Abstract
Purpose:
The aim of this study was to present the outcomes of the 2018 and 2020 Vitreo-retinal Society of India (VRSI) biosimilars of anti-vascular endothelial growth factor (VEGF) (VIBE) surveys.
Methods:
An online survey of members of VRSI was conducted in July 2018 and January 2020 regarding their practice-patterns on anti-VEGF biosimilars pertaining to safety, efficacy, pricing, and need for enhanced clinical trials before regulatory approval.
Results:
In 2018, 112 VRSI members participated, whereas in 2020, 98 society members participated. In both surveys, majority of respondents were aware of biosimilars (96%, 2018 vs. 100%, 2020; P = 0.9) and felt that approval of biosimilar drugs should be made more stringent with larger clinical trials (89%, 2018 vs. 91%, 2020; P = 0.93). An increase in use of ranibizumab-biosimilar (41%, 2018 to 56%, 2020; P = 0.2) and a simultaneous significant decline in use of bevacizumab-biosimilar (9%, 2018 to 2%, 2020; P = 0.04) was noted from 2018 to 2020. From 2018 to 2020, the proportion of respondents satisfied with safety (61% to 68%; P = 0.59) and efficacy (65% to 81%; P = 0.32) of ranibizumab-biosimilar increased. However, during the same period, we noted in reduction in satisfaction levels with safety of bevacizumab-biosimilar (30% to 25%; P = 0.54), whereas satisfaction with its efficacy was stable (29% vs 30%; P = 0.99). A substantial proportion of retina specialists considered that current cost of ranibizumab-biosimilar ($130) was sufficiently low for it to be used as a substitute for Avastin (37%, 2018 and 40%, 2020; P = 0.82).
Conclusion:
The VRSI surveys reveal that Indian vitreoretinal specialists are familiar with anti-VEGF biosimilars. There was a progressive trend favoring ranibizumab-biosimilar over bevacizumab-biosimilar. One-third of the participants deem the current price of ranibizumab-biosimilar as appropriate to replace Avastin. Simultaneously, the need for enhanced pharmacovigilance and larger clinical trials are warranted for regulatory approval of these agents.
Keywords: Bevacizumab, biosimilars, ranibizumab, Razumab, Vitreoretinal Society of India
Biologics are therapeutic proteins derived from living organisms through biotechnology-based processes.[1] Biologics that inhibit the actions of vascular endothelial growth factor (anti-VEGFs) have revolutionized the management of various chorioretinal disorders including neovascular age-related macular degeneration (nAMD), diabetic macular edema (DME), and macular edema due to retinal vein occlusions (RVO).[2] The recombinant anti-VEGF drugs bevacizumab (Avastin®; Genentech, S. San Francisco, CA/Roche, Basel, Switzerland), ranibizumab (Lucentis?®; Genentech, S. San Francisco, CA/Roche, Basel, Switzerland), aflibercept (Eylea®, Regeneron, Tarrytown, NY), and brolucizumab (Beovu®; Novartis, Basel, Switzerland) are used widely throughout the world.[3,4,5] Ranibizumab, aflibercept, and brolucizumab are approved by the US Food and Drug Administration (FDA) for the management of retinal conditions,[3,4,5] whereas bevacizumab, which is approved for the treatment of several advanced solid malignancies, is used off-label by ophthalmologists.[6]
Treatment of chorioretinal vascular conditions usually requires that intravitreal injections be repeated over the course of many years, thereby incurring high cumulative drug costs. This has prompted physicians, insurers, and health systems to search for less expensive alternatives such as biosimilars. Biosimilars are produced with reverse engineering techniques in living cell lines, with safety, efficacy, structure, pharmacodynamics, and pharmacokinetic features that resemble approved biologics.[7] A biologic drug usually takes 10–15 years to develop at a cost of US $1.2-1.5 billion, as less research and development (R&D) is required to produce biosimilars, and fewer clinical trials are necessary to obtain regulatory approval, they can be produced within a period of 5–10 years at a cost of US $120–150 million.[7,8] As a result, the unit cost of a biosimilar is considerably less than the innovator molecule.[9]
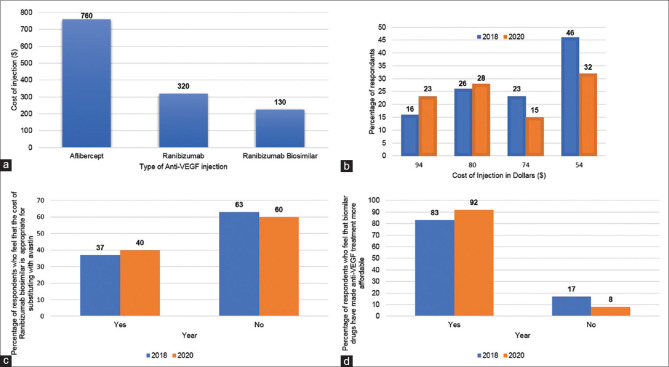
Cost is an important determinant of medical decision making and drug selection in most countries and this makes a shift towards less expensive biosimilars an attractive strategy. India's enormous market for biologics, valued at more than US $4 billion (the third highest in the Asia-Pacific region),[8] drove the development of Razumab® (Intas Pharmaceuticals, Ahmedabad, India), the first and only biosimilar of Lucentis.[10] Razumab was approved by the Drug Controller General of India (DGCI) in 2015 after a phase 3 trial showed its efficacy in 103 eyes with nAMD.[11] Razumab costs US$130 per dose, compared to Lucentis (Branded Accentrix) ($320) and Eylea ($760) in India [Fig. 1, Graph 1a]. A bevacizumab biosimilar is available but far fewer physicians use it.
