Graphical abstract
Abbreviations: AqE, Aqueous extract; CRP, 1.1 CORESTA Reference Product 1.1; H292, Human bronchial epithelial cells; HGF, Human gingival fibroblasts; LDH, Lactate dehydrogenase assay; MOP, Modern oral product; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NRU, Neutral red uptake assay; RTCA, Real Time Cell Analysis
Keywords: Cytotoxicity, In vitro, Tobacco-free modern oral tobacco, Nicotine, Risk assessment, RTCA, Tobacco-free nicotine pouches
Highlights
-
•
Tobacco-free nicotine pouches are an emerging nicotine product category.
-
•
RTCA provides a real-time assessment of the potential cytotoxicity of these products.
-
•
Gingival fibroblasts and epithelial cells showed comparable responses.
-
•
The BAT products assessed showed little to no cytotoxicity, with increasing flavours and nicotine strengths.
-
•
RTCA could differentiate between a commercial product and a reference snus product.
Abstract
In many regulated industries there is an increasing pressure to provide timely and robust risk assessment data to support product launches. Real-time cell analysis (RTCA) is a tool that allows for the fast and relatively labour-free cytotoxic assessment of test compounds, compared to traditional methods. Here, we propose an application for the RTCA platform to provide a screening approach, to evaluate the cytotoxic potential of tobacco-free nicotine pouches, also termed modern oral product (MOP), to determine the contribution of differing nicotine strengths (4−11 mg) and a range of available flavour types from multiple markets, on overall product toxicity.
Aqueous extracts were prepared for all products using 1 pouch in 20 mL cell culture media and applied to the cell system for 24 h. Test extract nicotine concentrations reflected the increases in product nicotine strength; however, these changes were not present in the same magnitude in the cytotoxicity data obtained from both primary human gingival fibroblasts (HGF) and an NCI-H292 human bronchial epithelial continuous cell line. Furthermore, across the range of flavours and product nicotine strengths tested, H292 cells whilst not the target organ for oral product use, accurately predicted the results seen in HGFs and could be considered a useful surrogate for fast screening studies. H292 cells are more easily cultured and for longer periods, offering a more compatible test system.
In conclusion, the data demonstrate the utility of the RTCA platform for the quick assessment of a large range of product variants. Furthermore, for a cytotoxicity measure with this test product, the simple H292 cell line can predict outcomes in the more complex HGF and provide useful pre-clinical cytotoxicity screening data to inform the risk assessment of MOPs and the relative contribution of flavourings, nicotine and other components.
1. Introduction
The last decade has seen an increasing diversity of nicotine-containing products; including electronic cigarettes (e-cigarettes), Tobacco Heating Products (THPs) and more recently Modern Oral Products (MOPs), also referred to as tobacco-free nicotine pouches. These products are smokeless, comprising of a small fleece containing nicotine, flavourings, sweeteners and plant-based fibres. Like the more traditional oral product, snus, these products are intended to be placed under the upper lip to allow the release of nicotine for 30−60 min per use. Due to the lack of tobacco constituents there is the potential for these products to have a reduced risk status compared to snus, for which there is already epidemiological evidence to suggest reduced cancer incidences compared to smoking. The risk of using snus is believed to be only 1% of that of traditional cigarette smoke [1].
Multiple studies have evaluated the oral health risks associated with smoking, smokeless loose tobacco products and smokeless tobacco-based pouched snus products. Cigarette smoke has been associated with gum inflammation/disease, tooth loss, cancer and tooth staining [2,3]. Studies have shown there is minimal or no increased risk of oral cancer associated with smokeless tobacco use [[4], [5], [6], [7]]. In addition, a recent chemical analysis reported that MOPs have lower toxicant levels compared to Swedish-style snus products [8]. Recently, an in vitro assessment of the category showed limited biological activity of the MOP category using a variety of approaches compared to traditional snus and cigarette smoke extracts [9].
The changing nicotine product landscape highlights the requirement to diversify in vitro methods and expand into newer approaches over classical toxicological evaluation approaches, to provide timely and informative duty of care. This vision was propagated by the 2007 NRC report entitled Toxicity Testing in the 21 st Century (Tox21), the ensuing partnership between the National Toxicology Program (NTP), NIH Chemical Genomics Center (NCGC) and the Environmental Protection Agency (EPA) has aided the rapid advancement into novel high throughput in vitro methodologies [10].
Real Time Cell Analysis (RTCA) is one such technology, that has risen in popularity due to its versatility, ease of use and the ability to track cell status throughout the course of the experiment. RTCA is a non-invasive, label-free screening technique which reduces the labour and time required for traditional cell-based assays, whilst maximising the physiological relevance of the data.
As shown in Fig. 1, each well of the E-Plates contains microelectrodes to monitor changes in impedance caused by adherence or changes in cell morphology in real-time, reporting data via the matrix of Cell Index (CI). Many changes in cell status can be captured via CI such as cell adhesion, cell growth, cytotoxicity, modulation of barrier function or morphological dynamics. The RTCA instrument used in this study is the xCELLigence (ACEA Biosciences; California, US) Multi-Plate version which contains 6 cradles, allowing for 6 × 96 well E-Plates to be monitored at any one time with integrated (RTCA Software 2.1.0) software. This allows for the simultaneous assessment of multiple variables and test articles. Importantly, the instrument can be installed within a cell culture incubator allowing cells to be monitored in situ, with limited manipulation or movement from their optimal environment, and not limiting the time course of exposure. RTCA is commercially available and has been widely adopted in the scientific community, being utilised for many cell types and in a variety of research areas (Table 1).
Fig. 1.
Graphical representation of the experimental method using Real-Time Cell Analysis for the assessment of Modern Oral Products. A. shows xCELLigence with docking plate. B. example of an impedance measure C. example of RT trace data w/ phases growth. D. shows sample preparation for MOP and snus.
Table 1.
