Dear Editor,
Brolucizumab is a humanized single-chain variable fragment of an antibody, allowing delivery of greater molar dose compared to larger molecules.[1] Studies have demonstrated high penetration in retina resulting in better absorption of macular fluid in AMD.[2] It was recently approved by the DCGI. There have been, however, reports about intraocular inflammation (IOI) and vasculitis after administering Brolicizumab.[3] In the HAWK and HARRIER study, brolucizumab 6 mg was shown to have 4% risk of IOI, compared to 1% with aflibercept 2 mg.[1] We report a case of intense immediate ocular inflammation with hypopyon after intravitreal injection of brolucizumab in a case of polypoidal choroidal vasculopathy.
A 62-year-old female had received 30 intravitreal injections in the right eye (23 Eylea (aflibercept), 4 Zaltrap (ziv-aflibercept), 2 Ozurdex (dexamethasone implant), and 1 Lucentis (ranibizumab) over the last 4 years. She had also undergone reduced fluence PDT in October 2017. The vision significantly deteriorated over the years to 20/800 due to significant damage to the outer retinal layers, subretinal scar, and cataract. She underwent cataract surgery in September 2020 along with intravitreal Zaltrap, after which her vision improved to 20/250. However, due to persistent significant intraretinal fluid on OCT at 1 month after the previous intervention, she was given brolucizumab injection in the right eye on 29 October 2020. On the same day, 6 hours after the injection, the patient developed loss of vision, redness and mild pain. When she presented on 30.10.2020, she had minimal congestion of the conjunctiva, no lid edema, 1 mm hypopyon, and no view of the fundus due to intense vitritis [Fig. 1]. Her vision was hand movements close to face in the right eye. She was started on hourly topical steroids, but the hypopyon did not reduce. As a precaution, she underwent vitrectomy biopsy with intravitreal ceftazidime and vancomycin. Microbiological testing of the vitreous fluid did not show any organism on smear, and there was no growth on culture. Polymerase chain reaction for Eubacteria was negative. She was started on topical Moxifloxacin 6 times daily, Prednisolone acetate 1 hourly, and Homatropine eye drops three times daily. On the fifth day after brolucizumab injection, the inflammation had significantly resolved and fundus details were visible upto third-order vessels [Fig. 2]. There were no exudates seen in the vitreous cavity with no evidence of vasculitis. The vision had improved to 20/600.
Figure 1.
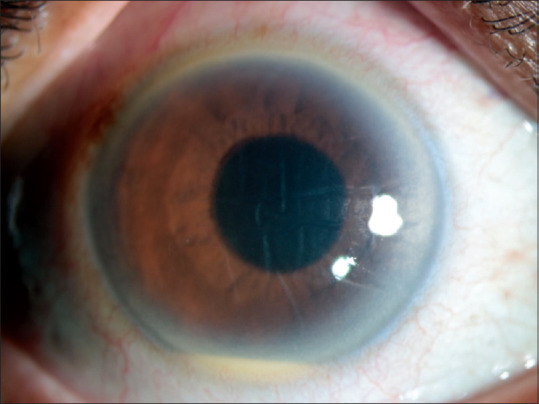
RE: Slit lamp photograph showing mild conjunctival congestion, corneal edema and a hypopyon of around 1 mm. The visual acuity of the patient had reduced to HM+ and retinal details were not visible due to intense vitritis. There was no lid edema
Figure 2.
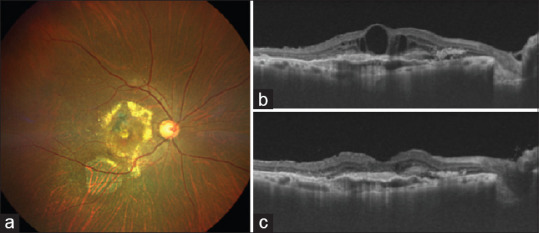
(a) Fundus photograph of the right eye 5 days after the brolucizumab injection. The vitritis had resolved and retinal details including 3rd order vessels and macula subretinal exudates due to PCV were visible. The OCT at this stage showed a marked resolution of SRF and IRF (c) as compared to the pre-Brolucizumab OCT (b). The visual acuity had improved to 20/600
Baumal et al. have reported IOI with retinal vasculitis as early as 14 days after brolucizumab injection.[3] Numerous causes of intraocular inflammation after brolucizumab injection have been postulated. Since brolucizumab is a single-chain antibody fragment that lacks the Fc region, it cannot activate the complement pathway or antibody-dependent cell-mediated cytotoxicity.[4] Local antibodies may form immune complexes leading to vasculitis through a mechanism of delayed hypersensitivity. Our case had immediate inflammation on the same day which has not been reported so far, and hence another possible mechanism could be related to endotoxins from E. coli. Our case was similar to Toxic Anterior Segment Syndrome (TASS).
In conclusion, while brolucizumab appears to be an effective drug in ARMD, including anti-VEGF resistant cases, it can cause serious inflammation which can be confused with endophthalmitis. Our case and other reports of IOI after brolucizumab injection calls for a modified protocol of examination and treatment compared to other anti-VEGF agents. All patients should be seen after day 1 with dilated fundus examination, and retina specialists should also avoid direct loading dose injections without a fundus examination every month. Brolucizumab should be used with caution after appropriate discussion with the patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dugel PU, Singh RP, Koh A, Ogura Y, Weissgerber G, Gedif K, et al. HAWK and HARRIER: Ninety-Six-Week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020 doi: 10.1016/j.ophtha.2020.06.028. S0161-6420 (20) 30570-4. [DOI] [PubMed] [Google Scholar]
- 2.Gaudreault J, Gunde T, Floyd HS, Ellis J, Tietz J, Binggeli D, et al. Preclinical pharmacology and safety of ESBA1008, a single-chain antibody fragment, investigated as potential treatment for age related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:3025. [Google Scholar]
- 3.Baumal CR, Spaide RF, Vajzovic L, Freund KB, Walter SD, John V, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127:1345–59. doi: 10.1016/j.ophtha.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–38. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]


