Abstract
Keratoconus is an ectatic corneal disease characterized by progressive stromal thinning, irregular astigmatism, and defective vision. It can be unilateral or bilateral with asymmetric presentation. It starts at puberty and either progresses rapidly to an advanced stage of the disease or stops in case of delayed onset and slow progression. Pediatric keratoconus is more aggressive than in adults and the management protocols differ because of various rationales such as accelerated progression, advanced stage of disease at the time of diagnosis and co-morbidities. It poses a burden to the society as it affects the quality of life, social, and educational development in children. Hence early diagnosis, recognition of progression, and timely intervention with collagen crosslinking is imperative to arrest the worsening. Association with systemic syndromes and ocular comorbidities can be of concern in pediatric keratoconus. Severe ocular allergy when associated hastens progress and complicates timely intervention of crosslinking treatment and compliance to contact lens wear. Keratoplasty in pediatric keratoconus has good outcomes but can encounter frequent suture-related concerns. This article discusses the epidemiology, etiopathogenesis, clinical challenges, and current perspectives of management of pediatric keratoconus.
Keywords: Allergy, atopy, corneal collagen crosslinking, DALK, genetics, keratoconus, pediatric, penetrating keratoplasty, vernal keratoconjunctivitis
Pediatric keratoconus (KC) is the corneal ectasia occurring in children less than 18 years or in adolescence between 10 and 19 years of age, though the disease can manifest in any age.[1]
KC in children [Fig. 1] exhibits several unique clinical features such as faster disease progression and severe visual impairment at the time of diagnosis creating a negative impact on their quality of life. This review provides an update on epidemiology, prevalence, clinical challenges, collagen crosslinking treatment and keratoplasty challenges and outcomes in pediatric keratoconus.
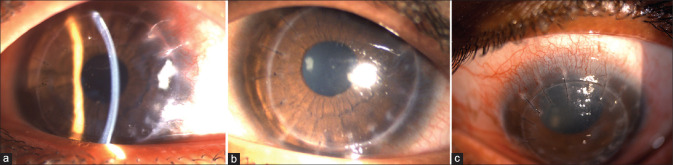
Figure 1.
Clinical picture of pediatric keratoconus showing (a) Munson's sign, (b and c) apical scar, and (d) advanced ectasia
Epidemiology
The prevalence of KC in the general population is 1 in 2000.[2] According to the Intelligence Research in Sight Registry of AAO, the prevalence of KC in pediatric population is 0.16%.[3] KC affects Indians, Pakistanis, Arabs, and Polynesians 4.4 times more than the Caucasians.[4] The prevalence rates of keratoconus exhibits a geographical variation. The prevalence of pediatric KC is 1.1% in Riyadh[5] and 4.79% in Saudi Arabia.[6] This difference in the incidence of KC [Table 1][7] is due to its variation in genetic, environmental factors, nutrition, and study tools employed in their studies. There is no clear evidence in the literature indicating a gender difference. The CLEK study[8] reported a mean age at diagnosis of 27.3 ± 9.5 years (10 to 39 years) with about 90% of patients being diagnosed by the age of 10 years. The youngest age of KC documented in the literature is a four-year-old girl with Down's syndrome.[9]
Table 1.
Incidence of KC in different countries
| Countries | Prevalence of Keratoconus |
|---|---|
| Russia | 0.3 per lakh |
| India | 2,300 per lakh |
| United states | 54.5 per lakh |
| Iran | 2,300 per lakh |
| Columbia | 3,900 per lakh |
A younger age of onset of KC tends to be associated with rapid progression and more advanced stage of disease at diagnosis.[10] Leoni-Mesplie et al.[11] report that 27.8% of patients were below 15 years with stage 4 KC (Amsler Krumeich classification) as against 7.8% with age more than 27 years at the time of diagnosis with male predominance. Chatzis et al.[12] observed KC progression in 88% of children by one year of diagnosis mandating an early advocation of CXL treatment in these pediatric eyes.
Etiopathogenesis
The pathophysiology of keratoconus is poorly understood. Keratoconus is a multifactorial disorder which involves genetic, metabolic, and environmental factors.[10] The time-honoured definition of keratoconus states that the condition is noninflammatory.[13] Current evidence, however, suggests a significant role for the inflammatory mechanisms.[11,13] Altered dynamics of pro- and anti-inflammatory cytokines is perhaps responsible for activating metalloproteinases and promoting apoptosis of keratocytes.[11,13] The end result is a reduction in collagen cross-linking, resulting in altered corneal rigidity or biomechanical strength.[14]
Causative factors
The various causative factors implicated in the pathogenesis of KC[15] include the following:
1) Genetic factors
2) Environmental factors
3) Role of inflammation
4) Role of enzymes
5) Role of oxidative stress
6) Role of hormones
-
Genetic Factors
KC has a genetically heterogenous distribution. It has been reported to be inherited as autosomal recessive, autosomal dominant, and sporadic[16] with 10% (range: 5-27.9%) of patients diagnosed with pediatric KC having a family history.[17] The evidences in support of the genetic basis of KC [Flowchart 1] include the following:
- a) Prevalence of KC in first-degree relatives (20.5%)[18]
- b) Twin studies in KC that indicate monozygotic twins to have higher concordance than dizygotic twins, with a greater similarity of phenotype[19]
- c) Associated genetic syndromes are Down's syndrome, Marfan's syndrome, osteogenesis imperfecta, Apert syndrome, Ehlers Danlos syndrome, Leber's congenital amaurosis[18]
The genes and mutations associated with keratoconus are elaborated in Table 2.[16,17]
-
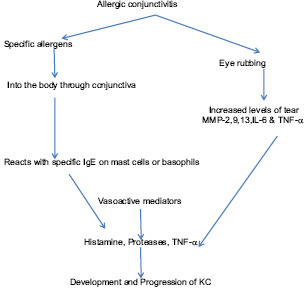
Environmental Factors
About 40% of children are affected by systemic allergies.[20] In the CLEK study,[8] 52.9% of patients with KC had hay fever or allergies, 14.9% had asthma, 8.4% had hatopic dermatitis and 27% had vernal keratoconjunctivitis (VKC), and 40% had abnormal topography. VKC is the most common associated ocular allergy with KC.[21] The complex mechanism of development of KC in VKC is due to the exposure to allergen, eye rubbing [Flow Chart 2], and the resultant microtrauma to epithelium[22] that induces increase in the levels of inflammatory mediators [such as matrix metalloproteinases (MMP-1), IL-6, and TNF-α] from both the epithelial and stromal cells, with IL-6 and TNF-α, upregulating MMP-1.[23,24] The pathogenesis [Flow Chart 3] involves the release of specific allergens into the body through conjunctiva, which react with specific IgE on mast cells or basophils. The released vasoactive mediators such as histamine, proteases, TNF-α, and interleukins seem to induce the development and progression of KC.[23] The eye rubbing in KC has been shown to increase MMP-13 in tears that have an important role in the apoptotic activity of the keratocytes.[20,21,22] Keratocyte apoptosis leads to stromal volume loss following degradation of extracellular matrix resulting in thinning and ectasia.[21]
There also seems to be a higher risk of acute hydrops (40%) in these eyes as compared to primary KC eyes (2.6%).[25] Early topography evaluations are essential in these eyes as higher corneal elevation, thinner pachymetry, and increased frequency of KC topography patterns are detected in these corneas as compared to normal.[26,27] Rubbing of eyes for 15 seconds results in 18.4% reduction in central and midperipheral corneal epithelial thickness which explains the association of chronic rubbing and most common site of cone formation (paracentral and inferonasal). Contact lens–induced allergies and eye rubbing can cause KC in genetically predisposed individuals.[28,29]
-
Role of Inflammation
Increased levels of IL-6, IL-1β, TNF-α, TNF-γ and reduced levels of IL-10 are noted in keratoconus eyes. The increased levels of gelatinases, collagenases, metalloproteinases, proteases, cytokines, TNF-α and TNF-β have been associated with progressive KC.[22,23] This upregulation of cytokines and proteases is perhaps due to the underexpression of lactoferrin, an antimicrobial and anti-inflammatory protein.[24] Enzyme-linked immunosorbent assay (ELISA) analysis of tears in keratoconus patients has shown the elevated levels of inflammatory markers IL-6, TNF-α, and MMP-9.[29] The tear biomarkers identified in KC are listed in Table 3.[30]
-
Role of Enzymes
MMP-9 is a gelatinase that has been found responsible for the degradation of denatured collagen fibrils.[31] Immunohistochemistry labelling has identified many biomarkers responsible for keratoconus.[32] Several studies have observed an upregulation of many MMPs, such as MMP-14, MMP-1, MMP-7, and MMP-2, that cause degradation of fibronectin, membrane glycoprotein, and type I and II collagen.[31]
Lysyl oxidase (LOX) belongs to the family of amino oxidases also known as lysine-6-oxidase, one of 5 LOX family members, located on chromosome 5q23.2.[32,33] LOX oxidizes peptide lysine and hydroxyl lysine residues in collagen to peptidyl alpha amino adipic delta semialdehyde, which spontaneously combines with vicinal peptidyl aldehydes and forms covalent cross linkages between collagen and elastin fibres.[32] The lower expression of LOXmRNA is significantly associated with loss of cohesion between collagen fibrils and corneal ectasia.[33] Copper deficiency has also been hypothesized to be associated with lower LOX activity that causes KC.[34]
-
Oxidative Stress
The antioxidants protecting ocular tissues against damage are superoxide dismutase enzymes (SOD), low molecular weight antioxidants such as ascorbic acid, ferritin, glutathione, high molecular weight antioxidants, catalase, and glutathione peroxidase.[35] The concept of oxidative stress has been related to KC with oxidative stress index said to be an indicator for KC progression.[35] The imbalance between the formation of free radicals and their removal by antioxidants results in the accumulation of aldehydes, peroxy-nitrites, causing the destruction of tissues. IL-1 that is released in tears following eye rubbing also inhibits the synthesis of SOD.[36]
-
Role of Hormones
The role of sex hormones and thyroid hormones in the pathogenesis of KC has been supported by the following explanations.[37,38,39]
- 1) Onset of disease at puberty following changes in hormonal levels
- 2) Progression of corneal ectasia during pregnancy
- 3) Presence of estrogen and progesterone receptors is high in children with vernal keratoconjunctivitis
- 4) Corneal hysteresis and corneal resistance factor measured with ocular response analyser has been noted to decrease during the different stages of menstrual cycles
- 5) Corneal thickness is found to be increased during ovulation
- 6) Higher levels of estrogen mRNA levels in tears
- 7) Reports of progressive KC after infertility treatment
- 8) T4 (Thyroxine) receptors are found in lacrimal gland. The T4 levels have been found to be elevated in tears of patients with KC.
