Abstract
Purpose:
COVID-19 infection, its treatment, resultant immunosuppression, and pre-existing comorbidities have made patients vulnerable to secondary infections including mucormycosis. It is important to understand the presentation, temporal sequence, risk factors, and outcomes to undertake measures for prevention and treatment.
Methods:
We conducted a retrospective, interventional study on six consecutive patients with COVID-19 who developed rhino-orbital mucormycosis and were managed at two tertiary ophthalmic referral centers in India between August 1 and December 15, 2020. Diagnosis of mucormycosis was based on clinical features, culture, and histopathology from sinus biopsy. Patients were treated with intravenous liposomal amphotericin B with addition of posaconazole and surgical debridement of necrotic tissue.
Results:
All patients were male, mean age 60.5 ± 12 (46.2–73.9) years, type 2 diabetics with mean blood glucose level of 222.5 ± 144.4 (86–404) mg/dL. Except for one patient who was diagnosed with mucormycosis concurrently with COVID-19, all patients received systemic corticosteroids for the treatment of COVID-19. The mean duration between diagnosis of COVID-19 and development of symptoms of mucor was 15.6 ± 9.6 (3–42) days. All patients underwent endoscopic sinus debridement, whereas two patients required orbital exenteration. At the last follow-up, all six patients were alive, on antifungal therapy.
Conclusion:
Mucormycosis is a life-threatening, opportunistic infection, and patients with moderate to severe COVID-19 are more susceptible to it. Uncontrolled diabetes mellitus and use of corticosteroids increase the risk of invasive fungal infection with mucormycosis which can develop during the course of the illness or as a sequelae. High index of suspicion, early diagnosis, and appropriate management can improve survival.
Keywords: Corticosteroids, COVID-19, diabetes mellitus, mucormycosis
Rhino-orbital infection with mucorales species of fungus is a dreaded condition with mortality rate of approximately 50% even with treatment. It is an opportunistic infection with invasion of the blood vessels by fungal hyphae, infarction, and necrosis of host tissue.[1] Mucormycosis has long been known to affect immunocompromised patients particularly those with uncontrolled diabetes. There are very few reported cases of concurrent mucormycosis and COVID-19 infection.[1,2,3] However, it is clear that several factors are responsible for the spike in incidence of rhino-orbito-cerebral mucormycosis. We present a series of cases with the hope of defining the demography of the population at risk, early diagnosis with typical clinical features, necessary investigations, and recommendations for the management of mucormycosis in patients with COVID-19.
Methods
We performed a retrospective interventional study of patients presenting at two tertiary referral centers with clinically and microbiologically proven cases of mucormycosis and concurrent or past history of COVID-19 infection. The demographic and clinical data were collected with the consent of the patients and with approval by the Institutional Review Board. The diagnosis of COVID-19 was based on RT-PCR test on nasopharyngeal/oropharyngeal swabs. Proven mucormycosis was defined as histopathologic, cytopathologic or direct microscopic examination showing fungal hyphae in biopsy specimen with associated tissue damage, or a positive culture result. Probable mucormycosis was concluded as the presence of combined host factors and clinical criterion with mycological evidence and if only the criteria for a host factor and a clinical criterion were met but mycological criteria were absent, possible mucormycosis was diagnosed.[4]
In the ophthalmology outpatient clinic, a deep nasal swab was sent for KOH mount and fungal culture based on the clinical suspicion. Magnetic resonance imaging (MRI) orbit, brain, and paranasal sinuses with or without computed tomography (CT) was performed for assessing extent of the disease. Based on the initial report of nasal swab and radiographic features, systemic antifungals were initiated in conjunction with otorhinolaryngology and infectious disease specialists. Liposomal amphotericin B (5 mg/kg/day, up to a maximum of 10 mg/kg/day for CNS infections; avoiding slow escalation) was given intravenously (IV) with monitoring of renal parameters.[5] Endoscopic sinus debridement with biopsy was performed and specimen was sent for histopathology, microbiology for culture, and sensitivity test. Oral antifungal, posaconazole (loading dose 300 mg twice a day on the first day, maintenance dose 300 mg orally once a day, starting on the second day) was initiated based on culture and histopathology report. Orbital exenteration was performed by the eyelid sparing technique with transverse blepharorrhaphy in patients with suboptimal response to systemic antifungals in 72 hours. Postoperatively, long term oral antifungal treatment was continued.
Results
There were six patients who presented between August and December 2020. All of them were men, with a mean age of 60.5 ± 12 (range 46.2 to 73.9) years. The geographic profile was limited to the Indian states of Maharashtra, Telangana, and Andhra Pradesh, each of them being referred cases. All of them suffered from type 2 diabetes mellitus for a mean duration of 5.9 ± 4.9 (median 6.5) years with two being diagnosed with the onset of COVID-19. Five patients had been initiated on insulin during the course of treatment for COVID-19 for uncontrolled blood sugars. At presentation, the average fasting blood sugar was 222.5 ± 144.4 (86-404) mg/dL. Fundus examination of Case 2 showed moderate nonproliferative diabetic retinopathy with diabetic macular edema. History of diabetic ketoacidosis was available from hospital records of three patients during COVID-19 treatment.
