Abstract
The chicken egg yolk, which is abundant with lipids, proteins, and minerals, is the major nutrient resource for the embryonic development. In fact, the magnitude and type of yolk nutrients are dynamically changed during the chicken embryogenesis to meet the developmental and nutritional requests at different stages. The yolk nutrients are metabolized and absorbed by the yolk sac membrane and then used by the embryo or other extraembryonic tissues. Thus, understanding the metabolites in the yolk helps to unveil the developmental nutritional requirements for the chicken embryo. In this study, we performed ultra high performance liquid chromatography/tandem mass spectrometry (UHPLC-MS/MS) analysis to investigate the change of metabolites in the egg yolk at embryonic (E) 07, E09, E11, E15, E17, and E19. The results showed that 1) the egg yolk metabolites at E07 and E09 were approximately similar, but E09, E11, E15, E17, and E19 were different from each other, indicating the developmental and metabolic change of the egg yolk; and 2) most of the metabolites were annotated in amino acid metabolism pathways from E11 to E15 and E17 to E19. Especially, arginine, lysine, cysteine, and histidine were continuously increased during the embryonic development, probably because of their effects on the growth promotion and oxidative stress amelioration of the embryo. Interestingly, the ferroptosis was found as one of major processes occurred from E15 to E17 and E17 to E19. Owing to the upregulated expression of acyl-CoA synthetase long-chain family member 4 detected in the yolk sac, we assumed that the ferroptosis of the yolk sac was perhaps caused by the accumulation of reactive oxygen species, which was induced by the large amount of polyunsaturated fatty acids and influx of iron in the yolk. Our findings might offer a novel understanding of embryonic nutrition of broilers according to the developmental changes of metabolites in the egg yolk and may provide new ideas to improve the health and nutrition for prehatch broiler chickens.
Key words: chicken, embryogenesis, yolk, metabolomics
Introduction
The embryonic development takes more than 30% (21 d) of the whole lifespan of modern commercial broilers, which are usually slaughtered for market at 5 to 7 wk after hatch (Cherian, 2015). In the last decades, the productivity of commercial broiler breeds was greatly improved because of the advanced nutritional formula and technology applied in broiler industry, which rapidly accelerated the nutritional supplementation and health for posthatch broiler chickens. However, only a few researches were carried out to investigate the importance of chicken embryonic nutrition, which has been widely accepted to determine the embryonic health, hatchability, and hatching quality of the broiler chickens.
During the embryogenesis, chicken embryo mainly relies on the nutrients and antibodies that are originated from the yolk and albumin to support its growth and health (Hatta et al., 2007). The egg yolk, which is considered as the primary nutrient supplier for the embryo, is derived from the lipid and protein deposition from liver to ovary in hens (Vieira, 2007). The lipid content of egg yolk accounts for 31 to 33%, and most of them are in the form of very-low-density lipoproteins, which takes about 66% of the yolk dry matter (Yamamoto et al., 1997). The majority of the yolk lipid are neutral lipid (65%), phospholipid (25%), and cholesterol (5%) (Powrie and Nakai, 1986). The phosphatidylcholine and polyunsaturated fatty acid (PUFA) are highly enriched in fertilized egg yolk during the early embryogenesis because of their important functions related to neural and brain development (Duan et al., 2013). Yolk protein is another essential component and possesses about 16 to 17% of the total yolk weight (Powrie and Nakai, 1986). The proteins in the yolk are also generated from liver and transported to ovary associated with the lipids (Shen et al., 1993). Phosvitin and lipovitellin are 2 major yolk proteins that are hydrolyzed from the vitellogenin and play critical roles in binding metals for supplying trace minerals for the embryo (Richards and Steele, 1987; Hatta et al., 2007). Compared with egg albumin, the egg yolk has much greater content of minerals and vitamins, especially the fat-soluble vitamins (Vieira, 2007).
In fact, the nutrients in the egg yolk are dynamically changed because of the continuous metabolism of yolk sac membrane, which begins to enclose the yolk content around embryonic day (E) 04 and forms the mature membrane structure at E07. The yolk sac membrane metabolizes the nutrients in the yolk by 2 potential ways: 1) The yolk sac is able to synthesize and secrete digestive or catalytic enzymes into yolk to complete the metabolic reactions (Yadgary et al., 2011; Speier et al., 2012; Zhang and Wong, 2017); and 2) the yolk sac ingests the nutrients and metabolizes them intracellularly before releasing the metabolites into the yolk (Karlheinz, 2008; Bauer et al., 2013; Zhang and Wong, 2019). The egg yolk helps the embryo at different developmental stages to develop and grow through offering specific nutrients and functional molecules that are metabolized by the yolk sac membrane coordinately. Also, the extraembryonic tissues, such as yolk sac, amnion, and allantois, can use the egg yolk to maintain their development and degradation. Thus, it is critical to investigate the metabolites in the yolk at different developmental stages during the chicken embryogenesis, to understand the potential functions of the metabolites associated with the embryonic nutrition and development of broilers. In this study, we collected yolk samples from fertilized eggs from E07 to E19 and performed LC-MS/MS metabolomic analysis to explore the metabolic change of yolk nutrients during the chicken embryogenesis.
Material and methods
Sample Collection
The total of 100 Ross 308 fertilized eggs were purchased and incubated at 37.5°C with 70% relative humidity. All the eggs were candled at E05 to check the fertility and eliminated for the unfertilized eggs. At each sampling day, the egg was gently opened from air cell by using a clean scissors. The egg shell membrane was slowly peeled off by using a sterilized tweezer to expose the embryo and yolk sac membrane. Five-milliliter yolk samples were collected by using a syringe to puncture through the yolk sac membrane and homogenized before freezing the samples in liquid nitrogen. Six yolk samples at E07, E09, E11, E15, E17, and E19 were collected for further untargeted metabolomic analysis. All animal procedures in this work are approved by the institutional animal care and use committee at Hunan Agricultural University.
Metabolites Extraction
The fresh frozen yolk samples including the yolk nutrients and water (100 mg) were individually grounded with liquid nitrogen, and the homogenate was resuspended with prechilled 80% methanol and 0.1% formic acid by well vortex. Thus, each metabolite that was tested in the yolk sample represented the current concentration of this metabolite. The samples were incubated on ice for 5 min and then were centrifuged at 15,000 rpm and 4°C for 5 min. Some of supernatant was diluted to the final concentration containing 53% methanol by LC-MS grade water. The samples were subsequently transferred to a fresh Eppendorf tube and then were centrifuged at 15,000 g and 4°C for 10 min. Finally, the supernatant was injected into the LC-MS/MS system for further analysis.