Flow Chart 1.
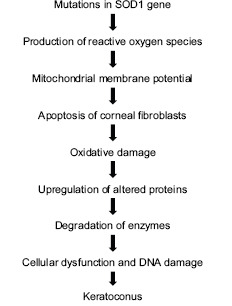
Genetic mechanism involved in Keratoconus
Table 2.
Genetic mutations involved in keratoconus
| GENE | CHROMOSOME | VARIATIONS | DISEASES |
|---|---|---|---|
| SOD1 (Superoxide Dismutase 1) | 21q22 | 7-base deletion intron-2 | KC |
| ZNF469 (Zinc Finger Protein 469) | 16q42 | Frame shift mutations/Missense mutations | KC Brittle cornea syndrome type 1 |
| PRMD5 (PR/SET Domain 5) | PR domain containing protein 5 | Homozygous mutations linked with ZNF469 | Brittle cornea syndrome type 2 |
| TGFBI (Transforming Growth Factor Beta) | 5q31 encodes big-h3 | c. 1603G4T mutation located in exon12 | Corneal dystrophies KC |
| DOCK9 (Dedicator of cytokinesis 9) | Located in 13q32 along with IPO5 (importin5, OMIM 602008) and STK24 (serine/ threonine kinase 24, OMIM 604984) | Mutations | strong candidate gene for keratoconus |
| MiRI184 (micro RNA) | 13q32 | mutations altering the miRI (184) seed region | KC & congenital anterior polar cataract |
| VSX1 (Visual System Homeobox 1) | 20p11-q11 | Involved in keratocytes-fibroblastic transformation | KC |
Flow Chart 2.
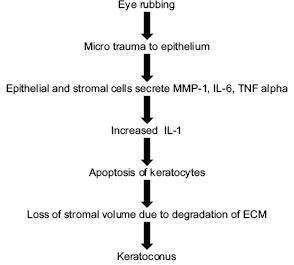
Pathogenesis of eye rubbing causing Keratoconus
Flow Chart 3.
Pathogenesis of keratoconus in ocular allergies
Table 3.
Tear biomarkers in Keratoconus
| Protein | Expression |
|---|---|
| BY ELISA ANALYSIS | |
| IL-6 | Increased |
| TNF-ALPHA | Increased |
| MMP-9 | Increased |
| MMPs-1,3,7,13 | Increased |
| IL-4,5,6,8 | Increased |
| TNF-alpha, beta | Increased |
| SFRP1 | Decreased |
| BY LCMS ANALYSIS (Liquid chromatographytandem Mass spectrometry) | |
| SCGB2A1 | Increased |
| MMP1 | Increased |
| AZGP1 | Decreased |
| LTF | Decreased |
| CYSTATINS | Decreased |
| LCN | Increased |
| AZGP1 | Increased |
| PIP | Increased |
Ocular and Systemic Associations
A number of ocular and systemic disorders are associated with pediatric keratoconus Table 4.[16,17]
Table 4.
Ocular and Systemic conditions associated with paediatric KC
| Ocular Conditions | Systemic Conditions |
|---|---|
| Atopy | Apert syndrome |
| Aniridia | Atopy |
| Blue sclera | Crouzon’s syndrome |
| Congenital cataracts | Down’s syndrome |
| Ectopia lentis | Ehlers Danlos Syndrome |
| Microcornea | False chordae tendinae |
| Leber’s congenital amaurosis | Leber’s congenital amaurosis |
| Retinitis pigmentosa | Marfan’s syndrome |
| Retinopathy of prematurity | Mitral valve prolapse |
| Vernal keratoconjunctivitis | Noonan’s syndrome |
| Fuchs dystrophy | Osteogenesis imperfecta |
| Posterior polymorphous dystrophy | Raynaud’s syndrome |
| Syndactyly | |
| Xeroderma pigmentosa |
Ocular associations
Various ocular associations with KC include, blue sclera, ectopia lentis, cataract, retinal detachment, retinitis pigmentosa, macular coloboma, Leber's congenital amaurosis, aniridia, spring catarrh, and atopic keratoconjunctivitis.[39]
Leber's congenital amaurosis (LCA),[40,41] shows a higher association with KC, the identified gene loci in LCA being 17p13. Other than genetic association, the oculo-digital sign or the Franceschetti sign comprising of eye poking, pressing, and rubbing is also responsible for KC development. The incidence of KC is high in the age group of 15–45 years (30%) as compared to children <14 years (2%) with LCA.[42]
Systemic disorders
Among the genetic disorders, Down's syndrome has the highest association with keratoconus, with reported incidence ranging from 0.5 to 15% (10–300 times more common than in the general population).[43]
Multiple factors have been implicated in the pathogenesis of KC in Down's syndrome such as genetic predisposition, dryness, blepharitis, persistent styes, infections, punctal agenesis, psychogenesis, mental stress, emotional stress, and blepharoptosis predisposing to eye rubbing and KC.[42] Delay in the diagnosis of KC in Down syndrome children is due to the unnoticed change in vision by parents and due to the thin cornea due to which CXL could not be done.
Among connective tissue disorders the most commonly associated is Ehlers Danlos syndrome with KC patients displaying joint hypermobility in comparison to age-matched controls (12%).[44,45] Mutations in COL5A1 or COL5A2, that codes for Type V collagen - α1 and α-2, are responsible for ocular abnormalities (eyelid and conjunctival abnormalities, keratoglobus, corneal thinning, keratoconus, dry eyes, pathologic myopias, angiod streaks, retinal detachment, scleral atrophy, and globe perforation). Collagen V accounts for 10%–20% of the total collagen in cornea.
Histopathology
Histopathological abnormalities have been documented in every layer of the KC cornea.[46] Immunohistochemical studies reveal characteristic histological findings such as thinned out epithelium with irregular loss of basal cells and presence of apoptotic cells over the cone. Breaks and fibrillations are seen in Bowman's layer. Stroma shows altered orientation of collagen fibrils and loss of lamellae. Increased visibility of nerve fibers is due to corneal thinning. The architecture of sub basal nerve plexus are altered with fragmentations and reduced density. Localized thickening of nerve fibers with wrapping of anterior keratocytes around the nerve is also a characteristic finding in KC. Keratocytes are fewer in number due to apoptosis. DM ruptures and folds are also seen in KC. Endothelium is normal, but may have intracellular dark structures, pleomorphism, and elongation of cells in few cases.
Clinical Concerns in Pediatric Keratoconus
Although KC in children differs from adults in few distinct clinical features, particularly time of presentation, rate of progression of the disease and treatment protocols, children share most of the common signs and symptoms of adult KC. The symptoms include, mild blurring of vision, which improves on squeezing of both eyelids to improve the precision of visual acuity, polyopia, distorted images and defective vision. Clinical signs of pediatric KC[37] are tabulated [Table 5]. The difference of pediatric KC from adult onset KC is elaborated in Table 6.[17,21,39] In contrast to adult KC, pediatric KC tends to present early, usually in the latter part of first decade of life, a positive family history, systemic syndromic association, ocular allergy and atopy with presence of significant eye rubbing, more significant rapid progression, and the presence of some amount of amblyopia resulting from the early onset of the disease causing visual deprivation, especially in cases with asymmetrical involvement or unilateral presentation.[47] Sometimes acute corneal hydrops may be the presenting feature in children, with the KC going unnoticed earlier [Fig. 2]. Reliable clinical diagnostic testing also becomes a difficulty in pediatric cases. Pediatric keratoconus occurring in patients with severe allergic keratoconjunctivitis present with higher association of ocular co-morbidities such as corneal scaring [Fig. 3], steroid induced cataract and glaucoma.
