Highlights
-
•
We comprehensively analyzed both BRAF mutation status and MLH1 promoter methylation status in MLH1-deficient patients from a large consecutive CRC cohort, which may help to modify strategy of LS screening for Chinese MLH1-deficient CRCs.
-
•
To our knowledge, this study is the first to systematically evaluate the characteristic of family history associated with LS patients harboring somatic MLH1 promoter methylation in Chinese population.
-
•
Our results contribute to a better understanding of the role of these two methods in LS screening among CRCs with a lower incidence rate of BRAF V600E mutation in some races.
Keywords: Lynch syndrome, colorectal cancer, BRAF mutation, MLH1 promoter methylation, screening strategy
Abstract
Background
The low prevalence of the BRAF V600E mutation in colorectal cancers (CRCs) in Chinese populations has stimulated concern about the efficacy of BRAF mutation analysis for Lynch syndrome (LS) screening.
Methods
In total, 169 of 4104 consecutive CRC patients with absent MLH1 staining were analyzed to compare the utility of the BRAF V600E mutation testing with MLH1 promoter methylation analysis in the Chinese population. Germline genetic testing was performed in patients with wild-type BRAF/methylated MLH1.
Results
Compared with BRAF genotyping, the use of MLH1 methylation testing alone to evaluate patients with MLH1 deficiency reduced referral rates for germline testing by 1.8-fold (82.8% vs. 47.1%). However, 6 patients harboring MLH1 promoter methylation were verified to have LS through germline genetic testing. It is notable that all 6 patients had a family history of CRC in at least 1 first-degree relative (FDR) or second-degree relative (SDR). The combination of MLH1 promoter methylation analysis and a family history of CRC could preclude significantly more patients from germline genetic testing than from BRAF mutation testing alone (45.5% vs. 17.2%, p<0.001) and decrease the number of misdiagnosed LS patients with MLH1 promoter methylation.
Conclusion
The combination of a family history of CRC with MLH1 promoter methylation analysis showed better performance than BRAF mutation testing in the selection of patients in the Chinese population for germline genetic testing.
Introduction
Lynch syndrome (LS) is the most common hereditary colorectal cancer (CRC) syndrome caused by germline pathogenic variants in any of four DNA mismatch repair (MMR) genes (MSH2, MLH1, PMS2 or MSH6) or a deletion in the EPCAM gene, which leads to methylation of the adjacent MSH2 promoter [1,2]. Mutations in MMR genes may lead to tumor DNA microsatellite instability (MSI) and deficient MMR (dMMR) [3], [4], [5].
Although LS accounts for only 2–3% of all CRC cases [6], pathogenic MMR gene mutation carriers have a high risk of developing multiple cancers, including colorectal, endometrial, ovarian, stomach, and urinary tract cancers. A recent study based on a prospective LS database reported that the calculated cumulative incidence of any cancer at age 70 years was 75% in females and 58% in males and that CRC was the most common [7]. More importantly, LS patients are susceptible to the development of subsequent cancers, including CRC (22% to 74%) [8,9] after first onset, and periodic colonoscopic surveillance can reduce CRC-related mortality [10]. However, routine germline genetic testing for hereditary cancers in the clinic is rarely available and is expensive in many areas of China. Therefore, it is essential to increase the efficiency of screening and reduce unnecessary patient referral to germline genetic testing. Particularly for patients with MLH1 deficiency, a proportion of those with sporadic disease can be excluded before germling testing through BRAF mutation and/or MLH1 promoter methylation testing.
Sporadic CRC with MLH1 deficiency commonly results from methylation of the cytosine-phosphate-guanine (CpG) island in the MLH1 gene promoter, thus causing transcriptional silencing of the MLH1 gene [11]. This pathologic phenotype is associated with the serrated neoplasia carcinogenetic pathway and highly correlated molecular characteristics, including the CpG island methylator phenotype (CIMP), the BRAF V600E mutation and methylation of the MLH1 gene promoter [11,12]. Therefore, testing of the BRAF mutation and MLH1 promoter methylation is now widely used to discriminate patients with sporadic dMMR among those with MLH1-deficient CRC in LS screening [13,14].
Previous reports showed evidence of somatic MLH1 methylation, along with the recent proposal of a constitutional MLH1 epimutation in a patient with suspected LS [15,16], being a second hit in some LS tumors and cast doubt on the performance of MLH1 methylation as a definite negative predictor of pathogenic MLH1 variants. In contrast, BRAF mutations are virtually absent in LS-associated tumors; approximately 1% of cancer patients with BRAF V600E mutations (and loss of MLH1) have LS [17]. The National Comprehensive Cancer Network (NCCN) guidelines recommend that for MLH1-deficient patients displaying a BRAF mutation, no further tests are required except in those with a young age of onset or a significant family history [18]. In addition, immunohistochemistry (IHC) has been proposed as an alternative method to detect the BRAF V600E mutation status, making this detection strategy efficient and effective in clinical practice [19,20]. Therefore, BRAF mutation testing is currently the most widely used test to exclude sporadic CRCs with MLH1 deficiency.