Figure 1.
Economics of anti-VEGF biosimilars. (a). Price of anti-VEGF injection in India (US dollars; $); (b). Graph showing ewillingness to payf price of ranibizumab biosimilar (Razumab) at which the respondents would switch from Avastin. (c). Graph showing similar proportion of respondents in 2018 and 2020 who are of the opinion that the current price of the ranibizumab biosimilar warranted a switch from Avastin. (d). Graph showing that a majority of respondents believe that biosimilars have made anti-VEGF treatment more affordable
Through March 2020, 120,582 Razumab injections had been administered in India. Razumab use had been probably limited by clusters of sterile endophthalmitis cases reported from a few cases used in the earlier batches.[10] Intas modified the manufacturing process to lower the risk of inflammation, but some retina specialists remained hesitant to use the drug.
To better understand the attitudes of Indian vitreo-retina specialists toward biosimilars, the Vitreo-retinal Society of India (VRSI) conducted online surveys in 2018 and 2020. Questions regarding drug safety, efficacy, and economic impact were designed to address physician's perceptions and experience with anti-VEGF biosimilars. The goals of this manuscript are to present the outcomes of the 2018 and 2020 VRSI biosimilars of anti-VEGF (VIBE) surveys and to describe changes in retinal physician's perception of biosimilar agents over the two-year period.
Methods
Electronic surveys were sent to members of the VRSI in July 2018, and January 2020, and recipients were asked to complete the online survey within 15 days. The survey was open for both regular and associate life members of VRSI. The membership criteria for both (Based on Article 5 of the VRSI Constitution) include:
Regular life members
All Ophthalmologists (with degree recognized by MCI) with documented fellowship training in the field of Retina and Vitreous residing in India will be eligible. Applicants should have completed fellowship training of at least 1 year, or senior residency of 2 years in a Retina Unit of a medical college.
Associate life members
All Ophthalmologists with Secondary interest in the field of Retina Vitreous and Allied disciplines and Scientists, Health personnel engaged in research in the field and residing in India will be eligible.
The biosimilars survey assessed members' perceptions of efficacy, safety, pricing (Indian rupee; INR), and the need for more clinical trials before drugs could be approved. In the survey, selection of appropriate pricing for Razumab was a forced choice. The options were based on the variations in the injection procedure cost, derived from a VRSI survey on anti-VEGF in 2018. Although the selections were mentioned as INR in the survey, for standardization purposes we are providing the corresponding United States dollars (USD) values, derived from INR values, in the manuscript ($54/INR 4000; $74/INR 5500; $80/INR 6000 and $94/INR 7000). The questionnaire is available as Supplemental Appendix 1.
Results are presented in the form of descriptive statistics, frequency tables, and doughnut charts. Most responses are reported as nominal data, whereas the question on appropriate pricing is reported as ordinal data. Data was analyzed using Excel (Microsoft, Richmond, USA) and STATA 16.1 (STATA Corp, LLC, College Station, Texas, TX, USA). Comparisons of 2018 and 2020 data were performed using the Chi-square test. Variables with P value <0.05 were considered as statistically significant.
Results
In 2018, 112 members (out of 700 e-mails delivered) of the VRSI participated in the biosimilar survey and in 2020, 98 society members (out of 826 e-mails delivered) participated.