The main applications of the RTCA platform and its published uses in differing research fields and cell types.
| RTCA application | Research Area | Cell type | Reference |
|---|---|---|---|
| Cell viability and cytotoxicity | Dental | Gingival fibroblasts | [11] |
| [12] | |||
| Nicotine | H292 HGF |
[9] | |
| Cosmetics | HepaRG | [13] | |
| Particle toxicology | BEAS-2B CHO HEK293 |
[14] | |
| Review | Summary of multiple cell lines | [15] | |
| Cell proliferation, adherence and migration | Co-culture models | HP62 Mesenchymal stem cells |
[16] |
| Oncology | H292 | [17] | |
| H292 MIA PaCa-2 HCT-116 MDA-MB-231 |
[18] | ||
| Extracellular matrix | HEK293 | [19] | |
| Morphological dynamics | Karyotyping | HeLa | [20] |
| Tissue injury/ disease | BSMC HLF |
[21] | |
| Virology | RD cells | [22] | |
| Vero E6 Vero CCL81 HEK293T |
[23] | ||
| Immunotherapy | WO-19 WO-6 PDX WO-12 |
[24] | |
| Cardiotoxicity | Safety assessment | iCell Cardiomyocytes® | [25] |
| Barrier function | Pharmaceutical | HMECs | [26] |
The application of the RTCA platform described in this paper forms a part of a larger proposed pre-clinical assessment framework [27] which is especially important when assessing a new category, where little information exists. Such a framework builds a weight of evidence on the category and facilities quicker and more accurate assessments for future product iterations, such as read across.
Fig. 2 illustrates a proposed in vitro testing strategy that sits within a larger framework for the assessment of potentially reduced risk products. It starts with duty of care assessments. The identification and removal of any potential chemical alerts such as those with identified Carcinogenic, Mutagenic and Reprotoxic (CMR) activities and the establishment of toxicological thresholds of concern. Such duty of care approaches have been described for cigarette smoke and e-cigarette risk assessments [28,29]. Once the duty of care assessments are completed, we propose to screen the products using RTCA as detailed in this manuscript. This ensures that biologically these products meet the standard required as identified by the various risk assessments. At this stage, any flags in terms of cytotoxicity can be highlighted and addressed through product development. For the purpose of this study the ISO guideline for cytotoxicity assays was employed, where a loss of 30 % viability or more is required to deem a product cytotoxic [30]. This represents the quickest way to screen products, albeit from a cytotoxicity perspective. Following this study, a more comprehensive in vitro biological assessment is conducted, focusing on disease mechanisms and endpoints, such as oxidative stress, protein damage, DNA damage and endothelial impairment, which may indicate potential risks that have not been captured in the formal risk assessment process. Such responses, if observed, could be linked to adverse outcome pathways. In addition to in vitro disease modelling, classical genotoxicological assessments conducted to OECD standards [31] can also be employed to meet regulatory guidelines. Results from this stage of assessment will capture the interactions of the extracted chemicals coming from the source material with those added to the product for flavouring purposes. Finally, the use of three-dimensional cell culture models such as those commercially available, could prove a valuable link to clinical and in vivo methods when coupled with more clinically relevant endpoints and potentially next generation sequencing. Such approaches are consistent and in-line with the 3Rs (refine, replace, and reduce) and Tox21 initiatives [32].
Fig. 2.
A schematic representation of a proposed MOPs in vitro assessment strategy.
2. Materials and methods
2.1. Study design
In this manuscript RTCA is discussed as a potential screening tool. Such an approach would be a precursor to an in vitro weight of evidence assessment programme and would support both product development (in terms of identifying potential flags) and assessment of multiple product variants in a time-efficient manner. In order to achieve this, the impact of cell type, test product nicotine strengths (4−11 mg) and a range of flavours (Table 3) were assessed and compared to commercial comparators and a reference snus product to determine the sensitivity of responses within similar product categories, as shown in Table 2.
Table 3.
Summary of extraction efficiency results and osmolarity measures.
| Unique product identifier | Product name |
Extraction efficiency (% ± SD) |
Osmolarity (mOsm) |
|---|---|---|---|
| LYFT_BF04 | Berry Frost | 74.75 ± 18.13 | 321.67 |
| LYFT_PM04 | Polar Mint | 97.25 ± 25.13 | 318.67 |
| LYFT_TB04 | Tropic Breeze | 79 ± 2.82 | 311.00 |
| LYFT_BF06 | Berry Frost | 100.71 ± 2.79 | 320.00 |
| LYFT_TB06 | Tropic Breeze | 85.30 ± 1.64 | 338.33 |
| LYFT_LC10 | Liquorice | 72.18 ± 8.65 | 339.33 |
| LYFT_UV10 | Urban Vibe | 66.05 ± 3.30 | 329.33 |
| LYFT_IC10 | Ice Cool | 79.86 ± 15.86 | 337.00 |
| LYFT_TB11 | Tropic Breeze | 77.73 ± 7.69 | 317.00 |
| LYFT_FZ11 | Freeze | 77.43 ± 14.13 | 343.00 |
| LYFT_WC11 | Winter Chill | 76.33 ± 4.48 | 339.33 |
| NDSP_BW06 | Bergamot & Wildberry | 86.79 ± 13.24 | 371.33 |
| NDSP_MN06 | Mint | 75.83 ± 13.85 | 385.67 |
| CRP1.1 | CORESTA Reference Product 1.1 | 74.5 ± 3.93 | 454.67 |
Table 2.
