Abstract
Purpose:
To compare the efficacy and safety of Alcaftadine 0.25%, Olopatadine hydrochloride 0.2%, and Bepotastine besilate 1.5% ophthalmic solutions in the treatment of allergic conjunctivitis.
Methods:
This is a prospective, observer-masked, comparative study of 180 patients with mild to moderate allergic conjunctivitis, randomized into three groups of 60 patients each. Each group was assigned to be treated with one of the three treatment options namely Alcaftadine 0.25%, Olopatadine hydrochloride 0.2% and Bepotastine besilate 1.5% ophthalmic solutions. Patients were followed-up at regular intervals with relief and resolution of symptoms and signs noted using Total Ocular Scoring System (TOSS) and hyperaemia scale.
Results:
All three topical medications were effective in resolving symptoms of the patients with mild to moderate allergic conjunctivitis. Baseline mean TOSS scores for Alcaftadine group, Olopatadine group and Bepotastine besilate group were (7.68±2.32), (7.65±2.32) and (7.45±2.27) respectively as compared to the corresponding TOSS scores on 14th Day (4th visit) which were (0.2 ± 0.43), (0.4 ± 0.56) and (0.1 ± 0.36) respectively. The resolution of symptoms in the Bepotastine and Alcaftadine groups was significantly profound as compared to the Olopatadine group (p = 0.008). Bepotastine and Alcaftadine groups significantly reduced allergic conjunctivitis symptoms compared to Olopatadine group (p = 0.008).
Conclusion:
All three topical ophthalmic medications used in the study are safe and effective in the treatment of allergic conjunctivitis. However, Bepotastine and Alcaftadine appear to outweigh Olopatadine in resolving the symptoms of allergic conjunctivitis.
Keywords: Alcaftadine, allergic conjunctivitis, Bepotastine besilate, hyperaemia scale, olopatadine, Total ocular symptom score (TOSS)
The conjunctiva of the eye is continually exposed to a variety of airborne antigens that can lead to inflammation, termed allergic conjunctivitis,[1] which is an ocular surface inflammatory disease that affects approximately 40% of the global population.[2] It is predominantly Ig E-mediated Type I hypersensitivity reaction where allergen binds to specific Ig E molecules, triggers mast cell degranulation and subsequent increase in histamine leading to activation of both H1 and H2 types of histamine receptors.[3]
Pharmacological treatment of allergic conjunctivitis includes H1 receptor blockade, mast cell stabilization, and blocking of cytokine production and prostaglandin formation.[4]
Currently, Alcaftadine 0.25% and Olopatadine hydrochloride 0.2% are approved once-daily and Bepotastine besilate 1.5%, twice daily dual-acting antiallergic agents for allergic conjunctivitis which includes inhibition of histamine receptor activation directly and reduction of allergic responses by stabilizing mast cells indirectly.[5] Olopatadine hydrochloride is a selective histamine H1 receptor antagonist and mast-cell stabilizer. It also has anti-inflammatory effects which include suppression of interleukins (IL) 6 and 8 production by inhibiting histamine related signalling pathways.[1,5]
Alcaftadine is an anti-allergic agent that provides relief from ocular itching by inverse agonistic effects on H1, H2 and H4 receptors in early phase and also stabilizes mast cells by inhibiting release of mediators such as cytokines and lipid mediators in the late phase of an ocular allergic response and decreases chemotaxis, eosinophil activation thereby exerts anti-inflammatory property.[6,7] Bepotastine besilate 1.5% ophthalmic solution is the dual-action agent, which combines strong antihistaminic activity with mast cell-stabilizing properties to provide both rapid and long-lasting relief in allergic conjunctivitis.[8] Considering the paucity of comparative studies between long-acting anti-histamines, Alcaftadine 0.25% and Olopatadine hydrochloride 0.2% and Bepotastine besilate 1.5% in Allergic conjunctivitis with regard to efficacy and safety amongst Indian patients, this study was undertaken.
Methods
The study was an observer-masked, randomized, prospective, parallel-group study conducted at the Department of Ophthalmology, Minto RIO, Bangalore Medical College and Research Institute, Bengaluru. The protocol was approved by the Ethics Committee of our Institute and adhered to the tenets of the Declaration of Helsinki.