Nevertheless, the genetic variability among different ethnic groups must be seriously considered, especially when screening for hereditary diseases. Previous reports indicated that the BRAF mutation frequency varied among patients with different ethnicities, especially between Asians and individuals in most European and American studies. The latter studies reported that BRAF mutations accounted for approximately 15% of all CRCs, and 44.9 − 57.7% of patients with MLH1-deficient CRCs harbored a BRAF mutation [21], [22]. In contrast, in Asian countries and some eastern European countries, such as Japan, Russia, Israel and China, the BRAF mutation frequencies in CRCs are much lower than those in Western countries, ranging from 3–6% [23], [24], [25], [26], [27], [28]. This frequency increases slightly to 15.4%, even in CRCs with loss of the expression of MLH1/PMS2 [29]. Therefore, the low occurrence of the BRAF mutation in such populations has led to clinical concern regarding its superiority to MLH1 promoter methylation as a negative predictor of germline MLH1 mutations.
To our knowledge, however, the diagnostic role of BRAF mutation testing and MLH1 methylation analysis in LS prescreening among patients with MLH1-deficient CRC in China is unclear. Therefore, the aim of this study was to compare the efficacy of these two diagnostic methods in a large consecutive cohort of Chinese patients with CRC and to investigate the feasibility and effectiveness of these methods for screening LS among Chinese patients.
Materials and methods
Patient cohort
We reviewed the pathology database for 4104 consecutive patients who had undergone radical surgery for CRC between June 2011 and December 2014 at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The immunohistochemical analysis of the 4 MMR proteins (MLH1, MSH2, MSH6 and PMS2) was performed routinely during the postoperative pathological examination at the Department of Pathology. CRC patients with a deficiency in at least one MMR protein were identified as being dMMR. Of these patients, those with MLH1-deficient CRC were enrolled in the study, and further tests were conducted as described below.
DNA extraction
Formalin-fixed and paraffin-embedded tumor tissues were used for BRAF V600E gene mutation and MLH1 promoter methylation analyses. Normal colorectal mucosal tissues were collected for germline genetic testing of the MLH1 gene. Genomic DNA was extracted by using a QIAamp DNA Mini Kit (Qiagen, Germany) according to the manufacturer's instructions. The quality and concentration of the DNA samples were assessed on a NanoDrop and Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Carlsbad, CA, USA).
BRAF V600E mutation analysis
The BRAF V600E mutational status was assessed by real-time PCR using a multiplex allele-specific PCR-based kit (ACCB, Beijing, China) on a Stratagene Mx3000P system (Agilent Technologies Inc., Santa Clara, CA) in the Molecular Pathology Laboratory of the Department of Pathology, Cancer Hospital Chinese Academy of Medical Sciences, as previously reported [30].
MLH1 promoter methylation analysis
Methylation-specific PCR (MS-PCR) was used to distinguish unmethylated DNA from methylated DNA based on sequence alterations produced by chemical treatment with sodium bisulfite with the EZ DNA Methylation-Gold Kit (Zymo Research, CA, USA). In brief, the methylation status was identified by subsequent PCR using primers specific to the methylated or unmethylated DNA. The analyzed fragment was located in the ‘C’ region, as described previously [31,32]. The primer sequences for the amplification of unmethylated MLH1 were 5′-TGAATTAATAGGAAGAGTGGATAGT-3′ (sense) and 5′-TCCCTCCCTAAAACAACTACTACCCA-3′ (antisense); and for the amplification of methylated MLH1, 5′-AATTAATAGGAAGAGCGGATAGC-3′ (sense) and 5′-CCTCCCTAAAACGACTACTACCCG-3′ (antisense). The PCR product was directly loaded onto 2% agarose gels and visualized under UV illumination. The representative MS-PCR results are shown in Supplementary Figure S1.
Panel next-generation sequencing (NGS) germline genetic testing
In total, 200 ng of normal genomic DNA was fragmented by sonication using a Covaris M220 (Covaris Inc., Massachusetts, USA). After the fragmentation process, end repair, A-tailing, adapter ligation, PCR and target enrichment were performed following the manufacturer's recommended protocols for the SureSelect-XT Low Input Target Enrichment System and ClearSeq Inherited Disease panel, which included our genes of interest: EPCAM, MLH1, PMS2, MSH2 and MSH6 (Agilent Technologies, Santa Clara, CA, USA). Final libraries were quantified with a Qubit High Sensitivity Kit (Invitrogen, Carlsbad, CA, USA). Products were sequenced on a HiSeq 2500 to at least an average depth of 100-fold coverage with WuXi NextCODE. Reads were aligned to the reference human genome GRCh37. Germline variations, including point mutations and short indels (<30 base pairs), were called with GATK, SAMtools and Picard tools. Annotations were defined using ANNOVAR. Variants were reviewed as pathogenic, likely pathogenic, a variant of uncertain significance (VUS), likely benign, or benign based on the database of the International Society of Gastrointestinal Hereditary Tumors (InSiGHT) (http://insight-group.org/variants/classifications/). If no pathogenicity data were available, the significance of the variants was assessed by integrating the in silico prediction results from Align GVGD, SIFT, MutationTaster, PolyPhen-2, Human Splicing Finder, etc., with other criteria according to consensus guidelines of the American College of Medical Genetics and Genomics (ACMG) [33].