Products assessed in this study. Products were assessed in two waves; Detailing the product type, flavour and nicotine classification.
| Testing wave | Product classification | Product Name | Unique product identifier | Nicotine strength (dots & mg) |
|---|---|---|---|---|
|
Wave 1 HGF and H292 (to confirm parity of response) |
British American Tobacco | Berry Frost | LYFT_BF04 | 1 dot (4 mg) |
| Polar Mint | LYFT_PM04 | |||
| Tropic Breeze | LYFT_TB04 | |||
| LYFT_TB06 | 2 dot (5.6 mg) | |||
| LYFT_TB11 | 4 dot (10.8 mg) | |||
| Commercial comparator (Nordic Spirit) | Bergamot & Wildberry | NDSP_BW06 | 2 dot (5.6 mg) | |
| Mint | NDSP_MN06 | |||
| Reference | CORESTA Reference Product 1.1 | CRP1.1 | 8 mg | |
|
Wave 2 H292 only |
British American Tobacco | Berry Frost | LYFT_BF06 | 2 dot (5.6 mg) |
| Liquorice | LYFT_LC10 | 3 dot (9.8 mg) | ||
| Urban Vibe | LYFT_UV10 | |||
| Ice Cool | LYFT_IC10 | |||
| Freeze | LYFT_FZ11 | 4 dot (10.8 mg) | ||
| Winter Chill | LYFT_WC11 | |||
In our previous study [9], we saw similar cytotoxicity responses for human gingival fibroblasts (HGF) and the lung derived H292 cells and have further explored these responses in this study. The continuous H292 cell line offers many advantages for a fast and robust screening approach. Therefore, we tested H292 in parallel with HGF in Wave 1 testing to determine whether the basic cytotoxicity response of the simpler culture model would predict and show parity with the more complex HGF cells. To ensure that the extraction efficiency between products tested was consistent, nicotine was used a marker for extraction efficiency, assuming that all constituents of the MOPs (such as flavourings and sweeteners) would be equally transferred. Finally, the results from multiple products are compared contextualised against a reference Swedish-style snus and commercially available MOPs.
2.2. Materials and reagents
Unless otherwise stated all materials and reagents were purchased from Fisher Scientific (Loughborough, UK).
2.3. Test products
The test products used in this study are detailed in Table 2. These include nine LYFT variants, two commercial comparator variants and a reference snus. Depending on the geographical location, British American Tobacco (BAT) markets MOPs under the name LYFT and VELO depending on the geographical location, European LYFT products are being transitioned to a global VELO brand. For ease, we have termed all BAT products LYFT in this study (Table 2).
2.4. Test sample generation
As previously determined [9] in order to compare between the relative toxicity of MOP variants, aqueous extracts (AqE) were generated for each MOP as shown in Table 2. Briefly, all aqueous extracts were generated by removing the external fleece from 1 pouch, placing the contents into a conical flask with 20 mL complete cell culture media and incubating at 37⁰C with 150 RPM shaking for 1 h. Following shaking, particulate was removed by centrifugation and supernatant filtered at 5 μm and 0.2 μm. 1 mL aliquot of each extract was used for nicotine analysis. The method of test article extraction has been previously detailed [9].
2.5. Determination of nicotine concentration
To quantify the concentration of nicotine in the product extracts 495 μL extract media were spiked with 5 μL of d4-nicotine (10 ng/mL final concentration), and resuspended in 5% acetonitrile in water before quantification by UPLC-MS/MS, as adapted from [33]. Subsequent extract dilutions were calculated from this 100 % value and averaged across 3 independent replicates.
2.6. AqE osmolality measurements
The osmolality of the AqE samples was measured using a Löser Micro-osmometer Type OM806 (Löser Messtechnik, Berlin, Germany). The osmometer was calibrated prior to use within the range of 0–900 milli osmoles (mOsm). Distilled water (diH20) was used as a 0 mOsm calibration and osmometer calibration solutions with concentration 300 mOsm/kg and 900 mOsm/kg (Löser Messtechnik, Berlin, Germany) were used for 300 mOsm and 900 mOsm respectively. Extracts were assessed in triplicate and data averaged.
2.7. Cell culture
Human bronchial epithelial cells (NCI-H292) (American Type Culture Collection (ATCC), were cultured at 37 °C in a 5% CO2 humidified atmosphere in Roswell Park Memorial Institute (RPMI) 1640 media supplemented with 10 % Foetal Bovine Serum (FBS), 2 mM Lglutamine and 50 U/mL penicillin and 50 μg/mL streptomycin. Cells were frozen in a cell bank at passage 79. Cyropreserved H292 were recovered and cultured for a further 21 passages before disposal.
Human gingival fibroblasts (HGF; ATCC, Middlesex, UK) were maintained at 37 °C in a 5% CO2 humidified atmosphere. Cells (ATCC, Lot #6440682) were cultured in fibroblast basal media supplemented with low serum growth kit (ATCC) which includes: rh FGF b (5 ng/mL), l-glutamine (7.5 mM), Ascorbic acid (50 μg/mL), Hydrocortisone Hemisuccinate (1 μg/mL), rh Insulin (5 μg/mL) and Fetal Bovine Serum (2%). Cells were frozen in a cell bank at passage p4. Cryopreserved HGFs were recovered and cultured for a further 2–3 passages before disposal, these were either seeded into new flasks, or into 96-well plates for assays and grown to confluency before use.
2.8. Real-Time Cell Analysis (RTCA)
For real-time cell analysis (RTCA), the xCELLigence® multi-plate (MP) instrument (ACEA Biosciences; California, US) with integrated (RTCA Software 2.1.0) software was used to determine Cell Index (CI) which is considered a proxy for cytotoxicity. The MP RTCA instrument has 6 cradles which allows 6 plates to be run simultaneously, with 3 samples per 96 well plate. The bottom of each 96 well E-Plate® contains a set of gold microelectrodes, attached to each well which allows the passing of an electric current. CI measures the electrode impedance at the electrode/solution interface, which is influenced by the presence of cells cultured in the wells and thus impeding electron flow.
As described in the manufacturer’s protocol, E-Plates® were seeded (20,000 cells/well H292 s and 10,000 cells/well HGFs [11] and allowed to settle for 30 min before a background measurement was taken. Cells were allowed to proliferate for 24 h before dosing with test article for 24 h. Impedance (Cell Index) measurements were taken throughout the 48 h time period, producing a cell viability curve once analysed. 0.25 % (v/v) concentration of Triton-X100 was used to anchor curves when full cytotoxicity was not gained by the test article.
