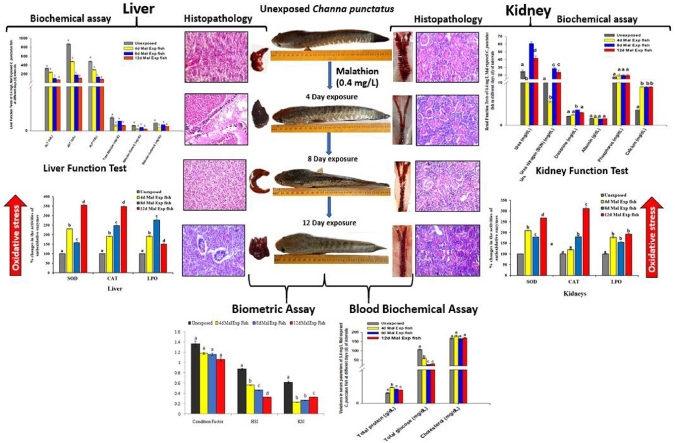
Graphical abstract
Keywords: Channa punctatus (Bloch), 96-h LC50, Malathion, Hepatorenal toxicity, Transaminases activity, Antioxidative assay, Histopathology
Highlights
-
•
The toxic manifestation of a mild dose of 0.4 mg/L malathion on Channa punctatus fish (Bloch) was evaluated after different time intervals.
-
•
The morphometric indices (K, HSI, KSI and hepatorenal tissue weight) decreased progressively with increased malathion exposure periods.
-
•
Significantly altered serum glucose, protein, urea, BUN, creatinine and Ca2+ values revealed physiological abnormality in the exposed fish.
-
•
Reduced serum transaminases and increased hepatorenal antioxidative enzymes activities suggest hepatorenal dysfunction in the exposed fish.
-
•
Long term exposure caused severe histopathological damage in the hepatorenal tissues of the malathion exposed fish indicating its toxic nature.
Abstract
The intensive application of pesticides without proper disposal management has led their excess residues to reach the neighbouring aquatic ecosystem and its inhabitants mainly fish. In natural water body pesticides get diluted, and therefore to study the silent toxic effect, a low dose of malathion (0.4 mg/L; 1/20th of 96-h LC50 value) for the different duration (1, 4, 8, 12 days) was evaluated through biochemical and histopathological biomarkers of the blood and hepatorenal tissues of Channa punctatus. With the increase in pesticide exposure periods, the biometric indices: Condition Factor (K), HSI and KSI and hepatorenal tissues weight decreased. Among the biochemical alterations in malathion exposed fish, serum glucose levels reduced by 72.23 % while protein amounts increased by 29.03 % in 12 days malathion exposed fish. Other parameters, viz., cholesterol, albumin, and phosphorous, remained the same as control fish after malathion exposure. Though serum bilirubin (total and direct) followed a biphasic response, it reduced by 60 % after 12 days of malathion exposure compared to control. Biochemical changes are reflecting the induction of compensatory energy mechanism to cope up with the malathion stress. The transaminases and ALP biomarker enzymes used for liver functionality test declined in the order of AST > ALP > ALT in a time-dependent manner in malathion exposed fish serum, indicating liver injuries in fish due to malathion. The elevated levels of urea, BUN, creatinine, and Ca2+ in the serum of 12 days of malathion exposed fish revealed renal dysfunction. In the treated fish, antioxidative (SOD and CAT) and LPO activities were significantly higher in the liver followed by the kidney than their controls. Further, histological examination registered progressive damages in the hepatorenal tissues of malathion exposed fish with the increased exposure periods compared to control. Thus, even a small dose of malathion in water could severely deteriorate the structure and function of tissue on its prolonged exposure, and therefore utmost care should be taken to prevent their seepage into the water bodies.
1. Introduction
Pesticides are the diverse group of synthetic chemicals applied commonly to control pests in agriculture and vector-borne disease eradication programs to enhance crop production and protect public health, respectively [[1], [2], [3], [4], [5]]. Globally, the organophosphate class of pesticides (OPPs) consumption alone has reached almost 50 % out of the various kinds of insecticides available worldwide [123]. Such intensive application of OPPs with a lack of proper disposal management has led their excess residues to accumulate in the air [6,7], water [[8], [9], [10]], soil [[11], [12], [13], [14]], and biological components [[15], [16], [17], [18], [19], [20]]. However, heavy load of pesticides in the aquatic ecosystem is a major ecological concern because their bioaccumulation in the non-target organism tissues can induce deleterious health impacts on them, particularly to fish and their consumers, including humans [[21], [22], [23], [24], [25]]. For instance, malathion (O, O′-dimethyl S-(1, 2-dicarbethoxyethyl) phosphorodithioate) is one such OPPs whose intoxication has been reported to cause severe pathologies like neuronal [26,27], hepatorenal [28,29], metabolic, [21,30,31], reproductive and development dysfunctions [32,33] in the aquatic organisms. Although malathion pesticide can cause genotoxicity and cancer in humans (Group 2A) [[34], [35], [36], [37]], it remains one of the most generally applied pesticides worldwide. Literatures studies have also revealed that the most important water resources are now tainted with malathion. For instance, rivers of the Central Amazon [38,39], U.S. urban streams [8,40], Mediterranean coastal zones [41], the Jiulong River estuary in China [42], the Babolrood River in Iran [43], the Nile River in Egypt [44], the Llobregat River in Catalonia, Spain [45,46] and the Ganga River in India [12,9,13] are all polluted with malathion now. Furthermore, investigations have shown that fish are also now contaminated with the malathion [12,47,13,2,48]. Consequently, accidental consumption of malathion contaminated fish by humans has potentially increased the risk of health hazard associated with malathion toxicity in them such as Parkinson's disease, neurocognitive disorders, weight gain, and its allied metabolic disorders [25,[49], [50], [51], [52], [53]]. Therefore, there is an urgent need for the surge to determine malathion toxicity in fishes. Although, the harmful impact of some of the insecticides have been investigated in some of the fish species [[54], [55], [57], [58], [59], [60]]. There is minimal evidence on the effects of low concentration of malathion on fish, and most of the studies are limited to lower invertebrates [28,38,[61], [62], [63]]. Therefore, efforts have been made to analyse the toxic manifestation of low concentration of commercial malathion on the economically important fish Channa punctatus using various biochemical and histopathological bioassays. Since fish are highly susceptible to any changes made in the water quality, batteries of tests are employed at their tissue levels to determine the toxic manifestation of xenobiotics on aquatic biota [38,64,65]. The fish’s blood and hepatorenal tissues were chosen for the present study because blood acts as a carrier medium for distributing the toxicants as well as metabolites to different parts of the body while the liver and kidneys are the major organs of accumulation, biotransformation, detoxification, and excretion [47,57,58,60,66]. Literature survey also revealed that analyses of histopathological and biochemical deformities in the blood and hepatorenal tissues of fish could act as a sensitive indicator for deciding early signs of pollutants stress over the analytical techniques [67,68]. The analytical tools often failed to detect the low concentration of chemicals in the aquatic ecosystem, thereby failing to analyse their ecological impacts [69,70]. Furthermore, because OPPs are known to induce oxidative stress, they may alter fish tissues' antioxidant status [71,72]. Therefore, estimation of activities of certain antioxidant stress marker enzymes, i.e., viz., superoxide dismutase (SOD), catalase (CAT) and lipid peroxidation (LPO), was also aimed to be conducted in the hepatorenal tissues of malathion exposed fish.
2. Materials and methods
2.1. Fish sampling
Irrespective of sex, healthy specimen of C. punctatus (Bloch) fish of almost uniform size (23 ± 3 cm) and weight (wt) (120 ± 10 g) (Family: Channidae; Order: Perciformes) were collected from a single population from the Dubbaga fish market, Lucknow, Uttar Pradesh (U.P), India during the month January 2019 and were brought to the Laboratory at BBAU, Lucknow. They were acclimatized for a month in glass aquaria (100 L capacity) containing fresh tap water (pH-7.2 ± 0.2, DO-7.0–7.4 Temperature-18 ± 2 °C, photoperiod-12 h (h) light:12 h dark). Ten such aquaria with 20 fish each were maintained. After every 24 h, they were fed ad libitum with mined goat liver. Routine cleaning of aquaria was performed after every 48 h to remove the excretory waste and food debris.
2.2. Chemicals and reagents
Commercial-grade malathion (50 % emulsified concentration, Saral Crop Science, Uttar Pradesh, India), was procured from the local market Lucknow, Uttar Pradesh, India. The bovine serum albumin (BSA), cholesterol, Folin and Ciocalteu’s phenol reagent, hexokinase, glucose-6-dehydrogenase, L-lactate dehydrogenase, L-malate dehydrogenase, p-nitrophenyl phosphate (PNPP), β-nicotinamide-adenine-dinucleotide reduced disodium salt hydrate (NADH) were purchased from Sigma-Aldrich. Routinely used other chemicals were of analytical grades and were procured from Thermo Fischer and SRL Pvt. Ltd. (Mumbai) from the local supplier, Lucknow, India.
The lysis buffer used was 0.1 M phosphate buffer (pH 7–7.4). For the total protein estimation BSA standard (1 mg/mL) was prepared. Alkaline solution used for protein estimation contained 2% Na2CO3, 0.1 N NaOH, 1% KNaC4H4O6·4H2O and 0.5 % CuSO4·5H2O, For the glucose analysis hexokinase reagent was used. It contained 100 mmol/liter Tris‐HCl buffer (pH 7.8), 1 mmol/L magnesium acetate, 0.66 mmol/L NAD+ and 0.40 mmol/L ATP, hexokinase 0.66 U/mL and glucose‐6‐phosphate dehydrogenase (0.66 U/mL). For the total cholesterol measurement, standard cholesterol solution prepared in absolute ethanol (0.4 mg/mL) and Liebermann-Burchard reagent containing chilled acetic anhydride, conc. sulfuric acid and glacial acetic acid were used. The diazo reagent was used for bilirubin determination. It contained sulphanilic acid (1 g), conc. HCl (15 mL), sodium nitrite (0.5 g) and distilled water. ALP substrate contained p-nitrophenyl phosphate (PNPP 20 mM/L) prepared in 0.1 M bicarbonate buffer (pH 10). ALT substrate contained L-alanine and 2-oxoglutaric acid prepared in phosphate buffer. AST substrate contained L-aspartic acid and 2-oxoglutaric acid. The sodium hypochlorite (0.02 M), sodium aquopentacyanoferrate (AqF) solution (0.005 g of Na3[Fe(CN)5H2O]O·H20 in 100 mL distilled water) and chlorophenol stock (1 M, 11.5 g of 2-chlorophenol in 95 % ethanol, 100 mL) were used for urea measurement. The bromocresol green reagent (0.12 mmol/L, pH 4.2) and albumin standard (5 g/dL) were utilized for albumin estimation. The calcium reagent solution contained 250 mg of sodium naphthalhydroxamic acid, 5 mL of ethanolamine, 2 g of D-tartaric acid and 9 g of NaCl. Alkaline EDTA solution contained 2 g of EDTA and 0.1 N NaOH. Colour reagent solution contained 60 g Fe(NO₃)₃ and 15 mL of concentrated HNO3. Standard calcium solution contained 100 mg of CaCO3 and 0.1 N HCl (30 mL). The SDS (10 %), ammonium 1-anilinonaphthalene-8-sulfonate (ANSA, 0.025 % w/v) and acid molybdate reagent were utilized for phosphorous determination. The acid molybdate reagent contained 2.5 % ammonium molybdate prepared in 5 N sulfuric acid. The TBA reagent contained SDS (8%), acetic acid in 5 N NaOH (20 %, pH 3.5), 0.8 % aqueous solution of thiobarbituric acid (TBA) and 0.8 % butylated hydroxy toulene (BHT) in ethanol. The L-methionine (20 mM), hydroxylamine hydrochloride (HAC, 10 mM), EDTA (50 μM), Triton X-100 (1%), riboflavin (100 μM) and Greiss reagent were utilized for SOD assay. Greiss reagent contained naphthylethylene diamine (0.1 % NED), 1 % sulphanilamide and 5% orthophosphoric acid. The phosphate buffer (50 mM), hydrogen peroxide (H202, 60 mM), absolute ethanol and Triton X-100 (10 %) were used for CAT analysis. The Bouin’s fixative contained 2% picric acid saturated aqueous solution (750 mL), 40 % formaldehyde (250 mL) and glacial acetic acid (50 mL). The 10 % neutral formalin contained 100 mL formaldehyde (37–40 %) and 900 mL distilled water.
2.3. Experimental design
2.3.1. Experimental setup I for malathion LC50 estimation
Based on literature studies, acclimatized C. punctatus fish were subjected to six randomly chosen concentrations (1.0, 2.0, 4.0, 6.0, 8.0, and 10 mg/L) of malathion for 24, 48, and 96 h respectively to determine the value of 96-h median lethal concentration (LC50) of malathion using an arithmetic procedure of trimmed Spearman-Karber method as described by Hamilton et al. [73]. The stock solution of different concentrations of malathion was prepared in acetone. The feeding was stopped 24 h before the experiment's initiation and was not fed during entire experimental periods as per guidelines of the Organization for Economic Co-operation and Development recommended for testing the effects of chemicals on aquatic organisms [74]. Control group of fish were not exposed to malathion. The experiment was conducted in triplicate with a sample size of 5 in each concentration selected for the investigation.