Case 1 was the only patient who presented with rhino-orbital-cerebral mucormycosis concurrently with COVID-19 infection and did not receive systemic steroids. The rest of the five cases were COVID-19 recovered patients, having been treated elsewhere for moderate to severe infection, requiring hospital admission. The mean duration between the diagnosis of COVID-19 and development of symptoms of mucormycosis was 15.6 ± 9.6 (3-42) days in these five cases. All patients complained of pain, redness, and periocular swelling as initial symptoms. This was followed by acute, progressive, drooping of eyelids, limitation of ocular movements, and painful loss of vision. The progress was rapid, an average of two days from the onset. Case 3 was treated with gabapentin for headache for a week before being referred [Figs. 1 and 2]. Systemic steroids had been given to all the patients in the form of oral prednisolone, intravenous dexamethasone, or intravenous methylprednisolone as part of the management of COVID-19 infection. Case 4 was already diagnosed and on antifungals when he came to us three months after the onset of symptoms for persistent loss of vision as the complaint. Case 5 had undergone sinus debridement elsewhere before being referred for orbital symptoms. Case 6 developed symptoms during the course of treatment for COVID-19 but was referred to us after 4 weeks, after having been initiated on intravenous amphotericin B.
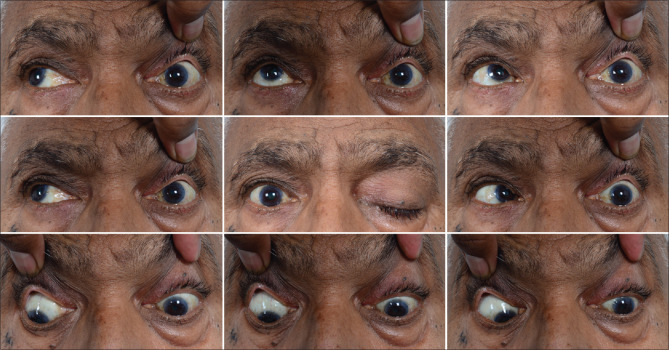
Figure 1.
Clinical pictures of Case 3 showing orbital apex involvement with complete ptosis and ophthalmoplegia of the left eye. The eye is quiet with no perception of light
Figure 2.
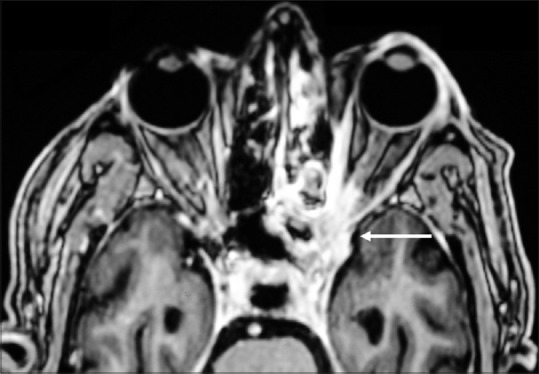
Axial scan of MRI orbit and brain of the same patient (Case 3) showing mild proptosis of the left eye, diffuse involvement of the ethmoid and sphenoid sinus, orbital apex (white arrow) and extension into the cavernous sinus
All the patients were conscious and oriented. At presentation to us, five patients denied perception of light, whereas Case 2 had best-corrected vision of 6/60 in the affected eye on the Snellen's chart. Except for Case 4, all patients had eyelid and periocular edema, complete ptosis, total ophthalmoplegia, proptosis and relative afferent pupillary defect. [Fig. 3] Black discharge or eschar was observed in the nasal cavity of Cases 1, 5, and 6. Palatal eschar was evident in two patients. Table 1 shows the clinical findings, management, and outcome of the six cases.
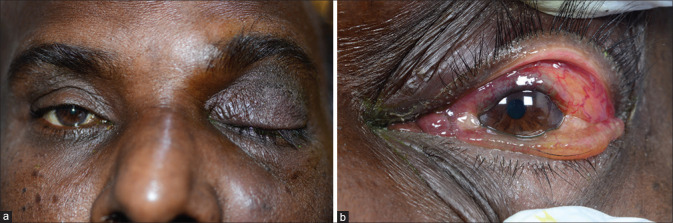
Figure 3.
Clinical picture of Case 2 with (a) left periocular edema, complete ptosis and (b) proptosis, conjunctival congestion, and severe chemosis
Table 1.