Diagnosis of allergic conjunctivitis was made clinically according to the presence of classical signs and symptoms. Total Ocular Symptom Scoring System (TOSS) scoring was used to grade the signs and symptoms. All patients aged between 18 and 60 years belonging to either gender, with mild-to-moderate allergic conjunctivitis [Table 1][9] presenting to outpatient department between July 2019 and September 2019 were included after obtaining written informed consent.
Table 1.
Classification of allergic conjunctivitis
| Mild | Moderate | Severe | Blinding | |
|---|---|---|---|---|
| Bulbar Conjunctiva | Congestion | Congestion | Thickening and Trantas spots | Granulomas |
| Tarsal Conjunctiva | Micro papillae | Macro (1 mm) papillae | Giant (>1 mm) papillae | Mega Cobblestones |
| Cornea | - | Micro erosions | Macro-erosions | Shield ulcer |
| Limbus | - | Focal (<180) degrees inflammation | Diffuse (>180) degrees Inflammation | Limbal deficiency |
Patients with severe allergic conjunctivitis, need for topical steroids or topical immunosuppressive, contact lens wearers, patients with an intra-ocular pressure of more than 21 mm Hg in either eye or any type of glaucoma, history of hypersensitivity to the study medications or their components (including benzalkonium chloride), history of an ocular herpetic infection, an active ocular infection, or any significant illness, taking systemic steroids or antihistamines currently or within 7 days prior to enrolment, pregnant, planning pregnancy, or nursing/lactating and use of any other topical ocular medications were excluded from the study. A total of 180 patients with mild or moderate allergic conjunctivitis were randomized into three groups with an allocation ratio of 1:1:1 using computer-generated random number sequence to receive topical anti-allergic medication for 14 days as follows:
Group 1: Topical 0.25% Alcaftadine eyedrops OD
Group 2: Topical 0.2% Olopatadine eyedrops OD
Group 3: Topical 1.5% Bepotastine besilate eyedrops BID.
Complete general, physical, and ophthalmologic examination was done. Patients were examined and their baseline symptoms and signs (TOSS) were recorded. Demographic data, ocular and medical histories, concomitant medications, physical examination, clinical examination, including recording of vital signs, Ophthalmological examination and details of drug prescribed by the treating ophthalmologist were recorded in the study pro forma at baseline visit (visit 1). Follow-up visits were on day 3 (visit 2), day 7 (visit 3) and day 14 (visit 4) after administering the study drugs. A deviation of ±1 a day for the first follow-up and ±2 days for subsequent follow-up was accepted. At each follow-up visit data on concomitant medications, ocular symptoms and ocular signs using hyperaemia score [Table 2][9] graded by slit-lamp examination by the investigator and adverse events (AEs) were collected. In case of relapse, the patient was asked to visit OPD on Day 21. Medication compliance was assessed with the help of a medication compliance card. Safety of study medications was assessed by ADRs.
Table 2.
TOSS and hyperaemia score grading
| Grading of symptoms - TOSS score | |
|---|---|
| TOSS Score - Grading of symptoms (Itching, tearing, redness and swelling) | |
| 0 | Indicating no symptoms |
| 1+ | Mild symptoms of discomfort which were just noticeable |
| 2+ | Moderate discomfort noticed most of the day but did not interfere with daily activities |
| 3+ | Severe symptoms interfering with daily activities |
| Hyperaemia score - Grading of signs | |
| 0 - No | Normal |
| 0.5 - Trace | Inconsistent rose red hyperaemia |
| 1 - Mild | Reddish color |
| 2 - Moderate | Bright red color |
| 3 - Severe | Bright and intense diffuse hyperaemia |
Statistical analysis
The sample size was calculated at a confidence level of 95%, the sample size determined was 60 subjects in each treatment group. All data were analyzed by Microsoft Excel and Statistical Package for Social Sciences (SPSS version 26.0). Continuous variables are presented as mean ± standard deviations (SD's) and the categorical variables as percentages. Comparison of TOSS and adverse effect scores between and within group at different time points (baseline, days 1, 3, 7 and 14) was performed by ANOVA with repeated measure analysis and with Bonferroni corrections. The value of p < 0.05 were considered to be statistically significant.