2.3.2. Experimental setup II
The sublethal toxicity of low concentration of malathion on the liver and kidneys of fish C. punctatus was determined by the static bioassay method following the OECD [74] guidelines. The 1/20th of 96-h LC50 value was randomly selected to investigate whether this dose of malathion can be considered NOEC (no observed effect concentration) for the fish. According to Sprague [75] and USEPA [76], NOEC is 1/10 or <1/10 of LC50 value of a toxicant that does not cause any visible adverse effects on fish pertains to today's aquatic ecosystems. The 1/20th of 96-h LC50 malathion (0.4 mg/L) was prepared by diluting the stock solution with distilled water. The acclimatized fish were divided into two groups: experimental and control. Malathion was not added in the control groups of fish, whereas the experimental fish were exposed to 1/20th of 96-h LC50 of malathion. The experiment was terminated after 12 days because fish started displaying abnormal behaviour {erratic swimming with increased opercular movement and surfacing frequency (not published)}. The experiments were conducted in triplicate with ten fish each. Aerators were used to maintain the levels of DO in both the control and experimental groups of fishes. After completing 24 h, 4, 8, and 12 days, the liver and kidneys tissues were dissected from both the experimental and control batches of fish. They were processed accordingly for biochemical and histopathological tests. Before the tissue collection, blood samples were obtained by cutting the caudal peduncle of fish.
2.3.3. Biometric assay
The fish fork length (cm) and weight (g) were measured to analyse the general health condition of fish, applying Fulton's condition factor (K). It was calculated following equation given below:
| (1) |
Where W is the weight of fish in g
L is the length of fish in cm
Hepatosomatic and kidneys somatic indices were measured according to the formula given below:
| (2) |
Where OSI in general stands for Organosomatic index
2.3.4. Serum biochemical assays
The serum was obtained by centrifuging the fish blood samples at 3500 rpm for 15 min and were stored at -20 °C. The biochemical parameter was analysed in the fish serum of both control and experimental groups of fish after 24 h, 4, 8, and 12 days of malathion exposure applying their standard protocols. The concentrations of total protein, glucose, and cholesterol were estimated following Lowry et al. [77], Schmidt [78], and Abell et al. [79], respectively. The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were measured by Bergmeyer et al. [80]. The urea [81], creatinine [82], albumin [83], bilirubin [84], calcium [85] and phosphorous [86] were determined with their respective methods. The activities of ALT, AST and ALP enzymes were represented in IU/L of serum.
2.3.5. Antioxidant enzymes assays
The liver and kidneys samples were dissected from the experimental and control (unexposed) fish and weighed. To analyse the antioxidant enzyme activities, tissue homogenates were prepared in ice-cold 0.1 M phosphate buffer saline (pH 7.0) using Potter-Elvehjem glass homogenizer fitted with Teflon pestle. They were initially centrifuged at 2500 rpm for 10 min at 4 °C. The supernatants were collected and redissolved in the buffer and recentrifuged at 12000 g for 20 min at 4 °C to obtain the post mitochondrial supernatant (PMS). The PMS was stored at -80 °C for further enzyme analyses. Their activities of superoxide dismutase (SOD), catalase (CAT) and lipid peroxidase (LPO) were measured by the methods of Das et al. [87] Aebi [88], and Ohkawa et al. [89], respectively. The SOD activity was expressed in unit/mg of protein, CAT in nano (n) mole of H2O2 decomposed/min/mg of tissue wt, and LPO in n mole of MDA formed/100 mg tissue wt respectively. The total protein concentration in the liver and kidneys were estimated at 660 nm using bovine serum albumin (BSA) as a standard, according to Lowry et al. [77].
2.3.6. Histopathological analyses
The tissues were pre-washed in fish saline (64 % NaCl) and blot dried before their fixation in Bouin's and 10 % neutral formalin, respectively, for 24 h and 48 h. Subsequently, tissue blocks were prepared, and sections were cut at 6 μm thickness using a rotatory microtome. Later, they were treated with different ethanol grades, stained with Ehrlich haematoxylin and eosin, and mounted with DPX. Finally, the slides were observed under the imaging microscope and were photographed.
2.3.7. Statistical analysis
Data were represented as mean ± standard error of means (SEM). The significant differences between the two mean values were analysed using one-way analysis of variance (ANOVA) followed by Duncan's multiple-range test (DMRT). Differences were considered significant at the level of p < 0.05. Since the variation in the biological parameters obtained for the controls (unexposed) and 24 h of malathion exposed fish were not significant, the results were not further discussed.
3. Results and discussion
3.1. Malathion LC50 value
The 96-h median lethal concentration (96-h LC50) of malathion for (50 % mortality) C. punctatus fish was determined 8.0 mg/L. Our results were comparatively higher than those of the 4.65, 6.65, and 5.93 mg/L 96-h LC50 values reported previously by Singh et al. [90], Pandey et al. [91], and Kumar et al. [92] for C. punctatus, respectively. While investigating the acute toxicity of insecticides on fish, several workers revealed that LC50 values of pesticides varied widely from fishes to fishes due to their differential rate in absorption, accumulation, metabolization, and excretion [55]. Further, the LC50 value of OPPs also depends on the rate of hydrolysis, which is a function of water's physicochemical properties [2]. In our experiment, the pH of the water range between 7−7.4. According to USEPA [2], the malathion hydrolyzes rapidly (half-lives of 6 days and 17 h) in neutral or alkaline water into several byproducts: malaoxon, α-malathion, diethyl thiomalate and O, O-dimethylphosphorodithioic acid that might exert higher toxicities to fish, leading to increase in LC50 values [93,125]. Further, El-Nahhal [39] explained that malathion might undergo metabolic biodegradation in fish tissues, resulting in smaller toxic fragments that might increase the LC50 values. Thus, the previous findings support our result showing a higher value of 96-h LC50 for malathion exposed C. punctatus fish.
3.2. Environmental relevance of the experimental dose of malathion used
The previous studies showed that the 96-h LC50 value of malathion varies with fish species differences and water physicochemical properties. Therefore, to prescribe the safe levels of malathion for fishes, malathion should be diluted in lower fractions. Mount and Stephen [94] have suggested that the safe levels of malathion for fishes lie somewhere between 1/15 and 1/45 of the 96-h LC50 concentration. Further, Singh et al. [90] recommended 0.070 to 0.117 mg/L range of malathion concentration to be safe for fish based on their estimated LC50 value. Paradoxically, few authors proposed that even the low concentrations of malathion may influence the fish's growth, metabolism, and survival on its long-term exposure [63,95]. Because pesticides containing agricultural runoff or floods ultimately reached into the water regime; therefore, it again becomes necessary to examine the detrimental effects of diluted concentration of malathion on fish. This study will help authorities regulate the amount of malathion that could be safely discharged into the neighbouring wetlands. A few authors have tried to investigate the impact of the low amount of malathion on some of the fish's biological parameters. Inbaraj and Haider [32] found significant (p < 0.05) dose-dependent inhibition in the activity of the brain AchE (acetylcholine esterase) and ovarian steroidogenesis after 120 days of exposure to low concentrations of 1/8, 1/16, and 1/24 of the 96-h LC50 of malathion (4.48 mg/L 96-h LC50) in C. punctatus fish. Further, Kumar et al. [92] revealed genotoxic effects of sub-lethal concentration (1/4, 1/8, and 1/10 of 5.93 mg/L of 96-h LC50) of malathion on the gills, kidneys, and blood profiles (lymphocytes) of fish C. punctatus at different periods (0, 1, 3, 7, 15, 22, and 29 days) using micronucleus test and comet assay. Their results demonstrated a concentration-dependent increase in DNA damage up to day 3, followed by a nonlinear decrease with increased exposure duration. However, to date, the biochemical, physiological, and histopathological effects of low concentration of malathion on the hepatorenal tissues of fish are scanty. Hence, the present study was undertaken to analyse the impact of a single dose of 1/20th of 96-h LC50 value of malathion (i.e., 0.4 mg/L) on fish's vital tissues.
3.3. Bioassays
3.3.1. Biometric assay and organosomatic indices
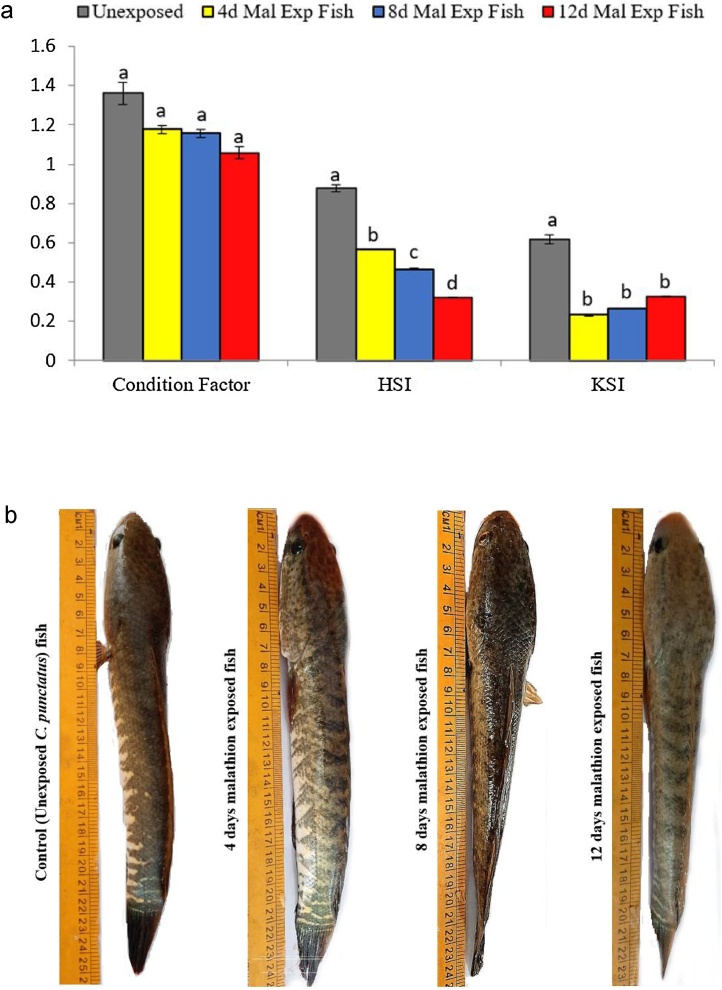
In general, the K and organosomatic indices (HSI and KSI) are applied to assess pollutants' deleterious effects on an individual's overall health and fitness [[96], [97], [98]]. Therefore, to determine the malathion effects on the general health status of C. punctatus fish, biometric indices were evaluated at different exposure intervals. The values obtained for the K, HSI, and KSI of both controls and 0.4 mg/L of malathion exposed fish were represented in Fig. 1. The mean value of K estimated for the untreated fish was 1.36 ± 0.056 while it was 1.17 ± 0.018, 1.15 ± 0.022, and 1.06 ± 0.03 for 0.4 mg/L malathion exposed fish after 4, 8, and 12 days respectively (Fig. 1). Though compared to the controls, a progressive decrease in the K values were noticed with an increase in the exposure periods up to 12 days, none of the alterations were significant (p < 0.05, DMRT) as evident by the insignificant changes in the body length (Fig. 1a and b). The HSI and KSI of control groups of fishes were found to be 0.877 ± 0.017 and 0.618 ± 0.024, respectively. Following 4 and 8 days of 0.4 mg/L of malathion exposure, the HSI of C. punctatus fish declined significantly (p < 0.05, DMRT) by 35.57 % and 46.97 % in comparison to their controls, respectively (Fig. 1). Similarly, compared to the untreated fish, the KSI values also significantly (p < 0.05, DMRT) deflate by 62.38 and 56.98 % after 4 and 8 days in 0.4 mg/L of malathion exposed fish, respectively (Fig. 1). Following 12 days of the malathion exposure, HSI and KSI of fish reduced by 63.13 % and 48.28 %, respectively, concerning controls (Fig. 1). Correspondingly, the weight of liver and renal tissues of 0.4 mg/L of malathion exposed C. punctatus fish were also found to be decreased by 62.43 % and 54.33 % after 12 days (Table 1). Agbohessi et al. [18] noticed a reduction in the K and HSI in the Guinean tilapia and African catfish inhabiting in cotton basin heavily impacted by pesticides in West Africa. Similarly, Gandar et al. [99], while evaluating the combined effects of seven pesticides with temperature on the behaviour and metabolism of Carassius auratus, also found insignificant differences between the K values of control and experimental groups of fish but noticed a significant decline in the HSI values of the fish exposed to 8.4 μg/L of pesticides mixture (S-metolachlor, isoproturon, linuron, atrazine-desethyl, aclonifen, pendimethalin and tebuconazole) at 22 °C with respect to controls. Further, Bacchetta et al. [98] also noticed insignificant changes in the K and HSI values of Piaractus mesopotamicus fish exposed individually to low concentrations of endosulfan (ED) and lambda-cyhalothrin (LC) and in contrast, a significant decline in the HSI values of fish exposed to their combined mixture compared to the controls. Velisek et al. [100] also revealed insignificant alterations in the biometric indices (K ad HSI) of common carp fish exposed to 0.06 μg/L simazine pesticide. Their study strikes out that the changes in the K and HSI values of fish depend on the pesticides' concentration, quantities, and water regime temperature. Thus, our results corroborate with the previous findings of Velisek et al. [100], Bacchetta et al. [98], Agbohessi et al. [18], and Gandar et al. [99]. According to them, the HSI values of fish facing pesticide stress decreased probably due to mobilization of its stored energy reserves biomolecules towards maintaining the homeostasis instead of utilizing them for their somatic growth. Thus, the evident decrease in hepatorenal indices of 0.4 mg/L of malathion exposed C. punctatus fish in the present study indicates that even a low amount of malathion could decline the weight of vital tissue in fish and might hamper their overall fitness and growth.
