Abstract
Introduction: Older adults are indisputably struck hard by the coronavirus disease 2019 (COVID-19) pandemic. The main objective of this meta-analysis is to establish the association between delirium and mortality in older adults with COVID-19.
Methods: Systematic literature searches of PubMed, Embase, and Scopus databases were performed up until 28 November 2020. The exposure in this study was the diagnosis of delirium using clinically validated criteria. Delirium might be in-hospital, at admission, or both. The main outcome was mortality defined as clinically validated non-survivor/death. The effect estimates were reported as odds ratios (ORs) and adjusted odds ratios (aORs).
Results: A total of 3,868 patients from 9 studies were included in this systematic review and meta-analysis. The percentage of patients with delirium was 27% [20%, 34%]. Every 1 mg/L increase in CRP was significantly associated with 1% increased delirium risk (OR 1.01 [1.00. 1.02], p=0.033). Delirium was associated with mortality (OR 2.39 [1.64, 3.49], p<0.001; I2: 82.88%). Subgroup analysis on delirium assessed at admission indicate independent association (OR 2.12 [1.39, 3.25], p<0.001; I2: 82.67%). Pooled adjusted analysis indicated that delirium was independently associated with mortality (aOR 1.50 [1.16, 1.94], p=0.002; I2: 31.02%). Subgroup analysis on delirium assessed at admission indicate independent association (OR 1.40 [1.03, 1.90], p=0.030; I2: 35.19%). Meta-regression indicates that the association between delirium and mortality were not significantly influenced by study-level variations in age, sex [reference: male], hypertension, diabetes, and dementia.
Conclusion: The presence of delirium is associated with increased risk of mortality in hospitalized older adults with COVID-19.
Keywords: Confusion, Delirium, Geriatric, SARS-CoV-2, Severe
1. Introduction
Older adults are indisputably struck hard by the coronavirus disease 2019 (COVID-19) pandemic. They are more susceptible to SARS-CoV-2 infection than the general population and frequently develop more severe forms of COVID-19 with high mortality rates. Managing critically-ill patients with COVID-19 during pandemic is challenging due to limited respiratory support and intensive care readiness; however, treating older adults in similar contexts is entirely on different levels of complexity due to multi-dimensional problems dilemmatic ethical decisions.(Martínez-Sellés et al., 2020; Truog et al., 2020)
Even though age increases risk of mortality in patients with COVID-19, age alone is insufficient for risk stratification. Risk stratification in the geriatric population can be improved by incorporating multi-faceted assessment using well-defined clinical scoring systems for prognostication in geriatric population with COVID-19, including frailty assessment (e.g. using clinical frailty scale) and comorbidities (e.g. Charlson comorbidity index).(Pranata et al., 2021; Kuswardhani et al., 2020) Another promising clinical predictor for poor prognosis in older adults with COVID-19, as recently shown by Ticinesi et al., is an acute confusional state, commonly known as delirium.(Ticinesi et al., 2020) Delirium is an acute disorder with a fluctuating course of altered consciousness or cognitive function, which commonly affects the hospitalized older adults.(Inouye et al., 2014) Whether the association of delirium and mortality in older adults with COVID-19 is independent or associated with other comorbidities is still uncertain.
The main objective of this meta-analysis is to establish the association between delirium and mortality in older adults with COVID-19.
2. Methods
This study follows the Meta-analyses Of Observational Studies in Epidemiology (MOOSE) reporting guidelines. The protocol for this study is registered in the PROSPERO (CRD42020223351).
2.1. Eligibility criteria
The inclusion criteria for this study were: 1) published prospective or retrospective observational studies, 2) Information on delirium, 3) mortality rate in COVID-19 patients stratified by the presence of delirium.
We excluded the paper if it fulfils one of the following criteria: 1) pre-prints, 2) abstract-only publication, 3) non-research letters, 4) reviews, 5) case reports, and 6) non-English language articles. We exclude preprints because of concern regarding the validity of the data.(Henrina et al., 2020)
2.2. Search strategy and study selection
Systematic literature searches of PubMed, Embase, and Scopus databases were performed with keywords “2019-nCoV” OR "COVID-19" OR "SARS-CoV-2" AND “Delirium” up until 28 November 2020. The PubMed (MEDLINE) search strategy was ((2019-nCoV or COVID-19 or SARS-CoV-2) and Delirium). After removal of duplicates, two authors independently screened the title/abstract of the records. Full-text of potentially eligible studies were assessed by applying the inclusion and exclusion criteria.