Table 5.
Clinical signs of keratoconus
| Clinical sign of KC | Clinical Details |
|---|---|
| External signs | Munson’s sign |
| Rizzotti’s sign | |
| Slit lamp findings | Stromal thinning |
| Posterior stress lines (Vogt’s) | |
| Iron ring (Fleischer’s ring) | |
| Epithelial/Sub epithelial scarring | |
| Retroillumination signs | Scissoring reflex |
| Oil droplet sign (Charleaux sign) | |
| Photokeratoscopy signs | Compression of mires inferotemporally (Egg shaped) |
| Compression of mires inferiorly and centrally | |
| Videokeratoscopy signs | Localised increase in surface corneal power |
| Inferior-superior asymmetry | |
| Skewing of steepest radial axes above and below horizontal meridian |
Table 6.
Differences between pediatric and adult keratoconus
| Paediatric Keratoconus | Adult keratoconus |
|---|---|
| Diagnosis of PKC is late due to scarcity of symptoms | Adult onset KC presents earlier than PKC |
| The Progression of PKC is explosive in nature, because the biochemical rigidity is inversely proportional to age | The progression is slower and stabilises after third or fourth decade |
| PKC patients are commonly associated with systemic and ocular diseases | Comparatively lower incidence of systemic and ocular associations |
| PKC usually associated with 8.8% to 36% of VKC in KC | Adult KC is usually associated with hormonal disturbances |
| PKP in Advanced PKC has poor prognosis | Comparatively better prognosis |
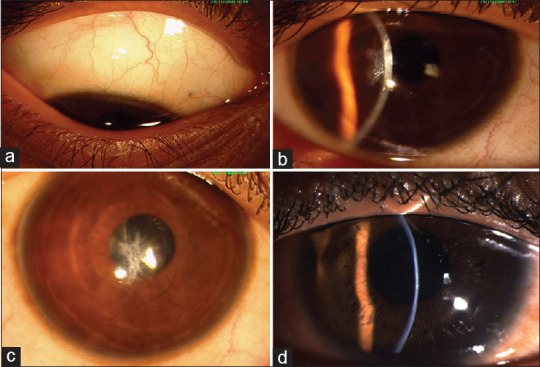
Figure 2.
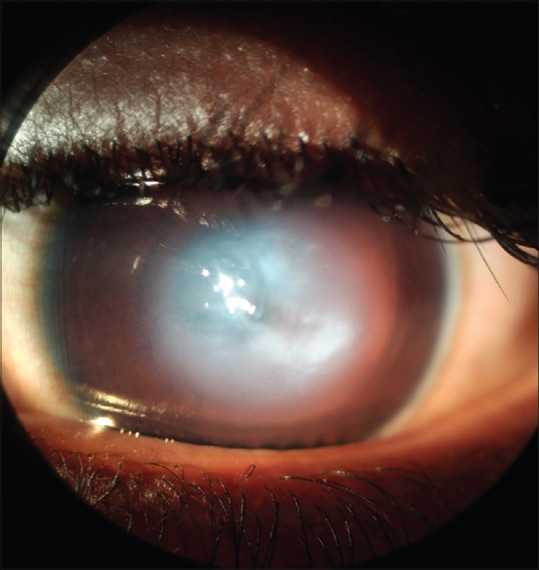
Acute hydrops in a case of pediatric keratoconus
Figure 3.
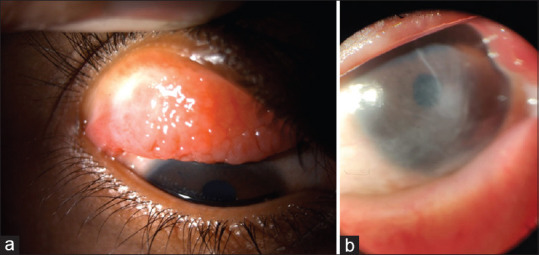
Clinical picture of GPC (a) and corneal opacity (b) in two different cases of pediatric keratoconus with vernal keratoconjunctivitis
Blurring of vision and polyopia are due to the ocular aberrations caused by irregular astigmatism in KC. It is compensated by the natural anatomy, the physiological optics of internal ocular structures, and the high accommodative power in most of the children.[48] Another hypothesis is that the irregular astigmatism is less due to centrally placed cone in children. Late presentation with corneal scarring requires early corneal transplantation.[49] The rate of progression of ectasia tends to be more aggressive in children compared to adult due to higher rate of corneal collagen remodelling and associated ocular allergies.[50] They are also at high risk of developing acute hydrops probably due to eye rubbing.
Progressive KC is defined by an increase of one or more dioptre of K-max over a period of 12 months.[51] The association of VKC, atopy, and eye rubbing in children are the risk factors for rapid progression.[20,21] Control of allergies with topical medications and early diagnosis of KC with topography is challenging in them. Topography imaging in children is challenging with poor repeatability of the testing, poor cooperation, and ability to focus, with decentration and shadows negatively affecting the image capture. Children with ocular allergy are also poor candidates for reliable topography imaging on serial follow-ups. Apart from ocular allergy, associated co-morbidities such as secondary glaucoma and cataract are to be managed. Counselling and education of the parents for a long-term follow-up, control of allergy with regular medications, and avoidance of eye rubbing remain crucial in successful rehabilitation of pediatric KC patients.
The Corneal Ectasia preferred Practice Pattern and the Pediatric Eye Evaluations Preferred Practice pattern of AAO recommend early screening and treatment in pediatric patients due to poor visual outcomes of unmanaged KC.[3,52]
Mass screening for KC is recommended for children with the following risk factors, for early diagnosis and treatment:
Allergic eye disease
Children living in high altitude/high UV exposure
Geographic areas with high incidence of KC
Children with Down's syndrome
Family history of KC
School children with myopia and myopic astigmatism.
The screening tools include retinoscopy, VKG, corneal tomography, wave-front aberrometers, ocular response analyzer, and ASOCT.
Differential diagnosis
Keratoconus has to be distinguished from other ectatic disorders such as pellucid marginal corneal degeneration disorder (PMCD), Terrein marginal corneal degeneration (TMCD), Brittle cornea syndrome and keratoglobus (KG).[53] PMCD and TMCD are common adult onset ectatic disorders, rarely occurring in children and present with distinct clinical features. KG is a congenital true ectatic disorder of cornea that needs to be differentiated from pediatric KC, since management and prognosis is different in both these conditions [Table 7].[51] Brittle cornea syndrome (BCS) is a rare autosomal recessive disorder characterized by deafness, joint hypermobility, and ocular features such as extreme corneal thinning, blue sclera, keratoconus, keratoglobus, and high myopia usually presents with recurrent spontaneous perforations [Fig. 4]. It is imperative to identify this condition early and manage with protective spectacles to prevent irreversible blindness.[54]
Table 7.
Differences between keratoglobus and keratoconus
| Keratoglobus (KG) | Keratoconus (KC) |
|---|---|
| Entire cornea is thinned out in KG | Central and paracentral thinning occurs in KC |
| Bilateral, presents since birth | Bilateral or Unilateral, presents at any age after birth usually in second or third decade |
| Non- Progressive disorder No association with Down’s syndrome commonly |
Progressive condition KC is common in Down’s syndrome children (incidence is 5.5% - 15%) |
| Associated with autosomal recessive pattern of inheritance | Usually isolated condition |
| Prone for rupture following trivial trauma | Corneal rupture is uncommon in KC |
| Optical rehabilitation with glasses is usually indicated in KG | Contact lens rehabilitation can be offered for visual rehabilitation in KC patients |
| Keratoplasty carries poor prognosis due to proximity of limbus. Recommended surgical techniques such as Tuck-in lamellar keratoplasty (TILK) are more demanding | Keratoplasty and DALK procedures carry the good prognosis in advanced cases |
Figure 4.
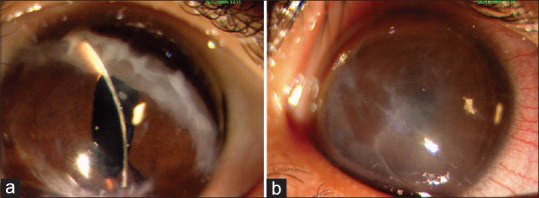
Slit lamp photograph of a case of Brittle cornea syndrome bilateral keratoglobus with multiple repaired corneal tears (a) and healed hydrops (b)
Management Protocol
Management of pediatric keratoconus centres on visual rehabilitation with contact lenses, rigid gas permeable in early cases, and multicurve contact lenses in moderate cases and corneo-scleral/scleral contact lenses in advanced cases. Spectacle correction often does not give best results except in early cases due to the irregular astigmatism. Treatment advice should highlight the importance of avoidance of eye rubbing, continued topical antiallergic therapy (in indicated cases), and frequent follow-up for topographic imaging to monitor progress. In pediatric keratoconus, several physicians prefer to offer early collagen crosslinking therapy in view of the aggressive nature of the disease.[55] There needs to be a cautious approach in view of the post crosslinking concerns related to sterile infiltrates and infectious keratitis in pediatric KC cases. Active ocular allergy needs to be well controlled before considering crosslinking treatment in pediatric KC cases associated with allergic keratoconjunctivitis. Early surgical intervention may be essential in cases with advance ectasia and incompatibility to contact lens rehabilitation due to ocular allergy.
