors and the overall survival of patients with NSCLC. Furthermore, in vivo experiments are required in prospective studies to confirm the results presented here.
In conclusion, the results of the present study demonstrated that miR-1301-3p expression was significantly upregulated in NSCLC tissues and cells, which was closely associated with the TNM stage and lymph node metastasis of patients. In addition, miR-1301-3p expression, advanced TNM stage and lymph node metastasis served as independent prognostic factors for NSCLC. Overexpression of miR-1301-3p significantly promoted the proliferation, migration and invasion of NSCLC cells by targeting thy-1. MiR-1301-3p was identified as a tumor promoter in NSCLC by regulating thy-1, which requires further in vivo validation.
Acknowledgements
Not applicable.
Funding Statement
The present study was funded by Binzhou Medical University Research Program and Research Startup Fund (grant no. BY2014KJ48).
Funding
The present study was funded by Binzhou Medical University Research Program and Research Startup Fund (grant no. BY2014KJ48).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
All authors contributed to the conception and design of the present study. LX, NN and PH performed the experiments. LX contributed to analysis and interpretation of the data. HG revised the manuscript for important intellectual content. All authors drafted the initial manuscript, and have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Binzhou Medical University Hospital (Binzhou, China; approval no. 201212), and written informed consent was provided by all patients prior to tissue collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kumar M, Ernani V, Owonikoko TK. Biomarkers and targeted systemic therapies in advanced non-small cell lung cancer. Mol Aspects Med. 2015;45:55–66. doi: 10.1016/j.mam.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Bilfinger T, Keresztes R, Albano D, Nemesure B. Five-year survival among Stage IIIA lung cancer patients receiving two different treatment modalities. Med Sci Monit. 2016;22:2589–2594. doi: 10.12659/MSM.898675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Marra A, Richardsen G, Wagner W, Muller-Tidow C, Koch OM, Hillejan L. Prognostic factors of resected node-positive lung cancer: Location, extent of nodal metastases, and multimodal treatment. Thorac Surg Sci. 2011;8:Doc01. doi: 10.3205/tss000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 7.Yu SL, Koo H, Lee SI, Kang J, Han YH, Yeom YI, Lee DC. A synthetic CPP33-conjugated HOXA9 active domain peptide inhibits invasion ability of non-small lung cancer cells. Biomolecules. 2020;10:1589. doi: 10.3390/biom10111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mielgo-Rubio X, Calvo V, Luna J, Remon J, Martín M, Berraondo P, Jarabo JR, Higuera O, Conde E, De Castro J, et al. Immunotherapy moves to the early-stage setting in non-small cell lung cancer: Emerging evidence and the role of biomarkers. Cancers (Basel) 2020;12:3459. doi: 10.3390/cancers12113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mustachio LM, Roszik J. Current targeted therapies for the fight against non-small cell lung cancer. Pharmaceuticals (Basel) 2020;13:374. doi: 10.3390/ph13110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis. 2015;35:3–11. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141:1202–1207. doi: 10.1016/j.jaci.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rupaimoole R, Slack FJ. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 13.Romano G, Veneziano D, Acunzo M, Croce CM. Small non-coding RNA and cancer. Carcinogenesis. 2017;38:485–491. doi: 10.1093/carcin/bgx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng X, Yan B, Shen Y. MiR-1301-3p inhibits human breast cancer cell proliferation by regulating cell cycle progression and apoptosis through directly targeting ICT1. Breast Cancer. 2018;25:742–752. doi: 10.1007/s12282-018-0881-5. [DOI] [PubMed] [Google Scholar]
- 15.Song XL, Huang B, Zhou BW, Wang C, Liao ZW, Yu Y, Zhao SC. miR-1301-3p promotes prostate cancer stem cell expansion by targeting SFRP1 and GSK3β. Biomed Pharmacother. 2018;99:369–374. doi: 10.1016/j.biopha.2018.01.086. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Zhao Y, Xu M, Zhou F, Yan J. Serum miR-1301-3p, miR-335-5p, miR-28-5p, and their target B7-H3 may serve as novel biomarkers for colorectal cancer. J BUON. 2019;24:1120–1127. [PubMed] [Google Scholar]