2.3. Data Extraction
Data extraction was performed by two independent authors, these data include first author, year of publication, design, delirium, criteria for delirium, timing for delirium diagnosis, age, male (gender), hypertension, diabetes, chronic obstructive pulmonary disease, dementia, and mortality.
The population in this study was patients with diagnosis of COVID-19 established by reverse transcriptase polymerase chain reaction (RT-PCR).
The exposure in this study was the diagnosis of delirium using clinically validated criteria. Delirium might be in-hospital, at admission, or both. The main outcome was mortality defined as clinically validated non-survivor/death. The effect estimates were reported as odds ratios (ORs) and adjusted odds ratios (aORs).
The risk of bias and quality of the included studies were assessed using the Newcastle-Ottawa Scale (NOS) by two independent authors. Any discrepancies that aroused were resolved by discussion.
2.4. Statistical analysis
We used STATA 16 (StataCorp LLC, Texas, US) to perform meta-analysis. Meta-analysis of proportion was performed to calculate the prevalence of delirium in the included studies. Restricted-maximum likelihood (REML) random-effects meta-analysis was used to generate pooled ORs and aORs along with their 95% confidence interval. P-values were considered as significant if they are ≤0.05. Inter-study heterogeneity was assessed using the I-squared (I2) and Cochrane Q test, an effect estimate was considered as significant for heterogeneity if the value was >50% or <0.10. To assess publication bias and small-study effects, we performed funnel plot analysis and Egger's test. Trim-and-fill analysis using run estimator was performed to account for publication bias. Meta-regression analysis using REML model was performed to assess whether the association between proportion; exposure and outcome varies by age, gender (male), hypertension, diabetes, chronic obstructive pulmonary disease, and dementia. Subgroup analysis for delirium “at admission” was performed.
3. Results
3.1. Baseline Characteristics of the Included Studies
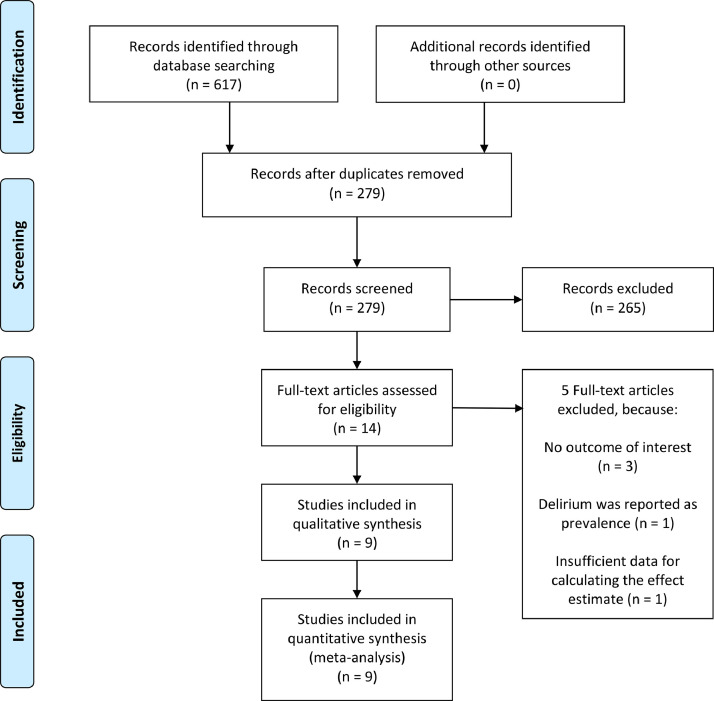
A total of 3,868 patients from 9 studies were included in this systematic review and meta-analysis [Fig. 1 ].(Garcez et al., 2020; Kennedy et al., 2020; Knopp et al., 2020; Marengoni et al., 2020; Mcloughlin et al., 2020; Mendes et al., 2020; Poloni et al., 2020; Ticinesi et al., 2020; Zerah et al., 2020) The baseline characteristics of the included studies can be seen in Table 1 . Risk of bias assessment based on NOS and adjustment for each multivariate analysis can be seen in Table 2 . The percentage of patients with delirium was 27% [20%, 34%], and it does not vary with age (p=0.122), male (p=0.286), hypertension (p=0.669), diabetes (p=0.215), chronic obstructive pulmonary disease (p=0.636), and dementia (p=0.787). Every 1 mg/L increase in CRP was significantly associated with 1% increased delirium risk (OR 1.01 [1.00. 1.02], p=0.033).