UHPLC-MS/MS Analysis
UHPLC-MS/MS (ultra high performance liquid chromatography/tandem mass spectrometry) analysis was performed using a Vanquish UHPLC system (Thermo Fisher, Germany) coupled with an Orbitrap Q ExactiveTM HF mass spectrometer (Thermo Fisher, Germany) in Novogene Co., Ltd. (Beijing, China). Samples were injected onto a Hypesil Gold column (100 × 2.1 mm, 1.9 μm) using a 17-min linear gradient at a flow rate of 0.2 mL/min. The eluents for the positive polarity mode were eluent A (0.1% FA in water) and eluent B (methanol). The eluents for the negative polarity mode were eluent A (5-mM ammonium acetate, pH 9.0) and eluent B (methanol). The solvent gradient was set as follows: 2% B, 1.5 min; 2-100% B, 12.0 min; 100% B, 14.0 min;100-2% B, 14.1 min;2% B, 17 min. A Q ExactiveTM HF mass spectrometer was operated in positive or negative polarity mode with a spray voltage of 3.2 kV, capillary temperature of 320°C, sheath gas flow rate of 40 arb, and aux gas flow rate of 10 arb.
Data Processing and Metabolite Identification
The raw data files generated by UHPLC-MS/MS were processed using the Compound Discoverer 3.1 (CD3.1; Thermo Fisher) to perform peak alignment, peak picking, and quantitation for each metabolite. The main parameters were set as follows: retention time tolerance, 0.2 min; actual mass tolerance, 5 ppm; signal intensity tolerance, 30%; signal:noise ratio, 3; and minimum intensity, 100,000. After that, peak intensities were normalized to the total spectral intensity. The normalized data were used to predict the molecular formula based on additive ions, molecular ion peaks, and fragment ions. And then peaks were matched with the mzCloud (https://www.mzcloud.org/), mzVault, and MassList databases to obtain the accurate qualitative and relative quantitative results. Statistical analyses were performed using the statistical software R (R version R-3.4.3), Python (Python 2.7.6 version), and CentOS (CentOS release 6.6). When data were not normally distributed, Log2 normal transformation was attempted.
Real Time Quantitative PCR
Total RNA was extracted from the yolk sac tissue, and SYBR green (Applied Biosystems) real-time quantitative PCR was performed by using Applied Biosystems 7500 Fast Real-time PCR system (Thermo Fisher Scientific). The primer information of glutathione peroxidase 4 (GPX4), acyl-CoA synthetase long-chain family member 4 (ACSL4), RPL4, and β-actin was listed in the Supplementary Table 1. The relative gene expression was normalized by the geometric average of RPL4 and β-actin Ct values. The 2−ΔΔCt method was used to calculate the gene expression, accordingly.
Data Analysis
All the metabolites were annotated using the KEGG database (https://www.genome.jp/kegg/pathway.html), HMDB database (https://hmdb.ca/metabolites), and LIPID Maps database (http://www.lipidmaps.org/). Principal components analysis (PCA) and sparse partial least squares discriminant analysis (sPLSDA) were performed at metaX (a flexible and comprehensive software for processing metabolomics data). One-way ANOVA was performed to calculate the significant change of each metabolite across different embryonic age. We applied Fisher's LSD to calculate the statistical significance for multiple comparisons. The differential metabolites were identified by 2 group comparisons, and the significance was judged by t test. The metabolites with VIP > 1, a P value < 0.05, and fold change ≥ 2 were considered to be differential metabolites.
The functions of the differential metabolites and metabolic pathways were studied using the KEGG database. The metabolic pathways enrichment of differential metabolites was performed; when ratio was satisfied by x/n > y/N, metabolic pathways were considered as enrichment, and when P value of metabolic pathway < 0.05, metabolic pathways were considered as statistically significant enrichment.
Results and discussion
Summary of the Metabolites Identified in Egg Yolk During Chicken Embryogenesis
A total of 1,256 metabolites were detected at each embryonic age in the egg yolk, which contained 708 metabolites identified under positive polarity mode and 548 metabolites identified under negative polarity mode. The metabolite feature data from either positive or negative mode were normalized as Log2 transformation (Supplementary Figure 1 and 2). The abundance of 516 (72.9%) metabolites in positive mode and 362 (66.1%) metabolites in negative mode showed significant change during the embryogenesis by one-way ANOVA (Supplementary Figure 3 and 4). Of all the detected metabolites, 273 metabolites were annotated with putative IDs, and 65 of them were found to be associated with the amino acid metabolism (Table 1).
Table 1.
Summary of basic descriptive statistics related to the yolk metabolites.