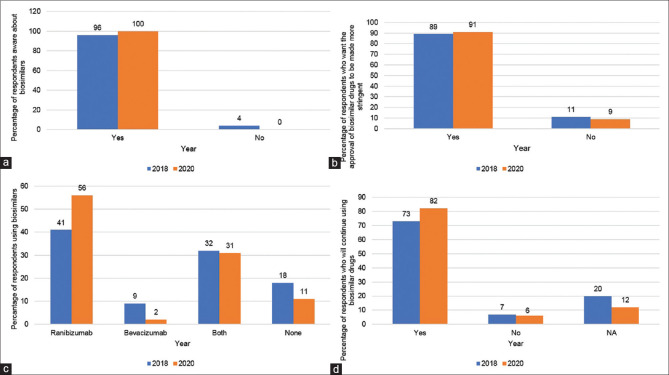
In both 2018 and 2020, the majority of respondents were aware of anti-VEGF biosimilars (108 (96%) vs.; 98 (100%); P = 0.9) [Fig. 2, Graph 2a]. Most participants believed that biosimilar drugs should be approved only after the completion of clinical trials that are larger and better designed than those already performed (89% in 2018 vs. 91% in 2020; P = 0.93) [Fig. 2, Graph 2b].
Figure 2.
Outlook towards anti-VEGF biosimilars. Bulk of the respondents in the VIBE surveys were aware regarding biosimilars (a) and feel that they require more stringent procedure for approval (b). From 2018 to 2020, there was a rise in proportion of respondents using ranibizumab biosimilar, while simultaneously, the usage of bevacizumab biosimilars declined (c). Similar trend was also noted when the respondents favored use ranibizumab biosimilar (d)
Choice of anti-VEGF biosimilars
The proportion of respondents using a ranibizumab biosimilar increased from 41% in 2018 to 56% in 2020 (P = 0.2), whereas those using a bevacizumab biosimilar decreased from 9% to 2% (P = 0.04) [Fig. 2, Graph 2c]. When respondents were asked if they would continue using biosimilars in the future, they were more likely to use a ranibizumab biosimilar (increase from 73% to 82%) compared to a bevacizumab biosimilar (decrease from 7% to 6%) (P = 0.31) [Fig. 2, Graph 2d].
Safety and efficacy of anti-VEGF biosimilars
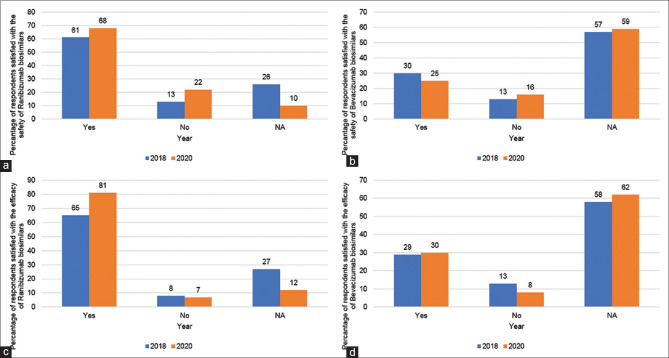
The proportion of respondents who were satisfied with the safety of ranibizumab biosimilar increased from 61% in 2018 to 68% in 2020 (P = 0.59), [Fig. 3 Graph 3a], whereas those satisfied with the safety of the bevacizumab biosimilar decreased from 30% to 25% (P = 0.54) [Fig. 3, Graph 3b]. From 2018 to 2020, there was a substantial increase in the proportion of respondents satisfied with the efficacy of the ranibizumab biosimilar (65% vs. 81%; P = 0.32) [Fig. 3, Graph 3c], whereas the proportion of respondents satisfied with the efficacy of the bevacizumab biosimilar did not change (29% to 30%; P = 0.99) [Fig. 3, Graph 3d].
Figure 3.
Safety and efficacy of anti-VEGF biosimilars. From 2018 to 2020, we noted an increase in satisfaction levels with the safety (61% to 68%) and efficacy (65% to 81%) of ranibizumab biosimilar amongst the participants (a and c). Yet, during the same period, the number of respondents satisfied with the safety of bevacizumab biosimilar (30% to 25%) reduced, whereas satisfaction with its efficacy remained almost identical (29% vs 30%) (b and d)
Economics of anti-VEGF biosimilars
In 2020, 92% of respondents felt that biosimilar drugs have made anti-VEGF treatment more affordable to the general population, compared to 83% of respondents in 2018 (P = 0.62) [Fig. 1, Graph 1d]. When the respondents were asked if the price of ranibizumab biosimilar was sufficient to switch from branded bevacizumab (Avastin®), similar proportions of respondents from 2020 (40%) and 2018 (37%) (P = 0.82) were in favor [Fig. 1, Graph 1c].