Fig. 1.
PRISMA Flowchart
Table 1.
Baseline Characteristics of the Included Studies
| Authors | Design | Sample | Delirium | Patients Characteristics | ||||||||
| Prevalence (%) | Diagnosis | Timing | Age (years) | Male (%) | Hypertension (%) | Diabetes (%) | COPD (%) | CI/Dementia (%) | CRP (mg/L) | |||
| Garcez 2020 | RO | 707 | 33.1 | CHART-DEL | In-hospital | 66 | 57 | 68 | 42 | 10 | 4 (Dementia) | 132 |
| Kennedy 2020 | RO | 817 | 27.7 | CAM | At Admission | 77.7 | 47 | N/A | 38 | 27 | 30 (CI/Dementia) | N/A |
| Knopp 2020 | PO | 217 | 29.5 | N/A | At Admission | 80 | 62 | N/A | N/A | N/A | 33 (Dementia) | 92 |
| Marengoni 2020 | RO | 91 | 27.5 | 4AT, DSM-V | At Admission + In-hospital | 79.5 | 60.4 | N/A | N/A | N/A | N/A | N/A |
| Mcloughlin 2020 | O | 71 | 43.7 | 4-AT, DSM-IV | Single day | 64.9 | 74.4 | N/A | N/A | N/A | 12.8 (Dementia) | N/A |
| Mendes 2020 | RO | 235 | 12.3 | CAM | At Admission | 86.3 | 43.4 | 71.5 | 23 | 10.6 | 50.6 (Cognitive Disorders) | 66.3 |
| Poloni 2020 | RO | 57 | 36.8 | CAM | At Admission | 82.8 | 33.3 | 54.4 | 19.3 | 14 | 100 (Dementia) | 152.4 |
| Ticinesi 2020 | RO | 852 | 11.0 | CAM | At Admission | 75.7 | 52.8 | 58.5 | 21 | 12 | 18.3 (Dementia) | 99.2 |
| Zerah 2020 | RO | 821 | 25.0 | CAM | At Admission | 86 | 58 | 67 | 25 | 12 | 54 (Dementia) | 113 |
CAM: Confusion Assessment Method, CHART-DEL: Chart-Based Delirium Identification Instrument, CI: Cognitive Impairment, COPD: Chronic Obstructive Pulmonary Disease, DSM-IV: Diagnostic and Statistical Manual of Mental Disorders 4, DSM-V: Diagnostic and Statistical Manual of Mental Disorders 5, O: Observational, PO: Prospective Observational, RO: Retrospective Observational, N/A: Not Available
Table 2.
Assessment of outcome analysis of each studies
| Authors | Adjustment | NOS |
| Garcez 2020 | Age, sex, literacy, previous diagnoses, Charlson comorbidity index, polypharmacy, days of symptoms, oxygen support, temperature, mean arterial pressure, lymphocyte count, C-reactive protein, glomerular filtration rate, D-dimer, and albumin | 8 |
| Kennedy 2020 | Age older than 75 years, the presence of 4 or more comorbid conditions, living in nursing home or assisted living, and prior psychoactive medications. | 8 |
| Knopp 2020 | Unclear | 7 |
| Marengoni 2020 | N/A | 6 |
| Mcloughlin 2020 | Age, sex and Clinical Frailty Scale score | 7 |
| Mendes 2020 | N/A | 7 |
| Poloni 2020 | N/A | 7 |
| Ticinesi 2020 | Age, multimorbidity and baseline respiratory conditions | 8 |
| Zerah 2020 | N/A | 7 |
NOS: Newcastle-Ottawa Scale.
3.2. Delirium and Mortality
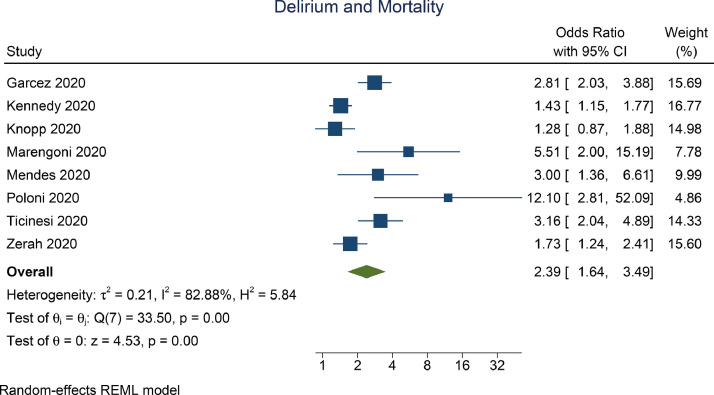
Delirium was associated with mortality (OR 2.39 [1.64, 3.49], p<0.001; I2: 82.88%, p<0.001) [Fig. 2 ]. Subgroup analysis on delirium assessed at admission indicate independent association (OR 2.12 [1.39, 3.25], p<0.001; I2: 82.67%, p<0.001). Subgroup analysis on delirium based on CAM assessment (OR 2.43 [1.48, 4.00], p<0.001; I2: 83.2%, p=0.001) showed significant association.