Collagen crosslinking
Collagen crosslinking (CXL) is a technique that uses ultraviolet A (UV-A) light and riboflavin (photosensitizer, vitamin B2). The goal of therapeutic collagen cross-linking treatment of the cornea increases the biomechanical rigidity of the cornea and arrests the progression of the ectasia.
Riboflavin is non-toxic, precursor of various co-enzymes, that increases the absorption of UV-A by the corneal stroma [Table 8]. When exposed to UV-A the resulting excitation of riboflavin generates reactive oxygen such as singlet oxygen and superoxide anions. These react with available groups with the generation of additional chemical bonds between amino acid residues.[56,57] This increases cross-linking between proteoglycans and collagen[54] with the resultant photopolymerization of collagen fibrils improving biomechanical properties. Both Young's modulus and corneal rigidity increase after collagen cross-linking.[56] Histopathologically, there is an increase in the diameter of the collagen fibrils.
Table 8.
Collagen Crosslinking Protocols
| Procedure | Riboflavin Concentration | Riboflavin saturation | UV-A application | Fluence |
|---|---|---|---|---|
| Standard Dresden Protocol |
Iso osmolar 0.1% solution (10 mg riboflavin-5-phosphate in 20% dextran) Ricrolin | 30 mins (every 2 min for 30 mins) | 30 mins riboflavin (every 2 mins for 30 mins) | 3 mw/cm2 5.4 J/cm2 |
| Accelerated protocols epi-off | Ricrolin | 10-30 mins every 2 mins | 30 mw/cm2, 3 mins | 5.4 J/cm2 |
| Trans epithelial | 0.1% riboflavin in 15%dextran with trometamol (Ricrolin+) | 10 mins | 30 mins | 3 mw/cm |
| Transepithelial iontophoresis assisted CXL | 0.1% riboflavin, no dextran, trometamol, EDTA | 10 mins | 30 mins | 3 mw/cm |
| Hypotonic | 0.5% riboflavin No dextran | 30 mins | 30 mins | 3 mw/cm |
The original protocol was based on the removal of epithelium followed by the application of riboflavin and then ultraviolet light in a sequential manner (Dresden protocol).[57] Long-term stabilization of KC in the “epi-off” procedure is well-documented both in adult & pediatric KC.[57] However, the alternate method of achieving the same result without the removal of the epithelium; the “epi-on” technique, cites potential advantages such as reduced post-operative pain, reduced risk of infection, and the potential to perform the procedure as an outpatient technique. Moreover, de-epithelialization has potential risks such as corneal haze, 2.8%[57] ulceration, infection, reactivation of herpes keratitis, and sterile infiltrates.
However, there exist many pertinent issues regarding the efficacy of epi-on cross-linking. The epithelial barrier function restricts the entry of substances with a molar mass greater than 100 g/mole. Riboflavin has a molar mass of 376 g/mole. It cannot penetrate the tight junctions of the epithelium. Moreover, while riboflavin is hydrophilic the epithelium is lipophilic. The riboflavin in the epithelium can also absorb the UV-A, thus decreasing the actual UV-A power within the stroma. The concentration of riboflavin is therefore uneven in the stroma. Oxygen is an important component of the crosslinking process and an intact epithelium restricts the entry of oxygen. In the epi-on procedure, 0.1% riboflavin in 15% dextran solution is supplemented with trometamol and EDTA (Ricrolin-TE), whereas in the epi-off procedure iso-osmolar 0.1% solution (10 mg riboflavin-5-phosphate in 20% dextran) (Ricrolin; Sooft, Montegiorgio, Italy) is used. In both procedures, riboflavin loading is done for 30 minutes followed by the application of UVA 370 nm at 3 mW/cm2 for 30 minutes.[58,59,60,61]
A number of studies have documented the efficacy of epi-off in pediatric KC. On follow-up of 77[58] and 152[59] patients (10 – 18 years) for a period of 36 and 48 months, respectively (Siena protocol) in two studies, Caporossi et al. noted visual improvement in 80% of patients and[58] 90% stabilization achieved in four years. Zotta et al.'s long-term results of 20 eyes of (14.34 ± 2.14 years) followed for a period of 89 months noted that K1, K2 and the topographic cylinder remained stable at 7.5 years.[62] Vinciguerra et al.[63] in a prospective, interventional study of 40 patients (mean age 14.2 ± 1.7 years) over two years follow-up noted that the significant improvement in both UDVA and CDVA with both K1 and K2 decreasing with the reduction in K-max being more significant. Significant reduction in total, corneal, higher-order, and astigmatic wave-front aberrations were also observed. The epi-off procedure also seems to be effective in steep corneas. In a study of 43 pediatric patients, 25 eyes having K-max values of 60 dioptres (D) or greater showed significant K-max reduction from 64.94 ± 4.99D to 62.25 ± 4.42D at 2 years follow-up.[64]
Variable results of transepithelial collagen cross-linking for pediatric KC has been reported. Caporossi et al. noted the stability in 24 patients who underwent transepithelial crosslinking (TE-CXL) to last only for a year.[65] In contrast, Salman's excellent results over a 12-month period with reduction in K-max of 2.3D and flattening of the anterior elevation as well as the increased stability of the cornea[66] prompted him to recommend epi-on procedure in the pediatric age group. Buzzonetti in a study of efficacy of TE-CXL in the pediatric age group (mean age 14.4 ± 3.7 years) spanning 18 months noted worsening of K-max showing that it does not effectively halt the KC progression compared to standard CXL.[67]
The general consensus is that the epi-on procedure is of doubtful; it does not appear to stop the progression of KC. The procedure may be considered in uncooperative patients and when pachymetry values are less than 400 microns. However, given some of the stated advantages, efforts have been made to improve the penetration of riboflavin by various innovations such cross-hatched grid pattern for epithelial debridement, benzalkonium (BAK), and benzoate application, iontophoresis, contact lens–assisted crosslinking etc., Not much work has been done in these areas in the pediatric population. BAK-assisted TE- CXL has been reported by Koppen et al.[68] in the pediatric age group with significant improvement in CDVA at six and 12 months in progressive, but observed progression of K-max throughout the study.
Recently, accelerated cross-linking is been accepted worldwide to shorten surgical time. For pediatric KC, accelerated CXL is not much effective with increase in K-max and posterior elevation values noted at 36 months post procedure.[69]
Soeters et al. has observed that in pediatric KC, thinner corneas =450 microns and centrally located cones responded better to cross-linking treatment.[69] However, it is almost imperative to note that the longest period of follow-up available in this age group is 7.5 years. Stability has been noted but there were no changes in the keratometry values.[70] However, a number of studies document corneal steepening noted to begin three years after the procedure.[12] An indirect extrapolation as to the efficacy of cross linking can be made from the fact that the number of transplants for KC is decreasing as evidenced by data from transplant registers. Italy's Corneal Transplant Epidemiological Study noted a 27-percent reduction in corneal grafting for keratoconus over a six-year period starting from 2002.[71] Caporossi et al.'s observed the need for re epi-off treatment to manage progression in 50% of children who had epi-on CXL earlier[72] points towards the need for robust follow-up post cross-linking. Reports of retreatments both in the adult and pediatric population are scarce.
VKC with KC is a relative contraindication for CXL. It would be advisable to control the allergic process before cross-linking. Nonhealing epithelial defects and predisposition to infection are more likely, if uncontrolled. Currently, standard CXL is advised if the corneal thickness is more than 400 microns and the hypotonic variant, if the pachymetry is at least 360 microns.
It is evident that a large number of protocols and options exist for cross-linking. Collagen cross-linking with hypo-osmolar riboflavin is reportedly successful in children but long-term data is lacking. Future directions in cross-linking in pediatric KC would be directed toward establishing the safety and efficacy of current protocols, and the relevance of protocols such as epi-off pulsed accelerated crosslinking, where higher irradiation applied in on-off mode would not only shorten treatment times but would obtain comparable efficacy as the standard Dresden protocol.[56,57] To summarize, the current consensus is that KC progresses much faster in children and therefore the procedure can be offered as soon as progression is documented to obviate the need for transplantation.
Concerns in surgical interventions in pediatric keratoconus
In cases where contact lens wear is not possible due to ocular allergy, and in advanced ectasia, surgical intervention is recommended. KC progresses rapidly in children (88%) compared to adults (8%).[11,12,73] KC is the most common acquired nontraumatic indication of 23%–67% of pediatric keratoplasty.[73] In Indian children with acquired nontraumatic causes, requiring penetrating keratoplasty, KC (37%) follows postinfectious keratitis adherent leukoma (63%) as the leading indications for surgery.[74] However, a recent study from Eastern China[75] noted KC (67.2%) to be the most common indication for pediatric keratoplasty.