The clinical profile, management and outcome of patients with rhino-orbito-cerebral mucormycosis associated with or following COVID-19 infection. *Nasal swab was not sent for Cases 1 and 5 as FESS had already been done elsewhere and specimen sent for microbiological tests when the patient was referred to us. Case 4 presented to us after treatment by otorhinolaryngologists
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Age (years) | 46.2 | 60.9 | 73.9 | 72.9 | 62 | 47 |
| Gender | M | M | M | M | M | M |
| Systemic illness | DM | DM, HTN | DM, HTN, CAD | DM | DM, HTN | DM, CAD |
| Duration of Diabetes (years) | 12 | 10 | 7 | 6 | 2 months | 1 month |
| FBS at presentation (mg/dL) | 400 | 404 | 96 | 157 | 86 | 192 |
| HbA1c | NA | 10.5 | 5.6 | NA | NA | 12 |
| COVID-19 treatment with corticosteroids | No | IV methylprednisolone, oral prednisolone | IV dexamethasone, oral prednisolone | Oral prednisolone | IV dexamethasone | IV dexamethasone |
| Duration between COVID-19 and rhino-orbital symptoms (days) | 0 | 17 | 30 | 14 | 42 | 3 |
| Duration of symptoms (days) | 4 | 10 | 42 | 42 | 14 | 30 |
| Eye | OD | OS | OS | OD | OS | OD |
| Vision | No PL | 6/60 | No PL | No PL | No PL | No PL |
| Ocular movements | Total ophthalmoplegia | Total ophthalmoplegia | Total ophthalmoplegia | Recovered | Total ophthalmoplegia | Total ophthalmoplegia |
| Pupil | No view | Regular, reacting to light | RAPD | fixed | fixed | No view |
| Ptosis | Yes | Yes | Yes | Resolved | Yes | Yes |
| Proptosis (mm) | 5 | 7 | 4 | Resolved | 4 | 3 |
| Conjunctival chemosis | Yes | Yes | No | No | Yes | Yes |
| Fundus | No view | Disc pallor, NPDR, DME | Disc hyperemic | Disc pallor | Normal | No view |
| Nasal sign | Yes | No | No | No | Yes | Yes |
| Palatal eschar | No | No | No | No | Yes | Yes |
| CNS extension | Right cavernous sinus, frontoparietal lobe | Absent | Left cavernous sinus, perygopalatine fossa | Orbital apex, cavernous sinus | SOV thrombosis, pachymeningitis | Cavernous sinus thrombosis, temporal lobe abscess, partial thrombosis of ICA |
| Nasal swab | NA* | Negative | Negative | NA* | NA* | Negative |
| FESS | Yes | Yes | Yes | Yes | Yes | Yes |
| Exenteration | Yes | No | No | No | Yes | No |
| Culture for mucor | No | Yes | No | Yes | Yes | Yes |
| Histopathology for mucor | Yes | Yes | No | Yes | Yes | Not done |
| Duration of follow-up (months) | 3 | 0.9 | 1.3 | 1.3 | 1.6 | 0.3 |
| Life salvage | Yes | Yes | Yes | Yes | Yes | Yes |
| Eye salvage | No | Yes | Yes | Yes | No | Yes |
| Vision salvage | No | No | No | No | No | No |
| Mucormycosis [4] | Proven | Proven | Possible | Proven | Proven | Proven |
CNS= Central Nervous System, CAD= Coronary artery disease, DM= Diabetes mellitus, DME= Diabetic macular edema, FESS= Functional Endoscopic Sinus Surgery, HTN= Hypertension, ICA= Internal carotid artery, M= male, NA= Not available, NPDR= non proliferative diabetic retinopathy, OD= Right eye, OS= Left eye, PL= Perception of light, RAPD= relative afferent pupillary defect, SOV= Superior ophthalmic vein)
A deep nasal swab from the affected side was sent for fungal culture for three patients. However, there was no growth from any of the specimen. Imaging revealed pansinusitis with orbital involvement. Intracranial extension with cavernous sinus involvement was present in all except Case 2. All patients underwent functional endoscopic sinus surgery (FESS) with sinus debridement by otorhinolaryngologists. Intraoperatively, necrotic tissue was noted consistent with mucormycosis. The culture report was positive for mucor in Cases 2, 4, 5, and 6. There was histopathological evidence of fungal hyphae typical of mucor with tissue invasion in all cases except Case 3 which was diagnosed as possible mucor and Case 6 for which histopathological examination was not done. Oral posaconazole was initiated in 5 patients (except case 6). The antifungals were prescribed and monitored by infectious disease specialists.