| Polarity mode | # of metabolites detected | # of significant metabolites | # of metabolites with putative IDs (KEGG) | # of annotated metabolites related to amino acid metabolism | # of annotated metabolites related to sugar metabolism | # of annotated metabolites related to lipid metabolism |
|---|---|---|---|---|---|---|
| Positive | 708 | 516 | ||||
| Negative | 548 | 362 | ||||
| Total | 1,256 | 878 | 273 | 65 | 10 | 48 |
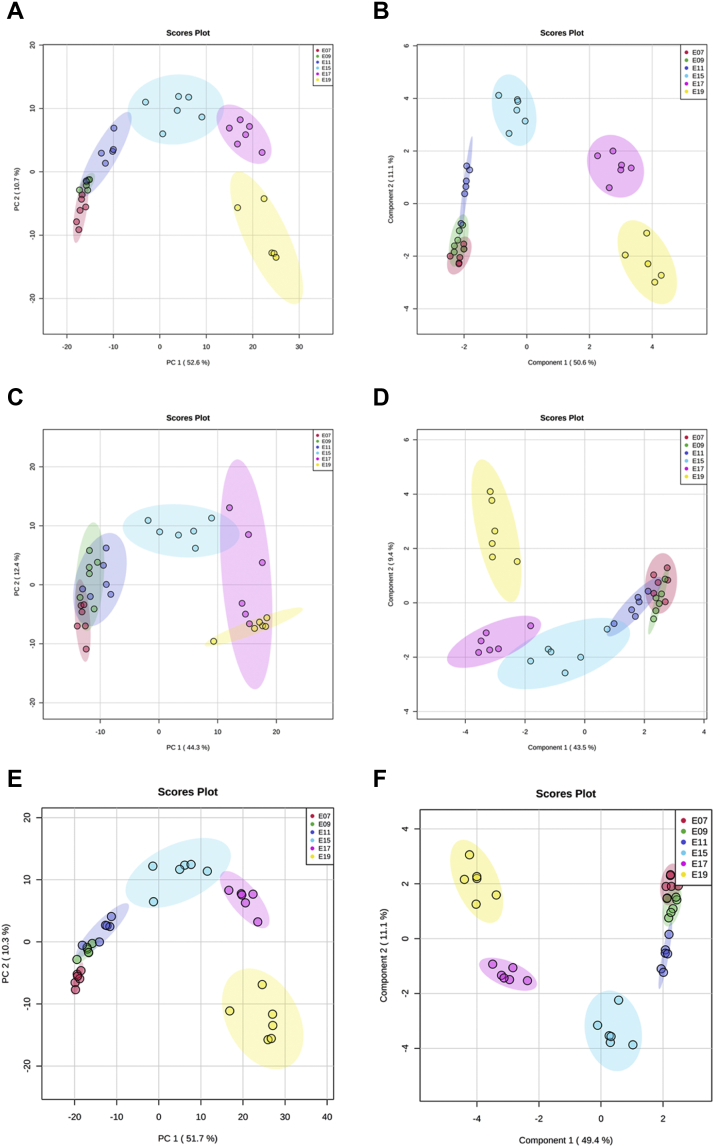
The PCA and sPLSDA were performed with the total metabolites that were obtained from both positive and negative modes and significantly changed during the embryonic development (Figure 1). Under positive mode (Figures 1A and 1B), both PCA and sPLSDA indicated that the abundance of metabolites detected in samples at E07 and E09 were similar to each other, while yolk samples at E11, E15, E17, and E19 were clustered into different groups. In addition, all the samples at each embryonic day were clustered closely, suggesting the appropriate representativity of the samples. In order to unify our data, we generated the PCA and sPLSDA by using the metabolites that were combined from positive and negative modes (Figures 1E and 1F). The results showed a similar clustering pattern with the positive mode alone, which indicated that the metabolites in egg yolk at E07, compared with those at E09, did not change much. But, from E07 to E11, with the increase of the whole metabolic rate in chicken egg, the abundance of metabolites detected in the yolk gradually accumulated. In addition, owing to the fast development of yolk sac and embryo from E11 to E15 and the degradation of yolk sac and massive energy requirement for the hatching process at the end of the incubation, the metabolic reactions in the egg yolk may be rapidly proceeded to meet these intensive physiological changes (Yadgary et al., 2010, 2013). Thus, in our results, it is reasonable that E11, E15, E17, and E19 were clustered into distinct groups according to their metabolites features.
Figure 1.
The principal component analysis (PCA) and sparse partial least squares discriminant analysis (sPLSDA) plots of the metabolites that were significantly changed during the chicken embryogenesis. Each plot was generated from the z-scored data of metabolite abundances that were significantly changed during the embryogenesis. The 2 major components that accounted for the most variation of the metabolite abundance were used to plot. Each dot in the figure represented each sample, and different colors indicated the embryonic stage. (A) Positive mode data, PCA plot. (B) Positive mode data, sPLSDA plot. (C) Negative mode data, PCA plot. (D) Negative mode data, sPLSDA plot. (E) Combined data, PCA plot. (F) Combined data, sPLSDA plot.
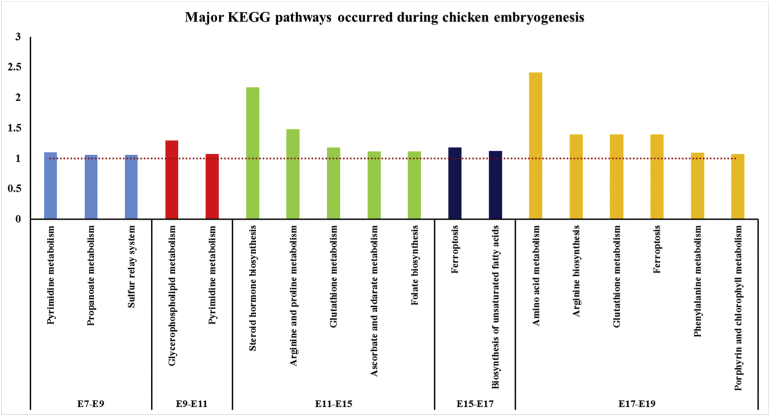
Metabolic Events Occurred at Different Stages of Chicken Embryogenesis
Based on PCA clustering results, the abundance of metabolites in egg yolk showed specific patterns at different stages of embryogenesis. This may suggest that the yolk nutrients were dynamically metabolized by the yolk sac membrane, which encloses the yolk and functions as the intestine to digest and absorb the nutrients in the intestinal lumen. In order to understand the change of nutrients in the egg yolk during the embryogenesis, major metabolic pathways associated with the detected metabolites were scanned at different stages of embryonic development (Figure 2). From E07 to E09 and E09 to E11, identified metabolites in the yolk were highly (P < 0.1) enriched in pyrimidine metabolism, propanoate metabolism, sulfur relay system, and glycerophospholipid metabolism, which indicated the rapid DNA synthesis, cell propagation, and energy storage during this stage. Owing to the structural and functional maturation of yolk sac including fast weight gain and its endodermal epithelial cells growth from E07 to E11 (Bauer et al., 2013; Yadgary et al., 2013), yolk sac may use or generate nutrients in the yolk for cell proliferation and genetic component synthesis. From E11 to E15, the major metabolic events were hormone, amino acid, and vitamin metabolism. Owing to the maturation of yolk sac around E11, it may begin to synthesize and absorb more nutrients, such as hormone, amino acid, and vitamins, to support the growth and development of the embryo (Speier et al., 2012; Yadgary et al., 2014). From E15 to E19, as the hematopoietic and nutritional functions of the yolk sac gradually diminished (Guedes et al., 2014; Yadgary et al., 2014), ferroptosis was found as the critical metabolic pathway during this stage. In addition, amino acid metabolism and synthesis of unsaturated fatty acid were highlighted from E15 to E19, which may indicate the critical neural and physiological requirements of the embryo at this stage prepared for the hatching.
Figure 2.
Major KEGG pathways enriched at different embryonic stages. KEGG pathways were searched based on the differential metabolites identified from each pair of embryonic days, such as E7-E9 means that the differential metabolites were calculated and enriched for KEGG pathway analysis when compared between E7 and E9. The cutoff (red dotted line) was selected as P < 0.1. The y axis means the -LOG (P value). Different colors of the bars represent the different pair group of embryonic days.