2.9. Statistical methods
Cytotoxicity data across the concentration range were averaged across the experimental replicates and then averaged across the concentration to provide a global mean viability value (%) per product, in each cell system tested. By removing extract concentration, it allowed for comparison of larger numbers of product variants.
Each product extract was assessed on a minimum of three independent occasions, using 8 concentrations of extract (0–100 %), applied in triplicate wells per experiment.
Resultant data were analysed for statistical significance using Minitab 17® statistical software, where data was normally distributed (as determined by the normality test function) either a two-sample t-test, one-way ANOVA or GLM ANOVA test was used to determine significance. Where data was not normally distributed non-parametric tests were used.
3. Results
3.1. Test article characterisation
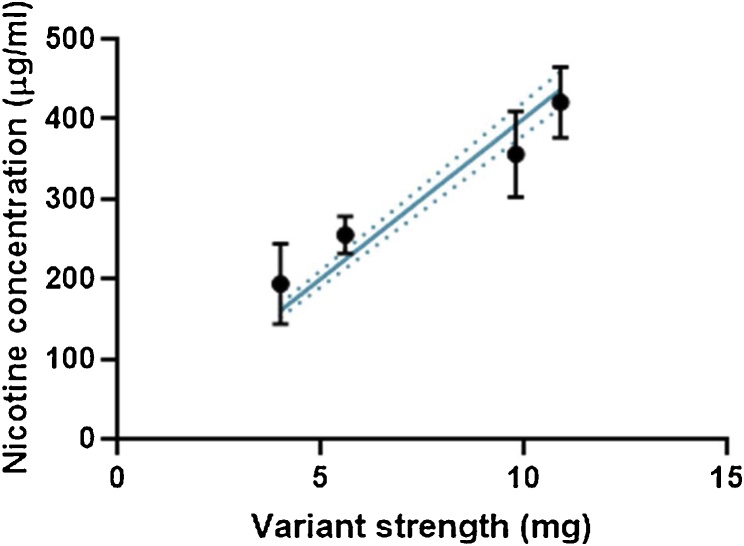
In order to characterise the extracts generated in this study, nicotine concentration was used both as a marker for dose, and as a quality control marker for extract generation. Fig. 3 demonstrates that with increasing product nicotine strength that there is a proportional increase in extract nicotine concentration. This indicates that the test article, even at the highest nicotine level (∼11 mg), are extracting equally as well as lower strength products, with no saturation of the test matrix. The relationship between product strength and extract concentration was highly linear (r2 = 0.98) and variations in extract concentration were relatively consistent regardless of nicotine strength.
Fig. 3.
Recovered nicotine concentration in all extracts used in this study across all variants. Solid line represents regression; dotted line is 95 % confidence intervals.
3.2. Cell system comparisons
In order to assess the effects of cell lines sensitivity to MOP extracts, lung-derived NCI-H292 and human gingival fibroblasts (HGF) cells were compared. HGF were chosen to provide a physiologically relevant route of exposure for MOPs. As primary cells, HGFs have some disadvantages for use in routine screening assays; with fewer useable passages, experiments often spread across multiple sub-cultures, increasing variability, exhibiting a longer population doubling time, and growing at lower confluency producing overall fewer cells per passage which can be rate limiting for 96-well based assays. H292 s, although not primary cells and not target organ specific, have been widely used for toxicity screening purposes for other tobacco and nicotine products [[34], [35], [36], [37]].
H292 s can be used for an extended number of passages per vial and grow to a monolayer creating a larger number of useable cells per passage. Due to their continuous nature, it is also feasible to suggest that we could expect less variability with H292s compared to the primary HGF s.
To determine parity of response between these two cell types, RTCA was employed to determine the relative toxicity for three product variants (consisting of different flavours and nicotine strengths). As shown in Fig. 4 both cell systems showed similar results with approximately a 10–20 % reduction in cell viability following 24 h incubation with the test extract. HGF showed slightly more variability in their response to MOPs, and slightly lower sensitivity compared to H292 s, although neither observations change the outcome, that these products were deemed non-toxic using both cells systems. Statistical analysis of the data using a t-test (p value = 0.01), confirmed that H292 s and HGFs were statistically different although the difference between the two cell lines was only 7 %. This observed statistical difference could be due to the low variability within the dataset and could be argued whether such a small change in viability should be consider biologically relevant. Future assessments utilised H292 s due to the decrease in variability of response and observed increase in sensitivity, providing a worst-case scenario assessment.
Fig. 4.
Cytotoxicity in H292 vs HGF cells as determined by RTCA for 3 different flavours (Berry Frost 4 mg nicotine (BF04), Polar Mint 4 mg nicotine (PM04), Tropical Breeze 6 mg nicotine (TB06)). Data are global mean ± SD (average cell viability across concentration range tested) from 3 independent experiments. Upper dotted line represents HGF averaged response and lower dotter line represents H292 averaged response.
3.3. Increasing nicotine strengths
The effect of nicotine strength on the cytotoxicity of LYFT variants were investigated. In this experiment the same flavour group was used, with varying nicotine strength (4, 6 and 11 mg). As Fig. 5 demonstrates, increasing the nicotine strength from 4 to 11 mg did not have a significant impact on the cytotoxic profile of the products. Statistical analysis by way of a one-way ANOVA analysis, showed that there were no significant differences between the products (p = 0.097). The mean viability difference between the three groups was only 2.9 %. Based on these results, nicotine was deemed not to have a significant impact on cytotoxicity. Future testing could, therefore, focus on the cytotoxic impact of differing flavour groups rather than nicotine strengths. Moreover, as nicotine has no impact on resultant cytotoxicity, we could justifiably compare between different nicotine strengths, when comparing flavour groups.
Fig. 5.