The participants were asked to estimate price of ranibizumab biosimilar that would convince them to switch from Avastin. In 2018, the surveyed participants selected the switching price as $54 (42%), followed by $80 (23%), $74 (21%) and $94 (14%); in 2020, a plurality of participants also chose $54 (33%), fewer chose $74 (15%), but more chose $80 (29%) and $94 (23%) [Fig. 1, Graph 1b].
Discussion
Razumab is the first and only biosimilar approved for intraocular use in India.[11] The approval was based on favorable results of its 12-weeks' phase 3 clinical trial (RE-ENACT) establishing its efficacy in 103 eyes with nAMD and subsequently in 160 eyes with RVO.[11,12] Subsequently, the RE-ENACT-2 trial in nAMD showed significant improvement in BCVA, central subfield thickness, intraretinal and subretinal fluid at 48 weeks, whereas the analogous RE-ENACT-2 trial in RVO also showed Razumab as an effective treatment option in RVO by significantly improving the BCVA and reducing the macular thickness at 48 weeks.[13,14] Biosimilars of bevacizumab (Zybev®, Zydus Cadilla, Ahmedabad, India; Bevatas®, Intas Pharmaceuticals, Ahmedabad, India) are less often used as off-label by retina specialists. Our survey aimed to assess physicians' views and usage patterns of these biosimilars. From 2018 to 2020, physicians reported a significant increase in the use of the ranibizumab biosimilar, whereas the use of the bevacizumab biosimilars decreased significantly [Fig. 2, Graph 2c]. When the respondents were asked to predict their future use of biosimilars, they anticipated using more ranibizumab biosimilar but not bevacizumab biosimilar [Fig. 2, Graph 2d].
Producing a biosimilar is complicated because complete information about the molecular structure of the original biologic agent is not available; the manufacturer of the biologic must then determine this through reverse engineering. Regulatory authorities have formulated manufacturing and approval guidelines for biosimilars that focus on showing structural similarity with the parent molecule and establishing the safety and efficacy of the biosimilars through clinical trials. Despite these guidelines, the validation of biosimilars for unrestricted clinical use may require additional studies as nine of 10 retina specialists (89% in 2018 and 91% in 2020; Fig. 2, Graph 2b) from our survey believed that larger, better designed clinical trials should be performed before biosimilars are approved.
Safety and efficacy of anti-VEGF drugs is important to physicians and patients, particularly when it comes to selecting biosimilars. Biosimilars are capable of inciting immunologic reactions after intraocular injections. Cases of sterile endophthalmitis were reported after injections from the initial batches of Razumab in 2015 and after subsequent batches in 2017 and 2019. In response to the inflammation, Intas promptly recalled all vials from the implicated batches, temporarily halted further production, and refined the manufacturing process before releasing new vials into the market. Although these episodes of sterile endophthalmitis were being investigated, VRSI advised its members to stop using Razumab (August 2015, March 2017, February 2019). Once the safety of the newly manufactured batches was confirmed, members were notified that they could carefully begin using Razumab again. Utilization data suggested that members were initially hesitant to begin using Razumab again, so the VRSI decided to repeat its biosimilar survey in 2020 after the last reported cluster of sterile endophthalmitis cases (February 2019). The repeat survey provided data to compare physicians' current perceptions and drug use with those from 2018.
The survey done in 2020 found that physicians became increasingly satisfied with the safety and efficacy of Razumab from 2018. During the same period respondents reported a reduction in satisfaction with the efficacy of bevacizumab biosimilar, whereas satisfaction with its safety was essentially unchanged. Interestingly more than twice as many respondents are satisfied with the safety and efficacy of Razumab compared to the bevacizumab biosimilar, and despite episodes of Razumab-related sterile endophthalmitis, there is a trend among retina specialists in India to increasingly use this drug in their practice. Furthermore, respondents are more comfortable with Razumab in 2020 despite the sterile endophthalmitis cluster of 2019.