Fig. 2.
Delirium and Mortality (Unadjusted)
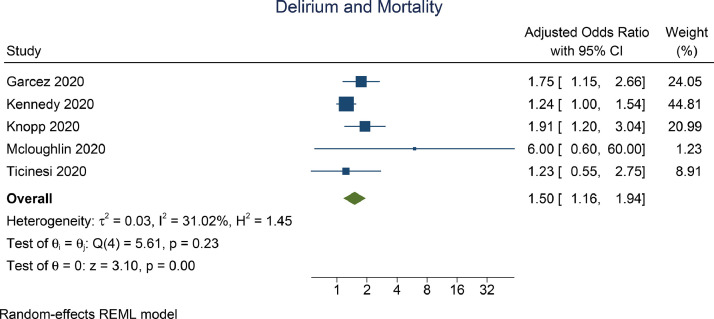
Pooled adjusted analysis indicate that delirium was independently associated with mortality (aOR 1.50 [1.16, 1.94], p=0.002; I2: 31.02%, p=0.230) [Fig. 3 ]. Subgroup analysis on delirium assessed at admission indicate independent association (OR 1.40 [1.03, 1.90], p=0.030; I2: 35.19%, p=0.252). Subgroup analysis on delirium based on CAM assessment (OR 1.24 [1.07, 1.81], p<0.001; I2: 0%, p=0.985) showed significant association.
Fig. 3.
Delirium and Mortality (Adjusted)
3.3. Publication Bias
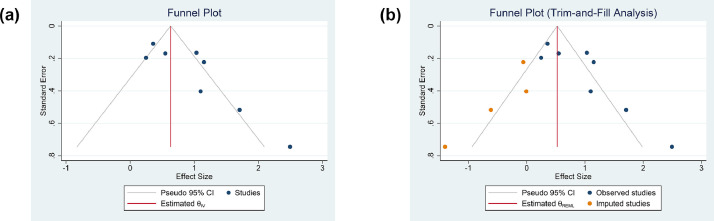
The funnel plot for the association between delirium and mortality was asymmetrical [Fig. 4 A]. Trim-and-fill analysis (run estimator) by imputation of 5 studies on the left side resulted in OR of 1.70 [1.49, 1.92] [Fig. 4B]. Egger's test showed indication of small-study effects (p=0.002).
Fig. 4.
Funnel-plot analysis (A) and Trim-and-fill analysis (B)
3.4. Sensitivity Analysis
Sensitivity analyses were performed by excluding Garcez 2020 (in-hospital delirium and using CHART-DEL), Mcloughlin 2020 (single day delirium), and Poloni 2020 (100% dementia); in turn. The pooled unadjusted and adjusted effect estimates remained significant after sensitivity analyses [Table 3 ].
Table 3.
Sensitivity Analyses
| Pooled Effect Estimate | Unadjusted Model (OR with 95% CI) | Adjusted Model (OR with 95% CI) |
| Full Model | 2.39 [1.64, 3.49], p<0.001; I2: 82.9%, | 1.50 [1.16, 1.94], p=0.002; I2: 31.02% |
| Exclusion of Garcez 2020 | 2.39 [1.52, 3.78], p<0.001; I2: 84.9% | 1.44 [1.05, 1.98], p<0.001; I2: 30.7% |
| Exclusion of Garcez 2020 + Mcloughlin 2020* | 2.39 [1.52, 3.78], p<0.001; I2: 84.9% | 1.40 [1.03, 1.90], p<0.001; I2: 35.2% |
| Exclusion of Garcez 2020 + Mcloughlin 2020 + Poloni 2020# | 2.07 [1.41, 3.04], p<0.001; I2: 79.8% | 1.40 [1.03, 1.90], p<0.001; I2: 35.2% |
Garcez 2020 (in-hospital delirium and using CHART-DEL)
Mcloughlin 2020 (single day delirium)
Poloni 2020 (100% dementia).