Samples of the same flavour (Tropical Breeze) in H292 as determined by RTCA for 3 different nicotine strengths. Data are mean ± SD from 3 independent experiments. Dotted line represents the averaged response.
3.4. Flavour assessment
The final assessment was to compare products across the smokeless nicotine category to determine the effect of flavours on cytotoxic potential, whilst contextualising against a competitor product and a Swedish-style snus reference benchmark. LYFT flavours assessed in this study ranged from mint to fruit variants, as shown in Table 1. In addition to assessing flavours in LYFT, two commercial comparators consisting of similar flavourings and nicotine strengths to LYFT were assessed. Finally, as no reference standard exists for the modern oral category, a reference snus product was used CRP1.1 [38]. Fig. 6A shows the mean cytotoxicity across the concentration tested for each product.
Fig. 6.
A. Cytotoxic assessment of LYFT flavour variants, commercial comparator and snus reference product (CRP1.1). Data are mean viability at highest concentration tested ± SD from 3 independent experiments (CRP1.1 response was adjusted for osmolarity). B. Osmolarity measures for all products tested, dotted lines indicate the physiological range of 280-400 mOsm.
Increases in toxicity were observed with commercial comparator brand, for both flavour variants. To eliminate the possibility of increased sweetener content (found in most tobacco-free nicotine products in a varying amounts) causing an osmotic shift, osmolarity of all extracts was recorded (Fig. 6B) with all extracts determined to be within physiological range except from CRP 1.1. The response for CRP1.1 was corrected for doses within physiological range (AqE concentrations of above 60 % exceeded physiological osmolarity and were excluded, the cytotoxicity at the highest concentration whilst maintaining physiological osmolarity was 60.32 %). Table 3 shows the extraction efficiency and osmolarity data for all the products tested. The testing of these products in ranges exceeding physiological conditions could be a confounding factor for increased cytotoxicity and the relationship between product constituents and osmolarity warrants further investigation.
All LYFT variants (at 100 % extract) induced up to 10–20 % toxicity, and therefore based on a 30 % toxicity threshold, can be deemed non-cytotoxic in an acute exposure model [30]. In contrast, the commercial comparator brand induced up to 86–91 % toxicity at 100 % extract. For LYFT variants, when the data across the nicotine strengths were compiled, there was no discernible increase in cytotoxicity for the higher strength products, confirming the result shown in Fig. 5.
In this instance neither the inclusion of flavour(s) or nicotine strength had an adverse effect on the base toxicity of the product. To understand the inclusion of different flavours into the product, LYFT products containing menthol were compared to those of a fruit flavour, and no statistical differences were observed (p = 0.095). When compared to the other products, the commercial comparators showed a statistical difference from LYFT products, as determined by the Tukey method. This observation indicates that the products are not equivalent in their toxicity and that the approach used is sensitive enough to pick up subtle but significant differences in the products on the market. CRP 1.1 (adjusted for osmolarity) was also considered to be significantly comparable to LYFT in terms of toxicity.
4. Discussion
The aim of this study was to determine the suitability of Real Time Cell Analysis (RTCA) as a screening tool for Modern Oral Products (MOPs) also known as tobacco-free nicotine pouches. Multiple parameters were assessed in order to optimise the approach, including assessment of cell types, determination of extract nicotine concentration, osmolarity, and cytotoxicity acceptance criteria. Using this approach 11 LYFT variants containing different flavours and nicotine strengths were compared to two commercially available competitor products and contextualised against a reference Swedish-style snus product.
The RTCA platform was selected for this screening approach, due to its real-time and semi-automated nature compared to classical cytotoxicity assays such as MTT, lactate dehydrogenase assay (LDH) and neutral red uptake (NRU), which all require a degree of downstream processing. RTCA measures electrical impedance in a real-time trace, from which cytotoxicity and other mechanistic and morphological changes can be derived [11,21]. Previous research has shown that RTCA can provide comparable data to the well-established NRU assay in a more time-efficient manner [9,39,40]. Furthermore, continuous monitoring of each well allows for easy detection of anomalies such as missed wells, inconsistent cell seeding or dosing errors, which in standard assays wouldn’t be detected until the end of the assay. The obvious advantages provided by this platform have led to it being increasingly used for the assessment of cell viability [15,16] suggesting that newer technologies are becoming more commonplace for high throughput cytotoxicity screening.
In order to characterise and quality control the extracts generated in this study, the nicotine extract concentration was determined, and extraction efficiency calculated (Table 3). In our previous study [9] there was a concern that, when attempting to increase test extract concentration (from one 4 mg pouch to two 4 mg pouches per 20 mL) the nicotine level was saturated. By testing a range of product strengths in this study we aimed to further elucidate the boundaries of our extraction method. The extracts prepared in this study did not show any signs of nicotine saturation, there was a highly linear relationship between product and subsequent extract nicotine concentrations up 10.8 mg. The saturation observed in the previous study must be have due to the doubling of pouch material in the extract affecting the transfer of nicotine rather than the nicotine itself being saturated [9]. For the purpose of this study, we have assumed that all flavourings and sweeteners would transfer into the extract at the same rate as nicotine. The main product constituent other than nicotine is the natural fibres from the pouch material, however, this material is not present in the final extract due to the centrifugation and filtration steps. The only other product constituents that could be analytically determined to further characterise the extracts would be flavour components, but this would be restrictive to comparisons within flavour families rather than across the whole product line. Adding a complementary dosimetry marker will be the subject of future investigation. Average extraction efficiency in this study was 79.3 %, to align with our previous study we did not include the fleece material in the extract preparation, however, it is our intention to include this in future studies to increase the overall extraction efficiency.
In order to complete the testing of multiple flavour variants and comparator products, the use of a high throughput-compatible cell line such as H292, that could be easily cultured producing large numbers of plates per passage was essential. From the data presented, the cytotoxic responses of H292 show a great deal of parity with those of HGF following wave 1 testing and therefore allowed the continuation of testing into wave 2, with an extended range of variants. Multiple oral cells were considered but HGFs were chosen based on ease of procurement, growth and use in other RTCA cytotoxicity studies [11,12]. EpiOral™ or EpiGingival™ 3D cell types will be explored, among others, in future projects.