Initial FESS carried out for Case 1 showed aspergillus as the infective fungus on microbiology culture. The patient was treated with liposomal amphotericin B with voriconazole. In view of worsening orbital and sinus involvement, an eyelid sparing orbital exenteration with transverse blepharorrhapy was performed under general anesthesia on day 7 along with a repeat endoscopic sinus debridement. Intraorbital irrigation with amphotericin B was done for 1 week. The drain was removed after four postoperative days. The histopathology and microbiology were consistent with mucormycosis with superadded pseudomonas infection. The patient also received appropriate antibiotics (cefoperazone + sulbactam) as per the sensitivity report. Postoperatively Case 1 developed a sino-cutaneous fistula through the exenteration wound. The patient is on follow-up with serial MRI for the stable intracranial component.
Case 4 had received treatment on the same line elsewhere. His ptosis, ocular movements, and proptosis had resolved on presentation [Figs. 4 and 5]. There was residual and progressive sinusitis. Case 5 underwent an eyelid sparing exenteration on day 14 for persistent, extensive orbital disease. The patient, however, developed eyelid necrosis with wound gape which healed secondarily with granulation. At the time of the last follow-up, all patients are alive and stable on liposomal intravenous amphotericin B with or without oral posaconazole with regular monitoring.
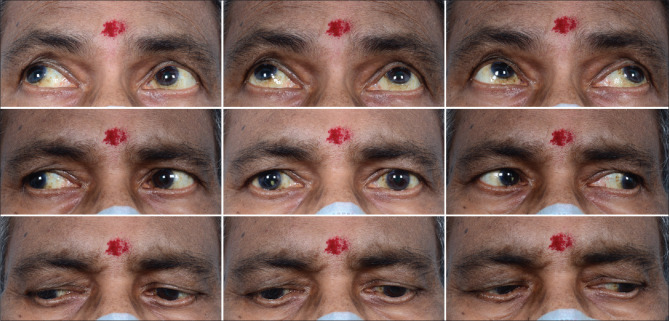
Figure 4.
Clinical picture of Case 4 after treatment with antifungals and surgical debridement with resolution of orbital inflammation and recovery of ocular movements of the right eye
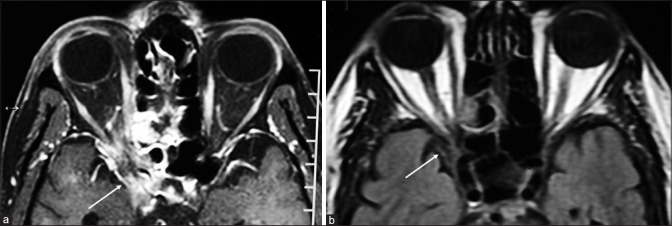
Figure 5.
T1 Axial section of MRI orbit with brain showing (a) invasive fungal infection involving the ethmoid sinus, orbital apex and ipsilateral cavernous sinus (white arrow). (b) Post treatment with antifungals and endoscopic sinus debridement showing minimal residual lesion (white arrow)
Discussion
Mucormycosis of the orbit is a vision-threatening and potentially fatal infection resulting in angioinvasion, mycotic thrombosis, and ischemic necrosis of tissues. Mucor, Rhizopus, Rhizomucor, Abidia, Apophysomyces, Saksenaea, and Cunninghumella are the common organisms of the Mucorales order responsible for the infection.[6,7] The incidence varies from 0.005 to 1.7 per million population and the global case fatality rate is as high as 46%.[1,8] Depending on the site of infection it is classified as rhinocerebral/sino-orbital, pulmonary, cutaneous, gastrointestinal, and disseminated.[9]
The factors predisposing to the development of the infection are uncontrolled diabetes mellitus, neutropenia, elevated free iron levels, deferoxamine, hematological malignancies, stem cell transplants, and organ transplant patients on immunosuppressants.[9] The spores are inhaled into the nasal or oral cavity from where they make their way into the paranasal sinuses. The orbit is accessible through the thin lamina papyracea of the ethmoid bone, infratemporal fossa, inferior orbital fissure, or orbital apex. The cribriform plate of ethmoid, supraorbital fissure, and perineural invasion are potential gateways to intracranial extension. Cavernous sinus thrombosis, sagittal sinus thrombosis, carotid occlusion, cerebral infarction, intracranial aneurysm/hemorrhage and cerebral abscesses are direct consequences.
Although all the sequelae and complications of COVID-19 are yet to be documented and described, spike in secondary infections are being increasingly recognized worldwide. Patients with COVID-19 are more vulnerable to fungal infection because of the compromised immune system with decreased CD4+ and CD8+ lymphocytes, associated comorbidities such as diabetes mellitus which potentiates both the conditions, decompensated pulmonary functions, and the use of immunosuppressive therapy for the management in moderate to severe cases.[2] The infections are also more likely in patients with severe COVID-19 disease and in those requiring intensive care unit admission or mechanical ventilation. The rate of secondary infection during hospital stay in patients with COVID-19 (bacterial and fungal) has been reported to be 8%.[2,10] In a review, Song et al. noted that the fungal infections are more likely to develop during the middle and later stages of COVID-19 infection.[11] The mortality rate is also higher (53% with vs 31% without invasive fungal infection) amongst the patients of COVID-19 with secondary fungal infection.[2]
Till date, there are three isolated case reports of sino-orbital mucormycosis reported in the world.[1,2,3] The patients also had uncontrolled diabetes. However, in these cases, the patient either presented with orbital symptoms or developed during the course of treatment for COVID-19. Patients received systemic antibiotics for sepsis and liposomal amphotericin B either empirically or after confirmation but all patients succumbed.