Mcloughlin 2020 only reported adjusted outcome
Poloni 2020 only reported unadjusted outcome
3.5. Meta-regression
Meta-regression indicates that the association between delirium and mortality were not significantly influenced by study-level variations in age (p=0.879), sex [reference: male] (p=0.227), hypertension (p=0.196), diabetes (p=0.312), dementia (p=0.393), and CRP (p=0.297). A statistically non-significant trend towards variation by chronic obstructive pulmonary disease was observed (coefficient: -0.039, p=0.075).
4. Discussion
Delirium was shown to increase mortality in patients with COVID-19 both unadjusted and adjusted models. This meta-analysis provides evidence and reinforces the previous well-established independent association of the impact of delirium on mortality,(Witlox et al., 2010) but at the present time is in the context of COVID-19.
The disparity of heterogeneity in the unadjusted model and adjusted model might be explained by the diverse definition of delirium used in the included studies. While the majority of studies used confusion assessment method (CAM) in defining delirium (Table 1), in the rest of the studies, delirium was diversely defined based on the following : 4AT, Chart-Based Delirium Identification Instrument (CHART-DEL), Diagnostic and Statistical Manual of Mental Disorders 4 and 5 (DSM-IV and DSM-V). Another possible cause of heterogeneity was the various confounders in the studies, which was reduced by the adjustment to confounders. Dementia was shown to be associated with mortality in patients with COVID-19.(July & Pranata, 2020) Poloni et al. study was exclusively on patients with dementia, they demonstrated a very high OR compared to other studies. However, Poloni et al. study has a small sample and wide confidence interval, thus only contributing to a small weight to the pooled analysis. In contrast, Garcez et al., who has a small number of patients with dementia, has a higher OR than several studies with a higher prevalence of dementia. Thus, our meta-regression analysis did not show statistical significance for dementia. However, the analyses were based on dementia as a diagnosis, not graded by severity. This is due to inadequate reporting of dementia grade by the studies. Sensitivity analyses indicate that after removing Garcez et al., Mcloughin et al., and Poloni et al. studies, the pooled effect estimate remained statistically significant, indicating statistical robustness.
COVID-19 patients living in the nursing home or assisted living, prior use of psychoactive medications, hearing and vision impairment, and stroke were shown to be associated with delirium.(Kennedy et al., 2020; Ticinesi et al., 2020) Our regression analysis indicates that the presence of delirium did not vary significantly by age, however, the finding might be due to a narrow age variation among the studies. Although not included in our outcome of interest, several notable findings are found. in patients with delirium. Delirium was associated with the increased length of hospital stay, the need for intensive care, and mechanical ventilation.(Garcez et al., 2020) Additionally, patients with delirium have a substantially poorer functional and cognitive outcome post-discharge.(Knopp et al., 2020; Mcloughlin et al., 2020)
Altered consciousness as a neurologic manifestation of COVID-19, ranging from somnolence to confusion, delirium, stupor, and coma, occurs in nearly 15% of hospitalized COVID-19 patients in the early report.(Mao et al., 2020) Altered consciousness may depend on the direct effects of SARS-CoV-2 infection, possible viral invasion to the central nervous system (CNS), secondary encephalopathy due to systemic inflammation, or other precipitating factors, including prolonged duration of hospital stay, urinary retention, constipation, pain, and various metabolic abnormalities that occur during severe infection.(Beach et al., 2020; Zambrelli et al., 2020) The presence of comorbidities, including advanced age, excessive body mass index (BMI), hypertension, diabetes mellitus, cardiovascular and cerebrovascular disease, chronic kidney disease, and lung disease, place patients at a higher risk of developing severe form of COVID-19, including the need for mechanical ventilation and admission to intensive care unit (ICU).(R. Pranata et al., 2020) Neurotransmitter imbalance, pro-inflammatory cytokines, tissue hypoxia, and sleep deprivation play a significant role in delirium pathomechanism.