Results
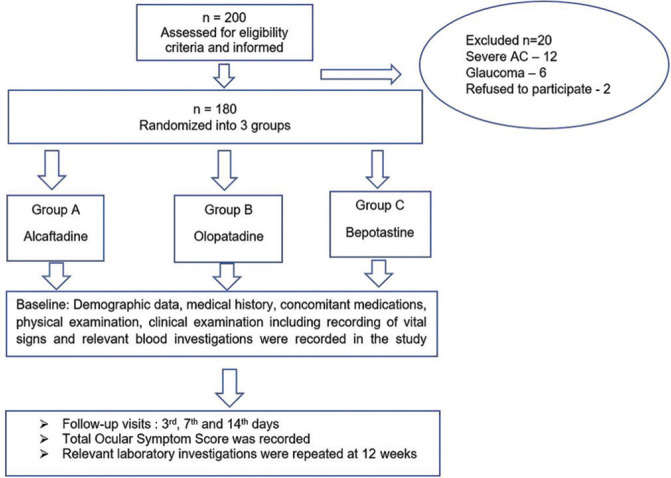
A total of 200 patients were screened for the study of whom 180 patients with mild or moderate allergic conjunctivitis, who met the required inclusion and exclusion criteria were enrolled in the study. The flow chart of recruitment, randomization, and follow-up is depicted in Fig. 1. Age, gender, and TOSS and hyperaemia scores were matched at baseline [Table 3]. Table 3 represents the demographic profile of the patients included in the study. Both the treatment groups were matched with respect to baseline demographic characteristics.
Figure 1.
Flowchart of recruitment, randomization and follow up
Table 3.
Baseline demographic characteristics
| Group A Alcaftadine (n=60) | Group B Olopatadine (n=60) | Group C Bepotastine (n=60) | P | |
|---|---|---|---|---|
| Age (years) (Mean±SD) | 28.66±9.12 | 28.66±9.12 | 29.01±8.92 | 0.25 |
| Gender - n (%) | 0.28 | |||
| Male | 38 (63.3%) | 32 (53.3%) | 45 (75%) | |
| Female | 22 (36.7%) | 28 (46.7%) | 25 (25%) | |
| Total Ocular Symptom Score (TOSS) | 7.68±2.32 | 7.65±2.32 | 7.45±2.27 | 0.8 |
The four major complaints recorded by patients were itching (60 patients, 100℅), redness (44 patients, 73%), tearing (48 patients, 80%), and swelling (20 patients, 33.3%). The total ocular symptom score (TOSS) showed a consistent decrease in subsequent visit in all the Groups and it was statistically significant, when compared from baseline to 14th day in all the groups (p = 0.0008) [Table 4 and Fig. 2]. The difference in mean TOSS between (Group A) Alcaftadine and (Group C) bepotastine treatment groups was observed at the third day of follow-up. This showed early relief of allergic conjunctivitis symptoms by bepotastine (4.8 ± 1.58) compared to Alcaftadine (mean (5.3 ± 1.59) and olopatadine (5.3 ± 1.58) but this was not statistically significant.
Table 4.
Total ocular symptom score at different visits
| Variable | Group A Alcaftadine (n=60) Mean (SD) | Group B Olopatadine (n=60) Mean (SD) | Group C Bepotastine (n=60) Mean (SD) | P* |
|---|---|---|---|---|
| Day 1 (Baseline) | 7.6 (2.32) | 7.6 (2.32) | 7.4 (2.27) | 0.8 |
| Day 3 | 5.3 (1.59) | 5.3 (1.58) | 4.8 (1.58) | 0.13 |
| Day 7 | 2.3 (1.04) | 2.4 (0.91) | 2.2 (1.04) | 0.33 |
| Day 14 | 0.2 (0.43) | 0.4 (0.56) | 0.1 (0.36) | 0.0008 |
*One-way ANOVA for significance
Figure 2.