Fig. 1.
(a) Changes in the Condition Factor (-K), hepatosomatic index (HSI) and kidney somatic index of C. punctatus fish exposed to 0.4 mg/L of malathion after 4, 8, and 12 days of exposure.
Values were represented as Mean ± SEM.
Significant differences between mean values at different stages of exposure were analysed by One-way analysis of variance followed by Duncan’s multiple range test (DMRT) at the level of p < 0.05.
Significant data is shown by small progressive alphabetic letters (a–d); similar consequent alphabetic letters present no significant changes between the two means.
(b) The images of control (unexposed) and malathion exposed C. punctatus fish at different durations.
Table 1.
Variations in the weight (g) of liver and kidneys tissues of C. punctatus fish exposed to 0.4 mg/L of malathion after 4, 8, and 12 days.
| Fish sample | Liver | Kidneys |
|---|---|---|
| Control | 0.9644 ± 0.0108a | 0.681 ± 0.001a |
| 4 days exposed fish | 0.527 ± 0.014b | 0.263 ± 0.003b |
| 8 days exposed fish | 0.431 ± 0.003c | 0.257 ± 0.002b |
| 12 days exposed fish | 0.362 ± 0.001d | 0.311 ± 0.001c |
Values were represented as mean ± SEM.
Superscript of different small alphabets (a–d) symbols on values represent the statistical difference between the different exposure periods at the level of p < 0.05 by ANOVA followed by DMRT.
3.3.2. Analysis of serum biochemical parameters
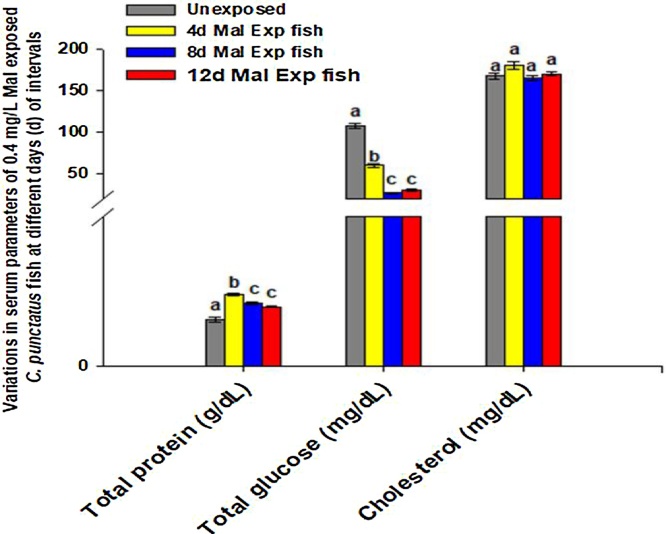
The biochemical profile of blood helps forecast the physiological disturbances that might occur in organisms either due to pathological or chemical stress [101,59,97,102]. Therefore, the toxic manifestation of a low dose of malathion was evaluated in terms of variation in the fish serum biochemical parameters such as total concentrations of glucose (mg/dL), protein (g/dL), and cholesterol (mg/dL), urea (mg/dL), creatinine (mg/dL), bilirubin (mg/dL), and activities of biomarker enzymes (ALT, AST, and ALP) in the serum of unexposed (controls) and 0.4 mg/L of malathion exposed C. punctatus fish after 4, 8, and 12 days of the experiment. Following the malathion exposure, fish serum total glucose level decreased while the total protein concentration enhanced with increased exposure periods (Fig. 2). The glucose levels in C. punctatus serum decreased (p < 0.05, DMRT) by 44.45 %, 75 %, and 72.23 % after 4, 8, and 12 days of 0.4 mg/L of malathion exposure respectively relative to their controls whereas the total protein levels in fish serum remains significantly higher than those of untreated control (Fig. 2). However, the serum protein amounts maximum enhanced by 54.84 % in 4 days of 0.4 mg/L malathion exposed fish compared to controls (Fig. 2). Also, the changes in the fish serum cholesterol levels were insignificant (p < 0.05) at all the periods of the experiment in comparison to controls (Fig. 2). It is well established that glucose monitoring in blood indicates an organisms' physiological health, therefore widely applied in ecotoxicological studies [97]. Since cholesterol and proteins are essential components of cell membranes, enzymes, and several stress marker hormones, determination of alteration in their concentrations in the fish blood could also provide critical information about liver and kidney tissue dysfunctions, impairment in metabolic pathways, and immune disorders. Besides, they may also substitute for the supplementary need of energy to accomplish the detoxification processes and maintain the organism's homeostasis when the supply of glucose gets exhausted in extreme stress conditions [101,97]. Venkataraman and Rani [103] revealed hypoglycemic conditions in the serum of Clarias batrachus fish exposed to 0.05 mg/L of malathion for 72 and 96 h. It was stated that hypoglycemic conditions in fish could arise due to the impairment in carbohydrate metabolism or increased conversion rate of glucose into lactate under anaerobiosis to meet the organism's energy demand under toxicants' stress. Similarly, Agrahari et al. [104] observed an 11 % and 50 % decrease in the serum glucose level of fish after 15 and 60 days of exposure to 1.86 mg/L monocrotophos, respectively. However, they noticed that a lower dose (0.96 mg/L) of the same pesticide did not cause any noteworthy changes in the fish serum glucose levels after the same exposure periods. Further, Sharma [105] also noted that 15 days of exposure to 4 mg/L of carbaryl caused a significant reduction of 24 % in the blood glucose levels in Clarias batrachus. The author suggests that since malathion toxicity is executed via cholinergic inhibitions in organisms, a drop in the blood glucose levels might reflect their utilization to cope with the general aftereffects of OPPs toxicity, such as hyperexcitability, tremors, and convulsions. Hence, serum hypoglycemic conditions of C. punctatus exposed to 0.4 mg/L of malathion could be due to the pesticide's toxic nature on its long-term exposure. Our results are in agreement with the findings of Sharma [105], Agrahari et al. [104], and Venkataraman and Rani [103], whom all suggested that hypoglycemic conditions in fish serum inhabiting in pesticide-contaminated water might occur to aid energy for performing normal physiological activities. Furthermore, the increased blood protein level in fish might be due to the synthesis of essential enzymes to detoxify the pesticides under stress. According to Sharma [105] and Saravanan et al. [106], the increase in protein synthesis is a general adaptation syndrome under toxicant stress. Similar results of disruptions in the biochemical compositions of blood were also observed in a few fish species due to malathion poisoning [92,101,39,107].
Fig. 2.
Changes in the serum biomolecules concentrations of C. punctatus fish exposed to 0.4 mg/L of malathion after 4, 8, and 12 days of exposure.
Values were represented as Mean ± SEM.
Significant differences between mean values at different stages of exposure were analysed by One-way analysis of variance followed by Duncan’s multiple range test (DMRT) at the level of p < 0.05.
Significant data is shown by small progressive alphabetic letters (a–d); similar consequent alphabetic letters present no significant changes between the two means.
3.3.3. Liver function test- (LFT)
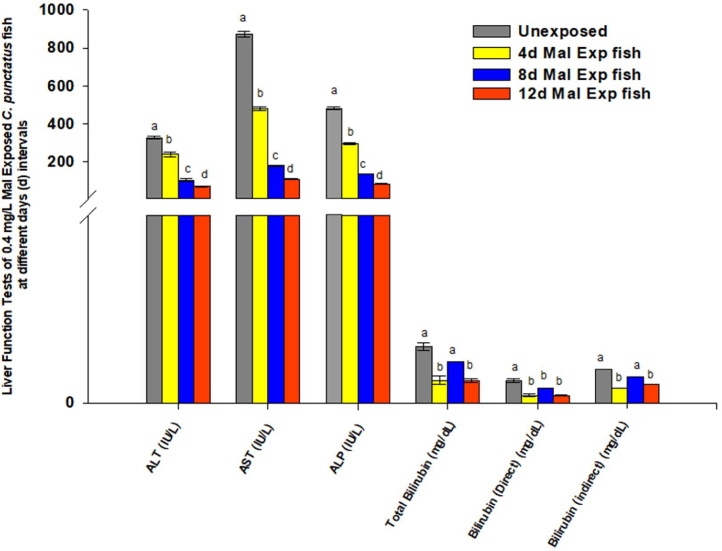
The transaminases (AST and ALT) and ALP regulate physiological processes, catalyzing trans-amination reactions to facilitate xenobiotics and other macromolecules' metabolism. Therefore, alterations in their activities allow direct identification of damages in liver and kidney functions and act as the markers to illustrate these tissues' integrity in the organisms [108,101,97]. Thus, in the present study, we also examined the enzyme activities (IU/L) of ALT, AST, and ALP in malathion exposed C. punctatus fish serum samples collected after 4, 8, and 12 days. It was noticed that the transaminases and ALP enzymes' activities in malathion exposed fish serum decreased in time dependent manner (Fig. 3). Further, compared to the controls, the enzyme activities in the malathion exposed fish serum reduced significantly (p < 0.05, DMRT) in order of AST > ALP > ALT (Fig. 3). The AST activities in malathion exposed fish serum declined by 45.02 %, 79.38 %, and 87.52 % after 4, 8, and 12 days respectively, relative to their controls (Fig. 3). Similarly, ALP and ALT's activity levels in fish serum also fall by 38.8 %, 71.57 % and 82.57 %, and 26.61 %, 67.89 %, and 77.98 %, respectively, after 4, 8, and 12 days of exposure to malathion compared to the unexposed (controls) fish (Fig. 3). Luskova et al. [109] and Bacchetta et al. [110] also recorded reductions in the activities of AST and ALT in fish serum following exposure to endosulfan and diazinon (OPPs) insecticides, respectively. Their study suggests that the pesticides' effects on fishes varied with the variation in the fish species and concentrations, durations, and types of insecticides applied for toxicity testing. Contrary to our findings, Sharma [105], Agrahari et al. [104], Velisek et al. [100], Banaee et al. [124], and Karmakar et al. [71] noticed increased activities of AST, ALT and ALP in the blood and liver tissues of fish exposed respectively to various concentrations of carbaryl, monocrotophos, deltamethrin, diazinon, - and malathion for different time intervals. They revealed that ALT and AST activities might increase in the blood of fish exposed to pesticides due to induction of metabolic shifts in protein catabolism or lysis of hepatic cell membrane and leakage of these enzymes into the bloodstream of fish. Thus, it is suggested that further examination of the effects of low concentration of pesticides on various fish species will help to describe the kinds of metabolic disorders develop to conquer the insecticides toxic stress. Moreover, Toledo-Ibarra et al. [111] and Stoyanova et al. [112] explained that the decline in ALP activities of fish tissues depends on the dose and time of insecticide exposure. Thus, in the present study, the apparent reason for the declined ALP activity levels in C. punctatus fish serum might be due to the low dose of malathion applied for examining their effects on fish metabolism. In addition, a biphasic mode of alteration was observed in the concentration (mg/dL) of total bilirubin, bilirubin (direct), and bilirubin (indirect) in fish serum following malathion exposure to different exposure periods. However, at the end of 12 days of 0.4 mg/L of malathion exposure, the fish serum total bilirubin levels decreased significantly by 60 % concerning to their controls (Fig. 3). Contrary to our findings, Jyothi and Narayan [113] found hyperbilirubinemia conditions in the blood of Clarias batrachus (Linn.) following exposure to carbaryl and phorate (OPPs). The probable reason stated by the author for hyperbilirubinemia was obstruction of the bile ducts in the pesticide exposed fish.
Fig. 3.
Changes in the liver function test in blood tissue of C. punctatus fish exposed to 0.4 mg/L of malathion after 4, 8, and 12 days of exposure.
Values were represented as Mean ± SEM.
Significant differences between mean values at different stages of exposure were analysed by One-way analysis of variance followed by Duncan’s multiple range test (DMRT) at the level of p < 0.05.