There are very few studies that have reported the use of ICRS for pediatric KC. ICRS, although safe and effective in children with poor visual acuity, it does not seem to be preferred in pediatric cases with VKC due to the risk of aggressive nature of the disease, tendency for eye rubbing and risk of extrusion.[73]
Improvement in refractive and visual outcomes following four years of ICRS and CXL, which has been done one month apart, has been reported.[76] Kera ring implantation with accelerated CXL using femto-laser for tunnel creation, showed increased visual outcomes in 42.9% of children.[77] Complications include extrusion and migration of the device, infections, and KC progression.[77]
Penetrating keratoplasty (PK) [Fig. 5a] can be challenging in children due to low scleral rigidity and increased vitreous positive pressure. However, well-controlled general anesthesia, low intraocular pressure by massage and intravenous mannitol, and use of Flieringa scleral ring can render the surgery safer.[78] Same sized grafts are preferred in PK or DALK in children. Rapid wound healing causes early suture loosening which mandates suture replacements or removal. Hence, 10.o' nylon interrupted sutures are to be performed.[77,78,79]
Figure 5.
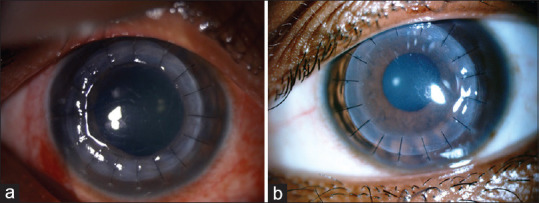
Postoperative clinical picture of penetrating keratoplasty (a) and deep anterior lamellar keratoplasty (b) in pediatric keratoconus cases
Deep anterior lamellar keratoplasty (DALK) [Fig. 5b] has become the preferred procedure of choice because of better structural stability and less immune rejection.[80] DALK can be performed with mechanical trephining or as Femtosecond laser-assisted procedure.[80,81] The stromal dissection can be done by manual method up to the pre-Descemet's layer as in pre-Descemetic DALK (pd-DALK) or by barring Descemet's membrane (DM) (d-DALK) either by air (Anwar's big bubble technique), hydro, or visco dissection.[82,83] DALK is technically challenging, and intraoperative DM perforation can lead to a double anterior chamber and interface problems such as infection or vascularization with poor visual outcome and graft failure. Conversion to PK may also be required.[84]
The reported success rate with big bubble range was around 75% to 80% in KC corneas that underwent DALK in children.[84,85] Feizi et al.[85] had done successful big bubble in 75% of the eyes and the rest were pd-DALK. DM perforation was noted in 11.4% (5 eyes) of DALK procedure, either while air injection or deep stromal dissection. Urretts Zavalia Syndrome (UZS) is a well-recognized but rare postoperative complication following keratoplasty for KC patients. The low rigidity and intrinsic abnormality of keratoconic eyes allows the occlusion of vessels at the root of the iris within the sclera during surgery.[86] Feizi et al.[85] reported UZS in 2.3% (one eye) of pediatric DALK maintained clear graft with BCVA 20/30 after appropriate management. Arora et al. attempted big bubble DALK in 20 eyes of 16 patients (mean age of 14.4 years) with advanced keratoconus.[87]
Another common concern that is frequently faced in eyes with ocular allergy that undergo PK or DALK is the predisposition for a significantly rapid suture loosening which tends to recur despite repeated suture replacements [Fig. 6]. This necessitates earlier suture removal or the resultant focal thinning at this graft host junction region of repeated affliction causing a higher postoperative astigmatism. It is imperative to ensure that the ocular surface allergy is well controlled before considering surgical intervention in cases of KC with VKC.
Figure 6.
Slit lamp photograph of post.DALK (at 3 months postoperative period) in a pediatric patient of VKC with keratoconus showing early suture loosening (a) [(note that differential wound healing had necessitated prior removal of few sutures (b)]; a different case of DALK in pediatric keratoconus with loose sutures (c)
Postoperative management for monitoring and repeated examinations to assess wound healing, intraocular pressure, posterior segment, and to manage suture related concerns needs general anaesthesia in children. Traumatic graft dehiscence, immune graft rejection [Fig. 7], secondary glaucoma, or cataract due to long term steroid use, are also expected to have a higher occurrence in pediatric cases. Topical steroids are needed to control inflammatory reaction, which are to be tapered and replaced with low potency steroids by 3-6 months. Steroid sparring immune-modulators like cyclosporine A 2%, are ideal for long term use to address immune graft rejection. Early suture removal and refractive correction is also needed to achieve optimum visual rehabilitation. Teaching the children as well as parents or caretakers to strictly follow the postoperative regimen, follow-up visits, and to use protective eye gears are imperative.[79,80,81,82,88]
Figure 7.
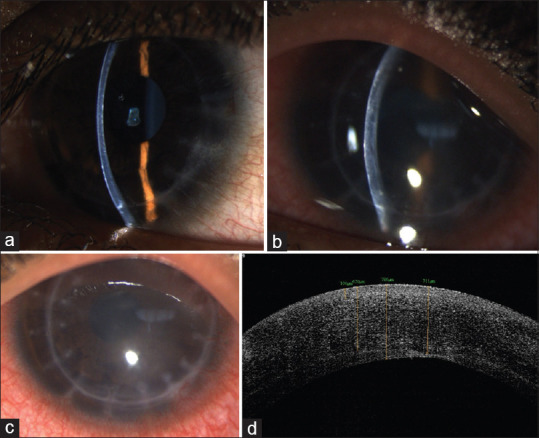
Slit lamp photograph of DALK of a case of pediatric keratoconus (a) showing increased stromal edema (b), corneal haze (c) due to stromal graft rejection; (d) ASOCT picture of the same eye showing the increased stromal thickness due to edema as a result of immune rejection
Patel et al. noted a significant visual improvement with BCVA equal to or better than 0.3 Log MAR in 86.4% of their PK cases of mean age 10.6 years ± 4.3 years (range: 2 weeks to 14 years) in 65 pediatric patients, at the final examination.[89] McClellan et al. also reported visual acuity better than 6/12 at the last follow-up in pediatric KC eyes that underwent PK.[90] In their comparison of big bubble (BB) DALK to pd-DALK, though Feizi et al.[82] noted postoperative BCVA was better in the BB-DALK subgroup, the difference was not found to be significant. In cases where big bubble is not achievable after several air injections, the surgical technique conversion to pd-DALK will be safe given the comparable postoperative visual and refractive outcomes.[90,91] Ashar et al.[81] reported that 75% of their DALK grafts performed for keratoconus were clear in with a visual acuity of > 20/80 which is comparable to PK for KC in children.
Buzzonetti et al. compared refractive outcome at 2 years follow-up post DALK by mechanical trephine and femtosecond laser assisted procedure in KC children (mean age 11.2 ± 2.2 years; range: 8–16 years), with standardized suturing technique and suture removal protocol.[92] The corrected distance visual acuity and manifest astigmatism were comparable in both groups, whereas spherical equivalent was statistically less in laser-assisted DALK. Better host-donor matching and interlocking in fs-DALK perhaps enable superior refractive outcome in DALK cases.[92]
VKC, despite being quiescent at the time of keratoplasty, can complicate the postoperative course by causing graft epithelial problems, vascularization, graft opacification, and rejection leading to graft failure.[85] Feizi et al. have reported graft epitheliopathy and superficial punctate keratitis in 3 out of 44 eyes in their series.[85] Arora et al. observed reactivation of VKC and appearance of shield ulcers in two of their patients resulting in subsequent graft opacification.[87] This importance of optimal medical management of VKC before surgery and prompt initiation of treatment of any inflammation after surgery needs to be greatly emphasized in pediatric KC.
Among all other indications for pediatric keratoplasty, KC carries the best prognosis for long-term graft survival. Older studies reporting results in a series of 164 pediatric PK (mean age at diagnosis - 30 months), noted a good survival rate of 80%, with grafts performed for KC being the most successful in terms of survival.[92] Similar results with keratoplasty performed for KC showing excellent prognosis with a reported 1-year survival rate of 90% by Patel et al. in their series of 58 eyes (mean age 10.6 ± 4.3 years; range 2 weeks – 14 years) from New Zealand.[89] Gulias-Cañizo et al. found in their recent retrospective review of a large series of 574 pediatric keratoplasties, KC showed the best graft survival at 60-months follow-up of 85%, as compared to other indications.[93] The Australian Registry Study on corneal transplantation in KC in all age groups observed significantly better outcomes with the first PK for KC to have a significantly better survival of 89% at 10 years in comparison to 49% at 20 years.[92]
Graft rejection remains the most important cause for graft failure in keratoplasty for keratoconus, especially in PK. In bilateral transplants, there is no increased risk of graft rejection, when the second eye was grafted after 1 year.[25] Long-term graft survival beyond 23 years cannot be assured and in pediatric eyes requiring surgery, there still exists the need for more than one graft in lifetime.[94] Pediatric DALK can also experience subepithelial and stromal graft rejection. Early recognition is imperative to initiate prompt therapy in order to salvage the graft and vision in these children.