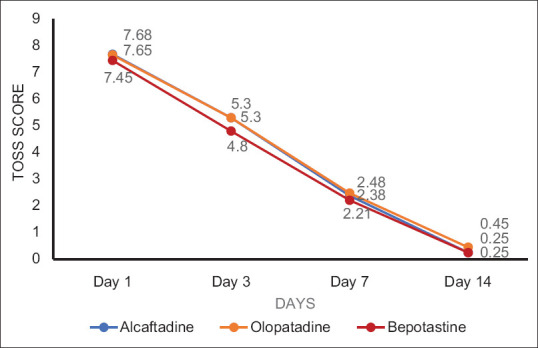
Graphical plot of total ocular symptom score (TOSS) at different follow-up
Total ocular symptom score at 14th-day visit with post hoc Tukey HSD test showed mean of Alcaftadine group vs mean of olopatadine group – p < 0.05, mean of olopatadine group vs mean of bepotastine group – p < 0.01, which were statistically significant whereas mean of Alcaftadine group vs mean of bepotastine group showed nonsignificant difference. Alcaftadine was found to be better than olopatadine in reducing the Allergic Conjunctivitis symptoms using TOSS score at 14th-day visit (p < 0.5). Although there is no significant difference between bepotastine and Alcaftadine groups, bepotastine showed a better reduction of symptoms compared to Olopatadine group using TOSS score at 14th-day visit (p < 0.1). Conjunctival hyperaemia had reduced in all the treatment groups but there was a significant reduction in Alcaftadine and Bepotastine treatment groups at 14th day compared to olopatadine group (p = 0.0037, ANOVA––post hoc Tukey's analysis) [Table 5 and Fig. 3]. No systemic or ocular serious adverse events were reported. Most common adverse events were burning sensation (3) in Alcaftadine group and taste impairment (3) in bepotastine group, followed by headache (2) in Alcaftadine group, dizziness (2) in olopatadine and mild redness (2) in bepotastine group were noted [Fig. 4]. No significant difference in the number of adverse events was noted among the three groups.
Table 5.
Conjunctival hyperaemia score at different visits
| Variable | Group A Alcaftadine (n=60) Mean (SD) | Group B Olopatadine (n=60) Mean (SD) | Group C Bepotastine (n=60) Mean (SD) | P* |
|---|---|---|---|---|
| Day 1 (Baseline) | 1.3 (0.88) | 1.4 (0.89) | 1.4 (0.83) | 0.7 |
| Day 3 | 0.8 (0.60) | 0.8 (0.60) | 0.8 (0.57) | 0.9 |
| Day 7 | 0.3 (0.28) | 0.3 (0.28) | 0.3 (0.28) | 0.8 |
| Day 14 | 0.008 (0.06) | 0.05 (0.15) | 0.008 (0.06) | 0.0037 |
*One-way ANOVA for significance
Figure 3.
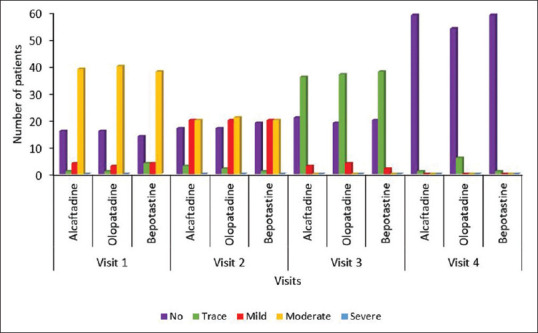
Graphical plot of hyperaemia degree at different visits
Figure 4.
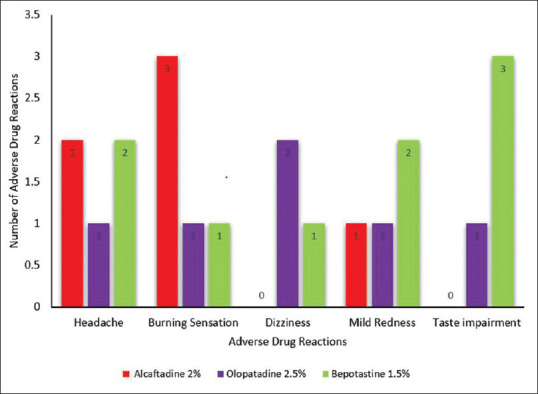
Adverse drug reactions of treatment groups
Discussion
Ocular allergy is a commonly encountered pathology in clinical practice, with an increase in the number of patients noticed in the last decade with a prevalence of approximately 40% of the population globally. Avoidance of allergens plays a key role in the prevention of allergic conjunctivitis. Addition of anti-histamine reduces inflammation, whereas mast cell stabilizers prevent mast cell degranulation on an exposure to allergens. Topical corticosteroids are the most potent agents to control inflammatory symptoms of allergic conjunctivitis but there is a risk of many side-effects.