COVID-19-associated primary encephalopathies may develop through similar pathways as other (non-CNS) infectious and non-infectious causes (e.g. toxic/metabolic encephalopathy). SARS-CoV-2 is thought to possess a similar capability to SARS-CoV, in invading the brain via a chain connected by synapses that extend from receptors in the lung to the medullary cardiorespiratory center, eventually causing acute respiratory failure.(Beach et al., 2020) Besides, the expression of angiotensin-converting enzyme 2 (ACE2) in the CNS (e.g. glial cells in brain, spinal neurons), which serves as an entry point for SARS-CoV-2, and the nature of its interaction with the nicotinic acetylcholine receptor (nAChR) increase the likelihood of viral invasion of the CNS.(Ahmad & Rathore, 2020) Direct endothelial infection and blood-brain-barrier (BBB) breakdown allows diapedesis of infected leukocytes into the brain. As seen in herpes simplex virus (HSV) encephalitis, abnormalities in magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) analysis were seen in some COVID-19 patients regardless of the presence of neuropsychiatric symptoms.(Beach et al., 2020) Another possible mechanism is viral invasion of the olfactory bulb toward the uncinate fasciculus until it reaches anterior cingulate cortex and basal forebrain, which is consistent with the high incidence of anosmia and ageusia in COVID-19 patients.(Ahmad & Rathore, 2020; Baller et al., 2020) In patients with concomitant systemic sclerosis and systemic lupus erythematosus (SLE) diagnosis, the decrease in the sense of smell correlates with neurocognitive dysfunction, inflammation, and hippocampi and amygdala volumes.(Beach et al., 2020)
COVID-19-associated secondary delirium may be caused by the impacts of acute respiratory distress syndrome (ARDS), including hypoxemia and oxidative stress due to diffuse alveolar damage, pulmonary emboli (PE) and cardiovascular accidents (CVA) due to hypercoagulability and disseminated intravascular coagulation (DIC), as well as hypoperfusion, uremia, and other metabolic disarray due to multi-organ dysfunction.(Ahmad & Rathore, 2020; Beach et al., 2020; Lim et al., 2020; Wijaya et al., 2020) Another potential mechanism is the development of secondary encephalopathy through the hyperactivation of immune response and cytokine storm, characterized by activation of T lymphocytes, macrophages, and endothelial cells and excessive release of pro-inflammatory cytokines such as interleukin, C-reactive protein, D-dimer, procalcitonin, and ferritin.(Ahmad & Rathore, 2020; Beach et al., 2020; Huang et al., 2020; Yonas et al., 2020) Endothelial transfer and penetration of inflammatory cytokines via the damaged BBB are likely to induce encephalopathy. One of the extreme manifestations is acute hemorrhagic necrotizing encephalopathy (AHNE) caused by SARS-CoV-2 infection and possibly related to cytokine storm. Lastly, a hybrid model, in which the novel coronavirus causes primary, secondary, or combined encephalopathy, is also possible.(Beach et al., 2020)
Routine investigation of MRI, CSF analysis, and electroencephalography (EEG) in COVID-19 patients with neuropsychiatric symptoms may not be practical considering the clinical status, cost, availability of resources, and staff exposure.(Beach et al., 2020) However, delirium appears to be a crucial sequela of COVID-19 and likely to present with other neuropsychiatric manifestations, including restlessness, agitation, hyperactivity, rigidity or increased tone, myoclonus, alogia, abulia, and other systemic inflammatory response.(Ahmad & Rathore, 2020; Baller et al., 2020) Therefore, all medical providers should expand diagnostic workup criteria to include CNS manifestations, including altered mental status. Guidelines should include delirium as a possible presenting feature, screening should be a standard of care, and non-pharmacological approaches to prevent and manage delirium need to be addressed.(Beach et al., 2020; O'Hanlon & Inouye, 2020)
The management of delirium in COVID-19 is pretty challenging, given that no guidelines are available so far. Compared to benzodiazepines and antipsychotics, melatonin is a safer option that should be considered in the prevention and treatment of sleep-wake rhythm disorders and impaired consciousness, including delirium, in COVID-19 patients.(Zambrelli et al., 2020) The use of exogenous melatonin or melatonin receptor agonist (MRA), such as ramelton, has been associated with improved sleep quality, reduced prevalence of delirium, and shortened duration of ICU stay.(Zhang et al., 2019) In addition, these agents are also postulated to be protective against acute lung injury caused by infection or ventilator given their anti-oxidative, anti-inflammatory, and immune-enhancing properties.(Wu et al., 2020)
The major limitation of the current meta-analysis was that all studies were retrospective observational in nature, which renders a high risk of bias. Moreover, cautious interpretation of this meta-analysis should take into account the possible presence of publication bias and small-study effects. Participants of the different studies have considerable variety regarding their criteria for example: percentage of home residents. Lastly, this meta-analysis did not prove causality, the results showed that delirium is an important alarm symptom associated with poor prognosis. Whether the association is causal requires further investigation.