To our knowledge this is the largest in vitro cytotoxicity assessment of MOP flavour variants and contributes valuable information towards a risk assessment framework on the contribution of flavours and nicotine strength to overall product toxicity. This study demonstrates that flavour (Table 3) and nicotine strength (4−11 mg) do not increase the overall toxicity of MOPs in a short-term assay. Flavoured nicotine products have a key role to play in providing alternatives for smokers, as demonstrated by mainstream oral nicotine replacement therapy products and e-cigarettes [41,42]. Electronic cigarettes for example, have played an important role in reducing smoking rates in UK, where non-tobacco flavours as just as popular as tobacco flavours, fruit (29 %), tobacco (27 %) and menthol/mint (25 %) [41].
We acknowledge there are several limitations to the conclusions that can be drawn from this study due to its single endpoint, short-term exposure and minimal responses. However, the next phase of the assessment framework will be to consider any genotoxicological effects, leading to a repeated dose study to model any potential longer-term toxic effects of product use in a more physiologically relevant 3D tissue system such as EpiOral™ or EpiGingival . RTCA platform can also be used in repeated dosing studies [13,25], in order to determine more long-term cytotoxicity and extending the reach of the current approach. In addition, osmolarity must also be considered as a confounding variable when analysing the data due to the varying levels of sweetener in the products.
For the current study in order to provide a worst-case scenario in vitro assessment, extracts were used from 0% to undiluted (100 %), based on extracts made on a per pouch (single use) basis. To contextualise this in terms of human daily exposure estimates, we can extrapolate from pharmacokinetic studies. A MOP-based pharmacokinetic study has recently been published using a similar product to LYFT (non-tobacco-based nicotine pouch, ZYN 3 mg and 6 mg), where the peak plasma concentrations following product use was approximately 16 ng/mL [43]. A total nicotine exposure of 80 μg was calculated from a single use, assuming a human blood volume of 5 L. Extracts in this study has approximately 70x the amount of total nicotine compared to the estimated human exposure, therefore cells in our model (at the 100 % concentration) received closer to a weekly exposure at 10 pouches/day, in a 24 h period. MOPs human consumption data has yet to be published, however human Swedish-style snus data reports average use at 11.7 (± 6.5) snus pouches/day [44], and it likely that MOP consumption will follow the same trends.
5. Conclusions
In summary, we propose that RTCA is a reliable and efficient screening technique for MOPs. This technique provides reproducible data and can discriminate between products in this category. By exposing the in vitro system to concentrations higher than the predicted daily exposure we can quickly identify variants that may pose risks and require further in vitro investigation with repeated use.
The reduced harm potential of Swedish-style snus has been clearly demonstrated [[4], [5], [6], [7]], by extension, tobacco free-MOPs, with nicotine levels comparable to levels found in Swedish-style snus, could be expected to have similar or greater harm reduction potential and therefore, this study contributes to the underpinning science used to determine the risk profile of the emerging MOPs category as an alternative to smoking. The data produced in this study demonstrate that LYFT are less cytotoxic, across multiple flavour variants and nicotine strengths, than an equivalent reference snus product and comparator products which can be replicated in both H292 s and HGFs. H292 s and HGFs showed parity of response to MOPs extracts and the higher throughput compatible H292 were chosen to screen wave 2 variants. Data also eliminated higher nicotine level as a contributing factor of acute cytotoxicity, allowing for a reduction in test sample number when performing future RTCA screening. Finally, consumer safety in this new and emerging category is paramount. Through the use of food purity flavourings, ingredients and pharmaceutical grade nicotine this should be assured [45], coupled with a robust pre-clinical assessment framework to continually evaluate tobacco free-MOPs and their risk profile.
Author’s contribution
EB, DB, DT and MG designed the study. NE and EB conducted the study under the supervision of MG. EB led the project and NE drafted the manuscript with support from EB, and DT. All authors approved the final version.
Author’s statement
All authors jointly designed the study. Nicole East and Emma Bishop conducted the study under the supervision of Marianna Gaca. Emma Bishop led the project and Nicole East, Emma Bishop and David Thorne drafted the manuscript with support from all authors. All authors approved the final version.
Declaration of Competing Interest
BAT funded the project and all authors were employees of BAT at the time of the study conduct. LYFT tobacco-free modern oral nicotine pouches are manufactured and supplied by BAT.