The Change of Amino Acids in Egg Yolk During Embryogenesis
The amino acids metabolism was annotated as the critical nutritional process occurred in the fertilized egg yolk. In addition, the change of amino acid content in egg yolk must be associated with the functional requirement of chicken embryo at different embryonic stages. Thus, we categorized the amino acids according to their functional groups and compared the change of each amino acid family to investigate the potential nutritional needs of chicken embryo at different developmental stages (Figure 2 and Table 2).
Table 2.
Changes of 20 amino acid abundance in the chicken egg yolk during the embryonic development.
| Name | E07 | E09 | E11 | E15 | E17 | E19 |
|---|---|---|---|---|---|---|
| Arginine | 2991283c | 2793610c | 2963049c | 4128909b | 6788224a | 6177125a |
| Cystine | 9377318c | 9148146c | 10650699c | 12861594c | 24424781b | 46462434a |
| Glycine | 21563919b | 20424628b | 21936243b | 28263401a,b | 31420423a | 25737461a,b |
| Serine | 65290177c | 58638602c | 85779913b,c | 131353109a | 114464403a,b | 66682818c |
| Histidine | 84014314b,c | 74762187c | 90564723b,c | 104726810b,c | 155569608a,b | 200505600a |
| Asparagine | 193227048a | 183493258a | 183954899a | 96192021b | 108288014b | 70340393b |
| Alanine | 264704470a,b | 284032270a,b | 330925741a | 210916290b | 117205829c | 95354230c |
| Aspartic acid | 277494971a | 224086282b | 222410275b | 140641727c | 104952000c | 41303566d |
| Proline | 381854472a | 309127653a | 356986249a | 192605100b | 102321227b | 129232862b |
| Leucine | 557188578a | 482518666a,b | 568265835a | 391891535b | 212421496c | 59368052d |
| Threonine | 563777088a | 638746230a | 700342140a | 651428079a | 368703676b | 196728104c |
| Glutamine | 961553642a | 858188701a | 990523880a | 1024468004a | 948622829a | 422883102b |
| Valine | 1423471723a | 1398353415a | 1462963058a | 1429732352a | 807121848b | 355157901c |
| Glutamic acid | 1876018393a,b | 1710972424b | 2395239981a | 1120433375c | 625617507c,d | 250408391d |
| Methionine | 1891370365a | 1713200462a | 1784051470a | 574754958b | 60509423c | 50943951c |
| Lysine | 2040160224c | 1915512073c | 1946634208c | 2283328709c | 4054964010b | 5324132852a |
| Isoleucine | 3902228212a | 3749472998a | 3879688038a | 3638257398a | 1807959565b | 574601972c |
| Phenylalanine | 9012994334a,b | 9510349368a,b | 11124785931a | 9259017113a,b | 5856609360b | 2217466360c |
| Tyrosine | 9359718441b | 9417132292b | 9488502308b | 11230969014a | 5465610212c | 2651667250d |
| Tryptophan | 11262036496b | 10790770744b | 11074670949b | 13212602555a | 9053491003c | 3904419730d |
a-dDifferent superscript lowercase letters in the same line indicated significant difference (P < 0.05), while the same shoulder notes in the same line indicated insignificant difference (P > 0.05). Each number represents the normalized peak area and the unit is counts∗min.
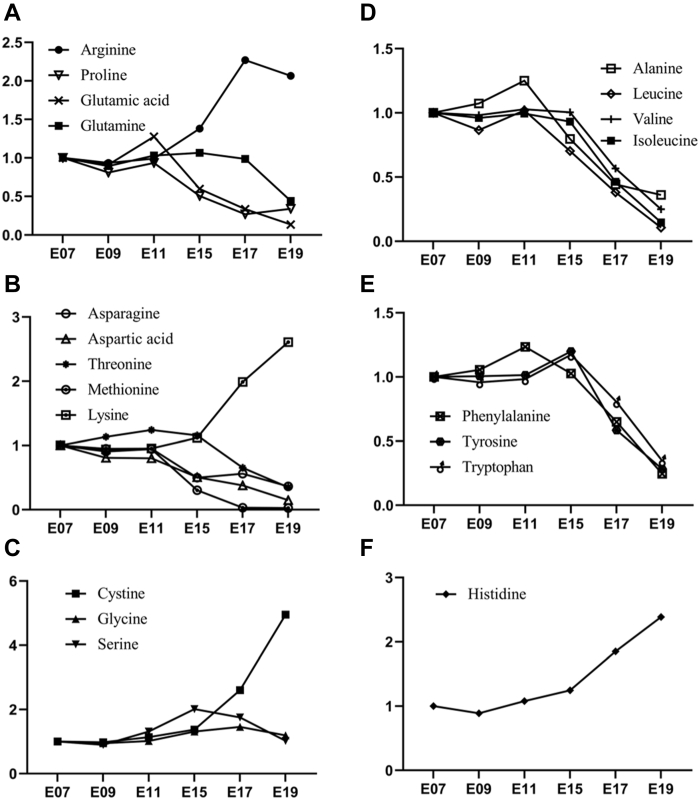
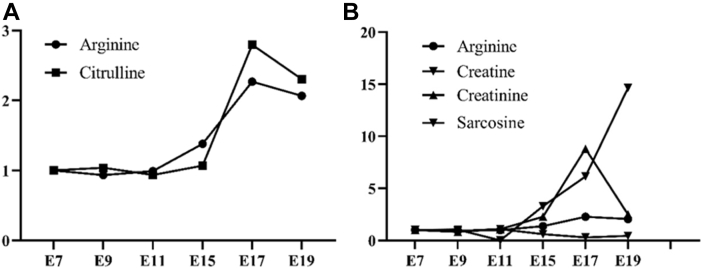
Arginine, proline, glutamic acid, and glutamine share the same skeleton with α-ketoglutaric acid, which means they are all able to be generated from the α-ketoglutaric acid through direct or indirect transamination (Wu, 2009). Our results showed that except for arginine that was increased from E11 to E17, and slightly decreased from E17 to E19, other 3 amino acids were absorbed by the embryo, so they declined gradually from E11 to E19 (Figure 3A). Arginine serves as an essential component to synthesize ornithine and generate urea in the last step of the ornithine cycle, which is a process to metabolize ammonia and prevent the toxic accumulation of ammonia in cells (Morris, 2002). The increase of arginine in egg yolk from E11 to E17 might indicate the high amount of ammonia that are produced by the deamination of amino acids for the energy production at this stage. In addition, when comparing the metabolites in the urea cycle, we found that the change of arginine and citrulline in the egg yolk was almost coordinated with each other, which might support that the yolk-metabolized arginine was involved in the process of ammonia metabolism (Figure 4A). With the synthesis of citrulline from arginine, this process generates an important neurotransmitter nitric oxide, which functions to protect the integrity of the vascularendothelium (Gornik and Creager, 2004; Ströhle et al., 2016), corresponding to the evidence of the fast vascularization of chicken yolk sac and muscle growth during E11 to E17 (Nagai and Sheng, 2008; Guedes et al., 2014). In addition, arginine is able to produce the creatine to satisfy the embryonic muscle and brain development (Wu et al., 2009; Joncquel-Chevalier Curt et al., 2015). In our results, arginine and creatine both increased in the egg yolk from E07 to E17 (Figure 4B). This might support that part of the arginine went into the arginine-creatine pathway to produce creatine for the embryo muscle growth and brain development.