In our series, all, except one (Case 1) patient presented after recovering from COVID-19 with documented RT-PCR negative reports. Cases 2-6 had received intravenous and/or oral steroids and all were diabetics. Uncontrolled diabetic status was evident by presence of diabetic retinopathy, history of diabetic ketoacidosis, and available blood sugar reports. Approximately 70% of rhino-orbital-cerebral mucormycosis is seen in patients with uncontrolled diabetes and most of them have ketoacidosis at the time of presentation.[1] What is interesting to note in our series is that symptoms of rhino-orbital mucormycosis developed as late as 30–42 days after the diagnosis of COVID-19.
For successful management of mucormycosis, a high index of clinical suspicion, low threshold for diagnosis in patients with risk factors, neuroimaging, and specific diagnostic tests with a coordinated effort from a multidisciplinary team including ophthalmology, otorhinolaryngology, infectious diseases, neurosurgery, critical care, microbiology, and pathology department is crucial. As majority of COVID-19 patients with moderate-to-severe disease are attended by pulmonary and intensive care teams, it is important to sensitize them about the early signs and symptoms to prevent delayed diagnosis and ensure timely referrals. A delay of even six days in initiating treatment doubles the 30-day mortality from 35% to 66%.[1] Radiological findings may be non-specific initially. Thickened mucosal lining and sinus opacification are universally present. Serial radiological investigations are required to assess progression and extent.[9] It has also been observed that following treatment, the radiological features may take longer to resolve than the clinical signs possibly because of a persistent inflammation. PET-CT is a useful tool for detection and assessment of response to treatment, limited by cost of repeated imaging.[12,13,14]
Surgical debridement (FESS and/or orbital exenteration) not only reduces the disease burden, allows better penetration of intravenous drugs, and limits further spread of the disease but also allows intraoperative diagnosis with characteristic necrotic tissue and provides sample for histopathological and microbiological confirmation.[8] There are multiple tests available to diagnose fungal infection[11]:
Direct microscopy and culture- Culture should be done separately at 30°C and 37°C. Mucor colonies are typically cottony white or greyish black.
Histopathology is considered more precise for the diagnosis than culture. Hematoxylin and eosin, Periodic acid-Schiff stain, and Gomori's methenamine silver stains show broad, non or pauci-septate fungal hyphae with tissue invasion.
Serology- antigen and b-antibody, (1,3) D-Glucan and Galactomannan detection by serum for suspected cases.
Polymerase chain reaction (PCR) based molecular identification. DNA sequencing based on bar codes 18S, ITS, 28s, rDNA, MALDI-TOF are available in limited laboratories but offer the advantage that they can detect fungal DNA in serum and paraffin-embedded tissues. PCR is also a rapid test as compared to histopathology and culture.
Orbital exenteration is done in cases with no visual potential, where the disease is limited to the orbit without or minimal extension to the cavernous sinus. The decision to exenterate lies with the treating physician because there is no firm consensus regarding the indications and timing of exenteration.[15] No significant difference has been found in survival with or without orbital exenteration. Several case reports have illustrated the management of sino-orbital mucormycosis without exenteration. Some series have even found exenteration to be detrimental to survival and allowing further dissemination of the disease.[16,17] A retrospective case series showed that for limited sino-nasal disease, surgical sino-nasal debridement achieved success in 94.4% of the cases. On the other hand, patients with the rhino-orbital disease treated with exenteration along with sinus debridement had a treatment failure with progression/mortality in 88.9% of the cases albeit the worse systemic and more severe disease.[18] However, it is an important management modality especially in patients with extensively involved, necrotic orbital tissue.[7]
Antifungals are the mainstay of management along with surgical debridement. The global guidelines for diagnosis and management of mucormycosis in 2019 by the European Confederation of Medical Mycology ECMM and Mycoses Study Group Education and Research Consortium (MSGERC) strongly recommends an early complete surgical treatment whenever possible in addition to systemic antifungal treatment.[5] Amphotericin B lipid complex, liposomal Amphotericin B and Posaconazole oral suspension are treated as first-line antifungals monotherapy and isavuconazole is supported as salvage therapy.[5,10] Posaconazole is often combined with liposomal amphotericin B with refractory mycosis or those who are intolerant to amphotericin B. Irrigation of orbit and sinuses with amphotericin B (1 mg/ml) increases the local concentration of the drug and has been shown to improve outcomes.[7] Retrobulbar and intraorbital injection of amphotericin B can be given in patients who are unable to undergo aggressive surgical debridement (1 ml of 3.5 mg/ml with the antecedent retrobulbar injection of anesthetic).[7,19] Hyperbaric oxygen therapy has also shown favorable survival outcomes, but it was not used in any of our patients.