Statistical analyses
Statistical analyses were performed with SPSS 25.0 software (SPSS, Chicago, IL). To evaluate the statistical significance of differences observed between groups, the χ2 test was applied. Specifically, Pearson's χ2 test, Fisher's exact test and continuity correction were used as appropriate. All P-values represent two-sided statistical tests, and only those less than 0.05 were considered significant.
Results
Patients
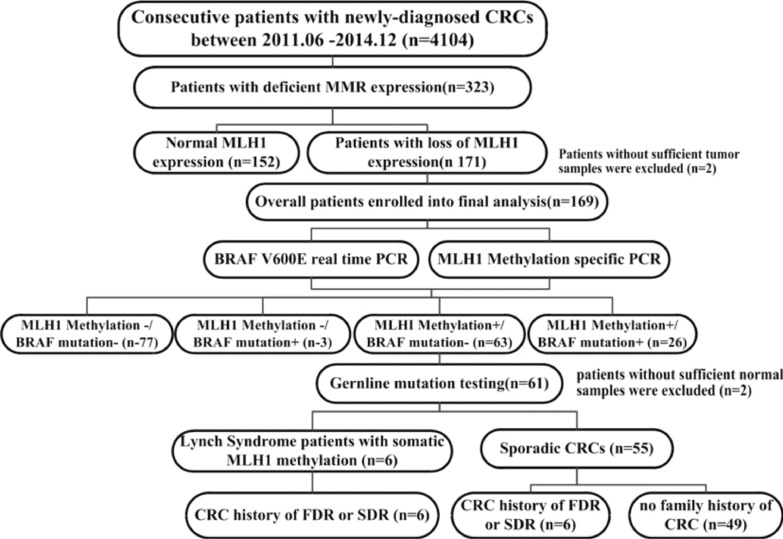
Of the 4104 CRC patients, 323 (7.9%) were identified as being dMMR. Of these patients, 171 (52.9%, 171/323) were MLH1 deficient. Two of these patients were excluded due to a lack of tumor samples. Therefore, a total of 169 patients were included in the final analysis (Fig. 1).
Fig. 1.
Flow diagram of the analytic strategy and main results of the study.
The clinicopathological features of the patients in this study are summarized in Supplementary Table S1. Tumors were characterized by certain clinicopathological features, consistent with those previously reported for dMMR CRC, such as a tendency to be proximal and poorly differentiated, to contain mucinous or signet ring cell components, and to exhibit few lymph node and distant metastases despite a late stage in the primary sites (T3, T4). Thirteen patients (7.7%, 13/169) had a personal history of CRC.
Family histories of cancer, namely, LS-related, possibly LS-related and other histories according to the revised Bethesda guidelines, are shown in Supplementary Table S2. Forty-four patients had a family history of CRC, which was top in the list of LS-related cancers. Stomach cancer was the second most commonly observed (11 patients), followed by biliary and urinary cancers, whereas endometrial cancer was less commonly observed.
MLH1 promoter methylation and BRAF V600E mutation statuses
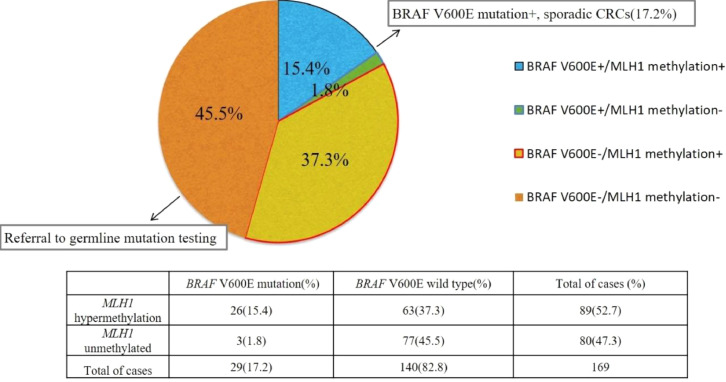
MLH1 promoter methylation was observed in 89 (52.7%) of 169 MLH1-deficient patients. However, only 29 (17.2%, 29/169) patients harbored the BRAF V600E mutation. 63 patients (37.3%, 63/169) presented wild-type BRAF and MLH1 promoter methylation. The comparisons of the BRAF mutation and MLH1 promoter methylation statuses are presented in Fig. 2. As expected, MLH1 promoter methylation was found in most patients harboring the BRAF V600E mutation (26/29).
Fig. 2.
Results of BRAF V600E mutation testing and MLH1 promoter methylation analysis. According to the guidelines of LS screening, 17.2% of patients with the BRAF V600E mutation could be precluded from germline genetic testing, while 45.5% of patients who displayed negative MLH1 promoter methylation were referred for germline genetic testing. The pathogenic variant status of the remaining 37.3% of patients needed to be clarified due to the possibility of coexisting MLH1 promoter methylation and a germline mutation.