Edited by Dr. A.M. Tsatsaka
References
- 1.Lee P.N. Summary of the epidemiological evidence relating snus to health. Regul. Toxicol. Pharmacol. 2011;59:197–214. doi: 10.1016/j.yrtph.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Stratton K., Shetty P., Wallace R., Bondurant S. Clearing the smoke: the science base for tobacco harm reduction—executive summary. Tob. Control. 2001;10:189–195. doi: 10.1136/tc.10.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergström J. Tobacco smoking and chronic destructive periodontal disease. Odontology. 2004;92:1–8. doi: 10.1007/s10266-004-0043-4. [DOI] [PubMed] [Google Scholar]
- 4.Roosaar A., Johansson A.L., Sandborgh-Englund G., Nyrén O., Axéll T. A long-term follow-up study on the natural course of snus-induced lesions among Swedish snus users. Int. J. Cancer. 2006;119:392–397. doi: 10.1002/ijc.21841. [DOI] [PubMed] [Google Scholar]
- 5.Weitkunat R., Sanders E., Lee P.N. Meta-analysis of the relation between European and American smokeless tobacco and oral cancer. BMC Public Health. 2007;7:334. doi: 10.1186/1471-2458-7-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J., Ye W., Zendehdel K., Adami J., Adami H.-O., Boffetta P., Nyrén O. Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: a retrospective cohort study. Lancet. 2007;369:2015–2020. doi: 10.1016/S0140-6736(07)60678-3. [DOI] [PubMed] [Google Scholar]
- 7.Schildt E.B., Eriksson M., Hardell L., Magnuson A. Oral snuff, smoking habits and alcohol consumption in relation to oral cancer in a Swedish case-control study. Int. J. Cancer. 1998;77:341–346. doi: 10.1002/(sici)1097-0215(19980729)77:3<341::aid-ijc6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Azzopardi D., Liu C., Murphy J. Chemical characterisation of tobacco-free’ modern’ oral nicotine products and their position on the toxicant and risk continuums. Food Chem. Toxicol. 2021 doi: 10.1080/01480545.2021.1925691. In Submission. [DOI] [PubMed] [Google Scholar]
- 9.Bishop E., East N., Bozhilova S., Santopietro S., Smart D., Taylor M., Meredith S., Baxter A., Breheny D., Thorne D., Gaca M. An approach for the extract generation and toxicological assessment of tobacco-free ‘modern’ oral nicotine pouches. Food Chem. Toxicol. 2020 doi: 10.1016/j.fct.2020.111713. [DOI] [PubMed] [Google Scholar]
- 10.Shukla S.J., Huang R., Austin C.P., Xia M. The future of toxicity testing: a focus on in vitro methods using a quantitative high-throughput screening platform. Drug Discov. Today. 2010;15:997–1007. doi: 10.1016/j.drudis.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urcan E., Haertel U., Styllou M., Hickel R., Scherthan H., Reichl F.X. Real-time xCELLigence impedance analysis of the cytotoxicity of dental composite components on human gingival fibroblasts. Dent. Mater. 2010;26:51–58. doi: 10.1016/j.dental.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Öztürk F., Malkoc S., Ersöz M., Hakki S., Bozkurt S.B. Real-time cell analysis of the cytotoxicity of the components of orthodontic acrylic materials on gingival fibroblasts. Am. J. Orthod. Dentofac. Orthop. 2011;140:e243–9. doi: 10.1016/j.ajodo.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Teng S., Tebby C., Barcellini-Couget S., De Sousa G., Brochot C., Rahmani R., Pery A.R.R. Analysis of real-time mixture cytotoxicity data following repeated exposure using BK/TD models. Toxicol. Appl. Pharmacol. 2016;305:118–126. doi: 10.1016/j.taap.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Vetten M.A., Tlotleng N., Tanner Rascher D., Skepu A., Keter F.K., Boodhia K., Koekemoer L.-A., Andraos C., Tshikhudo R., Gulumian M. Label-free in vitro toxicity and uptake assessment of citrate stabilised gold nanoparticles in three cell lines. Part. Fibre Toxicol. 2013;10:50. doi: 10.1186/1743-8977-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefanowicz-Hajduk J., Ochocka J.R. Real-time cell analysis system in cytotoxicity applications: usefulness and comparison with tetrazolium salt assays. Toxicol. Rep. 2020;7:335–344. doi: 10.1016/j.toxrep.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roshan Moniri M., Young A., Reinheimer K., Rayat J., Dai L.J., Warnock G.L. Dynamic assessment of cell viability, proliferation and migration using real time cell analyzer system (RTCA) Cytotechnology. 2015;67:379–386. doi: 10.1007/s10616-014-9692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raza M.Z., Cadassou O., Cros-Perrial E., Dumontet C., Jordheim L. 2018. Role of 5’-nucleotidases in the Biology of Human Lung Cancer Cell Lines. [Google Scholar]
- 18.Bricard G., Cadassou O., Cassagnes L.-E., Cros-Perrial E., Payen-Gay L., Puy J.-Y., Lefebvre-Tournier I., Tozzi M.G., Dumontet C., Jordheim L.P. The cytosolic 5’-nucleotidase cN-II lowers the adaptability to glucose deprivation in human breast cancer cells. Oncotarget. 2017;8:67380–67393. doi: 10.18632/oncotarget.18653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamidi H., Lilja J., Ivaska J. Using xCELLigence RTCA instrument to measure cell adhesion. Bio Protoc. 2017;7 doi: 10.21769/BioProtoc.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irelan J.T., Wu M.-J., Morgan J., Ke N., Xi B., Wang X., Xu X., Abassi Y.A. Rapid and quantitative assessment of cell quality, identity, and functionality for cell-based assays using real-time cellular analysis. J. Biomol. Screen. 2011;16:313–322. doi: 10.1177/1087057110397359. [DOI] [PubMed] [Google Scholar]