Corticosteroids are part of the guidelines for management of moderate to severe COVID-19 cases with proven benefit against the cytokine storm. The World Health Organization strongly recommends systemic corticosteroids (intravenous or oral) rather than no corticosteroids for the treatment of patients with severe and critical COVID-19. They conditionally recommend not to use systemic corticosteroids in the treatment of non-severe cases. The risk of superinfections was found to be low (RR 1.01, 95% CI 0.90–1.13).[20] The current guidelines in India recommend intravenous methylprednisolone 0.5–1 mg/kg/day or dexamethasone 0.1–0.2 mg/kg for 3 days for moderate cases (preferably within 48 hours of admission or if oxygen requirement is increasing and if inflammatory markers are elevated). IV methylprednisolone 1–2 mg/kg/day or dexamethasone 0.2–0.4 mg/kg for 5–7 days is advised for severe cases. Patients with progressive deterioration of oxygen indicators, rapid worsening on imaging, and excessive activation of body's inflammatory response, can be treated with glucocorticoids for a short period of time (3–5 days). (The dose should not exceed equivalent of methylprednisolone 1–2 mg/kg/day or dexamethasone 0.2–0.4 mg/kg/day).[21] The NIH recommends the use of injection dexamethasone 6 mg/day for a maximum of 10 days for patients who are ventilated or require supplemental oxygen.[2,6] The ECMM and MSGERC recommend prophylactic posaconazole in neutropenic patients, those with graft versus host disease or high-risk factor.[5] This may be considered in severe cases of COVID-19 with diabetes requiring corticosteroids.
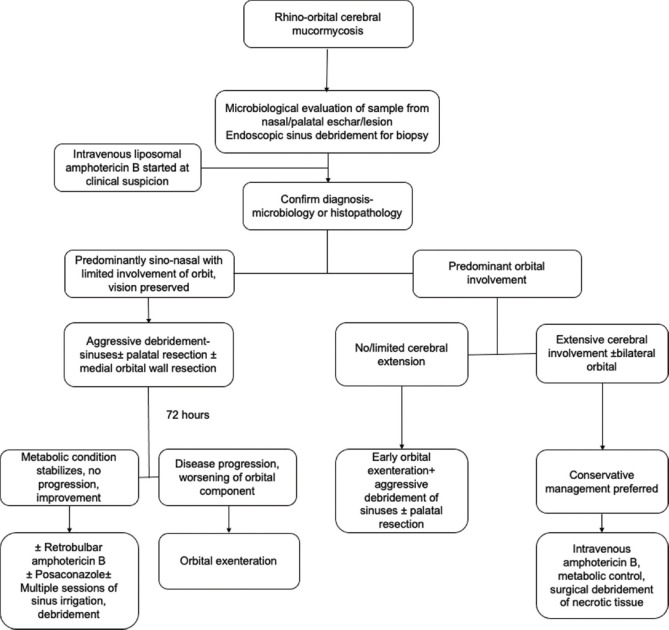
Song et al. have given a diagnostic and therapeutic pathway for invasive fungal co-infection in patients with COVID-19 and Fig. 6 shows the recommendations for invasive mucormycosis.[11] In the absence of clear, evidence-based indications, Fig. 7 gives a management protocol followed by us, which may be helpful for ophthalmologists in deciding the need and timing of orbital exenteration.
Figure 6.