Significant data is shown by small progressive alphabetic letters (a–d); similar consequent alphabetic letters present no significant changes between the two means.
3.3.4. Kidney function test- (KFT)
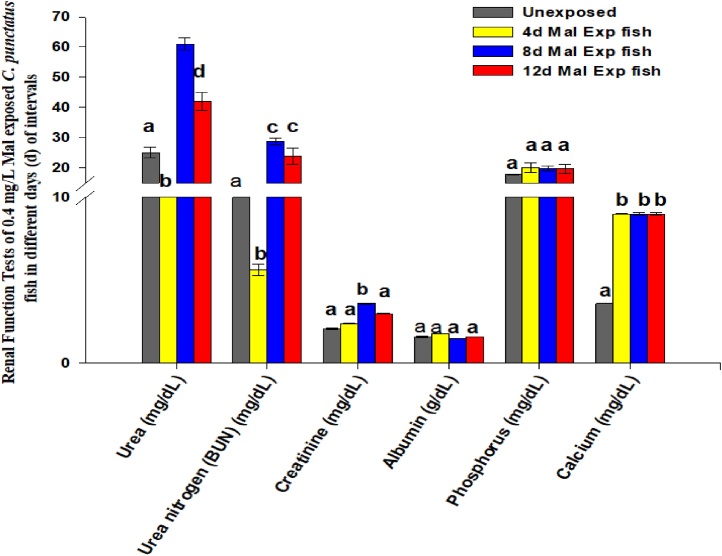
The renal function test- was determined in the blood samples of C. punctatus fish after 4, 8, and 12 days of 0.4 mg/L of malathion exposure to determine the physiological damages caused to kidneys (Fig. 4). Following 4 days of 0.4 mg/L of malathion exposure, the reduction in urea (mg/dL), urea nitrogen (mg/dL) and creatinine (mg/dL) were 52 %, 52 %, and 14 % respectively. On subsequent exposure to 8 days, there were significant (p < 0.05, DMRT) elevation of 144 %, 144 %, and 71.428 % in the urea, urea nitrogen (BUN), and creatinine quantities, respectively compared to the controls (Fig. 4). However, after 12 days of 0.4 mg/L of malathion exposure, their levels remained elevated by 68.00 %, 103.14 %, and 42.85 %, respectively, compared to controls (Fig. 4). Further, following 0.4 mg/L of malathion exposure, the calcium (Ca2+) level in blood samples of fish remained to be increased significantly by 150 % (p < 0.05, DMRT) each after 4, 8, and 12 days respectively relative to untreated fish (Fig. 4). Whereas, no significant alterations were observed in the levels of albumin (g/dL) and phosphorous (mg/dL) in fish serum following exposure to 0.4 mg/L of malathion compared to controls (Fig. 4). According to El-Nahhal [39], pesticide induces an energy crisis and altered protein metabolism. Alteration in deamination and transamination of amino acid are associated with nitrogen metabolism changes, which can be detected in terms of plasma nitrogenous metabolite levels. Extensive exposure to pesticides induces toxic changes in biochemical parameters in the liver and kidneys. Creatinine is a metabolic waste excreted with urine, and its proportion increases in blood with kidney malfunctions. Amin and Hashem [114] reported abnormally elevated levels of blood urea nitrogen and creatinine in deltamethrin exposed Clarias gariepinus. They explained that the increased concentrations of urea, BUN, and creatinine could result from glomerular dysfunction or increased renal tissue breakdown, or decreased urinary clearance by the kidney. Hence, in our study also, the significantly elevated concentrations of urea, BUN, and creatinine in the 12 days of 0.4 mg/L of malathion exposed fish serum corroborates the findings of the renal histomorphological deformities observed on continuous exposure to malathion that might hamper renal glomerular filtration. Further, the role of electrolytes in the organisms are well known for carrying out essential metabolic activities, neuromuscular irritability, intra and extracellular acidobasic equilibrium, and maintaining the osmolarity of body fluids [109]. While we observed significantly (p < 0.05, DMRT) increased concentration of Ca2+ ions in the malathion exposed fish serum, Luskova et al. [109] found significant reduction (p < 0.05) in the blood Ca2+ and phosphorous ions concentrations in the fish Cyprinus carpio following 4 days of exposure to 600 g/L of diazinon (OPPs). However, in the present study, the increased Ca2+ levels in the blood of malathion exposed fish might be explained on the basis of their participation in hyper nerve excitability and muscle convulsions induced by malathion toxicity. Furthermore, our study also confirms that the blood metabolites could be used as an effective bioindicator to assess pesticide toxicity in fish.
Fig. 4.
Changes in the kidney function test in blood tissue of C. punctatus fish exposed to 0.4 mg/L of malathion after 4, 8, and 12 days of exposure.
Values were represented as Mean ± SEM.
Significant differences between mean values at different stages of exposure were analysed by One-way analysis of variance followed by Duncan’s multiple range test (DMRT) at the level of p < 0.05.
Significant data is shown by small progressive alphabetic letters (a–d); similar consequent alphabetic letters present no significant changes between the two means.
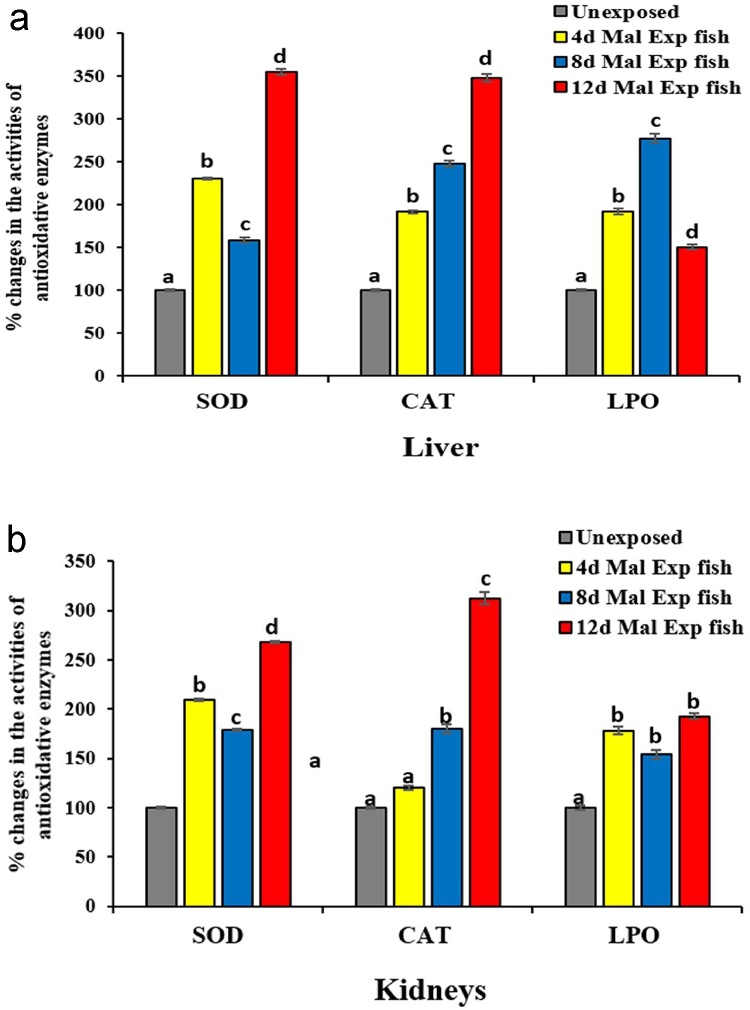
3.3.5. Effect of malathion on antioxidative enzymes (SOD, CAT, and LPO) in C. punctatus fish
OPPs such as malathion can induce oxidative damage in cells and tissues of organisms via generating a huge amount of reactive oxygen species (ROS), such as superoxide anion, peroxyl radicals, hydroperoxyl radical, hydrogen peroxide [64]. Fishes exhibit a strong antioxidative defense system to control ROS's abnormal levels raised due to oxidative stress [64,98,110]. The organs prominently involved in antioxidative responses are the liver and kidneys in fish. They are considered the potential target for xenobiotic induced toxicity because the liver is the principal metabolizing site for the biotransformation of OPPs and Kidneys' for its primary role in excretion. Further, both are metabolically highly active and can concentrate toxicants in higher magnitude than the rest of the body organs [108,22,114]. Ecotoxicological consequences of malathion, particularly its effects on antioxidative enzymes (SOD and CAT) and LPO in the liver and kidneys of fish, are not well understood, and only a few authors have demonstrated low concentrated malathion effects on the antioxidant system of fish [71,72,107]. Therefore, to evaluate the malathion toxicity, the variations in the activities of antioxidative marker enzymes such as SOD (IU/mg of protein), CAT (n mole of H2O2 decomposed/min/mg of tissue wt), and LPO (n mole of MDA formed/100 mg tissue wt) were measured in the hepatorenal tissues of C. punctatus fish following exposure to 0.4 mg/L of malathion for 4, 8 and 12 days (Fig. 5a and b). The fish liver and kidney tissues displayed a biphasic pattern of increments in the SOD activities following malathion exposure to 4, 8, and 12 days (Fig. 5a and b). Significantly (p < 0.05) enhanced values of 254 % and 167.69 % were found in the liver and kidneys' SOD activities of fish, respectively following 12 days of malathion exposure compared to the controls (Fig. 5a and b). However, almost linear increments were observed in the CAT activities of liver and kidney of malathion exposed fish concerning increased exposure durations (Fig. 5a and b). Compared to control, liver and kidneys' CAT activities reached 248.09 % and 212.39 %, respectively subsequent 12 days of malathion exposure (Fig. 5a and b). On the other hand, the LPO activities in the liver and kidneys tissues of malathion exposed fish showed a varied response at different time intervals of the experiment (Fig. 5a and b). In comparison to untreated fish, liver LPO activity inclined initially to 277.27 % following 8 days of malathion exposure, which after 12 days remained high with 150 %, while in the renal tissues, the activities of LPO increased (p < 0.05, DMRT) in a steady range of 162–205 respectively after 4, 8, and 12 days of the experiment (Fig. 5a and b). Thus, the activities of antioxidative enzymes in the liver of malathion exposed fish followed an order of SOD > CAT > LPO while the pattern of response observed in kidney was CAT > SOD > LPO (Fig. 5a and b). Since SOD and CAT antioxidant enzymes provide the first line of defense of organisms against ROS, therefore, the increased activities of liver and kidneys' antioxidant (SOD and CAT) enzymes were likely to be a species response to combat against the increased ROS levels generated by malathion toxicity [98]. Further, comparatively less elevated activities of LPO in the hepatorenal tissues in caparison to their SOD and CAT activities of C. punctatus fish following malathion treatment might be due to compensative role of SOD and CAT in effective cellular regeneration [108,71]. The elevated levels of LPO in the tissue also represent toxicant-induced oxidative stress in organisms [21]. Generally, the LPO mediates toxicity via generation of covalent adducts {thiobarbituric acid reactive substances (TBARS) and MDA} formed by the reaction between the protein (especially at their lysine residues) and carbonyl groups of the malondialdehyde (MDA) that eventually interact with the lipid bilayers of cells, causing severe cellular dysfunction or tissue damage [115,116]. Hence, in the present study, the elevated levels of MDA formed due to lipid peroxidation in hepatorenal tissues of malathion exposed C. punctatus fish clearly demonstrate that deformities in these tissues of fish are certainly due to poisonous nature of malathion. Thenmozhi et al. [117] found increased activity of CAT in the liver of Labeo rohita exposed to 5 mg/L of malathion for 15 days. Further, Yonar et al. [107], while analysing the effects of malathion on the activities SOD and CAT in gills and liver tissues of Cyprinus carpio fish, pointed out that increased activity of antioxidants in fish indicates an adaptive strategy to defend against the deleterious effects of ROS generated during insecticide metabolism. Similarly, Souza et al. [72] also noticed an increase of 1.1 and 1.75 folds in the hepatic SOD and CAT activities of Colossoma macropomum fish respectively, in comparison to their controls following 96 h of 7.30 mg/L of malathion exposure (one half of 96 h LC50 value 15.77 ± 3.30 mg/L). However, this author found insignificant alterations in the fish liver LPO activity following exposure to malathion relative to their controls. Furthermore, Toledo-Ibarra et al. [111] also did not find noteworthy enhancement in LPO activities of liver and gills of fish Nile tilapia on exposure to different concentrations (0.97, 1.95, 3.95 ppm) of diazinon (OPPs) for 12 or 24 h durations suggesting that probably longer exposure time is needed to oxidize lipids in the presence of diazinon. Thus, in the present study, comparatively reduced elevation in LPO activity of the hepatorenal tissues of fish might be apparently resulted from the compensatory response of the antioxidant defense systems (SOD and CAT) following low dose of malathion exposure [72]. On the contrary, some of the authors such as Huculeci et al. [108], Dinu et al. [64] and Karmakar et al. [71] noticed a prominent increase in the LPO activities in liver and kidneys tissues of fish exposed to OPPs in comparison to untreated fish. Huculeci et al. [108] demonstrated that liver LPO activities significantly increased while SOD and CAT activities were transitorily inhibited post three days of exposure to 0.05 mg/L of malathion in Carassius auratus gibelio fish. They pointed out that, in the kidney also CAT specific activity decreased in a time-dependent manner. Similarly, Dinu et al. [64] demonstrated decreased SOD and CAT activities and increased LPO activities in the liver of Carassius auratus gibelio exposed to 2 μg/L of deltamethrin. Karmakar et al. [71] also found a concentration-dependent increase in the levels of LPO activities in the liver, kidney, and gills tissues of fingerling of fish Labeo rohita with malathion exposure. According to the author, low doses of malathion failed to induce mortality in fishes but are sufficient to cause significant tissue damage via ROS generation, as manifested by the elevated levels of LPO activity in the kidney of malathion exposed Labeo rohita.