Newer topical agents have both anti-histamine and mast cell stabilization action. Their use can control acute symptoms and prevent relapses.[10] This study is a double-blinded, observer masked, randomized study directly comparing the efficacy of three topical anti-allergic medications, that is, Alcaftadine, olopatadine, and bepotastine in mild-to-moderate allergic conjunctivitis. These topical agents are FDA approved for use in allergic conjunctivitis, but trials compared these three medications are limited.
A comparative study done by Dudeja I, et al. concluded Alcaftadine 0.25%, olopatadine 0.2%, and bepotastine 1.5% eye drops have been proved to be safe and well-tolerated topical medication for allergic conjunctivitis.[9] This study resounded the same, and the medications were found to be safe, with minimal transient side effects of burning sensation and taste impairment noticed by a few patients (more in group 1 and group 3, respectively). Most patients responded to treatment and were willing to continue the eye drop, if indicated.
The efficacy of these anti-allergic medications over placebo has been proven in a study conducted by Donshik et al. All three medications showed significant relief in symptoms of redness and itching, which was proved statistically.[11] This study showed that all three study medications provide significant relief in symptoms from baseline to 14 days.
A study done by Ackerman S, et al. compared 0.25% Alcaftadine and 0.2% olopatadine using conjunctival allergen challenge found Alcaftadine superior to olopatadine at the earliest time point (3 min post-challenge). Alcaftadine showed significant relief in chemosis at 16 and 24 h post-instillation.[3] Another study done by McLaurin EB, et al., with 284 subjects found that subjects treated with Alcaftadine had a lower overall mean itch score of 3, 5, and 7 min than those treated with olopatadine.[5] This study results also showed Alcaftadine is better in reducing the Allergic conjunctivitis symptoms compared to Olopatadine at 14th day, which is statistically significant (p = 0.0008).
A comparative study done by McCabe et al. showed Bepotastine provided better relief of ocular allergy symptoms and nonocular symptoms associated with Allergic conjunctivitis, that is, runny nose compared to olopatadine. The study also found that a higher percentage of patients preferred bepotastine over olopatadine for treatment.[8] The current study indicates a greater significant relief of Allergic conjunctivitis symptoms with Bepotastine besilate than olopatadine group at 14th day, which is statistically significant (p = 0.0008).
Trials have been conducted at a cellular level, animals treated with Olopatadine and Alcaftadine showed similar efficacy and safety profiles. One such study done by Ono SJ, et al. found a decrease in expression of the junctional protein, ZO-1, which is caused by allergen challenge with Alcaftadine compared to olopatadine. In addition, Alcaftadine showed significantly lower conjunctival eosinophil infiltration caused by allergen challenge in animal studies.[12]
Clinical trials, thus, have proved the efficacy of all three medications for relief of symptoms of allergic conjunctivitis and found differences between medications in one or the other parameter. In our study, all three medications are effective in control of allergy symptoms with bepotastine group and Alcaftadine groups showing statistical significance as compared to olopatadine group in alleviating the allergic conjunctivitis symptoms.
Strengths
Three medications with standard doses were compared with an adequate sample size in a single randomized study. Randomization, blinding of the patients and evaluation of the effect of the study medications on clinical assessment of signs and symptoms provided an evidence-based option which was safe and effective.
Limitations
Since our study was conducted in a single Centre, the results cannot be compared with studies conducted in multicentered large subset study populations. Comparison of efficacy and safety of study medications could not be studied in patients with Severe Allergic Conjunctivitis as they were excluded from the study.
Conclusion
Newer antiallergic medications with combined anti-histamine and mast cell stabilization action can help reducing the use of topical steroids for a milder form of disease. All three study medications are safe and effective topical treatment modality for allergic conjunctivitis, whereas Bepotastine besilate and Alcaftadine groups appear to be better than the olopatadine group in reducing symptoms of Allergic Conjunctivitis. Conjunctival hyperaemia had reduced in all the treatment groups but there was a significant reduction in Alcaftadine and bepotastine treatment groups at the final visit compared to the olopatadine group.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Authors would like to thank Dr Praveen Panchaksharimath, Dr Bhavya Darshini M, Dr Kiran Warrier and Dr Akila K for their constant help and support for the project.