Figure 3.
Relative fold change of amino acid content in the chicken egg yolk during the embryonic development. Each section presents the different amino acid subfamily that is defined by the structure or function, such as α-ketoglutarate subfamily (A), oxaloacetate subfamily (B), glycerol-3-phosphate subfamily (C), branch-chained amino acid subfamily (D), aromatic amino acid subfamily (E), and histidine subfamily (F). The fold change (y axis) was calculated by dividing the abundance of each amino acid at E07, and the abundance of each amino acids in egg yolk is displayed in Table 2.
Figure 4.
The fold change of critical metabolites in urea cycle and arginine-creatine pathway in the egg yolk during the embryogenesis. (A) Change of arginine and citrulline. (B) Change of arginine and its downstream metabolites (creatine, creatinine and sarcosine).
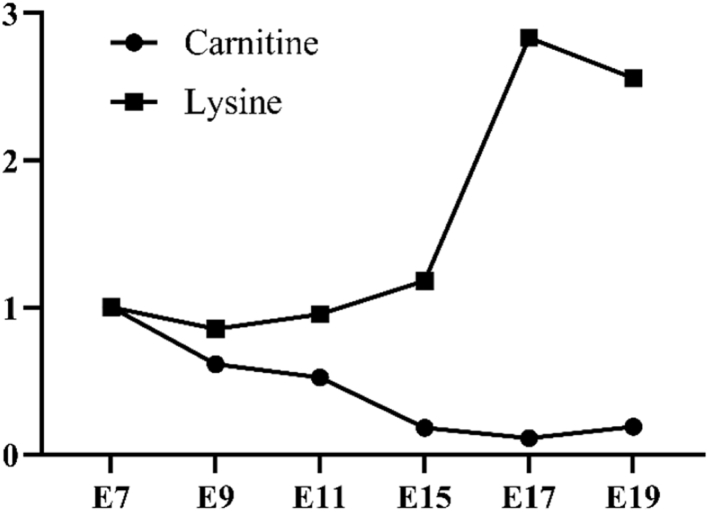
Aspartic acid, asparagine, methionine, lysine, and threonine are the aspartic acid family of amino acids, which are all derived from the oxaloacetic acid. In our results, the increasing tendency was observed for lysine content in the egg yolk, but other 4 amino acids were gradually decreased from E11 to E19 (Figure 3B). Lysine is one of the essential amino acids for animals and the second limiting amino acid for birds especially, which is involved in many critical metabolic and physiological processes such as the production of somatic proteins, synthesis of ketone body, and metabolism of glycolipid (Gous, 1998; Benevenga and Blemings, 2007). In this study, the accumulated production of lysine in the yolk was probably mainly to satisfy the intensive yolk lipid metabolism and body muscle and organ growth during the last stage of the chicken embryogenesis because the lysine serves as the precursor to synthesize the carnitine, which participates in the transport of the fatty acids (Pekala et al., 2011). However, the change of lysine and carnitine in the egg yolk was opposite (Figure 5). This might be because 1) lysine is multifunctional, thus accumulated lysine is necessary for other metabolic processes, and 2) the synthesis of carnitine may primarily occur in yolk sac epithelial cells; therefore, the decreased trend of carnitine was observed in the yolk. In addition, the high amount of lysine is able to facilitate the absorption of minerals and synthesis of collagens, which may help the growth of the bones and feathers for chicken embryo (Urdaneta-Rincon and Leeson, 2004; Yamauchi and Shiiba, 2008; Shi et al., 2019).
Figure 5.
The fold change of lysine and carnitine in the egg yolk during the embryogenesis.
The amino acids serine, cysteine, and glycine are all able to be produced by glycerol 3-phosphate, which is an intermediate of glucose metabolism. During the chicken embryonic development, the cysteine content in the yolk was found to increase exponentially from E11 to E19, while glycine and serine were merely fluctuated (Figure 3D). Cysteine and glycine are able to synthesize into important antioxidants and free radical scavenger glutathione with glutamate. Many free radicals produced in the body will damage the cell membrane; attack and malfunction macromolecules such as lipids, nucleic acids, and protein; promote aging; and induce generation of tumor or atherosclerosis (Go and Jones, 2011; Yin et al., 2016; Mailloux, 2019). At the end of the egg incubation, the yolk sac membrane that encloses the yolk nutrients rapidly metabolizes the lipids and absorbs the fatty acids in the form of the very-low-density lipoproteins (Bauer et al., 2013). These lipoproteins are big bulks which are extremely detrimental to the vascular endothelium and may lead to atherosclerosis (Nordestgaard and Tybjærg-Hansen, 1992; Nakajima and Tanaka, 2018). However, none of thrombi were detected in the yolk sac blood vessels. One of the reasons might be considered as the high amount of cysteine produced in the egg yolk to synthesize the glutathione, which mitigates the oxidative stress of the vascular endothelium.