Clinicopathological characteristics of patients with the BRAF V600E mutation or MLH1 promoter methylation
Compared with BRAF V600E-mutated CRC patients, BRAF V600E wild-type CRC patients were younger (median age: 64 years vs 54 years, respectively). In addition, more patients with BRAF V600E-mutated CRC than patients with BRAF V600E wild-type CRC had primary tumors located in the proximal colon (93.1% vs 57.1%, respectively; P<0.001). A similar pattern was observed between patients with MLH1 promoter methylation and patients without MLH1 promoter methylation (70.8% vs. 55.0%; p = 0.033). There were no significant differences in the clinicopathological features, such as the differentiation grade, presence of mucinous or signet ring cell components and personal tumor history, between patients displaying different statuses of the BRAF V600E mutation or MLH1 promoter methylation.
A previous study showed that a family history of CRC was a strong indicator of LS in patients with MLH1/MSH2 deficiency [4]. In our study, no patients with BRAF V600E-mutated CRC had a family history of CRC. Patients who had unmethylated MLH1 CRCs had a notably stronger family history of CRC than those who had methylated MLH1 CRCs. It is worth noting that 13.5% of the patients in the methylation group had a family history of CRC. The details are shown in Table 1.
Table 1.
Clinicopathological features of patients with different MLH1 promoter methylation or BRAF mutation statuses.
| Characteristic | Positive MLH1 promoter methylation n = 89 (%) | Negative MLH1 promoter methylation n = 80 (%) | p | Mutant BRAF n = 29 (%) | Wild-type BRAF n = 140 (%) | p |
|---|---|---|---|---|---|---|
| Age at onset (y) | ||||||
| Median | 63 | 52 | 64 | 54 | ||
| Mean | 60.3 | 49.5 | <0.001 | 66.2 | 52.9 | <0.001 |
| SD | 13.2 | 11.8 | 9.8 | 13.2 | ||
| Proximal location | 63 (70.8) | 44 (55.0) | 0.033 | 27 (93.1) | 80 (57.1) | <0.001 |
| Poor differentiation | 39 (43.8) | 27 (33.8) | 0.180 | 16 (55.2) | 50 (35.7) | 0.051 |
| Mucinous component | 46 (51.7) | 33 (41.3) | 0.175 | 12 (41.4) | 67 (47.9) | 0.525 |
| Signet ring component | 2 (2.2) | 1 (1.3) | 0.624 | 1 (3.4) | 2 (1.4) | 1.000 |
| Signet ring+ Mucinous component | 5 (5.6) | 8 (10.0) | 0.286 | 2 (6.9) | 11 (7.9) | 1.000 |
| Synchronic CRC | 3 (3.4) | 8 (10.0) | 0.081 | 1 (3.4) | 10 (7.1) | 0.749 |
| Lynch-related tumor | 3 (3.4) | 6 (7.5) | 0.395 | 0 | 9 (6.4) | 0.343 |
| FDR or SDR with CRC | 12 (13.5) | 32 (40) | <0.001 | 0 | 44 (31.4) | <0.001 |
| FDR or SDR with CRC aged <50 y | 3 (3.4) | 17 (21.3) | <0.001 | 0 | 19 (13.6) | 0.075 |
| FDR with CRC | 9 (10.0) | 27 (33.8) | <0.001 | 0 | 35 (25.0) | 0.002 |
| FDR with CRC aged <50 y | 3 (3.4) | 14 (17.5) | 0.002 | 0 | 16 (11.4) | 0.118 |
| FDR or SDR with a LS-related tumor | 17 (18.9) | 14 (17.5) | 0.788 | 6 (20.7) | 25 (17.9) | 0.720 |
| FDR or SDR with a LS-related tumor aged <50 y | 4 (4.5) | 6 (7.5) | 0.617 | 1 (3.4) | 9 (6.4) | 0.852 |
| Fulfillment of revised Bethesda guidelines | 42 (47.8) | 69 (86.3) | <0.001 | 8 (27.6) | 103 (73.6) | <0.001 |
CRC: colorectal cancer, FDR: first-degree relative, defined as a patient's parent (father or mother), full sibling (brother or sister) or child, SDR: second-degree relative, defined as a patient`s uncle, aunt, nephew, niece, grandparent, grandchild, half-sibling, and double cousin who shared 25% of a person's genes.
Family history of CRC could help discriminate LS patients with MLH1 promoter methylation
According to the NCCN guidelines, LS is rarely observed in CRCs harboring a BRAF mutation [18]. Therefore, germline genetic testing was conducted in 61 of 63 patients with wild-type BRAF and MLH1 promoter methylation (two patients were excluded because of poor DNA quality) to determine whether any LS patients in the MLH1 promoter methylation group failed to be identified. As expected, 6 patients were identified to have LS with germline pathogenic MLH1 variants (Table 2).
Table 2.