5. Conclusion
The presence of delirium is associated with increased risk of mortality in hospitalized older adults with COVID-19.
Authors Contribution
Raymond Pranata: This author helped in concept development, manuscript drafting, data acquisition, data analysis, and statistical analysis.
Ian Huang: This author helped in manuscript drafting, data acquisition, and data analysis
Michael Anthonius Lim: This author helped in manuscript drafting, data acquisition, and data analysis.
Emir Yonas: This author helped in manuscript review and editing
Rachel Vania: This author helped in manuscript review and editing
Raden Ayu Tuty Kuswardhani: This author helped in manuscript review and editing
The protocol for this study is registered in the PROSPERO (CRD42020223351).
Declaration of Competing Interest
None Declared
Acknowledgements
None
References
- Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: A literature review. Journal of Clinical Neuroscience : Official Journal of the Neurosurgical Society of Australasia. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baller E.B., Hogan C.S., Fusunyan M.A., Ivkovic A., Luccarelli J.W., Madva E., Nisavic M., Praschan N., Quijije N.V, Beach S.R., Smith F.A. Neurocovid: Pharmacological recommendations for delirium associated with COVID-19. Psychosomatics. 2020;61(6):585–596. doi: 10.1016/j.psym.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach S.R., Praschan N.C., Hogan C., Dotson S., Merideth F., Kontos N., Fricchione G.L., Smith F.A. Delirium in COVID-19: A case series and exploration of potential mechanisms for central nervous system involvement. General Hospital Psychiatry. 2020;65:47–53. doi: 10.1016/j.genhosppsych.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcez F.B., Aliberti M.J.R., Poco P.C.E., Hiratsuka M., Takahashi S.de F., Coelho V.A., Salotto D.B., Moreira M.L.V., Jacob-Filho W., Avelino-Silva T.J. Delirium and adverse outcomes in hospitalized patients with COVID-19. Journal of the American Geriatrics Society. 2020;68(11):2440–2446. doi: 10.1111/jgs.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrina J., Lim M.A., Pranata R. COVID-19 and misinformation: how an infodemic fuelled the prominence of vitamin D. British Journal of Nutrition. 2020:1–2. doi: 10.1017/S0007114520002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Therapeutic Advances in Respiratory Disease. 2020;14 doi: 10.1177/1753466620937175. 175346662093717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S.K., Westendorp R.G.J., Saczynski J.S. Delirium in elderly people. Lancet (London, England) 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- July J., Pranata R. Prevalence of dementia and its impact on mortality in patients with coronavirus disease 2019: A systematic review and meta-analysis. Geriatrics & Gerontology International. 2020 doi: 10.1111/ggi.14107. ggi.14107. [DOI] [PubMed] [Google Scholar]
- Kennedy M., Helfand B.K.I., Gou R.Y., Gartaganis S.L., Webb M., Moccia J.M., Bruursema S.N., Dokic B., McCulloch B., Ring H., Margolin J.D., Zhang E., Anderson R., Babine R.L., Hshieh T., Wong A.H., Taylor R.A., Davenport K., Teresi B., …, Inouye S.K. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Network Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp P., Miles A., Webb T.E., Mcloughlin B.C., Mannan I., Raja N., Wan B., Davis D. European Geriatric Medicine; 2020. Presenting features of COVID-19 in older people: relationships with frailty, inflammation and mortality. 2020.06.07.20120527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswardhani R.A.T., Henrina J., Pranata R., Lim Anthonius, M. Lawrensia, S, Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: A systematic review and meta-analysis. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(6):2103–2109. doi: 10.1016/j.dsx.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: Systematic review and Meta-analysis. Canadian Journal of Kidney Health and Disease. 2020;7 doi: 10.1177/2054358120938573. 205435812093857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengoni A., Zucchelli A., Grande G., Fratiglioni L., Rizzuto D. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age and Ageing. 2020;49(6):923–926. doi: 10.1093/ageing/afaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Sellés D., Martínez-Sellés H., Martinez-Sellés M. Ethical issues in decision-making regarding the elderly affected by coronavirus disease 2019: an expert opinion. European Cardiology. 2020;15:e48. doi: 10.15420/ecr.2020.