Fig. 5.
(a) Changes in the antioxidative enzymes activities in liver tissue of C. punctatus fish exposed to 0.4 mg/L of malathion after 4, 8, and 12 days of exposure.
Values were represented as Mean ± SEM.
Significant differences between mean values at different stages of exposure were analysed by One-way analysis of variance followed by Duncan’s multiple range test (DMRT) at the level of p < 0.05.
Significant data is shown by small progressive alphabetic letters (a–d); similar consequent alphabetic letters present no significant changes between the two means.
(b) Changes in the antioxidative enzymes activities in kidneys tissue of C. punctatus fish exposed to 0.4 mg/L of malathion after 4, 8, and 12 days of exposure.
Values were represented as Mean ± SEM.
Significant differences between mean values at different stages of exposure were analysed by One-way analysis of variance followed by Duncan’s multiple range test (DMRT) at the level of p < 0.05.
Significant data is shown by small progressive alphabetic letters (a–d); similar consequent alphabetic letters present no significant changes between the two means.
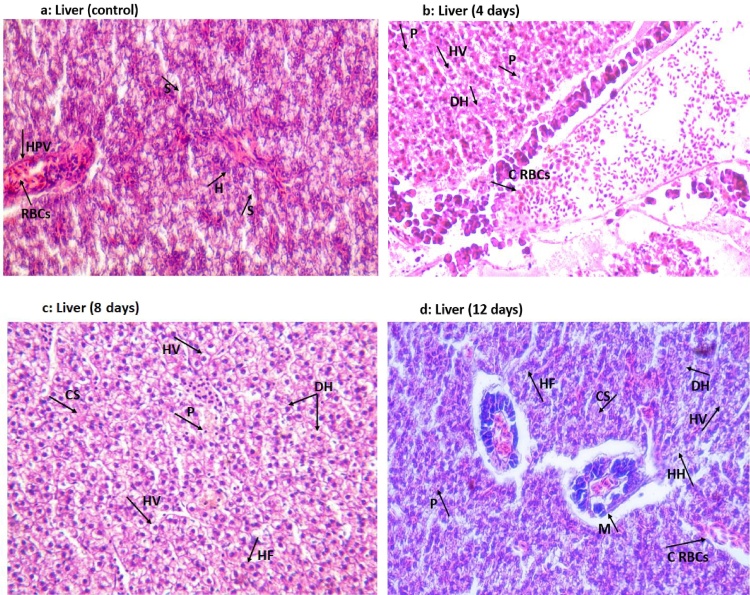
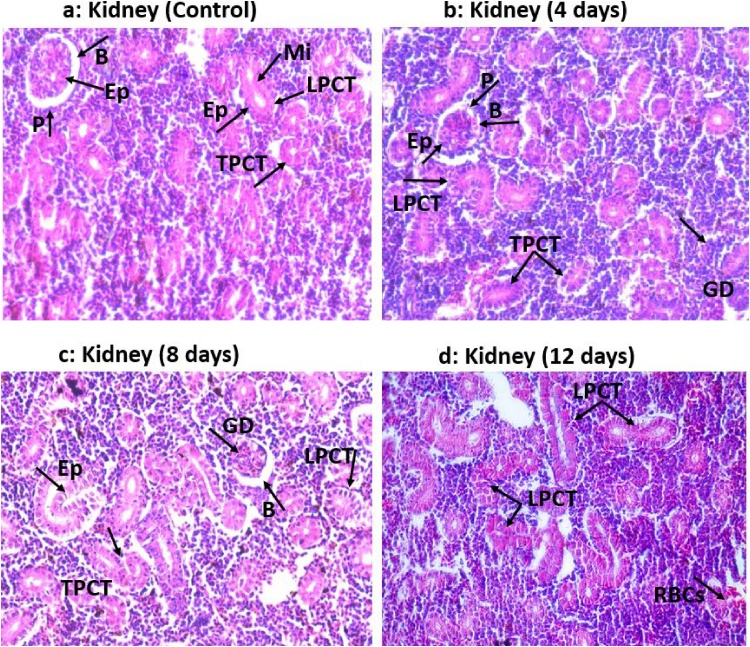
3.3.6. Histopathological effect of a low dose of malathion on liver and kidneys tissues
Following exposure to 0.4 mg/L of malathion for 4, 8, and 12 days, extensive damages in the hepatic and renal tissues of C. punctatus fish were noticed, whereas in the control groups, liver and kidney tissues exhibited typical histological structure (Figs. 6a-d and 7 a-d). Further, it was found that the tissue deterioration in fish increased progressively with an increase in the malathion exposure periods (Figs. 6b-d and 7 b-d). Moreover, no alterations were observed in the structure of these tissues of fish after 24 h of 0.4 mg/L of malathion exposure than controls, therefore, were not further discussed.
Fig. 6.
Histopathological alterations in the liver tissue of 0.4 mg/L of malathion exposed Channa punctatus at different days of intervals compared to control (unexposed) at 400 × .
(Fig. 6a) Liver (Control): H: Hepatocytes (polygonal in shape with a prominent nucleus), S: Sinusoids (fenestrated), HPV: Hepatic Portal Vein, RBCs: (Red Blood cells).
(Fig. 6b) Liver (4 days): HV: Vacuolization in hepatocytes, DH: Disintegration of hepatocytes cell membrane and oozing of cytoplasmic content, P: Pyknotic nuclei, C RBCs: Congestion of RBCs in HPV of hepatocyte.
(Fig. 6c) Liver (8 days): HV: Vacuolization in hepatocytes, DH: Disintegration of hepatocytes cell membrane, oozing of cytoplasmic content with lateral nuclei, P: Pyknotic nuclei, HF: Hepatocytes fusion; CS: Congested Sinusoids
(Fig. 6d) Liver (12 days): HV: Vacuolization in hepatocytes, DH: Disintegration of hepatocytes cell membrane and oozing of cytoplasmic content, P: Pyknotic nuclei, C RBCs: Congestion of RBCs in HPV of hepatocyte, HH: Hypertrophied Hepatocytes, HF: Hepatocytes fusion, M: melanomacrophages center, CS: Congested Sinusoids
Fig. 7.
Histopathological alterations in the kidney tissue of 0.4 mg/L of malathion exposed Channa punctatus at different days of intervals compared to control (unexposed) at 400 × .
(Fig. 7a) Kidney (Control): Typical structure of renal tubules with glomerulus and well-defined Bowman’s space (B) (inter-space between two layers), P: Parietal layer, Ep: Inner layer made up of epithelial cells, Mi: Microvilli protruding the lumen, TPCT: Transverse section of proximal convoluted tubule, LPCT: Longitudinal section of proximal convoluted tubule.
(Fig. 7b) Kidney (4 days): B: Decreased inter-space in Bowman’s capsule, P: Necrosis of parietal layer, Ep: Necrosis of epithelial cells of glomerular structure, GD: Glomerular destruction, LPCT and TPCT: Hypertrophied cells of tubules and occlusion of tubular lumen, vacuolization of inner epithelial cell and detached epithelium layer of the renal tubule
(Fig. 7c) Kidney (8 days): B: Increased Bowman’s space, GD: Glomerular destruction, LPCT: Necrosis of parietal layer of PCT, Ep: Hypertrophied and vacuolized epithelial cell of PCT, TPCT: Deformed renal tubules, shrinkage of glomeruli with narrow lumen of PCT.
(Fig. 7d) Kidney (12 days): LPCT: Extensive destruction of renal tubule with complete degeneration of the glomerular structure, hypertrophied and vacuolized epithelial cell of PCT, TPCT: Deformed renal tubules, shrinkage of glomeruli with narrow lumen of PCT and infiltration of RBCs in their interstitial fluid.
3.3.6.1. Liver
The control (unexposed) group of C. punctatus fish showed a normal structure of hepatocytes with clear hepatic cord (Fig. 6a) On subsequent exposure to 0.4 mg/L of malathion for 4 days, considerable anatomical changes in the liver tissue of experimental groups of C. punctatus fish such as dilation of sinusoids, hypertrophied hepatocytes, and congestion of erythrocytes along the central vein were noticed compared to the controls (Fig. 6a-b). In addition to that, vacuolization in cytoplasmic content of hepatocytes, pyknotic nuclei, rupturing of the hepatocyte's cell membrane and efflux of their cytoplasmic content was also observed in experimental groups of C. punctatus concerning unexposed controls (Fig. 6a-b). Deka and Mahanta [29] found swelling of hepatocytes with pyknotic nuclei in malathion exposed catfish Heteropneustes fossilis. According to Pugazhvendan et al. [66], Fahmy [21], and Mostakim et al. [59], damages in the liver tissue of fish occurred relatively higher than any other tissue probably because it is the metabolic centre for detoxification of toxicants and has a greater capacity of absorbing OPPs residues. After prolonged 8 and 12 days of 0.4 mg/L of malathion exposure, necrosis of liver cells, congestion of red blood cells (RBCs) into the hepatic portal vein and fibrosis of sinusoids were evident in experimental groups of fish in comparison to their controls (Fig. 6c-d). Similar results such as vacuolization in cytoplasmic content, degeneration, and infiltration of lymphocytes in hepatic tissue were also noted by Ibrahim [118] in fish Oreochromis niloticus on individual exposure to both 0.1 mg/L and 0.3 mg/L doses of malathion. Magar and Shaikh [119] also noticed degeneration, vacuolization of the cytoplasm of hepatocytes, and atrophy of liver cells in C. punctatus on subsequent exposure of 4 days to 0.8 mg/L of malathion. They suggested that hepatocytes' vacuolization might indicate an imbalance between the rate of synthesis and the rate of release of a substance in hepatocytes. Moreover, according to the Moore et al. [120] and Tchounwou et al. [102], pesticides, like other toxicants, induce the generation of free radicals that destruct the vital macromolecules constituents of the cells and therefore, the alterations in hepatocytes of organisms are the only reflections of the toxic effect of contaminants. Furthermore, El-Nahhal [39] revealed higher toxicity of malathion to liver tissue of Tilapia nilotica compared to the brain due to relatively more significant malathion deposition in hepatic tissue than brain where the blood-brain barrier prohibits malathion entry into it. They further explained that liver is more sensitive to malathion toxicity than brain because it might first lead to inhibition of carboxylesterase (CE) in the liver and later causes inhibition of AchE enzyme. Therefore, histopathological alterations in the liver could be used as the early signals for determining malathion toxicity in fishes [39]. The levels of injuries in tissues of malathion exposed fishes could differ from fish to fish because physiological activities of each fish species are different from others, and therefore pesticide toxicity varies from moderately toxic to some species of fish to highly toxic to other species [59,39]. Thus, our observation of damages in liver tissue of C. punctatus fish following exposure to 0.4 mg/L of malathion might be due to excessive malathion and its biotransformed metabolites accumulation in hepatocytes and greater inhibition of liver enzymes activities compared to other affected tissues. Furthermore, significant alterations in the vital biomacromolecules compositions and enzymatic activities (esterases) of liver and kidneys tissues of Tilapia nilotica and Oreochromis mossambicus fishes following exposure to varying concentration of malathion, respectively were also previously reported by Zhang et al. [42] and El-Nahhal [39]. Current investigations showed that even though a low dose of 0.4 mg/L of malathion (1/20th of 96-h LC50 value) would not cause the immediate mass death of C. punctatus fish, it could extensively deteriorate hepatocyte configuration of fish on its continuous prolonged exposure.