Characteristics of CRC patients with germline pathogenic MLH1 variants.
| No. | Sex | Age (y) | Germline MLH1 mutation | Mutation significance | Tumor location | Family history | Fulfillment of the revised Bethesda criteria |
|---|---|---|---|---|---|---|---|
| 1 | F | 62 | MLH1:c.793C>T (p.Arg265Cys) | Pathogenic | Ascending colon | Colon | N |
| 2 | M | 52 | MLH1:c.677G>A (p.Arg226Gln) | Pathogenic | Rectum | Breast, colon, ovary, endometrium | Y |
| 3 | F | 69 | MLH1:c.2179_2182del (p.His727fs) | Pathogenic | Ascending colon | Colon, endometrium | Y |
| 4 | M | 52 | MLH1:c.883A>G (p.Ser295Gly) | Pathogenic | Ascending colon | Stomach, colon | Y |
| 5 | M | 32 | MLH1:c.1845_1847del (p.615_616del) | Pathogenic | Transverse colon | Colon | Y |
| 6 | F | 34 | MLH1:c.678–2del (splicing) | Likely pathogenic | Sigmoid colon | Colon | Y |
CRC: colorectal cancer, F: female, M: male.
To further refine the clinical criteria for selecting patients who should be subjected to germline genetic testing, variables associated with germline pathogenic MLH1 variants were identified. Table 3 shows the parameters significantly associated with pathogenic variant carriers in the univariate analysis. A family history of CRC in at least 1 first- or second-degree relative (FDR/SDR) could discriminate all patients (6/6) with germline pathogenic variants. In other words, a family history of CRC in at least 1 FDR or SDR could increase the performance of MLH1 promoter methylation analysis in LS screening.
Table 3.
Predictors of germline pathogenic variants in patients with MLH1 hypermethylation/wild-type BRAF (univariate analysis).
| Characteristic | Patients with a (likely) pathogenic variant a (n = 6) | Patients without a pathogenic variant (n = 55) | p |
|---|---|---|---|
| Colorectal cancer in ≥1 FDR | 5 | 4 | <0.001 |
| Colorectal cancer in a FDR at age 50 y | 3 | 0 | 0.001 |
| Colorectal cancer in ≥1 FDR or SDR | 6 | 6 | <0.001 |
| Synchronous, metachronous colorectal, or other LS-related cancer | 2 | 5 | 0.136 |
| Colorectal cancer with dMMR diagnosed at age 60 y | 4 | 30 | 0.893 |
| FDR or SDR with a LS-related tumor | 2 | 11 | 0.816 |
| Fulfillment of revised Bethesda guidelines | 5 | 32 | 0.449 |
a. Patients with a pathogenic variant and a likely pathogenic variant were included; FDR, first-degree relative; SDR, second-degree relative; LS, Lynch syndrome; dMMR, mismatch repair deficiency.
Therefore, since no patient with BRAF mutations had a family history of CRCs, only 80 patients without MLH1 promoter methylation, along with 12 patients with MLH1 promoter methylation whose FDR or SDR had a history of CRC, required further germline genetic testing (92/169), while the number would have increased to 140 patients when using the BRAF V600E mutation alone as a negative predictor of germline pathogenic variants. The total costs of LS screening for all patients (n = 169) in our study were calculated based on the two strategies (Supplementary Figure 2). Use of the BRAF status alone as a selection criterion would require a total of ¥ 941,400 ($145,258.02), while the introduction of a family history in addition to MLH1 promoter methylation would reduce this cost by 30.6%.
Overall, when taking a family history of CRC into consideration, MLH1 promoter methylation analysis significantly reduced the number of patients referred to germline genetic testing compared with BRAF genotype testing (54.5% vs 88.2%, p < 0.001).
Discussion
The calculated cumulative incidence of CRC by age 70 years is 46% in patients harboring germline pathogenic MLH1 variants [7]; however, 70−80% of CRC patients displaying MLH1 loss are diagnosed with sporadic CRC. Therefore, a screening strategy with high sensitivity and specificity should be adapted to distinguish patients with possible LS from those with sporadic CRC for germline genetic testing.
According to the widely used NCCN guidelines, patients with MLH1 deficiency are recommended to undergo BRAF V600E and/or MLH1 methylation testing to exclude patients from germline testing; however, both methods have certain flaws. For example, BRAF V600E testing in populations with a low BRAF mutation frequency has low specificity, and MLH1 promoter testing can result in a missed diagnosis. Therefore, we aimed to find a screening flow that may increase the efficiency and accuracy of testing in the population with low incidence of BRAF V600E mutations.
BRAF mutation analysis has been well acknowledged as the strongest negative predictor of germline pathogenic MLH1 variants in MLH1-deficient tumors. Of note, the BRAF mutation frequency varies among individuals with different ethnicities. Parsons et al. reviewed 35 studies assessing the BRAF V600E mutation status in CRC patients mostly from Western countries and found that BRAF V600E mutations occurred in 63.5% of CRC patients displaying MLH1 promoter methylation or MLH1 protein loss (95% CI 46.98%−78.53%) [17]. However, in our study, BRAF mutations were found in only 17.2% of CRC patients with MLH1 expression loss, and only 29.2% (26/89) of CRC patients displaying MLH1 promoter methylation harbored BRAF mutations, which is much lower than the percentage of patients in Western countries.