Feizi et al. observed subepithelial graft rejection in 5 out of 44 eyes that underwent DALK (11.36%) at a mean interval of 7.6 ± 2.5 months (range 6–12 months) in 9.1% of eyes, which was successfully treated with topical corticosteroids in 3 – 6 weeks.[85] Arora et al. observed 1 patient who developed stromal rejection 3 months after surgery.[87] Elbaz et al. noted stromal rejection in 5 eyes (9.8%) of which four eyes responded completely to topical steroids and one graft failed.[84]
Buzzonetti et al.'s analysis of graft survival and difference in graft failure between PK and DALK in children below and above 5 years age observed 78% graft failure in the younger children (below 5 years of age) and 31% in the older children.[92] Though lesser overall graft failure was seen in DALK compared to PK, there was no statistically significant difference between the younger and older children but the DALK eyes showed a statistically significant longer survival time than PK eyes (588 vs. 406 mean days).[92,95,96]
With reported incidence of glaucoma in pediatric keratoplasties being as high as 25%,[28] but Feizi et al.[85] noted transiently elevated IOP in 18.2% post DALK for keratoconus eyes which responded well to the treatment of reduction of steroids and anti-glaucoma medications. The addition of topical cyclosporine can help reduce the long-term corticosteroid use required to alleviate graft rejection in children.[97] The Urret–Zavalia syndrome can occur, especially after DALK, with the prolonged anterior chamber filling with a tight air bubble, as with cases of long drawn of anterior lamellar dissection as in BB-DALK[93] or after tight pneumopexy for DM perforation.[82] Though the exact pathogenesis is not clear, pressure-induced ischemic necrosis is postulated to cause dilated and fixed pupil, iris atrophy, and even cataract.
Children are more prone to traumatic graft host dehiscence due to their higher physical activities where, in cases of PK, the entire anterior segment can be compromised as compared to DALK. Posttraumatic graft host dehiscence was reported in 4.8% post DALK eyes by Feizi et al.[85]
While keratoplasty for KC in children has become a much safer procedure than before, enabling an excellent anatomical and functional outcome in pediatric eyes, concerns do remain to be addressed for enhancing prognosis. DALK is preferable to penetrating keratoplasty because of better structural stability, preservation of host endothelium, and lesser rejection rates.
Conclusion
Pediatric keratoconus can present at an advanced stage, faster progression and associated ocular co-morbidities. Need for timely intervention to stop progression with collagen crosslinking therapy, optimal visual rehabilitation, management of associated ocular comorbidities is imperative. Optimal decision making in management of keratoconus involves a thorough knowledge of the clinical challenges that can occur during the course of treatment of this ectactic corneal condition.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chang H-YP, Chodosh J. The genetics of keratoconus. Semin Ophthalmol. 2013;28:275–80. doi: 10.3109/08820538.2013.825295. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267–73. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- 3.Moshirfar M, Heiland MB, Rosen DB, Ronquillo YC, Hoopes PC. Keratoconus screening in elementary school children. Ophthalmol Ther. 2019;8:367–71. doi: 10.1007/s40123-019-0199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson AR, Soneji B, Sarvananthan N, Sandford-Smith JH. Does ethnic origin influence the incidence or severity of keratoconus? Eye. 2000;14:625–8. doi: 10.1038/eye.2000.154. [DOI] [PubMed] [Google Scholar]
- 5.Torres Netto EA, Al-Otaibi WM, Hafezi NL, Kling S, Al-Farhan HM, Randleman JB, et al. Prevalence of keratoconus in paediatric patients in Riyadh, Saudi Arabia. Br J Ophthalmol. 2018;102:1436–41. doi: 10.1136/bjophthalmol-2017-311391. [DOI] [PubMed] [Google Scholar]
- 6.Assiri AA, Yousuf BI, Quantock AJ, Murphy PJ. Incidence and severity of keratoconus in Asir province, Saudi Arabia. Br J Ophthalmol. 2005;89:1403–6. doi: 10.1136/bjo.2005.074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonas JB, Nangia V, Matin A, Kulkarni M, Bhojwani K. Prevalence and associations of keratoconus in rural Maharashtra in central India: The central India eye and medical study. Am J Ophthalmol. 2009;148:760–5. doi: 10.1016/j.ajo.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, et al. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Invest Ophthalmol Vis Sci. 1998;39:2537–46. [PubMed] [Google Scholar]
- 9.Sabti S, Tappeiner C, Frueh BE. Corneal cross-linking in a 4-year-old child with keratoconus and down syndrome. Cornea. 2015;34:1157–60. doi: 10.1097/ICO.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 10.Gokhale NS. Epidemiology of keratoconus. Indian J Ophthalmol. 2013;61:382–3. doi: 10.4103/0301-4738.116054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Léoni-Mesplié S, Mortemousque B, Touboul D, Malet F, Praud D, Mesplié N, et al. Scalability and severity of keratoconus in children. Am J Ophthalmol. 2012;154:56–62e1. doi: 10.1016/j.ajo.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Chatzis N, Hafezi F. Progression of keratoconus and efficacy of pediatric [corrected] corneal collagen cross-linking in children and adolescents. J Refract Surg. 2012;28:753–8. doi: 10.3928/1081597X-20121011-01. [DOI] [PubMed] [Google Scholar]
- 13.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 14.Hovakimyan M, Guthoff RF, Stachs O. Collagen cross-linking: Current status and future directions. J Ophthalmol. 2012;2012:406850. doi: 10.1155/2012/406850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wojcik KA, Blasiak J, Szaflik J, Szaflik JP. Role of biochemical factors in the pathogenesis of keratoconus. Acta Biochim Pol. 2014;61:55–62. [PubMed] [Google Scholar]
- 16.Burdon KP, Vincent AL. Insights into keratoconus from a genetic perspective. Clin Exp Optom. 2013;96:146–54. doi: 10.1111/cxo.12024. [DOI] [PubMed] [Google Scholar]
- 17.Loukovitis E, Sfakianakis K, Syrmakesi P, Tsotridou E, Orfanidou M, Bakaloudi DR, et al. Genetic aspects of keratoconus: A literature review exploring potential genetic contributions and possible genetic relationships with comorbidities. Ophthalmol Ther. 2018;7:263–92. doi: 10.1007/s40123-018-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Rabinowitz YS, Rotter JI, Yang H. Genetic epidemiological study of keratoconus: Evidence for major gene determination. American journal of medical genetics. 2000;93:403–9. [PubMed] [Google Scholar]
- 19.Bechara SJ, Waring GO, Insler MS. Keratoconus in two pairs of identical twins. Cornea. 1996;15:90–3. [PubMed] [Google Scholar]
- 20.Abelson MB, Granet D. Ocular allergy in pediatric practice. Curr Allergy Asthma Rep. 2006;6:306–11. doi: 10.1007/s11882-006-0064-x. [DOI] [PubMed] [Google Scholar]
- 21.Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015;2015:795738. doi: 10.1155/2015/795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMonnies CW. Mechanisms of rubbing-related corneal trauma in keratoconus. Cornea. 2009;28:607–15. doi: 10.1097/ICO.0b013e318198384f. [DOI] [PubMed] [Google Scholar]
- 23.Balasubramanian SA, Pye DC, Willcox MDP. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: Relevance in keratoconus. Clin Exp Optom. 2013;96:214–8. doi: 10.1111/cxo.12038. [DOI] [PubMed] [Google Scholar]
- 24.Seppälä HPS, Määttä M, Rautia M, Mackiewicz Z, Tuisku I, Tervo T, et al. EMMPRIN and MMP-1 in keratoconus. Cornea. 2006;25:325–30. doi: 10.1097/01.ico.0000183534.22522.39. [DOI] [PubMed] [Google Scholar]
- 25.Tuft SJ, Gregory WM, Buckley RJ. Acute corneal hydrops in keratoconus. Ophthalmology. 1994;101:1738–44. doi: 10.1016/s0161-6420(94)31110-9. [DOI] [PubMed] [Google Scholar]
- 26.Karimian F, Aramesh S, Rabei HM, Javadi MA, Rafati N. Topographic evaluation of relatives of patients with keratoconus. Cornea. 2008;27:874–8. doi: 10.1097/ICO.0b013e31816f5edc. [DOI] [PubMed] [Google Scholar]