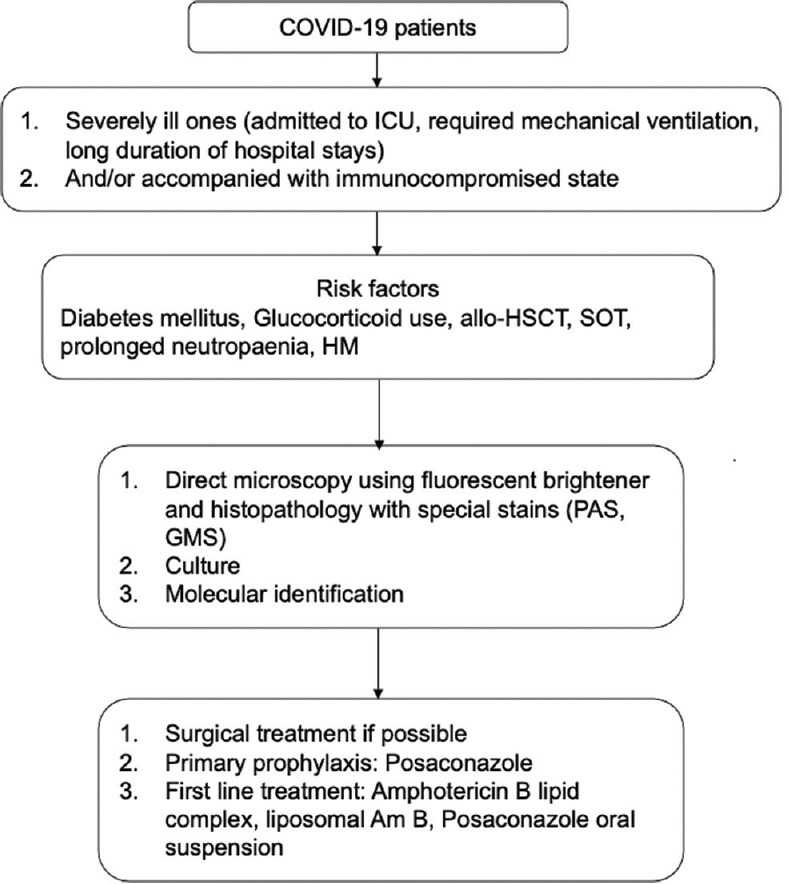
Guideline for diagnosis and treatment of mucormycosis in patients with COVID-19 (Modified from Song et al.[10]). Abbreviations: allo-HSCT = Allogenic hematopoietic stem cell transplant, GMS = Gomori's methenamine silver, HM = Hematopoietic malignancies, PAS = Periodic acid-Schiff stain, SOT = Solid organ transplant
Figure 7.
Proposed guideline for surgical debridement and orbital exenteration for rhino-orbito-cerebral mucormycosis based on disease extent
This is a series of cases of orbital mucormycosis that presented to us as a sequelae of COVID-19 and its management. Although the patients are still being actively managed, we believe that these must be reported on priority because there is an urgent need to sensitize specialist teams dealing with COVID-19 patients to look for early signs in high-risk cases so that there is no delay in diagnosis and referrals. The signs and symptoms of orbital mucormycosis are not different from those of mucormycosis in non-COVID-19 patients. Simple tests like vision, pupil, ocular motility, and sinus tenderness can be part of routine physical evaluation of a COVID-19 patient hospitalized with moderate to severe infection or diabetics with COVID-19 or those receiving systemic corticosteroids. Although tissue necrosis is a late sign, the palate can be examined for any eschar. A nasal swab for KOH mount and culture is a bedside procedure. Orbital exenteration for life-threatening infection is triaged as a Level A condition or an urgent condition requiring surgery within 4–72 hours as per the preferred practice pattern advised in our country during COVID-19.[22] Thus, appropriate surgery has to be undertaken with full personal protective equipment.
Based on the available literature, systemic corticosteroids are recommended for moderate to severe COVID-19 cases and have been shown to reduce mortality. But the treating physicians must be careful about the need and doses for specific severe cases with strict monitoring of blood sugar levels. Evidence-based guidelines for prophylactic use of antifungals for patients at risk is required while keeping in mind the risk of development of drug resistance. Patients should also be made aware about the risks involved with the treatment and the need for strict glycemic control. Development of unilateral facial or orbital pain, headache, periocular swelling, double vision or diminution of vision should prompt even the COVID-19 recovered patients to seek immediate medical attention. As majority of our patients developed symptoms of mucormycosis after recovering from COVID-19, follow-up of high-risk patients with COVID-19 for sequelae is imperative. In our series, a high index of suspicion, combined efforts from multiple departments, and timely surgical and medical management were critical aspects for ensuring survival. Visual prognosis continues to remain poor. Further studies are required to determine the significance and relative risk of each variable involved in the pathogenesis and survival outcome including other medications used for COVID-19, hematological parameters, and inflammatory markers apart from virus, corticosteroids, and diabetes. Other fungal infections of the orbit including isolated and coinfections with aspergillus have also seen a rise in numbers and their reporting is also important.
Our study is limited by short follow-up and geographical localization of cases, but the primary aim was to report the increasing incidence of mucormycosis following COVID-19 infection.
Conclusion
COVID-19 pandemic has resulted in widespread mortality, morbidity, social and economic upheavals of an unprecedented magnitude. Global scientific collaboration and reporting of new information related to this is of paramount importance to increase the knowledge with regard to the novel viral infection. The incidence of rhino-orbito-cerebral mucormycosis is likely to rise, both as a co-infection and as a sequelae of COVID-19. Early diagnosis and management with appropriate and aggressive antifungals and surgical debridement can improve survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to acknowledge the contributions of Dr Navela Philips, Dr Ramanna Lakshmi and Dr Aman Vaishya in the collection of clinical data.