Valine, leucine, and isoleucine are branched chain amino acids (BCAAs), which are important for body protein synthesis and cerebral activities (Harper et al., 1984; Sperringer et al., 2017). During the chicken embryogenesis, these BCAAs are barely changed but apparently decreased in the egg yolk from E11 to E19 (Figure 3D). This may indicate that the BCAAs were highly produced and absorbed by the yolk sac to stimulate the muscle growth of the embryo. Phenylalanine, tryptophan, and tyrosine are all aromatic amino acids, which are involved in the process of producing many different hormones and neurotransmitters. All 3 aromatic amino acids showed the same changing tendency in the yolk, which decreased gradually from E15 to E19 (Figure 3E). This decreasing pattern was different from energy provider glutamine, protein synthesis stimulator leucine, and reactive oxygen species (ROS) reliever methionine, which declined at E11 because of the fast embryonic body growth at this stage. Aromatic amino acids, such as tryptophan, which can be metabolized into 5-hydroxytryptamine, melatonin, niacin, and indoles to regulate the microvasoconstriction, blood pressure, development, and time rhythm, are mostly similar to the BCAAs to decrease at E15 when the embryonic organs are mature and the embryo is getting ready to hatch. Therefore, the fast utilization of aromatic amino acids and BCAAs at the end of the embryogenesis may be because they and their metabolites are important for the cognition, awakeness, food intake, and other behaviors of the embryo, which are the prerequisites of the hatching process (Ursin, 1976; Sperringer et al., 2017; Yamashita and Yamamoto, 2017).
One of the interesting findings was that the content of histidine in the egg yolk was increased from E09 to E19, which may indicate that the requirement of histidine for embryo development is elevated gradually. Histidine is the only amino acid which contains imidazole in its structure. The major function of histidine is to generate histamine, which serves as a vasodilative neurotransmitter to regulate the secretion of digestive enzymes, homeostatsis of pH, and synthesis of proteins (Mercer et al., 1990; Sakurai et al., 2009). It is reported that histamine was able to attenuate lipid metabolic disorder by increasing the lipase activity and relieve nonalcoholic fatty liver through histamine H2 receptor–initiated signaling pathway (Rong et al., 2009; Yamada et al., 2016). At the end of the chicken embryogenesis, the liver was found to use the remaining lipid by the intensive lipolysis and oxidative phosphorylation, which may result in the severe oxidative stress on the embryo hepatocytes and liver damage (Cogburn et al., 2018; Xu et al., 2019). Thus, the increased histamine in the egg yolk toward the end of the hatch might be to deal with the excessive metabolic burden on the liver of chicken embryo.
Ferroptosis Might Induce Yolk Sac Degradation at the End of Incubation
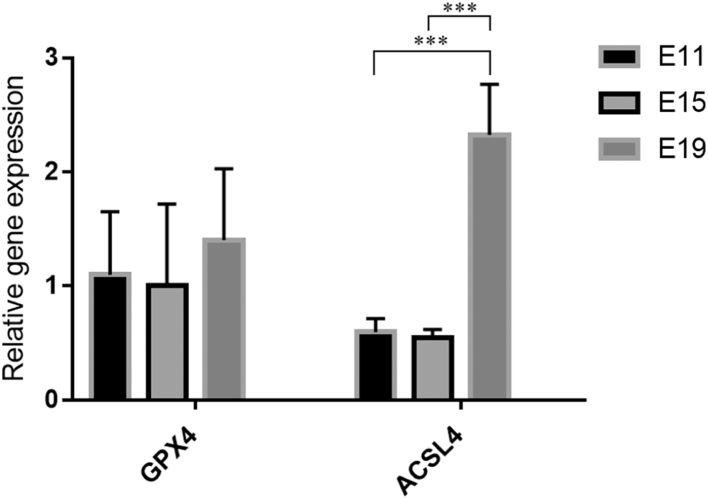
Notably, ferroptosis was the major pathway we identified when we compared the yolk metabolites between E15 to E17 and E17 to E19. Ferroptosis is a novel cell death pathway which was first identified at 2012 and differs from the apoptosis, necrosis, and autophagy (Hirschhorn and Stockwell, 2019). It is typically induced by the iron and PUFAs, which produce the ROS, perturb the permeability of the lipid cell membrane, and attack the cellular organelles or critical proteins (Mou et al., 2019). Right now, the beneficial effects of ferroptosis naturally are still in secret. Previously, the yolk sac degradation at the end of the egg incubation was considered as a process of apoptosis because of the downregulated gene expression of cytoskeleton proteins and collapse of the yolk sac blood islands that were observed (Yadgary et al., 2014). However, in our study, we speculated that the degradation of yolk sac at the end of the chicken embryogenesis probably was caused by the ferroptosis. One of the typically characters for ferroptosis is the unexpected accumulation of PUFA. The part of detected long-chain fatty acids are listed in Table 3. Most of the PUFA abundances were fluctuated during the embryonic development, but the content of arachidonic acid showed slight decrease in the yolk, which were consistent with the previous findings (Yadgary et al., 2014; Şahan et al., 2014). However, although the content of PUFA did not change much, many evidences pointed that heme was probably catabolized into biliverdin and iron around E17 (Yadgary et al., 2014). This fast influx of iron at the end of incubation in the yolk might activate the effect of PUFA left in the yolk and initiate the ferroptosis. In order to prove our assumptions, we tested the gene expression of key enzyme ACSL4, which is able to activate PUFA and cause the accumulation of ROS (Figure 6). Our results showed that ACSL4 was highly expressed at E19 compared with E11 and E15 in the yolk sac membrane. The ferroptosis also could be induced by the malfunction of GPX4, which blocks the synthesis of glutathione and causes the ROS accumulation (Figure 6). However, the expression of GPX4 did not show any significant change, which might suggest the ferroptosis of yolk sac mainly resulted from the PUFA-activated ROS accumulation stimulated by iron influx. Thus, it is possible for us to bridge the relationship between the ferroptosis and yolk sac residue, which is an important index to evaluate the health of the hatchlings.
Table 3.
Changes of important fatty acid content in egg yolk at different incubation stages.
| Name | E7 | E9 | E11 | E15 | E17 | E19 |
|---|---|---|---|---|---|---|
| Arachidonic acid (20:4n-6) | 18890406059a | 20208638092a | 19480151455a | 14613252146b | 9862391605c | 17602379490a,b |
| Oleic acid (18:1n-9) | 88433434a | 64689513b | 77432034a,b | 35081408c | 14825761c,d | 24458952d |
| α-Linolenic acid (18:3n-3) | 1984916397a | 2104613869a | 2087899803a | 1167752884b | 517873261c | 949373270b,c |
| Erucic acid (22:1) | 22510308b | 32986482a,b | 27129511a,b | 37774343c | 10412241c | 7569397c |
a-dDifferent superscript lowercase letters in the same line indicated significant difference (P < 0.05), while the same shoulder notes in the same line indicated insignificant difference (P > 0.05). Each number represents the normalized peak area and the unit is counts∗min.
Figure 6.
Relative gene expression of GPX4 and ACSL4 in chicken yolk sac at different embryonic days. The error bar represents standard deviation (SD) and the significance was represented as ∗∗∗ (P < 0.001).