The cost of an anti-VEGF injection regimen can be an important consideration when treatment is initiated. The need for repeated injections increases the cost of treatment regimens that limit physician's ability to treat large numbers of patients for long periods of time. The availability of less expensive biosimilars, which reduce treatment costs by 25% to 50% compared to branded drugs, could expand the use of anti-VEGF therapy while lowering total cost.[7] The approved branded medications in India, Lucentis ($320) and Eylea ($760), are more expensive than the ranibizumab biosimilar Razumab ($130), and a majority of respondents believed that biosimilars have made anti-VEGF treatment more affordable.
Respondents were asked to estimate the price of ranibizumab biosimilar that would increase its use instead of Avastin. More respondents chose $54 as the pivot price in both surveys, although the proportion decreased from 42% in 2018 to 33% in 2020. The surveys indicated that retina specialists are generally willing to accept a higher ranibizumab biosimilar price in 2020, as compared to 2018, to move patients away from Avastin. This 'willingness to pay' price is approximately one-third the actual cost of Lucentis and marginally lower than the current price of Razumab.
The lack of FDA approval coupled with unresolved safety and medicolegal concerns prevent bevacizumab and its biosimilars from being used widely in India. Incidents of compounding-related endophthalmitis led Indian regulatory authorities to temporarily ban the intravitreal use of bevacizumab in 2016.[6] Such events limit the availability of a low-cost anti-VEGF agent, which is a significant problem in the developing world. Approved low cost biosimilars such as Razumab, which is packaged in a single-use vial, have the ability to fill this void. Razumab clinical trials (RE-ENACT study and RE-ENACT 2 study) provided safety and efficacy data for the treatment of nAMD and RVO,[11,12] and as a result, Razumab sales have increased from 2,842 vials in 2015 to 49,914 vials in 2019 [Fig. 4].
Figure 4.
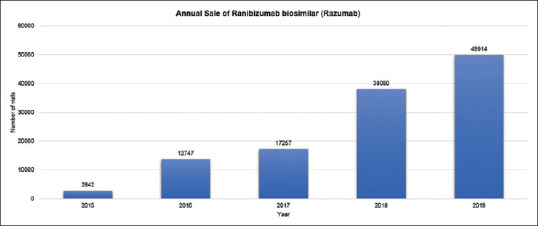
Graph showing annual growth in sales of ranibizumab biosimilar (Razumab
This study represents the only survey data on physicians' perceptions of anti-VEGF biosimilars in India. The 2-year interval between surveys both validates the original survey data and identifies trends. Unfortunately, the participation rate among Indian vitreoretinal specialists was small and data was obtained from only a minority of the VRSI's membership. This low participation rate limits the interpretation of results to ±5%. Additionally, we do not have the data regarding number of respondents in the 2020 survey who were also part of the 2018 survey cohort. Nonetheless, the comparative survey results are meant to be interpreted with regards to the overall trend in Biosimilar usage amongst the VRSI members rather than a direct comparison between same cohorts.
Conclusion
In conclusion, the introduction of biosimilars has given Indian retina specialists additional anti-VEGF drug options. The VRSI survey established that physicians are well aware of biosimilars and that there is an increasing trend toward prescribing a ranibizumab biosimilar. Physicians are generally satisfied with the safety and efficacy of biosimilars, but they believe that more rigorous trials should be conducted prior to regulatory approval. A third of the respondents feel that Razumab is appropriately priced but they acknowledge that a further price reduction would be necessary for Razumab to become the drug of first choice. The United States patents on Lucentis and Eylea will end in 2020, whereas the European patents expire in 2022 and 2025, respectively. With patents of these biologics about to expire and acceptance of biosimilars growing, a shift from branded drugs toward biosimilars in developed nations may occur.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Vitreoretinal Society of India (VRSI) biosimilars of anti-VEGF survey
Questionnaire
-
Are you aware of biosimilars for anti-VEGF?
- Yes
- No
-
Have you used any of the biosimilars?
- Ranibizumab
- Bevacizumab
- Both
- None
-
Are you satisfied with the efficacy of Ranibizumab biosimilar in your patients?
- Yes
- No
- Not applicable
-
Are you satisfied with Bevacizumab biosimilar efficacy in your patients?
- Yes
- No
- Not applicable
-
Are you satisfied with the safety of Ranibizumab biosimilar?
- Yes
- No
- Not applicable
-
Are you satisfied with the safety of Bevacizumab biosimilar?
- Yes
- No
- Not applicable
-
Do you think approval of biosimilar drugs should be made more stringent with larger clinical trials?
- Yes
- No
-
Do you think biosimilar drugs have made anti-VEGF treatment more affordable to the general population?