The variance in the BRAF mutation rate may be associated with the difference in serrated tumorigenic pathways according to ethnicity. A previous large cohort study showed that people of southern European origin had a lower risk of CRCs with CIMP and BRAF mutations than people of Anglo-Celtic origin, suggesting an ethnic difference in the development of BRAF mutation-related CRCs [34].
The low frequency of BRAF mutations among Chinese patients with CRC in this study raises concerns about the low specificity of BRAF V600E mutation testing as an exclusion strategy in LS screening. Under this clinical reality, more patients with MLH1 deficiency will be referred for germline genetic testing, which yields financial and emotional burdens on cancer patients and their relatives. Therefore, a modified screening strategy with increased specificity is urgently needed in Chinese patients with MLH1-deficient CRC.
In this study, the incidence of MLH1 promoter methylation was almost threefold that of the BRAF V600E mutation (47.3% vs. 17.2%), indicating a strong potency to preclude more sporadic CRC patients from germline genetic testing. It is notable that some rare cases of somatic MLH1 promoter methylation may be the second hit in some LS patients [15,35] and may lead to a missed diagnosis of LS when using MLH1 promoter methylation testing as an exclusive method. To tackle this problem, the NCCN guidelines recommend that for patients with wild-type BRAF V600E/MLH1 promoter methylation, no additional tests are needed except for those with a young age of onset or a significant family history [19]; however, they fail to specify criteria such as family history, and therefore, their potency is difficult to assess.
In our study, we identified 6 (9.8%, 6/61) LS patients with pathogenic MLH1 mutations among 61 patients displaying wild-type BRAF and MLH1 promoter methylation, similar to the results reported by Moreira (7.5%, 3/40) [36]. Previous studies showed that a family history of CRC in at least 1 FDR was an independent predictor of germline pathogenic MLH1 variants in the assessment of each clinicopathological parameter possibly related to LS [4]. Similarly, in our study, a family history of CRC in at least 1 FDR or SDR also showed outstanding performance for discriminating patients with possible LS from those with MLH1 promoter methylation, and no LS patient was misdiagnosed using such selection criteria.
The strengths of this study are as follows. First, our results contribute to a better understanding of the role of these two methods in LS screening among CRC patients of some races with a low incidence rate of the BRAF V600E mutation. We comprehensively analyzed both the BRAF mutation status and MLH1 promoter methylation status in MLH1-deficient patients from a large consecutive CRC cohort. Therefore, the different genetic profiles of CRC patients with MLH1 protein expression loss in this study may help to modify the strategy of LS screening for Chinese patients with MLH1-deficient CRC. Second, to our knowledge, this study is the first to systematically evaluate the characteristic of family history associated with LS patients harboring somatic MLH1 promoter methylation in Chinese population. Use of a history of CRC in at least 1 FDR or SDR as a selection criterion for the referral of patients displaying wild-type BRAF and MLH1 promoter methylation for germline genetic testing could significantly increase the efficiency of LS screening. The number of patients who needed germline genetic testing decreased compared with that using the BRAF V600E mutation alone. Unlike when MLH1 methylation analysis was used alone, no LS patient with MLH1 promoter methylation was missed.
In the past, despite guideline recommendations, MMR and germline testing were less prevalent in developing countries, including China, due to technical issues. Recently, the DNA sequencing technique has experienced rapid development, and immunotherapy has shown outstanding efficacy in cancer patients with dMMR, which means that more patients accept MSI/MMR testing to pursue effective treatment. As a result, the demand for LS screening is expected to increase in the next few years. Therefore, a more efficient and accurate screening algorithm would decrease the medical burden.
Nevertheless, our study has limitations. First, germline mutations were tested only in patients with wild-type BRAF V600E/MLH1 promoter methylation and not in the whole study population; therefore, the sensitivity and specificity of the traditional methods (BRAF V600E or MLH1 promoter testing alone) and the one we proposed (combined with a family history) could not be compared directly. In addition, the screening strategy suggested in this study awaits confirmation in another cohort of patients with CRC.
In conclusion, the BRAF V600E mutation rate in Chinese patients with MLH1-deficient CRC was significantly lower than that in patients from Western countries, thus leading to a decreased ability to rule out patients with sporadic disease from germline genetic testing.
A family history of CRC in at least 1 FDR or SDR should be considered a valuable clinical characteristic when referring MLH1-deficient patients with MLH1 promoter methylation to germline MLH1 genetic testing. MLH1 promoter methylation analysis combined with a family history of CRC in the LS screening algorithm is more efficient and cost effective than BRAF mutation testing alone in the Chinese population.
Author contributions
Ning Lyu: Conceptualization, Funding acquisition. Shuangmei Zou: Conceptualization, resources, Supervision. Lin Dong: Conceptualization, Methodology, Formal analysis. Wenmiao Wang:Data curation, Methodology, Formal analysis, Writing - original draft. Jianming Ying: Conceptualization, Supervision, Writing - review & editing. Susheng Shi: Supervision. Qiurong Ye: Methodology.