3.3.6.2. Kidneys
Histopathological examination of the kidneys tissue of the control group of fish showed a regular structure of tubules, glomerulus, and Bowman's capsule (Fig. 7a). Following 4 days of exposure to 0.4 mg/L of malathion, kidneys tissues of experimental groups of C. punctatus showed shrinkage of glomeruli and renal tubules while no abnormalities were observed in the control fish (Fig. 7a-b). After 8 days of 0.4 mg/L of malathion exposure, the epithelium layer of the renal tubule detached from the basement membrane (Fig. 7c). In addition to that, extensive vacuolization in the cytoplasm of renal cells, hypertrophy, and renal tubules’ degeneration were also noticed (Fig. 7b-d). Cristina et al. [122] reported cytoplasm vacuolization in renal cells, changes in cell and nuclear volumes, renal tubules necrosis, nuclear malformations of epithelial cells, anisogamy, nuclei pycnosis, and nuclei hypertrophy following 72 h of 0.05 mg/L of malathion exposure in Carassius carassius fish. Further, in the present study, after 12 days of exposure to 0.4 mg/L of malathion, the epithelial lining of renal tubules deteriorates extensively, the lumen of renal tubules narrows down and spaces in Bowman's capsule increased. In addition to that, excessive degeneration of renal tubules was found in the 12 days malathion exposed fish compared to 8 days (Fig. 7c-d). Such extensive damages in kidneys tissues of C. punctatus fish illustrates the toxic nature of the low amount of malathion. Uikey [121] also found marked degenerative changes in renal tubules such as necrosis and increased spaces in Bowman's capsule of fish Labeo rohita following exposure to sublethal concentration of 0.8 mg/L of malathion. Similarly, histopathological lesions in kidneys tissue were also noticed in common carp following 14 continuous days of exposure to 100 μg/L of chlorpyrifos pesticide [58]. Their observations were cellular and nuclear hypertrophy, narrowing of the tubular lumen, cytoplasmic vacuolation, nuclear degeneration, occlusion of tubular lumen, dilation of glomerular capillaries, degeneration of glomerulus, and haemorrhage in Bowman's space of renal tissue. According to Prashanth [57], cypermethrin induced severe histopathological changes in the kidney of Cirrhinus mrigala. Some of the alterations noted by them were necrosis of tubular cells and pyknosis of nuclei followed by aggregations of more and more leucocytes and macrophages around the tubules. According to Prashanth [57], Deka and Mahanta [29], and Sharma and Sharma [60] the differential sensitivity of animals to malathion toxicity is attributed to variations in the activity of competing for hepatic and renal activities and detoxifying enzymes. Thus, our findings of histological damages in the kidneys tissue of malathion exposed C. punctatus fish corroborates with the results of Cristina et al. [122], Uikey [121], and Pal et al. [58]. Further, the histomorphological alteration in the renal tissues of malathion exposed C. punctatus might be attributed to the elevated levels of antioxidative enzyme activities and comparatively greater retention of pesticide than the rest of the body organs till it gets eliminated out from the body [29,54,57,60].
4. Conclusion
The toxic manifestation of the low dose of 0.4 mg/L (1/20 of 8.0 mg/L of 96-h LC50 value) of malathion on C. punctatus fish was assessed using the specific morphometric, serum, and hepatorenal tissues functionality biomarkers on subsequent exposure to 4, 8, and 12 days. It was clear from the findings that this malathion dose was low to cause any significant alterations in the body form (insignificant changes in the body weight, length, K after 12 days of exposure). However, continuous exposure to the same dose of malathion, indeed induced distress symptoms in the fish. Following 4 and 8 days of malathion exposure, the HSI, KSI, hepatorenal tissue weight and glucose, bilirubin (total and direct), activities of AST, ALT, and ALP levels in the fish serum decreased significantly. In contrast, the serum concentration of total protein, urea, urea nitrogen (BUN), creatinine and Ca2+ elevated significantly compared to values of control. The levels of antioxidative enzyme activities (SOD, CAT, and LPO) and histological deformities enhanced significantly in the hepatorenal tissues of 4 and 8 days of malathion exposed fish concerning their controls. However, the percentage inhibition of transaminases (AST and ALT) and ALP activities in serum and elevations of antioxidative marker enzymes (SOD, CAT, and LPO) in hepatorenal tissues were more pronounced in 12 days malathion exposed fish than in 4 or 8 days. Further, the deterioration noticed in the serum biochemical and tissues' histopathological parameters was time and tissue-dependent. Besides, the concentrations of total cholesterol, albumin, and phosphorous in the serum of malathion exposed fish remained comparable to controls' values. Thus, our finding indicates that even a low dose of malathion might induce physiological abnormalities in the fish at its constant persistence. Therefore, seepage of even low amounts of malathion into nearby wetlands should be prohibited. Moreover, the consequences of lower concentrations of malathion on various fish species' biology need to be investigated further to determine their safe levels for the aquatic ecosystem.
CRediT authorship contribution statement
Sandhya Bharti: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing - original draft, Writing - review & editing. Fazle Rasool: Formal analysis, Investigation, Resources, Visualization, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
Thanks are due to Dr. Abha Mishra, Assistant Professor, Dept. of Zoology, BBAU, Lucknow for her kind support, and providing the facilities of microtome (Leica) and image microscope (Olympus inbuild with cannon camera). The author is also grateful to Mr. Fazle Rasool for fish sampling, feeding, taking care of the fish and maintenance of Fish Laboratory during the entire period of the experiment.
Edited by Dr. A.M Tsatsaka
Contributor Information
Sandhya Bharti, Email: sandhyabharti54@gmail.com.
Fazle Rasool, Email: Fazlerasol@gmail.com.
References
- 1.US Environmental Protection Agency (USEPA) U.S. environmental protection agency, office of prevention, pesticides and toxic substances, office of pesticide programs, U.S. government printing office; Washington, DC: 2006. Reregistration Eligibility Decision (RED) for Malathion; EPA 738-R-06-030. [Google Scholar]
- 2.US Environmental Protection Agency, Office of Pesticide Programs . 2009. Memorandum Registration Review – Preliminary Problem Formulation, Ecological Risk, Environmental Fate, and Endangered Species Assessments for Malathion. dated April 22, 2009, pp. 44, Available at: https://www.regulations.gov/ EPA-HQ-OPP-2009-0317-0002, last accessed May 5, 2017. [Google Scholar]
- 3.Assocham . 2007. Debunking Theory of India’s Pesticide Consumption Being Highest. A Report on “Pesticide Residues in Indian Food and Agricultural Products’’ Submitted to Govt. India.http://www.freshplaza.com/news_detail.asp?id=1265 Available from. [Google Scholar]
- 4.Kamaraju R., Sharma V.P., Vaishali V., Natarajan E., Kumar B.T., Mohan B.R., Prasad D.A. Temporospatial distribution of insecticide-resistance in Indian malaria vectors in the last quarter-century: need for regular resistance monitoring and management. J. Vector Borne Dis. 2017;54(2):111–130. [PubMed] [Google Scholar]
- 5.US Department of Agriculture (USDA, APHIS) John Stewart, Raleigh, NC-27606; 2018. Draft Human Health and Ecological Risk Assessment for Malathion in Exotic Fruit Fly Applications; pp. 1–56. [Google Scholar]
- 6.Bradman M.A., Harnly M.E., Goldman L.R., Marty M.A., Dawson S.V., Dibartolomeis M.J. Malathion and malaoxon environmental levels used for the exposure assessment and risk characterization of aerial applications to residential areas of southern California, 1989-1990. J Exp Anal Environ Epidemiol. 1994;4(1):49–63. [PubMed] [Google Scholar]
- 7.Poomagal S., Sujatha R., Kumar P.S., Vo D.V.N. A fuzzy cognitive map approach to predict the hazardous effects of malathion to environment (air, water and soil) Chemosphere. 2020;263 doi: 10.1016/j.chemosphere.2020.127926. [DOI] [PubMed] [Google Scholar]
- 8.Castillo L.E., De la Cruz E., Ruepert C. Ecotoxicology and pesticides in tropical aquatic ecosystems of Central America. Environ. Toxicol. Chem. 1997;16:41–51. [Google Scholar]
- 9.Agarwal A., Prajapati R., Singh O.P., Raza S.K., Thakur L.K. Pesticide residue in water—a challenging task in India. Environ. Monit. Assess. 2015;87(2):54. doi: 10.1007/s10661-015-4287-y. [DOI] [PubMed] [Google Scholar]
- 10.Iturburu F.G., Calderon G., Amé M.V., Menone M.L. Ecological risk assessment (ERA) of pesticides from freshwater ecosystems in the Pampas region of Argentina: legacy and current use chemicals contribution. Sci. Total Environ. 2019;691:476–482. doi: 10.1016/j.scitotenv.2019.07.044. [DOI] [PubMed] [Google Scholar]
- 11.Bhanti M., Taneja A. Contamination of vegetables of different seasons with organophosphorus pesticides and related health risk assessment in northern India. Chemosphere. 2007;69(1):63–68. doi: 10.1016/j.chemosphere.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 12.Aktar M.W., Paramasivam M., Sengupta D., Purkait S., Ganguly M., Banerjee S. Impact assessment of pesticide residues in fish of Ganga river around Kolkata in West Bengal. Environ. Monit. Assess. 2009;157(1–4):97–104. doi: 10.1007/s10661-008-0518-9. [DOI] [PubMed] [Google Scholar]
- 13.Aktar W., Sengupta D., Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip. Toxicol. 2009 doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joko T., Anggoro S., Sunoko H.R., Rachmawati S. Identification of soil properties and organophosphate residues from agricultural land in Wanasari sub-District, Brebes, Indonesia. E3S Web of Conferences. 2018;31:06010. doi: 10.1051/e3sconf/20183106010. [DOI] [Google Scholar]
- 15.Hill E.F., Burton G.A., Jr., Cairns J., Jr. Wildlife toxicology of organophosphorus and carbamate pesticides. In: Hoffman D.J., Rattner B.A., editors. Handbook of Ecotoxicology. Lewis Publishers; Boca Raton: 2003. pp. 281–312. [Google Scholar]
- 16.Hayes T.B., Case P., Chui S., Chung D., Haeffele C., Haston K. Pesticide mixtures, endocrine disruption, and amphibian declines: are we underestimating the impact? Environ. Health Perspect. 2006;114:40–50. doi: 10.1289/ehp.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenik J., Tankiewicz M., Biziuk M. Properties and determination of pesticides in fruits and vegetables. Trends Analyt. Chem. 2011;30(6):814–826. doi: 10.1016/j.trac.2011.02.008. [DOI] [Google Scholar]
- 18.Agbohessi P.T., Toko I.I., Ouedraogo A., Jauniaux T., Mandiki S.N.M., Kestemont P. Assessment of the health status of wild fish inhabiting a cotton basin heavily impacted by pesticides in Benin (West Africa) Sci. Total Environ. 2015;506-507:567–584. doi: 10.1016/j.scitotenv.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 19.Li S., Yang Y., Shi M.H., Wang J.F., Ran X.Q. miR-96-5p attenuates malathion-induced apoptosis of human kidney cells by targeting the ER stress marker DDIT3. J. Environ. Sci. Health B. 2020:1–7. doi: 10.1080/03601234.2020.1816092. [DOI] [PubMed] [Google Scholar]
- 20.Tzatzarakis M., Kokkinakis M., Renieri E., Goumenou M., Kavvalakis M., Vakonaki E., Chatzinikolaou A., Stivaktakis P., Tsakiris I., Rizos A., Tsatsakis A. Multiresidue analysis of insecticides and fungicides in apples from the Greek market. Applying an alternative approach for risk assessment. Food Chem. Toxicol. 2020;140 doi: 10.1016/j.fct.2020.111262. [DOI] [PubMed] [Google Scholar]
- 21.Fahmy G.H. Malathion toxicity: effect on some metabolic activities in Oreochromis niloticus, the tilapia Fish. Int. J. Biosci. Biochem. Bioinforma. 2012;2(1):52–55. [Google Scholar]
- 22.Deka S., Mahanta R. Malathion toxicity on fish - A Review. Int. J. Curr. Res. 2016;8(12):44120–44128. [Google Scholar]