Conclusions
The egg yolk is the major nutrient resource for the embryo development, but its metabolic change during the chicken embryogenesis is still unclear. Thus, we tested the metabolomic profile of the chicken egg yolk, and 1,256 metabolites were identified. The principal component analysis revealed the yolk metabolites were dynamically changed with the embryonic development and differential metabolites at different embryonic stages were enriched in the pathways including the metabolisms of pyrimidine, propanoate, glycerophospholipid, glutathione, and amino acid; biosynthesis of hormone, vitamin, and unsaturated fatty acids; sulfur relay system; and ferroptosis. Amino acid metabolism was found to be important metabolic events through the embryogenesis, and arginine, lysine, cysteine, and histidine were the amino acids significantly increased in the egg yolk, probably suggesting their indispensable function for the whole embryonic and yolk sac growth. Interestingly, the expression of ACSL4 mRNA was significantly increased from E11 to E19, which might indicate that the PUFA-induced ferroptosis was the potential cell death procedure to explain the yolk sac degradation close to the end of the egg incubation. Our new findings might provide better insights into nutrition and health of chicken embryo and hatchlings.
Acknowledgments
This study was supported by National Key R&D Program of Intergovernmental Key Projects of China (grant no.: 2018YFE0101700); support project for Scientific and technical talents in Hunan Province (2020 TJ-Q02); and Hunan Hundred Talents Program.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2021.01.036.
Disclosures
The authors declare no conflicts of interest.
Supplementary data
References
- Bauer R., Plieschnig J.A., Finkes T., Riegler B., Hermann M., Schneider W.J. The developing chicken yolk sac acquires nutrient transport competence by an orchestrated differentiation process of its endodermal epithelial cells. J. Biol. Chem. 2013;288:1088–1098. doi: 10.1074/jbc.M112.393090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevenga N.J., Blemings K.P. Unique aspects of lysine nutrition and metabolism. J. Nutr. 2007;137:1610S–1615S. doi: 10.1093/jn/137.6.1610S. [DOI] [PubMed] [Google Scholar]
- Cherian G. Nutrition and metabolism in poultry: Role of lipids in early diet. J. Anim. Sci. Biotechnol. 2015;6:28. doi: 10.1186/s40104-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogburn L.A., Trakooljul N., Chen C., Huang H., Wu C.H., Carré W., Wang X., White H.B. Transcriptional profiling of liver during the critical embryo-to-hatchling transition period in the chicken (Gallus gallus) BMC Genomics. 2018;19:695. doi: 10.1186/s12864-018-5080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X., Li M., Wu F., Yang N., Nikoo M., Jin Z., Xu X. Postfertilization changes in nutritional composition and protein conformation of hen egg. J. Agric. Food Chem. 2013;61:12092–12100. doi: 10.1021/jf403099q. [DOI] [PubMed] [Google Scholar]
- Go Y.M., Jones D.P. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic. Biol. Med. 2011;50:495–509. doi: 10.1016/j.freeradbiomed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornik H.L., Creager M.A. Arginine and endothelial and vascular health. J. Nutr. 2004;134:2880S–2887S. doi: 10.1093/jn/134.10.2880S. [DOI] [PubMed] [Google Scholar]
- Gous R.M. Making progress in the nutrition of broilers. Poult. Sci. 1998;77:111–117. doi: 10.1093/ps/77.1.111. [DOI] [PubMed] [Google Scholar]
- Guedes P.T., De Oliveira B.C.E.P.D., Manso P.P.D.A., Caputo L.F.G., Cotta-Pereira G., Pelajo-Machado M. Histological analyses demonstrate the temporary contribution of yolk sac, liver, and bone marrow to hematopoiesis during chicken development. PLoS One. 2014;9:e90975. doi: 10.1371/journal.pone.0090975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A.E., Miller R.H., Block K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- Hatta H., Kapoor M.P., Juneja L.R. Egg Bioscience and Biotechnology. John Wiley & Sons; Hoboken, NJ: 2007. Bioactive components in egg yolk; pp. 185–237. [Google Scholar]
- Hirschhorn T., Stockwell B.R. The development of the concept of ferroptosis. Free Radic. Biol. Med. 2019;133:130–143. doi: 10.1016/j.freeradbiomed.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joncquel-Chevalier Curt M., Voicu P.M., Fontaine M., Dessein A.F., Porchet N., Mention-Mulliez K., Dobbelaere D., Soto-Ares G., Cheillan D., Vamecq J. Creatine biosynthesis and transport in health and disease. Biochimie. 2015;119:146–165. doi: 10.1016/j.biochi.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Karlheinz M. Proteomic analysis of the chicken egg vitelline membrane. Proteomics. 2008;8:2322–2332. doi: 10.1002/pmic.200800032. [DOI] [PubMed] [Google Scholar]
- Mailloux R.J. Cysteine switches and the regulation of mitochondrial bioenergetics and ROS production. Adv. Exp. Med. Biol. 2019;1158:197–216. doi: 10.1007/978-981-13-8367-0_11. [DOI] [PubMed] [Google Scholar]
- Mercer L.P., Dodds S.J., Weber M.D., Dunn J.D. Histidine, histamine, and the neuroregulation of food intake: a review and hypothesis. Nutrition. 1990;6:273–277. [PubMed] [Google Scholar]
- Morris S.M. Regulation of enzymes of the urea cycle and arginine metabolism. Annu. Rev. Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- Mou Y., Wang J., Wu J., He D., Zhang C., Duan C., Li B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019;12:34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H., Sheng G. Definitive erythropoiesis in chicken yolk sac. Dev. Dyn. 2008;237:3332–3341. doi: 10.1002/dvdy.21746. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Tanaka A. Atherogenic postprandial remnant lipoproteins; VLDL remnants as a causal factor in atherosclerosis. Clin. Chim. Acta. 2018;478:200–215. doi: 10.1016/j.cca.2017.12.039. [DOI] [PubMed] [Google Scholar]
- Nordestgaard B.G., Tybjærg-Hansen A. IDL, VLDL, chylomicrons and atherosclerosis. Eur. J. Epidemiol. 1992;8:92–98. doi: 10.1007/BF00145358. [DOI] [PubMed] [Google Scholar]