- Yes
- No
-
If you have used biosimilar drugs, are you likely to continue using it in the future?
- Yes
- No
- Not applicable
-
If you have NEVER USED biosimilar drugs, are you likely to start using it in the near future?
- Yes
- No
- Not applicable
-
Is the present cost of Razumab Biosimilar ($130) appropriate if we want to substitute Avastin with the Biosimilar?
- Yes
- No, Razumab should be priced lower than $130
- No, Razumab could be priced higher than $130
-
The usage of Razumab compared to Avastin may increase if the current cost of Razumab comes down to
- $94
- $80
- $74
- $54
References
- 1.Weise M, Bielsky MC, De Smet K, Ehmann F, Ekman N, Narayanan G, et al. Biosimilars—why terminology matters. Nat Biotechnol. 2011;29:690–3. doi: 10.1038/nbt.1936. [DOI] [PubMed] [Google Scholar]
- 2.Yorston D. Anti-VEGF drugs in the prevention of blindness. Community Eye Health. 2014;27:44–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Li E, Donati S, Lindsley KB, Krzystolik MG, Virgili G. Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration? Cochrane Database Syst Rev. 2020;5:CD012208. doi: 10.1002/14651858.CD012208.pub2. doi: 10.1002/14651858.CD012208.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campa C, Alivernini G, Bolletta E, Parodi MB, Perri P. Anti-VEGF therapy for retinal vein occlusions. Curr Drug Targets. 2016;17:328–36. doi: 10.2174/1573399811666150615151324. [DOI] [PubMed] [Google Scholar]
- 5.Mansour SE, Browning DJ, Wong K, Flynn HW, Jr, Bhavsar AR. The evolving treatment of diabetic retinopathy. Clin Ophthalmol. 2020;14:653–78. doi: 10.2147/OPTH.S236637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain P, Sheth J, Anantharaman G, Gopalakrishnan M. Real-world evidence of safety profile of intravitreal bevacizumab (Avastin) in an Indian scenario. Indian J Ophthalmol. 2017;65:596–602. doi: 10.4103/ijo.IJO_992_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma A, Reddy P, Kuppermann BD, Bandello F, Lowenstein A. Biosimilars in ophthalmology: “Is there a big change on the horizon.”? Clin Ophthalmol. 2018;12:2137–43. doi: 10.2147/OPTH.S180393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal G, Nagpal M, Sharma A, Puri V, Dhingra GA. Upcoming drifts in bio-similars. Curr Clin Pharmacol. 2020 doi: 10.2174/1574884715666200507131943. doi: 10.2174/1574884715666200507131943. [DOI] [PubMed] [Google Scholar]
- 9.Grabowski H, Guha R, Salgado M. Biosimilar competition: Lessons from Europe. Nat Rev Drug Discov. 2014;13:99–100. doi: 10.1038/nrd4210. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A, Kumar N, Kuppermann B, Francesco B, Lowenstein A. Ophthalmic biosimilars: Lessons from India. Indian J Ophthalmol. 2019;67:1384–5. doi: 10.4103/ijo.IJO_430_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S, Khan MA, Chaturvedi A RE-ENACT Study Investigators Group. Real life clinical effectiveness of Razumab® (World's First BiosimilarRanibizumab) in wet age-related macular degeneration: A subgroup analysis of pooled retrospective RE-ENACT study. Int J Ophthalmol Eye. 2018;6:368–73. [Google Scholar]
- 12.Sharma S, Khan MA, Chaturvedi A RE-ENACT Study Investigators Group. Real-life clinical effectiveness of Razumab® (the World's First Biosimilar of Ranibizumab) in retinal vein occlusion: A subgroup analysis of the pooled retrospective RE-ENACT Study. Ophthalmologica. 2019;241:24–31. doi: 10.1159/000488602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma S, Khan M, Chaturvedi A RE-ENACT 2 Study Investigators Group. A multicenter, retrospective study (RE-ENACT 2) on the use of Razumab™ (World's first biosimilar ranibizumab) in wet age-related macular degeneration. Ophthalmol Ther. 2020;9:103–14. doi: 10.1007/s40123-019-00228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma S, Khan M, Chaturvedi A, RE-ENACT 2 Study Investigators Group A multicenter, retrospective study (RE-ENACT 2) on Razumab™ (World's first biosimilar ranibizumab) in retinal vein occlusion. Ophthalmol Ther. 2020;9:625–39. doi: 10.1007/s40123-020-00277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]