References
- 1.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.09.032. doi: 10.1016/j.ajem. 2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19? Cureus. 2020;12:e10726. doi: 10.7759/cureus.10726. doi: 10.7759%2Fcureus. 10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mekonnen ZK, Ashraf DC, Jankowski T, Grob SR, Vagefi MR, Kersten RC, et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plastic Reconstr Surg. 2020 doi: 10.1097/IOP.0000000000001889. doi: 10.1097/iop. 0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SC, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405–21. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah K, Dave V, Bradoo R, Shinde C, Prathibha M. Orbital exenteration in rhino-orbito-cerebral mucormycosis: A prospective analytical study with scoring system. Indian J Otolaryngol Head Neck Surg. 2019;71:259–65. doi: 10.1007/s12070-018-1293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AS, Lee PW, Allworth A, Smith T, Sullivan TJ. Orbital mycoses in an adult subtropical population. Eye. 2020;34:1640–7. doi: 10.1038/s41433-019-0733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox A, Janson B, Stiff H, Chung A, Benage M, Van Heukelom J, et al. A multidisciplinary educational curriculum for the management of orbital compartment syndrome. Am J Emerg Med. 2020;38:1278–80. doi: 10.1016/j.ajem.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Papadogeorgakis N, Parara E, Petsinis V, Vourlakou C. A case of successfully treated rhinocerebral mucormycosis: Dental implications. Int J Dent. 2010;2010 doi: 10.1155/2010/273127. doi: 10.1155/2010/273127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawson TM, Moore LS, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa530. doi: 10.1093%2Fcid%2Fciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: A clinical and diagnostic perspective from China. Mycopathologia. 2020:1–8. doi: 10.1007/s11046-020-00462-9. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukherjee B, Raichura ND, Alam MS. Fungal infections of the orbit. Indian J Ophthalmol. 2016;64:337–45. doi: 10.4103/0301-4738.185588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Wu H, Huang F, Fan Z, Xu B. Utility of 18F-FDG PET/CT in diagnosis and management of mucormycosis. Clin Nucl Med. 2013;38:e370–1. doi: 10.1097/RLU.0b013e3182867d13. [DOI] [PubMed] [Google Scholar]
- 14.Altini C, Ferrari C, Rubini D, Dicuonzo F, Rubini G. (18) F-FDG PET/CT contribution to diagnosis and treatment response of rhino-orbital-cerebral mucormycosis. Hell J Nucl Med. 2015;18:68–70. doi: 10.1967/s002449910167. [DOI] [PubMed] [Google Scholar]
- 15.Hargrove RN, Wesley RE, Klippenstein KA, Fleming JC, Haik BG. Indications for orbital exenteration in mucormycosis. Ophthalmic Plast Reconstr Surg. 2006;22:286–91. doi: 10.1097/01.iop.0000225418.50441.ee. [DOI] [PubMed] [Google Scholar]
- 16.Songu M, Unlu HH, Gunhan K, Ilker SS, Nese N. Orbital exenteration: A dilemma in mucormycosis presented with orbital apex syndrome. Am J Rhinol. 2008;22:98–103. doi: 10.2500/ajr.2008.22.3121. [DOI] [PubMed] [Google Scholar]
- 17.Pelton RW, Peterson EA, Patel BC, Davis K. Successful treatment of rhino-orbital mucormycosis without exenteration: The use of multiple treatment modalities. Ophthalmic Plast Reconstr Surg. 2001;17:62–6. doi: 10.1097/00002341-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Nithyanandam S, Jacob MS, Battu RR, Thomas RK, Correa MA, D'Souza O. Rhino-orbito-cerebral mucormycosis.A retrospective analysis of clinical features and treatment outcomes. Indian J Ophthalmol. 2003;51:231–6. [PubMed] [Google Scholar]
- 19.Hirabayashi KE, Kalin-Hajdu E, Brodie FL, Kersten RC, Russell MS, Vagefi MR. Retrobulbar injection of amphotericin B for orbital mucormycosis. Ophthalmic Plast Reconstr Surg. 2017;33:e94–7. doi: 10.1097/IOP.0000000000000806. [DOI] [PubMed] [Google Scholar]
- 20. [[Last accessed on 2020 Dec 17]]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 .
- 21. [[Last accessed on 2020 Dec 17]]. Available from: https://www.mohfw.gov.in/pdf/UpdatedClinical Management ProtocolforCOVID19 dated03072020.pdf .
- 22.Ali MJ, Hegde R, Nair AG, Bajaj MS, Betharia SM, Bhattacharjee K, et al. All India Ophthalmological Society-Oculoplastics Association of India consensus statement on preferred practices in oculoplasty and lacrimal surgery during the COVID-19 pandemic. Indian J Ophthalmol. 2020;68:974–80. doi: 10.4103/ijo.IJO_1415_20. [DOI] [PMC free article] [PubMed] [Google Scholar]