- 23.Nicolopoulou-Stamati P., Maipas S., Kotampasi C., Stamatis P., Hens L. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front. Public Health. 2016;4:148. doi: 10.3389/fpubh.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W., Lei-Lei Yang L.L., Luo S.M., Ma J.Y., Zhao Y., Shen W., Yin S. Toxic effects and possible mechanisms following malathion exposure inporcine granulosa cells. Environ. Toxicol. Pharmacol. 2018;64:172–180. doi: 10.1016/j.etap.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Shieh P., Jan C., Liang W. The protective effects of the antioxidant N-acetylcysteine (NAC) against oxidative stress-associated apoptosis evoked by the organophosphorus insecticide malathion in normal human astrocytes. Toxicology. 2019;417:1–14. doi: 10.1016/j.tox.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Jebali J., Banni M., Guerbej H., Almeida E.A., Bannaoui A., Boussetta H. Effects of malathion and cadmium on acetylcholinesterase activity and metallothionein levels in the fish Seriola dumerilli. Fish Physiol. Biochem. 2006;32(1):93–98. doi: 10.1007/s10695-006-0041-2. [DOI] [PubMed] [Google Scholar]
- 27.Chandra S. Toxic effect of malathion on acetylcholinesterase activity of liver, brain and gills of freshwater catfish Heteropneustes fossilis. Environ. Conserv. 2008;9(3):47–52. [Google Scholar]
- 28.Patil V.K., David M. Hepatotoxic potential of malathion in the freshwater teleost, Labeo rohita (Hamilton) Vet. Arh. 2009;79(2):179–188. [Google Scholar]
- 29.Deka S., Mahanta R. A study on the effect of organophosphorus pesticide malathion on hepato-renal and reproductive organs of Heteropneustes fossilis (Bloch) The Science Probe. 2012;1(1):1–13. [Google Scholar]
- 30.Lal B., Sarang M.K., Kumar P. Malathion exposure induces the endocrine disruption and growth retardation in the catfish, Clarias batrachus (Linn.) Gen Comp Endocr. 2013;181:139–145. doi: 10.1016/j.ygcen.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Ortiz-Delgado J.B., Funes V., Sarasquete C. The organophosphate pesticide -OP- malathion inducing thyroidal disruptions and failures in the metamorphosis of the Senegalese sole, Solea senegalensis. BMC Vet. Res. 2019;15(1):57. doi: 10.1186/s12917-019-1786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inbaraj R.M., Haider S. Effect of malathion and endosulfan on brain acetylcholinesterase and ovarian steroidogenesis of Channa punctatus (Bloch) Ecotoxicol. Environ. Saf. 1988;16(123-):128. doi: 10.1016/0147-6513(88)90025-5. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz-Delgado J.B., Scala E., Arellano J.M., Ubeda-Manzanaro M., Sarasquete C. Toxicity of malathion at early life stages of the Senegalese sole, Solea senegalensis (Kaup, 1858): notochord and somatic disruptions. Histol. Histopathol. 2018;33:157–169. doi: 10.14670/HH-11-899. [DOI] [PubMed] [Google Scholar]
- 34.Guyton K.Z., Loomis D., Grosse Y., El Ghissassi F., Benbrahim-Tallaa L., Guha N., Scoccianti C., Mattock H., Straif K. International agency for research on Cancer monograph working group, IARC, Lyon, France, carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015;16:490–491. doi: 10.1016/S1470-2045(15)70134-8. [DOI] [PubMed] [Google Scholar]
- 35.IARC (International Agency for Research on Cancer) Some Organophosphate Insecticides and Herbicides, vol. 12. IARC, Lyon, France, p. 464 pp. hepatotoxicity. J Royal Sci. 2015;1(1):10–15. [Google Scholar]
- 36.Selmi S., Rtibi K., Grami D., Sebai H., Marzouki L. Malathion, an organophosphate insecticide, provokes metabolic, histopathologic and molecular disorders in liver and kidney in prepubertal male mice. Toxicol. Rep. 2018;5:189–195. doi: 10.1016/j.toxrep.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pesticide Action Network International (PAN) International list of highly hazardous pesticides. Pestic. Action Net. Int. 2018:1–37. doi: 10.1016/j.icesjms.2004.07.004. [DOI] [Google Scholar]
- 38.Rico A., Waichman A.V., Geber-Correa R., Van den Brink P.J. Effects of malathion and carbendazim on Amazonian freshwater organisms: comparison of tropical and temperate species sensitivity distributions. Ecotoxicology. 2011;20:625–634. doi: 10.1007/s10646-011-0601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Nahhal Y. Toxicity of some aquatic pollutants to fish. Environ. Monit. Assess. 2018;190:449. doi: 10.1007/s10661-018-6830-0. [DOI] [PubMed] [Google Scholar]
- 40.Hoffman R.S., Capel P.D., Larson S.J. Comparison of pesticides in eight U.S. Urban streams. Environ. Toxicol. Chem. 2000;19:2249–2258. doi: 10.1002/etc.5620190915. [DOI] [Google Scholar]
- 41.Gomez-Gutierrez A.I., Jover E., Bodineau L., Albaies J., Bayona J.M. Organic contaminant loads into the Western Mediterranean Sea: estimate of Ebro River inputs. Chemosphere. 2006;65:224–236. doi: 10.1016/j.chemosphere.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z., Hong H., Wang X., Lin J., Chen W., Xu L. Determination and load of organo‐ phosphorus and organochlorine pesticides at water from Jiulong River estuary, China. Mar. Pollut. Bull. 2002;45:397–402. doi: 10.1016/s0025-326x(02)00094-2. [DOI] [PubMed] [Google Scholar]
- 43.Fadaei A., Dehghani M.H., Nasseri S., Mahvi A.H., Rastkari N., Shayeghi M. Organophosphorous pesticides in surface water of Iran. Bull. Environ. Contam. Toxicol. 2012;88(6):867–869. doi: 10.1007/s00128-012-0568-0. [DOI] [PubMed] [Google Scholar]
- 44.Malhat F., Nasr I. Organophosphorus pesticides residues in fish samples from the River Nile tributaries in Egypt. Bull. Environ. Contam. Toxicol. 2011;87(6):689–692. doi: 10.1007/s00128-011-0419-4. [DOI] [PubMed] [Google Scholar]
- 45.Koch-Schulmeyer M., Ginebreda A., Gonzalez S., Cortina J.L., Lopez, de Alda M., Barcelo D. Analysis of the occurrence and risk assessment of polar pesticides in the Llobregat river basin (NE Spain) Chemosphere. 2012;86:8–16. doi: 10.1016/j.chemosphere.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 46.Masia A., Campo J., Navarro-Ortega A., Barcelo D., Pico Y. Pesticide monitoring in the basin of Llobregat River (Catalonia, Spain) and comparison with historical data. Sci. Total Environ. 2015;503-504:58–68. doi: 10.1016/j.scitotenv.2014.06.095. [DOI] [PubMed] [Google Scholar]
- 47.Cengiz E.I., Unlu E. Histopathology of gills in mosquitofish, Gambusia affinis after long‐term exposure to sublethal concentrations of malathion. J. Environ. Sci. Health B. 2003;38(5):581–589. doi: 10.1081/PFC-120023516. [DOI] [PubMed] [Google Scholar]
- 48.Chatterjee N.S., Banerjee K., Utture S., Kamble N., Rao B.M., Panda S.K., Mathew S. Assessment of polyaromatic hydrocarbons and pesticide residues in domestic and imported pangasius (Pangasianodon hypophthalmus) fish in India. Sci. Food Agric. 2016;96:2373–2377. doi: 10.1002/jsfa.7352. [DOI] [PubMed] [Google Scholar]
- 49.Edwards J.W., Lee S.G., Heath L.M., Pisaniello D.L. Worker exposure and a risk assessment of malathion and fenthion used in the control of Mediterranean fruit fly in South Australia. Environ. Res. 2007;103:38–45. doi: 10.1016/j.envres.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Holtcamp W. Obesogens: an environmental link to obesity. Environ. Health Perspect. 2012;120:a62–a68. doi: 10.1289/ehp.120-a62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moretto A., Colosio C. The role of pesticide exposure in the genesis of Parkinson’s disease: epidemiological studies and experimental data. Toxicology. 2013;307:24–34. doi: 10.1016/j.tox.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 52.Ozkara A., Akyıl D., Konuk M. 2016. Pesticides, Environmental Pollution, and Health, Environmental Health Risk - Hazardous Factors to Living Species, Marcelo L. Larramendy and Sonia Soloneski, Intech Open.https://www.intechopen.com/books/environmental-health-risk-hazardous-factors-to-living-species/pesticides-environmental-pollution-and-health Available from: [Google Scholar]
- 53.Hernandez-Toledano D.S., Estrada-Muniz E., Vega L. Genotoxicity of the organophosphate pesticide malathion and its metabolite dimethylthiophosphate in human cells in vitro. Mutat. Res. 2020;856(857):503233. doi: 10.1016/j.mrgentox.2020.503233. [DOI] [PubMed] [Google Scholar]
- 54.Ortiz J.B., De Canales M.L.G., Sarasquete C. Histopathological changes induced by lindane (HCH) in various organs of fishes. Sci. Mar. 2003;67(1):53–61. [Google Scholar]
- 55.Wasu Y.H., Gadhikar Y.A., Ade P.P. Sublethal and chronic effect of carbaryl and malathion on Clarias batrachus (Linn.) J. Appl. Sci. Environ. Manage. 2009;13(2):23–26. [Google Scholar]
- 57.Prashanth M.S. Histopathological changes observed in the kidney of freshwater fish, Cirrhinus mrigala (Hamilton) exposed to cypermethrin. Recent Res. Sci. Technol. 2011;3(2):59–65. [Google Scholar]
- 58.Pal S., Kokushi E., Koyama J., Uno S., Ghosh A.R. Histopathological alterations in gill, liver and kidney of common carp exposed to chlorpyrifos. J. Environ. Sci. Health B. 2012;47(3):180–195. doi: 10.1080/03601234.2012.632285. [DOI] [PubMed] [Google Scholar]
- 59.Mostakim G.M., Zahangir Md M., Mishu M.M., Rahman Md K., Islam M.S. Alteration of blood parameters and histoarchitecture of liver and kidney of silver barb after chronic exposure to Quinalphos. J. Toxicol. 2015 doi: 10.1155/2015/415984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma K.K., Sharma J. Histopathological alterations in the liver, gills and kidneys of fish Labeo rohita after the exposure of endosulfan and lindane. Int J Adv Sci Eng Technol. 2019;4(3):237–240. [Google Scholar]
- 61.Barata C., Solayan A., Porte C. Role of B-esterases in assessing toxicity of organophosphorus (chlorpyrifos, malathion) and carbamate (carbofuran) pesticides to Daphnia magna. Aquat. Toxicol. 2004;66:125–139. doi: 10.1016/j.aquatox.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Androutsopoulos V.P., Hernandez A.F., Liesivuori J., Tsatsakis A.M. A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology. 2013;307:89–94. doi: 10.1016/j.tox.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Rebechi D., Richardi V.S., Vicentini M., Guiloski I.C., Silva de Assis H.C., Navarro- Silva M.A. Low malathion concentrations influence metabolism in Chironomus sancticaroli (Diptera, Chironomidae) in acute and chronic toxicity tests. Revista Brasileira de Entomologia. 2014;58(3):296–301. [Google Scholar]
- 64.Dinu D., Marinesco D., Munteanu M.C., Staicu A.C., Costache M., Dinischiotu A. Modulatory effects of deltamethrin on antioxidant defense mechanisms and lipid peroxidation in Carassius auratus gibelio liver and Intestine. Arch. Environ. Contam. Toxicol. 2010;58:757–764. doi: 10.1007/s00244-009-9401-0. [DOI] [PubMed] [Google Scholar]
- 65.Lopez-Lopez E., Sedeno-Diaz J.E. Biological indicators of water quality: The role of fish and macroinvertebrates as indicators of water quality. In: Armon R., Hänninen O., editors. Environmental Indicators. Springer; Dordrecht: 2015. [DOI] [Google Scholar]
- 66.Pugazhvendan S.R., Narendiran N.J., Kumaran R.G., Kumaran S., Alagappan K.M. Effect of malathion toxicity in the freshwater fish Ophiocephalus punctatus-A histological and histochemical study. World Journal of Fish and Marine Sciences. 2009;1(3):218–224. [Google Scholar]
- 67.Renieri E.A., Sfakianakis D.G., Alegakis A.A., Safenkova I.V., Buha A., Matovic V., Tzardi M., Dzantiev B.B., Divanach P., Kentouri M., Tsatsakis A.M. Nonlinear responses to waterborne cadmium exposure in zebrafish. An in vivo study. Environ. Res. 2017;157:173–181. doi: 10.1016/j.envres.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 68.Akter R., Pervin M.A., Jahan H., Rakhi S.F., Reza A.H.M.M., Hossain Z. Toxic effects of an organophosphate pesticide, envoy 50 SC on the histopathological, hematological, and brain acetylcholinesterase activities in stinging catfish (Heteropneustes fossilis) J Basic Appl Zool. 2020;81(47) doi: 10.1186/s41936-020-00184-w. [DOI] [Google Scholar]
- 69.Chovanec A., Hofer R., Schiemer F. Fish as bioindicators. In: Markert B.A., Breure A.M., Zechmeister H.G., editors. Bioindicators and Biomonitors. Elsevier; Burlington: 2003. pp. 639–676. [Google Scholar]
- 70.Yancheva V., Velcheva I., Stoyanova S., Georgieva E. Histological biomarkers in fish as a tool in ecological risk assessment and monitoring programs: a review. Appl. Ecol. Environ. Res. 2016;14(1):47–75. doi: 10.15666/aeer/1401_047075. [DOI] [Google Scholar]