- 27.Barreto J, Netto MV, Santo RM, José NK, Bechara SJ. Slit-scanning topography in vernal keratoconjunctivitis. Am J Ophthalmol. 2007;143:250–4. doi: 10.1016/j.ajo.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 28.Baum J. On the location of the cone and the etiology of keratoconus. Cornea. 1995;14:142–3. [PubMed] [Google Scholar]
- 29.Collier SA. Is the corneal degradation in keratoconus caused by matrix-metalloproteinases? Clin Experiment Ophthalmol. 2001;29:340–4. doi: 10.1046/j.1442-9071.2001.d01-17.x. [DOI] [PubMed] [Google Scholar]
- 30.Nishtala K, Pahuja N, Shetty R, Nuijts RMMA, Ghosh A. Tear biomarkers for keratoconus. Eye Vis (Lond) 2016;3:19. doi: 10.1186/s40662-016-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackiewicz Z, Määttä M, Stenman M, Konttinen L, Tervo T, Konttinen YT. Collagenolytic proteinases in keratoconus. Cornea. 2006;25:603–10. doi: 10.1097/01.ico.0000208820.32614.00. [DOI] [PubMed] [Google Scholar]
- 32.Dudakova L, Jirsova K. The impairment of lysyl oxidase in keratoconus and in keratoconus-associated disorders. J Neural Transm (Vienna) 2013;120:977–82. doi: 10.1007/s00702-013-0993-1. [DOI] [PubMed] [Google Scholar]
- 33.Shetty R, Sathyanarayanamoorthy A, Ramachandra RA, Arora V, Ghosh A, Srivatsa PR, et al. Attenuation of lysyl oxidase and collagen gene expression in keratoconus patient corneal epithelium corresponds to disease severity. Mol Vis. 2015;21:12–25. [PMC free article] [PubMed] [Google Scholar]
- 34.Avetisov SE, Mamikonian VR, Novikov IA. [The role of tear acidity and Cu-cofactor of lysyl oxidase activity in the pathogenesis of keratoconus] Vestn Oftalmol. 2011;127:3–8. [PubMed] [Google Scholar]
- 35.Olofsson EM, Marklund SL, Pedrosa-Domellöf F, Behndig A. Interleukin-1alpha downregulates extracellular-superoxide dismutase in human corneal keratoconus stromal cells. Mol Vis. 2007;13:1285–90. [PubMed] [Google Scholar]
- 36.Lema I, Durán JA. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology. 2005;112:654–9. doi: 10.1016/j.ophtha.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 37.Thanos S, Oellers P, Meyer Zu Hörste M, Prokosch V, Schlatt S, Seitz B, et al. Role of Thyroxine in the Development of Keratoconus. Cornea. 2016;35:1338–46. doi: 10.1097/ICO.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 38.Ayan B, Yuksel N, Carhan A, Gumuskaya Ocal B, Akcay E, Cagil N, et al. Evaluation estrogen, progesteron and androgen receptor expressions in corneal epithelium in keratoconus. Cont Lens Anterior Eye. 2019;42:492–6. doi: 10.1016/j.clae.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 40.Hameed A, Khaliq S, Ismail M, Anwar K, Ebenezer ND, Jordan T, et al. A novel locus for Leber congenital amaurosis (LCA4) with anterior keratoconus mapping to chromosome 17p13. Invest Ophthalmol Vis Sci. 2000;41:629–33. [PubMed] [Google Scholar]
- 41.Elder MJ. Leber congenital amaurosis and its association with keratoconus and keratoglobus. J Pediatr Ophthalmol Strabismus. 1994;31:38–40. doi: 10.3928/0191-3913-19940101-08. [DOI] [PubMed] [Google Scholar]
- 42.Kumaran N, Pennesi ME, Yang P, Trzupek KM, Schlechter C, Moore AT, et al. Leber congenital amaurosis/early-onset severe retinal dystrophy overview. InGeneReviews®[Internet] 2018 Oct 4. University of Washington, Seattle. [PubMed] [Google Scholar]
- 43.Shapiro MB, France TD. The ocular features of Down's syndrome. Am J Ophthalmol. 1985;99:659–63. doi: 10.1016/s0002-9394(14)76031-3. [DOI] [PubMed] [Google Scholar]
- 44.Woodward EG, Morris MT. Joint hypermobility in keratoconus. Ophthalmic Physiol Opt. 1990;10:360–2. doi: 10.1111/j.1475-1313.1990.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 45.Villani E, Garoli E, Bassotti A, Magnani F, Tresoldi L, Nucci P, et al. The cornea in classic type Ehlers-Danlos syndrome: Macro- and microstructural changes. Invest Ophthalmol Vis Sci. 2013;54:8062–8. doi: 10.1167/iovs.13-12837. [DOI] [PubMed] [Google Scholar]
- 46.Khaled ML, Helwa I, Drewry M, Seremwe M, Estes A, Liu Y. Molecular and histopathological changes associated with keratoconus. Biomed Res Int. 2017;2017:7803029. doi: 10.1155/2017/7803029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahebjada S, Fenwick EK, Xie J, Snibson GR, Daniell MD, Baird PN. Impact of keratoconus in the better eye and the worse eye on vision-related quality of life. Invest Ophthalmol Vis Sci. 2014;55:412–6. doi: 10.1167/iovs.13-12929. [DOI] [PubMed] [Google Scholar]
- 48.Schlegel Z, Lteif Y, Bains HS, Gatinel D. Total, corneal, and internal ocular optical aberrations in patients with keratoconus. J Refract Surg. 2009;25(10 Suppl):S951–7. doi: 10.3928/1081597X-20090915-10. [DOI] [PubMed] [Google Scholar]
- 49.Sray WA, Cohen EJ, Rapuano CJ, Laibson PR. Factors associated with the need for penetrating keratoplasty in keratoconus. Cornea. 2002;21:784–6. doi: 10.1097/00003226-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Naderan M, Rajabi MT, Zarrinbakhsh P, Bakhshi A. Effect of Allergic Diseases on Keratoconus Severity. Ocul Immunol Inflamm. 2017;25:418–23. doi: 10.3109/09273948.2016.1145697. [DOI] [PubMed] [Google Scholar]
- 51.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 52.Omar IAN. Keratoconus Screening Among Myopic Children. Clin Ophthalmol. 2019;13:1909–12. doi: 10.2147/OPTH.S225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maguire LJ. Ectatic corneal degenerations. In: Kaufmann HE, editor. The Cornea. Second edition. USA: 1997. pp. 525–43. [Google Scholar]
- 54.Wan Q, Tang J, Han Y, Xiao Q, Deng Y. Brittle cornea syndrome: A case report and review of the literature. BMC Ophthalmol. 2018;18:252. doi: 10.1186/s12886-018-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 56.McCall AS, Kraft S, Edelhauser HF, Kidder GW, Lundquist RR, Bradshaw HE, et al. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA) Invest Ophthalmol Vis Sci. 2010;51:129–38. doi: 10.1167/iovs.09-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wollensak G. Histological changes in human cornea after cross-linking with riboflavin and ultraviolet A. Acta Ophthalmol. 2010;88:e17–8. doi: 10.1111/j.1755-3768.2008.01474.x. [DOI] [PubMed] [Google Scholar]
- 58.Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32:279–83. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- 59.Scarcelli G, Kling S, Quijano E, Pineda R, Marcos S, Yun SH. Brillouin microscopy of collagen crosslinking: Noncontact depth-dependent analysis of corneal elastic modulus. Invest Ophthalmol Vis Sci. 2013;54:1418–25. doi: 10.1167/iovs.12-11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Denaro R, Balestrazzi A. Riboflavin-UVA-induced corneal collagen cross-linking in pediatric patients. Cornea. 2012;31:227–31. doi: 10.1097/ico.0b013e31822159f6. [DOI] [PubMed] [Google Scholar]
- 61.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Denaro R. Age-related long-term functional results after riboflavin UV A corneal cross-linking. J Ophthalmol. 2011;2011:608041. doi: 10.1155/2011/608041. doi.org/10.1155/2011/608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zotta PG, Diakonis VF, Kymionis GD, Grentzelos M, Moschou KA. Long-term outcomes of corneal cross-linking for keratoconus in pediatric patients. J AAPOS. 2017;21:397–401. doi: 10.1016/j.jaapos.2017.07.205. [DOI] [PubMed] [Google Scholar]
- 63.Vinciguerra P, Albé E, Frueh BE, Trazza S, Epstein D. Two-year corneal cross-linking results in patients younger than 18 years with documented progressive keratoconus. Am J Ophthalmol. 2012;154:520–6. doi: 10.1016/j.ajo.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 64.Knutsson KA, Paganoni G, Matuska S, Ambrosio O, Ferrari G, Zennato A, et al. Corneal collagen cross-linking in paediatric patients affected by keratoconus. Br J Ophthalmol. 2018;102:248–52. doi: 10.1136/bjophthalmol-2016-310108. [DOI] [PubMed] [Google Scholar]