- Pekala J., Patkowska-Sokola B., Bodkowski R., Jamroz D., Nowakowski P., Lochynski S., Librowski T. L-carnitine - metabolic functions and meaning in Humans Life. Curr. Drug Metab. 2011;12:667–678. doi: 10.2174/138920011796504536. [DOI] [PubMed] [Google Scholar]
- Powrie W.D., Nakai S. Egg Science and Technology. AVI Publishing Company; Westport, CT: 1986. The chemistry of eggs and egg products; pp. 97–139. [Google Scholar]
- Richards M.P., Steele N.C. Trace element metabolism in the developing avian embryo: a review. J. Exp. Zool. 1987;244:39–51. [PubMed] [Google Scholar]
- Rong R.H., Yao N., Wang M., Xue S.Y., Chin C.Y., Abe K., Xin S.Y., Kurihara H. Effects of histamine on lipid metabolic disorder in mice loaded with restraint stress. J. Pharmacol. Sci. 2009;111:117–123. doi: 10.1254/jphs.09090fp. [DOI] [PubMed] [Google Scholar]
- Şahan U., Ipek A., Sozcu A. Yolk sac fatty acid composition, yolk absorption, embryo development, and chick quality during incubation in eggs from young and old broiler breeders. Poult. Sci. 2014;93:2069–2077. doi: 10.3382/ps.2013-03850. [DOI] [PubMed] [Google Scholar]
- Sakurai E., Sakurai E., Watanabe T., Yanai K. Uptake of L-histidine and histamine biosynthesis at the blood-brain barrier. Inflamm. Res. 2009;58:34–35. doi: 10.1007/s00011-009-0656-8. [DOI] [PubMed] [Google Scholar]
- Shen X., Steyrer E., Retzek H., Sanders E.J., Schneider W.J. Chicken oocyte growth: receptor-mediated yolk deposition. Cell Tissue Res. 1993;272:459–471. doi: 10.1007/BF00318552. [DOI] [PubMed] [Google Scholar]
- Shi H., Ye X., He F., Ye J. Improving osteogenesis of calcium phosphate bone cement by incorporating with lysine: an in vitro study. Colloids Surf. B Biointerfaces. 2019;177:462–469. doi: 10.1016/j.colsurfb.2019.02.034. [DOI] [PubMed] [Google Scholar]
- Speier J.S., Yadgary L., Uni Z., Wong E.A. Gene expression of nutrient transporters and digestive enzymes in the yolk sac membrane and small intestine of the developing embryonic chick. Poult. Sci. 2012;91:1941–1949. doi: 10.3382/ps.2011-02092. [DOI] [PubMed] [Google Scholar]
- Sperringer J.E., Addington A., Hutson S.M. Branched-chain amino acids and brain metabolism. Neurochem. Res. 2017;42:1697–1709. doi: 10.1007/s11064-017-2261-5. [DOI] [PubMed] [Google Scholar]
- Ströhle A., Von Bibra H., Hahn A. L-Arginine and vascular health. Med. Monatsschr. Pharm. 2016;39:515–520. [PubMed] [Google Scholar]
- Urdaneta-Rincon M., Leeson S. Effect of dietary crude protein and lysine on feather growth in chicks to twenty-one days of age. Poult. Sci. 2004;83:1713–1717. doi: 10.1093/ps/83.10.1713. [DOI] [PubMed] [Google Scholar]
- Ursin R. The effects of 5-hydroxytryptophan and l-tryptophan on wakefulness and sleep patterns in the cat. Brain Res. 1976;106:105–115. doi: 10.1016/0006-8993(76)90076-7. [DOI] [PubMed] [Google Scholar]
- Vieira S.L. Chicken embryo utilization of egg micronutrients. Rev. Bras. Cienc. Avic. 2007;9:1–8. [Google Scholar]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Davis T.A., Kim S.W., Li P., Marc Rhoads J., Carey Satterfield M., Smith S.B., Spencer T.E., Yin Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–168. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E., Zhang L., Yang H., Shen L., Feng Y., Ren M., Xiao Y. Transcriptome profiling of the liver among the prenatal and postnatal stages in chickens. Poult. Sci. 2019;98:7030–7040. doi: 10.3382/ps/pez434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadgary L., Cahaner A., Kedar O., Uni Z. Yolk sac nutrient composition and fat uptake in late-term embryos in eggs from young and old broiler breeder hens. Poult. Sci. 2010;89:2441–2452. doi: 10.3382/ps.2010-00681. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Kedar O., Adepeju O., Uni Z. Changes in yolk sac membrane absorptive area and fat digestion during chick embryonic development. Poult. Sci. 2013;92:1634–1640. doi: 10.3382/ps.2012-02886. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Wong E.A., Uni Z. Temporal transcriptome analysis of the chicken embryo yolk sac. BMC Genomics. 2014;15:690. doi: 10.1186/1471-2164-15-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadgary L., Yair R., Uni Z. The chick embryo yolk sac membrane expresses nutrient transporter and digestive enzyme genes. Poult. Sci. 2011;90:410–416. doi: 10.3382/ps.2010-01075. [DOI] [PubMed] [Google Scholar]
- Yamada S., Guo X., Wang K.Y., Tanimoto A., Sasaguri Y. Novel function of histamine signaling via histamine receptors in cholesterol and bile acid metabolism: histamine H2 receptor protects against nonalcoholic fatty liver disease. Pathol. Int. 2016;66:376–385. doi: 10.1111/pin.12423. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Juneja L.R., Hatta H., Kim M. CRC Press, Boca Raton, FL; 1997. Hen Eggs: Their Basic and Applied Science. [Google Scholar]
- Yamashita M., Yamamoto T. Tryptophan circuit in fatigue: from blood to brain and cognition. Brain Res. 2017;1675:116–126. doi: 10.1016/j.brainres.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Yamauchi M., Shiiba M. Post-translational Modifications of Proteins. 2008. Lysine hydroxylation and cross-linking of collagen; pp. 95–108. Humana Press, Clifton, NJ. [DOI] [PubMed] [Google Scholar]
- Yin J., Ren W., Yang G., Duan J., Huang X., Fang R., Li C., Li T., Yin Y., Hou Y., Kim S.W., Wu G. l-Cysteine metabolism and its nutritional implications. Mol. Nutr. Food Res. 2016;60:134–146. doi: 10.1002/mnfr.201500031. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wong E.A. Spatial transcriptional profile of PepT1 mRNA in the yolk sac and small intestine in broiler chickens. Poult. Sci. 2017;96:2871–2876. doi: 10.3382/ps/pex056. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wong E.A. Expression of avian β-defensin mRNA in the chicken yolk sac. Dev. Comp. Immunol. 2019;95:89–95. doi: 10.1016/j.dci.2019.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.