- 71.Karmakar S., Patra K., Jana S., Mandal D.P., Bhattacharjee S. Exposure to environmentally relevant concentrations of malathion induces significant cellular, biochemical and histological alterations in Labeo rohita. Pestic. Biochem. Physiol. 2016;126:49–57. doi: 10.1016/j.pestbp.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 72.Souza S.S., Machado R.N., da Costa J.C., Campos D.F., da Silva G.S., de Almeida-Val V.M.F. Severe damages caused by Malathion exposure in Colossoma macropomum. Ecotoxicol. Environ. Saf. 2020;205 doi: 10.1016/j.ecoenv.2020.111340. [DOI] [PubMed] [Google Scholar]
- 73.Hamilton M.A., Russo C.R., Thurston V.R., Spearman-Karber Trimmed. Method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1977;59(11):714. [Google Scholar]
- 74.OECD . Environment Monograph 92. Environment Directorate; Paris, France: 1992. Guidance Document for Aquatic Effects Assessment. Organization for Economic Cooperation and Development. [Google Scholar]
- 75.Sprague J.B. Measurement of pollutant toxicity of fish, utilizing and applying bioassay results. Mars Res. 1973;4:3–32. [Google Scholar]
- 76.US Environmental Protection Agency (USEPA) EPA 600/R-99/064; Washington, DC: 2000. Methods for Measuring the Toxicity and Bioaccumulation of Sediment-associated Contaminants with Freshwater Invertebrates. [Google Scholar]
- 77.Lowry O.H., Rosenbrough N.J., Farr A.L., Randall B.J. Protein measurements with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 78.Schmidt F.H. Enzymatic determination of glucose and fructose simultaneously. Klin. Wochenschr. 1961;39:1244–1250. doi: 10.1007/BF01506150. [DOI] [PubMed] [Google Scholar]
- 79.Abell L.L., Levy B.B., Brodie B.B., Kendall F.E. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J. Biol. Chem. 1952;195:357–366. [PubMed] [Google Scholar]
- 80.Bergmeyer H.U., Horder M., Rej R. International federation of clinical chemistry (IFCC) scientific committee. J. Clin. Chem. Clin. Biochem. 1985;24:481–495. [PubMed] [Google Scholar]
- 81.Patton C.J., Crouch S.R. Enzymatic determination of urea. Anal. Chem. 1977;49:464–469. [Google Scholar]
- 82.Henry T.J. Principles and Techniques. edn. 2nd. Clin Chem.; New York: 1974. Harper and Row publishers. [Google Scholar]
- 83.Doumas B.T., Watson W.A., Biggs H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 84.Jendrassik L., Grof P. Vereinfachte photometrische methoden zur bestimmung des blutbilirubins. Biochemische Zeutschrift. 1938;297:82–89. [Google Scholar]
- 85.Trinder P. Colorimetric micro-determination of calcium in serum. Analyst. 1960;1017(85):889–894. [Google Scholar]
- 86.Fiske C.H., Subbarow Y. The colorimetric determination of phosphorous. J. Biol. Chem. 1925;66(2):375–400. [Google Scholar]
- 87.Das K., Samanta L., Chainy G.B.N. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Ind J Biochem Biophys. 2000;37:201–204. [Google Scholar]
- 88.Aebi H. Ctalase in vitro. In: Packer L., editor. Vol. 105. Academic; New York: 1984. pp. 673–684. (Methods in Ezymology). [Google Scholar]
- 89.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbutric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 90.Singh V.P., Gupta S., Saxena P.K. Evaluation of acute toxicity of carbaryl and malathion to freshwater teleosts, Channa punctatus (Bloch) and Heteropneustes fossilis (Bloch) Toxicol. Lett. 1984;20:271–276. doi: 10.1016/0378-4274(84)90159-0. [DOI] [PubMed] [Google Scholar]
- 91.Pandey S., Kumar R., Sharma S., Nagpure N.S., Srivastava S.K., Verma M.S. Acute toxicity bioassays of mercuric chloride and malathion on air-breathing fish Channa punctatus (Bloch) Ecotox Environ Saf. 2005;61:114–120. doi: 10.1016/j.ecoenv.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 92.Kumar R., Nagpure N.S., Kushwaha B., Srivastava S.K. Investigation of the genotoxicity of malathion to freshwater teleost fish Channa punctatus (Bloch) using the micronucleus test and comet assay. Arch Environ Con Tox. 2009;58(1):123–130. doi: 10.1007/s00244-009-9354-3. [DOI] [PubMed] [Google Scholar]
- 93.Newhart K. California Environmental Protection Agency Department of Pesticide Regulation Environmental Monitoring Branch; 2006. Environmental Fate of Malathion; pp. 1–20. [Google Scholar]
- 94.Mount D.I., Stephen C.E. A method for establishing acceptable toxicant limits for fish-malathion and butoxyethanol ester of 2,4-D. Trans. Am. Fish. Soc. 1967;21:185–193. [Google Scholar]
- 95.Subburaj A., Jawahar P., Jayakumar N., Srinivasan A., Ahilan B. Acute toxicity bioassay of malathion (EC 50%) on the fish, Oreochromis mossambicus (Tilapia) and associated histological alterations in gills. J. Entomol. Zool. 2018;6(1):103–107. [Google Scholar]
- 96.Arnold H., Pluta H.J., Braunbeck T. Simultaneous exposure of fish to endosulfan and disulfoton in vivo: ultrastructural, stereological and biochemical reactions in hepatocytes of male rainbow trout (Oncorhynchus mykiss) Aquat. Toxicol. 1995;33(1):17–43. [Google Scholar]
- 97.Banaee M. Stanislav Trdan, Intech Open; 2013. Physiological Dysfunction in Fish After Insecticides Exposure, Insecticides - Development of Safer and More Effective Technologies.https://www.intechopen.com/books/insecticides-development-of-safer-and-more-effective-technologies/physiological-dysfunction-in-fish-after-insecticides-exposure Available from: [Google Scholar]
- 98.Bacchetta C., Rossi A., Ale A., Campana M., Parma M.J., Cazenave J. Combined toxicological effects of pesticides: a fish multi-biomarker approach. Ecol. Indic. 2014;36:532–538. [Google Scholar]
- 99.Gandar A., Jean S., Canal J., Marty-Gasset N., Gilbert F., Laffaille P. Multistress effects on behavior and physiology of Carassius auratus. Environ Sci Pollut Res. 2016;24:3323–3331. doi: 10.1007/s12192-010-0223-9. [DOI] [PubMed] [Google Scholar]
- 100.Velisek J.J., Stastna K., Sudova E., Turek J., Svobodova Z. Effects of subchronic simazine exposure on some biometric, biochemical, hematological and histopatological parameters of the common carp (Cyprinus carpio L.) Neuroendocrinol Lett. 2009;30:236–241. [PubMed] [Google Scholar]
- 101.Fırat O., Hikmet Y., Cogun, Tuzin A., Yuzereroglu, Gok G., Fırat O., Kargin F., Kotemen Y. A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus. Fish Physiol. Biochem. 2011;37:657–666. doi: 10.1007/s10695-011-9466-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tchounwou P.B., Patlolla A.K., Yedjou C.G., Moore P.D. Marcelo L. Larramendy and Sonia Soloneski, Intech Open; 2015. Environmental Exposure and Health Effects Associated With Malathion Toxicity, Toxicity and Hazard of Agrochemicals. [DOI] [Google Scholar]
- 103.Venkataraman G.V., Rani P.N.S. Acute toxicity and blood profile of freshwater fish, Clarias batrachus (Linn.) exposed to malathion. J Academia Industrial Res. 2013;2(3):200–204. [Google Scholar]
- 104.Agrahari S., Pandey K.C., Gopal K. Biochemical alteration induced by monocrotophos in the blood plasma of fish, Channa punctatus (Bloch) Pestic Biochem phys. 2007;88:268–272. [Google Scholar]
- 105.Sharma B. Effect of carbaryl on some biochemical constituents of the blood and liver of Clarias batrachus, A fresh water teleost. J. Toxicol. Sci. 1999;24(3):157–164. doi: 10.2131/jts.24.3_157. [DOI] [PubMed] [Google Scholar]
- 106.Saravanan M., Kumar K.P., Ramesh M. Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: cypriniformes) during acute and chronic sublethal exposure to lindane. Pestic. Biochem. Phys. 2011;100:206–211. [Google Scholar]
- 107.Yonar S.M., Ural M.S., Silici S., Yonar E. Malathion-induced changes in the haematological profile, the immune response, and the oxidative/antioxidant status of Cyprinus carpio carpio: protective role of propolis. Ecotoxicol. Environ. Saf. 2014;102:202–209. doi: 10.1016/j.ecoenv.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 108.Huculeci R., Dinu D., Staicu A.C., Munteanu M.C., Costache M., Dinischiotu A. Malathion-induced alteration of the antioxidantdefence system in kidney, gill, and intestine of Carassius auratus gibelio. Environ. Toxicol. 2008:523–530. doi: 10.1002/tox.20454. [DOI] [PubMed] [Google Scholar]
- 109.Luskova V., Svoboda M., Kolarova J. The effect of diazinon on blood plasma biochemistry in carp (Cyprinus carpio L.) Acta Vet Brno. 2002;71:117–123. [Google Scholar]
- 110.Bacchetta C., Cazenave J., Parma M.J., Biancucci G.F. Biochemical Stress responses in tissues of the Cichlid Fish Cichlasoma dimerus exposed to a commercial formulation of endosulfan. Arch. Environ. Contam. Toxicol. 2011;61:453–460. doi: 10.1007/s00244-010-9635-x. [DOI] [PubMed] [Google Scholar]
- 111.Toledo-Ibarra G.A., Díaz Resendiz K.J.G., Ventura-Ramon G.H., González-Jaime F., Vega-Lopez A., Villanueva E.B., Pavón L., Girón-Pérez M.I. Oxidative damage in gills and liver in Nile tilapia (Oreochromis niloticus) exposed to diazinon. Comp Biochem Physiol Part A. 2016;200:3–8. doi: 10.1016/j.cbpa.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 112.Stoyanova S., Georgieva E., Velcheva I., Iliev I., Vasileva T., Bivolarski V., Tomov S., Nyeste K., Antal L., Yancheva V. Multi-Biomarker Assessment in common carp (Cyprinus carpio, Linnaeus 1758) liver after acute chlorpyrifos exposure. Water. 2020;12:1837. doi: 10.3390/w12061837. [DOI] [Google Scholar]
- 113.Jyothi B., Narayan G. Certain pesticide-induced carbohydrate metabolic disorders in the serum of freshwater fish Clarias batrachus (Linn.) Food Chem. Toxicol. 1999;37:417–421. doi: 10.1016/s0278-6915(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 114.Amin K.A., Hashem K.S. Deltamethrin-induced oxidative stress and biochemical changes in tissues and blood of catfish (Clarias gariepinus): antioxidant defense and role of alpha-tocopherol. BMC Vet. Res. 2012;8:45. doi: 10.1186/1746-6148-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stadtman E.R., Levine R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 116.Akhgari M., Abdollahi M., Kebryaeezadeh A., Hosseini R., Sabzevari O. Biochemical evidence for free radical-induced lipid peroxidation as a mechanism for sub chronic toxicity of malathion in blood and liver of rats. Hum. Exp. Toxicol. 2003;22:205–211. doi: 10.1191/0960327103ht346oa. [DOI] [PubMed] [Google Scholar]
- 117.Thenmozhi C., Vignesh V., Thirumurugan R., Arun S. Impacts of malathion on mortality and biochemical changes of freshwater fish Labeo rohita. Iran J. Environ. Health Sci. Eng. 2011;8(4):325–332. [Google Scholar]
- 118.Ibrahim A.T. 2019. Biochemical and Histopathological Response of Oreochromis niloticus to Malathion. [Google Scholar]
- 119.Magar R.S., Shaikh A. Effect of malathion toxicity on detoxifying organ of fresh water fish Channa punctatus. Trend in fisheries research: An International Peer Rev. J. 2013;3(3):723–728. [Google Scholar]
- 120.Moore P.D., Yedjou C.G., Tchounwou P.B. Malathion-induced oxidative stress, cytotoxicity, and genotoxicity in human liver carcinoma (HepG2) cells. Environ. Toxicol. 2010;25(3):221–226. doi: 10.1002/tox.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Uikey S. Effect of malathion toxicity on fresh water fish Labeo rohita. International Journal of Applied and Universal Research. 2015;2(4):10–15. [Google Scholar]
- 122.Cristina S.A., Cristina M.M., Anca D. Malathion induced histological modifications in gills and kidney of Carassius auratus gibelio. Lucrări stiinŃifice Zootehnie si Biotehnologii. 2008;41(1):448–453. [Google Scholar]
- 123.Atwood D., Paisley-Jones C. Market Estimates (USEPA), Biological and Economic Analysis Division, Office of Pesticide Programs, Office of Chemical Safety and Pollution Prevention. U.S. Environmental Protection Agency; Washington, DC: 2017. Pesticides Industry, Sales and Usage, 2008-2012. 20460. [Google Scholar]
- 124.Banaee M., Sureda A., Mirvaghefi A.R., Ahmadi K. Biochemical and histological changes in the liver tissue of rainbow trout (Oncorhynchus mykiss) exposed to sub-lethal concentrations of diazinon. Fish Physiol Biochem. 2013;39:489–501. doi: 10.1007/s10695-012-9714-1. [DOI] [PubMed] [Google Scholar]
- 125.Bender M.E., Westman J.R. The toxicity of malathion and its hydrolysisproducts to the eastern mudminnow, Umbra pygmaea(Dekay) Chesapeake Sci. 1976;17(2):125–128. doi: 10.2307/1351055. [DOI] [Google Scholar]