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcloughlin B.C., Miles A., Webb T.E., Knopp P., Eyres C., Fabbri A., Humphries F., Davis D. Functional and cognitive outcomes after COVID-19 delirium. European Geriatric Medicine. 2020;11(5):857–862. doi: 10.1007/s41999-020-00353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes A., Serratrice C., Herrmann F.R., Genton L., Périvier S., Scheffler M., Fassier T., Huber P., Jacques M.-C., Prendki V., Roux X., Di Silvestro K., Trombert V., Harbarth S., Gold G., Graf C.E., Zekry D. Predictors of in-hospital mortality in older patients with covid-19: the COVIDAge study. Journal of the American Medical Directors Association. 2020;21(11):1546–1554. doi: 10.1016/j.jamda.2020.09.014. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanlon S., Inouye S.K. Delirium: a missing piece in the COVID-19 pandemic puzzle. Age and Ageing. 2020;49(4):497–498. doi: 10.1093/ageing/afaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloni T.E., Carlos A.F., Cairati M., Cutaia C., Medici V., Marelli E., Ferrari D., Galli A., Bognetti P., Davin A., Cirrincione A., Ceretti A., Cereda C., Ceroni M., Tronconi L., Vitali S., Guaita A. Prevalence and prognostic value of delirium as the initial presentation of COVID-19 in the elderly with dementia: An Italian retrospective study. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Henrina J., Lim M.A., Lawrensia S., Yonas E., Vania R., Huang I., Lukito A.A., Suastika K., Kuswardhani R.A.T., Setiati S. Clinical frailty scale and mortality in COVID-19: A systematic review and dose-response meta-analysis. Archives of Gerontology and Geriatrics. 2021;93 doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B., Meyer M. Body mass index and outcome in patients with COVID-19: A dose–response meta-analysis. Diabetes and Metabolism. 2020 doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticinesi A., Cerundolo N., Parise A., Nouvenne A., Prati B., Guerra A., Lauretani F., Maggio M., Meschi T. Delirium in COVID-19: epidemiology and clinical correlations in a large group of patients admitted to an academic hospital. Aging Clinical and Experimental Research. 2020;32(10):2159–2166. doi: 10.1007/s40520-020-01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truog R.D., Mitchell C., Daley G.Q. The Toughest Triage — Allocating ventilators in a pandemic. New England Journal of Medicine. 2020;382(21):1973–1975. doi: 10.1056/NEJMp2005689. [DOI] [PubMed] [Google Scholar]
- Wijaya I., Andhika R., Huang I. The use of therapeutic-dose anticoagulation and its effect on mortality in patients with covid-19: a systematic review. Clinical and Applied Thrombosis/Hemostasis. 2020;26 doi: 10.1177/1076029620960797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witlox J., Eurelings L.S.M., de Jonghe J.F.M., Kalisvaart K.J., Eikelenboom P., van Gool W.A. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- Wu G.-C., Peng C.-K., Liao W.-I., Pao H.-P., Huang K.-L., Chu S.-J. Melatonin receptor agonist protects against acute lung injury induced by ventilator through up-regulation of IL-10 production. Respiratory Research. 2020;21(1):65. doi: 10.1186/s12931-020-1325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonas E., Alwi I., Pranata R., Huang I., Lim M.A., Yamin M., Nasution S.A., Setiati S., Virani S.S. Elevated interleukin levels are associated with higher severity and mortality in COVID 19 – A systematic review, meta-analysis, and meta-regression. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(6):2219–2230. doi: 10.1016/j.dsx.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrelli E., Canevini M., Gambini O., D'Agostino A. Delirium and sleep disturbances in COVID-19: a possible role for melatonin in hospitalized patients? Sleep Medicine. 2020;70:111. doi: 10.1016/j.sleep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerah L., Baudouin É., Pépin M., Mary M., Krypciak S., Bianco C., Roux S., Gross A., Toméo C., Lemarié N., Dureau A., Bastiani S., Ketz F., Boully C., de Villelongue C., Romdhani M., Desoutter M.-A., Duron E., David J.-P., …, Boddaert J. Clinical characteristics and outcomes of 821 older patients with sars-cov-2 infection admitted to acute care geriatric wards. The Journals of Gerontology: Series A. 2020:1–9. doi: 10.1093/gerona/glaa210. XX(Xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Gao F., Zhang S., Sun W., Li Z. Prophylactic use of exogenous melatonin and melatonin receptor agonists to improve sleep and delirium in the intensive care units: a systematic review and meta-analysis of randomized controlled trials. Sleep & Breathing = Schlaf & Atmung. 2019;23(4):1059–1070. doi: 10.1007/s11325-019-01831-5. [DOI] [PubMed] [Google Scholar]