Ethics statement
This study was approved by the Ethics Committee of NCC/CICAMS (NCC1790). The need for individual informed consent was waived because of the retrospective nature of the study. Patients were informed if they were determined to have Lynch syndrome.
Declaration of Competing Interest
All authors declare no conflicts of interest.
Funding
This work was funded by the National Key R&D Program of China (2016YFC0905300), the CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-001 and 2017-I2M-1-006), the Special Fund of Chinese Central Government for Basic Scientific Research Operations in Commonwealth Research Institutes (2016ZX310176) and the Beijing Hope Run Special Fund of Cancer Foundation of China (LC2017B14).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101049.
Contributor Information
Shuangmei Zou, Email: smzou@hotmail.com.
Lin Dong, Email: 18810446012@163.com.
Ning Lyu, Email: nlu03@126.com.
Appendix. Supplementary materials
References
- 1.Peltomaki P., Lothe R.A., Aaltonen L.A., Pylkkänen L., Nyström-Lahti M., Seruca R. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 1993;53:5853–5855. [PubMed] [Google Scholar]
- 2.Ligtenberg M.J., Kuiper R.P., Chan T.L., Goossens M., Hebeda K.M., Voorendt M. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1. Nat. Genet. 2009;41:112–117. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 3.Grilley M., Holmes J., Yashar B., Modrich P. Mechanisms of DNA-mismatch correction. Mutat. Res. 1990;236:253–267. doi: 10.1016/0921-8777(90)90009-t. [DOI] [PubMed] [Google Scholar]
- 4.Piñol V., Castells A., Andreu M., Castellví-Bel S., Alenda C., Llor X. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA. 2005;293:1986–1994. doi: 10.1001/jama.293.16.1986. [DOI] [PubMed] [Google Scholar]
- 5.Hampel H., Frankel W.L., Martin E., Arnold M., Khanduja K., Kuebler P. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J. Clin. Oncol. 2008;26:5783–5788. doi: 10.1159/000481413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popat S., Hubner R., Houlston R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 7.Møller P., Seppälä T., Bernstein I., Holinski-Feder E., Sala P., Evans D.G. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2017;66:464–472. doi: 10.1136/gutjnl-2015-309675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton K., Green K., Lalloo F., Evans D.G., Hill J. Colonoscopy screening compliance and outcomes in patients with Lynch syndrome. Colorectal Dis. 2015;17:38–46. doi: 10.1111/codi.12778. [DOI] [PubMed] [Google Scholar]
- 9.Järvinen H.J., Renkonen-Sinisalo L., Aktán-Collán K., Peltomäki P., Aaltonen L.A., Mecklin J.P. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J. Clin. Oncol. 2009;27:4793–4797. doi: 10.1200/JCO.2009.23.7784. [DOI] [PubMed] [Google Scholar]
- 10.Järvinen H.J., Aarnio M., Mustonen H., Aktan-Collan K., Aaltonen L.A., Peltomäki P. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118(5):829–834. doi: 10.1016/s0016-5085(00)70168-5. May. [DOI] [PubMed] [Google Scholar]
- 11.Weisenberger D.J., Siegmund K.D., Campan M., Young J., Long T.I., Faasse M.A. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat. Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins N.J., Bariol C., Ward R.L. The serrated neoplasia pathway. Pathology. 2002;34:548–555. [PubMed] [Google Scholar]
- 13.Vasen H.F., Blanco I., Aktan-Collan K., Gopie J.P., Alonso A., Aretz S. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013;62:812–823. doi: 10.1136/gutjnl-2012-304356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson J., Lacy J., Matloff E., Robert M. Microsatellite Instability Testing in Colorectal Carcinoma: a Practical Guide. Clin. Gastroenterol. Hepatol. 2014;12:171–176. doi: 10.1016/j.cgh.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Rahner N., Friedrichs N., Steinke V., Aretz S., Friedl W., Buettner R. Coexisting somatic promoter hypermethylation and pathogenic MLH1 germline mutation in Lynch syndrome. J. Pathol. 2008;214(1):10–16. doi: 10.1002/path.2263. Jan. [DOI] [PubMed] [Google Scholar]
- 16.Hitchins M.P., Ward R.L. Constitutional (germline) MLH1 epimutation as an aetiological mechanism for hereditary non-polyposis colorectal cancer. J. Med. Genet. 2009;46:793–802. doi: 10.1136/jmg.2009.068122. [DOI] [PubMed] [Google Scholar]
- 17.Parsons M.T., Buchanan D.D., Thompson B., Young J.P., Spurdle A.B. Correlation of tumor BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumor features for MMR variant classification. J. Med. Genet. 2012;49:151–157. doi: 10.1136/jmedgenet-2011-100714. [DOI] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal; version 3.2019. 25p. [DOI] [PubMed]