- 17.Zhang YQ, Wang WY, Xue JX, Xu Y, Fan P, Caughey BA, Tan WW, Cao GQ, Jiang LL, Lu Y, et al. MicroRNA expression profile on solid subtype of invasive lung adenocarcinoma reveals a panel of four miRNAs to Be associated with poor prognosis in Chinese patients. J Cancer. 2016;7:1610–1620. doi: 10.7150/jca.14923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huston SF, Abdelmalik AG, Nguyen NC, Farghaly HR, Osman MM. Whole-body 18F-FDG PET/CT: The need for a standardized field of view-a referring-physician aid. J Nucl Med Technol. 2010;38:123–127. doi: 10.2967/jnmt.109.073353. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10:760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn YH, Ko YH. Diagnostic and therapeutic implications of microRNAs in non-small cell lung cancer. Int J Mol Sci. 2020;21:8782. doi: 10.3390/ijms21228782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Aamri M, Yammouri G, Mohammadi H, Amine A, Korri-Youssoufi H. Electrochemical biosensors for detection of MicroRNA as a cancer biomarker: Pros and Cons. Biosensors. 2020;10:186. doi: 10.3390/bios10110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz-Quintero B. Extracellular MicroRNAs as intercellular mediators and noninvasive biomarkers of cancer. Cancers (Basel) 2020;12:3455. doi: 10.3390/cancers12113455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Liu L, Sun Y, Xue Y, Qu J, Pan S, Li H, Qu H, Wang J, Zhang J. miR-615-3p promotes proliferation and migration and inhibits apoptosis through its potential target CELF2 in gastric cancer. Biomed Pharmacother. 2018;101:406–413. doi: 10.1016/j.biopha.2018.02.104. [DOI] [PubMed] [Google Scholar]
- 25.Cui HW, Han WY, Hou LN, Yang L, Li X, Su XL. miR-1915-3p inhibits Bcl-2 expression in the development of gastric cancer. Biosci Rep. 2019;39:BSR20182321. doi: 10.1042/BSR20182321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Y, Wang Y, Zang A, Wang Z, Fang G, Hong D. miR-4766-5p inhibits the development and progression of gastric cancer by targeting NKAP. Onco Targets Ther. 2019;12:8525–8536. doi: 10.2147/OTT.S220234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei CH, Wu G, Cai Q, Gao XC, Tong F, Zhou R, Zhang RG, Dong JH, Hu Y, Dong XR. MicroRNA-330-3p promotes cell invasion and metastasis in non-small cell lung cancer through GRIA3 by activating MAPK/ERK signaling pathway. J Hematol Oncol. 2017;10:125. doi: 10.1186/s13045-017-0493-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Jia Y, Tan W, Zhou Y. Transfer RNA-derived small RNAs: Potential applications as novel biomarkers for disease diagnosis and prognosis. Ann Transl Med. 2020;8:1092. doi: 10.21037/atm-20-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parizi PK, Yarahmadi F, Tabar HM, Hosseini Z, Sarli A, Kia N, Tafazoli A, Esmaeili SA. MicroRNAs and target molecules in bladder cancer. Med Oncol. 2020;37:118. doi: 10.1007/s12032-020-01435-0. [DOI] [PubMed] [Google Scholar]
- 30.Li K, Xu Y, Yuan LN. Down-regulation of miR-5702 is associated with clinical progression and poor prognosis in patients with non-small-cell lung cancer. Eur Rev Med Pharmacol Sci. 2019;23:2047–2052. doi: 10.26355/eurrev_201903_17245. [DOI] [PubMed] [Google Scholar]
- 31.Wang N, Liu W. Increased expression of miR-552 acts as a potential predictor biomarker for poor prognosis of colorectal cancer. Eur Rev Med Pharmacol Sci. 2018;22:412–416. doi: 10.26355/eurrev_201801_14189. [DOI] [PubMed] [Google Scholar]
- 32.Wen J, Wang H, Dong T, Gan P, Fang H, Wu S, Li J, Zhang Y, Du R, Zhu Q. STAT3-induced upregulation of lncRNA ABHD11-AS1 promotes tumour progression in papillary thyroid carcinoma by regulating miR-1301-3p/STAT3 axis and PI3K/AKT signalling pathway. Cell Prolif. 2019;52:e12569. doi: 10.1111/cpr.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu G, Wang H, Yuan D, Yao J, Meng L, Li K, Zhang Y, Dang C, Zhu K. RUNX1-activated upregulation of lncRNA RNCR3 promotes cell proliferation, invasion, and suppresses apoptosis in colorectal cancer via miR-1301-3p/AKT1 axis in vitro and in vivo. Clin Transl Oncol. 2020;22:1762–1777. doi: 10.1007/s12094-020-02335-5. [DOI] [PubMed] [Google Scholar]
- 34.Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006;20:1045–1054. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 35.Shentu TP, Huang TS, Cernelc-Kohan M, Chan J, Wong SS, Espinoza CR, Tan C, Gramaglia I, van der Heyde H, Chien S, Hagood JS. Thy-1 dependent uptake of mesenchymal stem cell-derived extracellular vesicles blocks myofibroblastic differentiation. Sci Rep. 2017;7:18052. doi: 10.1038/s41598-017-18288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schliekelman MJ, Creighton CJ, Baird BN, Chen Y, Banerjee P, Bota-Rabassedas N, Ahn YH, Roybal JD, Chen F, Zhang Y, et al. Thy-1+ Cancer-associated fibroblasts adversely impact lung cancer prognosis. Sci Rep. 2017;7:6478. doi: 10.1038/s41598-017-06922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schubert K, Gutknecht D, Koberle M, Anderegg U, Saalbach A. Melanoma cells use Thy-1 (CD90) on endothelial cells for metastasis formation. Am J Pathol. 2013;182:266–276. doi: 10.1016/j.ajpath.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Hsu HH, Ko KH, Chou YC, Lin LF, Tsai WC, Lee SC, Chang H, Huang TW. SUVmax and tumor size predict surgical outcome of synchronous multiple primary lung cancers. Medicine (Baltimore) 2016;95:e2351. doi: 10.1097/MD.0000000000002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrovic M, Baskic D, Bankovic D, Ilic N. Neuroendocrine differentiation as an indicator of chemosensitivity and prognosis in nonsmall cell lung cancer. Biomarkers. 2011;16:311–320. doi: 10.3109/1354750X.2011.560281. [DOI] [PubMed] [Google Scholar]
- 40.Fukui M, Suzuki K, Matsunaga T, Oh S, Takamochi K. Importance of smoking cessation on surgical outcome in primary lung cancer. Ann Thorac Surg. 2019;107:1005–1009. doi: 10.1016/j.athoracsur.2018.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.