References
- 1.Gong L, Sun X, Qu J, Wang L, Zhang M, Zhang H, et al. Loteprednol etabonate suspension 0.2% administered QID compared with olopatadine solution 0.1% administered BID in the treatment of seasonal allergic conjunctivitis: A multicenter, randomized, investigator-masked, parallel group study in Chinese patients. Clin Ther. 2012;34:1259–1272. doi: 10.1016/j.clinthera.2012.04.024. e1. [DOI] [PubMed] [Google Scholar]
- 2.Chigbu DI, Coyne AM. Update and clinical utility of Alcaftadine ophthalmic solution 0.25% in the treatment of allergic conjunctivitis. Clin Ophthalmol. 2015;9:1215–25. doi: 10.2147/OPTH.S63790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackerman S, D'Ambrosio F, Greiner JV, Villanueva L, Ciolino JB, Hollander DA. multicenter evaluation of the efficacy and duration of action of Alcaftadine 0.25% and olopatadine 0.2% in the conjunctival allergen challenge model. J Asthma Allergy. 2013;6:43–52. doi: 10.2147/JAA.S38671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borazan M, Karalezli A, Akova YA, Akman A, Kiyici H, Erbek SS. Efficacy of olopatadine HCI 0.1%, ketotifen fumarate 0.025%, epinastine HCI 0.05%, emedastine 0.05% and fluorometholone acetate 0.1% ophthalmic solutions for seasonal allergic conjunctivitis: A placebo-controlled environmental trial. Acta Ophthalmol. 2009;87:549–54. doi: 10.1111/j.1755-3768.2008.01265.x. [DOI] [PubMed] [Google Scholar]
- 5.McLaurin EB, Marisco NP, Ackerman SL, Ciolono JB, Williams JM, Villanueva L, et al. Ocular itch relief with Alcaftadine 0.25% versus olopatadine 0.2% in allergic conjunctivitis: Pooled analysis of two multicentre randomized clinical trials. Adv Ther. 2014;31:1059–71. doi: 10.1007/s12325-014-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallois-Bernos AC, Thurmond RL. Alcaftadine, a new antihistamine with combined antagonistic activity at histamine H1, H2 and H4 receptors. J Receptor Ligand Channel Res. 2012;5:9–20. [Google Scholar]
- 7.Meena MK, Gupta V. Alcaftadine 0.25% versus olopatadine 0.2% in allergic conjunctivitis. Int J Sci Res. 2018;7:53–5. [Google Scholar]
- 8.McCabe CF, McCabe SE. Comparative efficacy of Bepotastine besilate 1.5% ophthalmic solution versus Olopatadine hydrochloride 0.2% ophthalmic solution evaluated by patient preference. Clin Ophthalmol. 2012;6:1731–8. doi: 10.2147/OPTH.S35431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudeja L, Janakiraman A, Dudeja I, Sane K, Babu M. Observer-masked trial comparing efficacy of topical olopatadine (0.1%), bepotastine (1.5%), and Alcaftadine (0.25%) in mild to moderate allergic conjunctivitis. Indian J Ophthalmol. 2019;67:1400–4. doi: 10.4103/ijo.IJO_2112_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra GP, Tamboli V, Jawla J, Mitra AK. Recent patents and emerging therapeutics in the treatment of allergic conjunctivitis. Recent Pat Inflamm Allergy Drug Discov. 2011;5:26–36. doi: 10.2174/187221311794474883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donshik PC, Pearlman D, Pinnas J, Raizman MB, Tauber J, Tinkelman D, et al. Efficacy and safety of ketorolac tromethamine 0.5% andlevocabastine 0.05%: A multicentre comparison in patients with seasonalallergic conjunctivitis. Adv Ther. 2000;17:94–102. doi: 10.1007/BF02854842. [DOI] [PubMed] [Google Scholar]
- 12.Ono SJ, Lane K. Comparison of effects of Alcaftadine and olopatadine on conjunctival epithelium and eosinophil recruitment in a murine model of allergic conjunctivitis. Drug Des Devel Ther. 2011;5:77–84. doi: 10.2147/DDDT.S15788. [DOI] [PMC free article] [PubMed] [Google Scholar]