- 19.Vakiani E., Yaeger R., Brooke S., Zhou Y., Klimstra D.S., Shia J. Immunohistochemical detection of the BRAF V600E mutant protein in colorectal neoplasms. Appl. Immunohistochem. Mol. Morphol. 2015;23(6):438–443. doi: 10.1097/PAI.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capper D., Voigt A., Bozukova G., Ahadova A., Kickingereder P., von Deimling A. BRAF V600E-specific immunohistochemistry for the exclusion of Lynch syndrome in MSI-H colorectal cancer. Int. J. Cancer. 2013;133(7):1624–1630. doi: 10.1002/ijc.28183. [DOI] [PubMed] [Google Scholar]
- 21.Lochhead P., Kuchiba A., Imamura Y., Liao X., Yamauchi M., Nishihara R. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J. Natl. Cancer Inst. 2013;105:1151–1166. doi: 10.1093/jnci/djt173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogino S., Nosho K., Kirkner G.J., Kawasaki T., Meyerhardt J.A., Loda M. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58(1):90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seppälä T.T., Böhm J.P., Friman M., Lahtinen L., Väyrynen V.M., Liipo T.K. Combination of microsatellite instability and BRAF mutation status for subtyping colorectal cancer. Br. J. Cancer. 2015;112(12):1966–1975. doi: 10.1038/bjc.2015.160. Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Natsume S., Yamaguchi T., Takao M., Iijima T., Wakaume R., Takahashi K. Clinicopathological and molecular differences between right-sided and left-sided colorectal cancer in Japanese patients. Jpn. J. Clin. Oncol. 2018;48(7):609–618. doi: 10.1093/jjco/hyy069. Jul 1. [DOI] [PubMed] [Google Scholar]
- 25.Hanna M.C., Go C., Roden C., Jones R.T., Pochanard P., Javed A.Y. Colorectal cancers from distinct ancestral populations show variations in BRAF mutation frequency. PLoS One. 2013;8:e74950. doi: 10.1371/journal.pone.0074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu T., Lu H., Guo L., Huang W., Ling Y., Shan L. Detection of BRAF mutation in Chinese tumor patients using a highly sensitive antibody immunohistochemistry assay. Sci. Rep. 2015;5:9211. doi: 10.1038/srep09211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanus G.A., Belyaeva A.V., Ivantsov A.O., ESh Kuligina, Suspitsin E.N., Mitiushkina N.V. Pattern of clinically relevant mutations in consecutive series of Russian colorectal cancer patients. Med. Oncol. 2013;30:686. doi: 10.1007/s12032-013-0686-5. [DOI] [PubMed] [Google Scholar]
- 28.Rozek L.S., Herron C.M., Greenson J.K., Moreno V., Capella G., Rennert G. Smoking, gender, and ethnicity predict somatic BRAF mutations in colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 2010;19:838–843. doi: 10.1158/1055-9965.EPI-09-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye J.X., Liu Y., Qin Y., Zhong H.H., Yi W.N., Shi X.Y. KRAS and BRAF gene mutations and DNA mismatch repair status in Chinese colorectal carcinoma patients. World J. Gastroenterol. 2015;21(5):1595–1605. doi: 10.3748/wjg.v21.i5.1595. Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W., Qiu T., Zhi W., Shi S., Zou S., Ling Y. Colorectal carcinomas with KRAS codon 12 mutation are associated with more advanced tumor stages. BMC Cancer. 2015;15:340. doi: 10.1186/s12885-015-1345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capel E., Fléjou J.F., Hamelin R. Assessment of MLH1 promoter methylation in relation to gene expression requires specific analysis. Oncogene. 2007;26(54):7596–7600. doi: 10.1038/sj.onc.1210581. Nov 29. [DOI] [PubMed] [Google Scholar]
- 32.Gausachs M.1., Mur P., Corral J., Pineda M., González S., Benito L. MLH1 promoter hypermethylation in the analytical algorithm of Lynch syndrome: a cost-effectiveness study. Eur. J. Hum. Genet. 2012;20(7):762–768. doi: 10.1038/ejhg.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.English D.R., Young J.P., Simpson J.A., Jenkins M.A., Southey M.C., Walsh M.D. Ethnicity and risk for colorectal cancers showing somatic BRAF V600E mutation or CpG Island Methylator Phenotype. Cancer Epidemiol. Biomarkers Prev. 2008;17(7):1774–1780. doi: 10.1158/1055-9965.EPI-08-0091. [DOI] [PubMed] [Google Scholar]
- 35.Hewish M., Lord C.J., Martin S.A., Cunningham D., Ashworth A. Mismatch repair deficient colorectal cancer in the era of personalized treatment. Nat. Rev. Clin. Oncol. 2010;7:197–208. doi: 10.1038/nrclinonc.2010.18. [DOI] [PubMed] [Google Scholar]
- 36.Moreira L., Muñoz J., Cuatrecasas M., Quintanilla I., Leoz M.L., Carballal S. Prevalence of somatic MutL Homolog 1 Promoter Hypermethylation in Lynch Syndrome Colorectal Cancer. Cancer. 2015;121(9):1395–1404. doi: 10.1002/cncr.29190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.