- 65.Caporossi A, Mazzotta C, Paradiso AL, Baiocchi S, Marigliani D, Caporossi T. Transepithelial corneal collagen crosslinking for progressive keratoconus: 24-month clinical results. J Cataract Refract Surg. 2013;39:1157–63. doi: 10.1016/j.jcrs.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 66.Salman AG. Transepithelial corneal collagen crosslinking for progressive keratoconus in a pediatric age group. J Cataract Refract Surg. 2013;39:1164–70. doi: 10.1016/j.jcrs.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 67.Buzzonetti L, Petrocelli G. Transepithelial corneal cross-linking in pediatric patients: Early results. J Refract Surg. 2012;28:763–7. doi: 10.3928/1081597X-20121011-03. [DOI] [PubMed] [Google Scholar]
- 68.Koppen C, Wouters K, Mathysen D, Rozema J, Tassignon MJ. Refractive and topographic results of benzalkonium chloride-assisted transepithelial crosslinking. J Cataract Refract Surg. 2012;38:1000–5. doi: 10.1016/j.jcrs.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 69.Tian M, Jian W, Zhang X, Sun L, Zhou X. Three-year follow-up of accelerated transepithelial corneal cross-linking for progressive paediatric keratoconus. Br J Ophthalmol. 2020 doi: 10.1136/bjophthalmol-2019-315260. doi: 10.1136/bjophthalmol-2019-315260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soeters N, van der Valk R, Tahzib NG. Corneal cross-linking for treatment of progressive keratoconus in various age groups. J Refract Surg. 2014;30:454–60. doi: 10.3928/1081597X-20140527-03. [DOI] [PubMed] [Google Scholar]
- 71.Frigo AC, Fasolo A, Capuzzo C, Fornea M, Bellucci R, Busin M, et al. Corneal transplantation activity over 7 years: Changing trends for indications, patient demographics and surgical techniques from the Corneal Transplant Epidemiological Study (CORTES) Transplant Proc. 2015;47:528–35. doi: 10.1016/j.transproceed.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 72.Caporossi A, Mazzotta C, Paradiso AL, Baiocchi S, Marigliani D, Caporossi T. Transepithelial corneal collagen crosslinking for progressive keratoconus: 24-month clinical results. J Cataract Refract Surg. 2013;39:1157–63. doi: 10.1016/j.jcrs.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 73.Mukhtar S, Ambati BK. Pediatric keratoconus: A review of the literature. Int Ophthalmol. 2018;38:2257–66. doi: 10.1007/s10792-017-0699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ganekal S, Gangangouda C, Dorairaj S, Jhanji V. Early outcomes of primary pediatric keratoplasty in patients with acquired, atraumatic corneal pathology. J AAPOS. 2011;15:353–5. doi: 10.1016/j.jaapos.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Zhao S, Le Q, Yao W, Xu J. Indications and techniques of pediatric keratoplasty in eastern China from 2008 to 2017. Cornea. 2019;38:1370–6. doi: 10.1097/ICO.0000000000002071. [DOI] [PubMed] [Google Scholar]
- 76.Abdelmassih Y, el-Khoury S, Dirani A, Antonios R, Fadlallah A, Cherfan C, et al. Safety and efficacy of sequential intracorneal ring segment implantation and crosslinking in pediatric keratoconus. Am J Ophthalmol. 2017;178:51–7. doi: 10.1016/j.ajo.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 77.Saleem MIH, Ibrahim Elzembely HA, AboZaid MA, Elagouz M, Saeed AM, Mohammed OA, et al. Three-year outcomes of cross-linking PLUS (combined cross-linking with femtosecond laser intracorneal ring segments implantation) for management of keratoconus? J Ophthalmol online. 2018;2018:6907573. doi: 10.1155/2018/6907573. doi: 10.1155/2018/6907573. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piñero DP, Alio JL. Intracorneal ring segments in ectatic corneal disease–A review. Clin Exp Ophthalmol. 2010;38:154–67. doi: 10.1111/j.1442-9071.2010.02197.x. [DOI] [PubMed] [Google Scholar]
- 79.Gloor P. Pediatric penetrating keratoplasty. In: Krachmer JH, Mannis M, Holland FJ, editors. Cornea. Vol 3. St Louis MO: Mosby; 1997. pp. 1731–56. [Google Scholar]
- 80.Vanathi M, Panda A, Vengayil S, Chaudhuri Z, Dada T. Pediatric keratoplasty. Surv Ophthalmol. 2009;54:245–71. doi: 10.1016/j.survophthal.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 81.Ashar JN, Pahuja S, Ramappa M, Vaddavalli PK, Chaurasia S, Garg P. Deep anterior lamellar keratoplasty in children. Am J Ophthalmol. 2013;155:570–4. doi: 10.1016/j.ajo.2012.09.029. e1. [DOI] [PubMed] [Google Scholar]
- 82.Tan DT, Por YM. Current treatment options for corneal ectasia. Current Opinion in Ophthalmology. 2007;18:279–83. doi: 10.1097/ICU.0b013e3281a7ecaa. [DOI] [PubMed] [Google Scholar]
- 83.Karimian F, Feizi S. Deep anterior lamellar keratoplasty: Indications, surgical techniques and complications. Middle East Afr J Ophthalmol. 2010;17:28–37. doi: 10.4103/0974-9233.61214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elbaz U, Kirwan C, Shen C, Ali A. Avoiding big bubble complications: Outcomes of layer-by-layer deep anterior lamellar keratoplasty in children. Br J Ophthalmol. 2018;102:1103–8. doi: 10.1136/bjophthalmol-2017-310962. [DOI] [PubMed] [Google Scholar]
- 85.Feizi S, Javadi MA, Najafi M, Abolhosseini M, Moshtaghion SM. Outcomes of big-bubble deep anterior lamellar keratoplasty for pediatric keratoconus. Int Ophthalmol. 2020;40:1253–9. doi: 10.1007/s10792-020-01291-x. [DOI] [PubMed] [Google Scholar]
- 86.Spierer O, Lazar M. Urrets-Zavalia syndrome (fixed and dilated pupil following penetrating keratoplasty for keratoconus) and its variants. Surv Ophthalmol. 2014;59:304–10. doi: 10.1016/j.survophthal.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Arora R, Jain P, Jain P, Manudhane A, Goyal J. Results of deep anterior lamellar keratoplasty for advanced keratoconus in children less than 18 years. Am J Ophthalmol. 2016;162:191–8. doi: 10.1016/j.ajo.2015.11.020. e2. [DOI] [PubMed] [Google Scholar]
- 88.Low JR, Anshu A, Tan ACS, Htoon HM, Tan DT. The outcomes of primary pediatric keratoplasty in Singapore. Am J Ophthalmol. 2014;158:496–502. doi: 10.1016/j.ajo.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 89.Patel HY, Ormonde S, Brookes NH, Moffatt LS, McGhee CN. The indications and outcome of paediatric corneal transplantation in New Zealand: 1991–2003. Br J Ophthalmol. 2005;89:404–8. doi: 10.1136/bjo.2004.053116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McClellan K, Lai T, Grigg J, Billson F. Penetrating keratoplasty in children: Visual and graft outcome. Br J Ophthalmol. 2003;87:1212–4. doi: 10.1136/bjo.87.10.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lowe MT, Keane MC, Coster DJ, Williams KA. The outcome of corneal transplantation in infants, children, and adolescents. Ophthalmology. 2011;118:492–7. doi: 10.1016/j.ophtha.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 92.Buzzonetti L, Petrocelli G, Valente P, Petroni S, Parrilla R, Iarossi G. Refractive outcome of keratoconus treated by big-bubble deep anterior lamellar keratoplasty in pediatric patients: Two-year follow-up comparison between mechanical trephine and femtosecond laser assisted techniques. Eye Vis (Lond) 2019;6:1. doi: 10.1186/s40662-018-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gulias-Cañizo R, Gonzalez-Salinas R, Hernandez-Zimbron LF, Hernandez-Quintela E, Sanchez-Huerta V. Indications and outcomes of pediatric keratoplasty in a tertiary eye care center: A retrospective review. Medicine (Baltimore) 2017;96:e8587. doi: 10.1097/MD.0000000000008587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dana MR, Moyes AL, Gomes JA, Rosheim KM, Schaumberg DA, Laibson PR, et al. The indications for and outcome in pediatric keratoplasty.A multicenter study. Ophthalmology. 1995;102:1129–38. doi: 10.1016/s0161-6420(95)30900-1. [DOI] [PubMed] [Google Scholar]
- 95.Kelly T-L, Williams KA, Coster DJ Australian Corneal Graft Registry. Corneal transplantation for keratoconus: A registry study. Arch Ophthalmol. 2011;129:691–7. doi: 10.1001/archophthalmol.2011.7. [DOI] [PubMed] [Google Scholar]
- 96.Buzzonetti L, Ardia R, Petroni S, Petrocelli G, Valente P, Parrilla R, et al. Four years of corneal keratoplasty in Italian paediatric patients: Indications and clinical outcomes. Graefes Arch Clin Exp Ophthalmol. 2016;254:2239–45. doi: 10.1007/s00417-016-3447-2. [DOI] [PubMed] [Google Scholar]
- 97.Cosar CB, Laibson PR, Cohen EJ, Rapuano CJ. Topical cyclosporine in pediatric keratoplasty. Eye Contact Lens. 2003;29:103–7. doi: 10.1097/01.ICL.0000062460.03555.32. [DOI] [PubMed] [Google Scholar]