- 21.Bravo D.D., Chernov-Rogan T., Chen J., Wang J. An impedance-based cell contraction assay using human primary smooth muscle cells and fibroblasts. J. Pharmacol. Toxicol. Methods. 2018;89:47–53. doi: 10.1016/j.vascn.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Teng Z., Kuang X., Wang J., Zhang X. Real-time cell analysis--a new method for dynamic, quantitative measurement of infectious viruses and antiserum neutralizing activity. J. Virol. Methods. 2013;193:364–370. doi: 10.1016/j.jviromet.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 23.Zost S.J., Gilchuk P., Chen R.E., Case J.B., Reidy J.X., Trivette A., Nargi R.S., Sutton R.E., Suryadevara N., Chen E.C., Binshtein E., Shrihari S., Ostrowski M., Chu H.Y., Didier J.E., Macrenaris K.W., Jones T., Day S., Myers L., Eun-Hyung Lee F., Nguyen D.C., Sanz I., Martinez D.R., Rothlauf P.W., Bloyet L.-M., Whelan S.P.J., Baric R.S., Thackray L.B., Diamond M.S., Carnahan R.H., Crowe J.E. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nat. Med. 2020;26:1422–1427. doi: 10.1038/s41591-020-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gitto S.B., Kim H., Rafail S., Omran D.K., Medvedev S., Kinose Y., Rodriguez-Garcia A., Flowers A.J., Xu H., Schwartz L.E., Powell D.J., Simpkins F. An autologous humanized patient-derived-xenograft platform to evaluate immunotherapy in ovarian cancer. Gynecol. Oncol. 2020;156:222–232. doi: 10.1016/j.ygyno.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhari U., Nemade H., Wagh V., Gaspar J.A., Ellis J.K., Srinivasan S.P., Spitkovski D., Nguemo F., Louisse J., Bremer S., Hescheler J., Keun H.C., Hengstler J.G., Sachinidis A. Identification of genomic biomarkers for anthracycline-induced cardiotoxicity in human iPSC-derived cardiomyocytes: an in vitro repeated exposure toxicity approach for safety assessment. Arch. Toxicol. 2016;90:2763–2777. doi: 10.1007/s00204-015-1623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bischoff I., Hornburger M.C., Mayer B.A., Beyerle A., Wegener J., Fürst R. Pitfalls in assessing microvascular endothelial barrier function: impedance-based devices versus the classic macromolecular tracer assay. Sci. Rep. 2016;6 doi: 10.1038/srep23671. 23671-23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy J., Gaca M., Lowe F., Minet E., Breheny D., Prasad K., Camacho O., Fearon I., Liu C., Wright C., Mcadam K., Proctor C. Assessing modified risk tobacco and nicotine products: Description of the scientific framework and assessment of a closed modular electronic cigarette. Regul. Toxicol. Pharmacol. 2017;90 doi: 10.1016/j.yrtph.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Costigan S., Meredith C. An approach to ingredient screening and toxicological risk assessment of flavours in e-liquids. Regul. Toxicol. Pharmacol. 2015;72:361–369. doi: 10.1016/j.yrtph.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Baker R.R., Massey E.D., Smith G. An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food Chem. Toxicol. 2004;42:53–83. doi: 10.1016/j.fct.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 30.ISO . 2009. ISO 10993-10995:2009. Biological Evaluation of Medical Devices — Part 5: Tests for in Vitro Cytotoxicity. [Google Scholar]
- 31.OECD . 2017. Overview on Genetic Toxicology TGs. [Google Scholar]
- 32.National Research Council N. The National Academies Press; Washington, DC: 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. [Google Scholar]
- 33.Adamson J., Li X., Cui H., Thorne D., Xie F., Gaca M.D. Nicotine quantification in vitro: a consistent dosimetry marker for e-Cigarette aerosol and cigarette smoke generation. Appl. In Vitro Toxicol. 2017;3:14–27. [Google Scholar]
- 34.Taylor M., Carr T., Oke O., Jaunky T., Breheny D., Lowe F., Gaça M. E-cigarette aerosols induce lower oxidative stress in vitro when compared to tobacco smoke. Toxicol. Mech. Methods. 2016;26:465–476. doi: 10.1080/15376516.2016.1222473. [DOI] [PubMed] [Google Scholar]
- 35.Azzopardi D., Patel K., Jaunky T., Santopietro S., Camacho O.M., Mcaughey J., Gaça M. Electronic cigarette aerosol induces significantly less cytotoxicity than tobacco smoke. Toxicol. Mech. Methods. 2016;26:477–491. doi: 10.1080/15376516.2016.1217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adamson J., Jaunky T., Thorne D., Gaça M.D. Characterisation of the borgwaldt LM4E system for in vitro exposures to undiluted aerosols from next generation tobacco and nicotine products (NGPs) Food Chem. Toxicol. 2018;113:337–344. doi: 10.1016/j.fct.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Breheny D., Thorne D., Baxter A., Bozhilova S., Jaunky T., Santopietro S., Taylor M., Terry A., Gaça M. The in vitro assessment of a novel vaping technology. Toxicol. Rep. 2020;7:1145–1156. doi: 10.1016/j.toxrep.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CORESTA . 2016. CORESTA Smokeless Tobacco Reference Product CRP1.1 - Swedish Snus [Online]https://www.coresta.org/sites/default/files/pages/2016_CRP1.1.pdf [Google Scholar]
- 39.Moe B., Gabos S., Li X.F. Real-time cell-microelectronic sensing of nanoparticle-induced cytotoxic effects. Anal. Chim. Acta. 2013;789:83–90. doi: 10.1016/j.aca.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Yan G., Du Q., Wei X., Miozzi J., Kang C., Wang J., Han X., Pan J., Xie H., Chen J., Zhang W. Application of real-time cell electronic analysis system in modern pharmaceutical evaluation and analysis. Molecules. 2018;23 doi: 10.3390/molecules23123280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mcneil A., B. L, Calder R., Bauld L., Robson D. Public Health England; London: 2018. Evidence Review of e-cigarettes and Heated Tobacco Products 2018. A Report Commissioned by Public Health England. [Google Scholar]
- 42.Hartmann-Boyce J., Mcrobbie H., Lindson N., Bullen C., Begh R., Theodoulou A., Notley C., Rigotti N.A., Turner T., Butler A.R., Et Al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2020;(10) doi: 10.1002/14651858.CD010216.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lunell E., Fagerström K., Hughes J., Pendrill R. Pharmacokinetic comparison of a novel non-tobacco-Based nicotine pouch (ZYN) with conventional, tobacco-based Swedish snus and American moist snuff. Nicotine Tob. Res. 2020;22(10):1757–1763. doi: 10.1093/ntr/ntaa068. PMID: 32319528. [DOI] [PubMed] [Google Scholar]
- 44.Digard H., Errington G., Richter A., Mcadam K. Patterns and behaviors of snus consumption in Sweden. Nicotine Tob. Res. 2009;11:1175–1181. doi: 10.1093/ntr/ntp118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robichaud M.O., Seidenberg A.B., Byron M.J. Tobacco companies introduce ‘tobacco-free’ nicotine pouches. Tob. Control. 2020;29:e145–e146. doi: 10.1136/tobaccocontrol-2019-055321. [DOI] [PMC free article] [PubMed] [Google Scholar]