Abstract
Autophagy is frequently induced in the hypoxic tumour microenvironment. Accumulating evidence reveals important functions of autophagy at the tumour‐immune interface. Herein, we propose an update on the roles of autophagy in modulating tumour immunity. Autophagy promotes adaptive resistance of established tumours to the cytotoxic effects of natural killer cells (NKs), macrophages and effector T cells. Increased autophagic flux in tumours dampen their immunogenicity and inhibits the expansion of cytotoxic T lymphocytes (CTLs) by suppressing the activation of STING type I interferon signalling (IFN‐I) innate immune sensing pathway. Autophagy in suppressive tumour‐infiltrating immune subsets maintains their survival through metabolic remodelling. On the other hand, autophagy is involved in the antigen processing and presentation process, which is essential for anti‐tumour immune responses. Genetic deletion of autophagy induces spontaneous tumours in some models. Thus, the role of autophagy is context‐dependent. In summary, our review has revealed the dichotomous roles of autophagy in modulating tumour immunity. Broad targeting of autophagy may not yield maximal benefits. The characterization of specific genes regulating tumour immunogenicity and innovation in targeted delivery of autophagy inhibitors into certain tumours are among the most urgent tasks to sensitize cold cancers to immunotherapy.
Keywords: autophagy, immune cell, tumour cell, tumour immunity
1. BACKGROUND
Autophagy serves as an evolutionarily conserved physiological phenomenon to maintain cellular homeostasis and survival during nutrient deprivation. The initiation of autophagic response is briefly presented as the encapsulation of excessive or damaged cellular components and organelles into autophagosome leading to enzymatic degradation. 1 , 2 According to the various delivering routes and contents to lysosomes, autophagy is generally categorized into macroautophagy (predominant form generally termed as autophagy), microautophagy and chaperon‐mediated autophagy. 3 Autophagy is also frequently altered under pathological circumstances, namely hypoxia, endoplasmic reticulum (ER) stress, nutrient deficiency, radiation and chemotherapy. 4 , 5 , 6 Aside from its direct effect on cancer cell response to environmental challenges, recent studies show that autophagy in cancer cells regulates tumour‐immune interactions, depending upon the context of cancer types, metabolic alterations in the tumour microenvironment (TME) and the stage of cancers. 7 Genetic evidence showed that autophagy is a critical mechanism suppressing tumour initiation, 8 however, in established tumours autophagy contributes to adaptive resistance. 9 Of note, mitophagy, another form of autophagy, plays a similar role in regulating tumour development by adjusting tumour immune response. 10 In this review, we seek to summarize recent evidence characterizing the functions of autophagy, including mitophagy, in regulating tumour‐immune interactions (Figures 1, 2, 3; Table 1).
FIGURE 1.
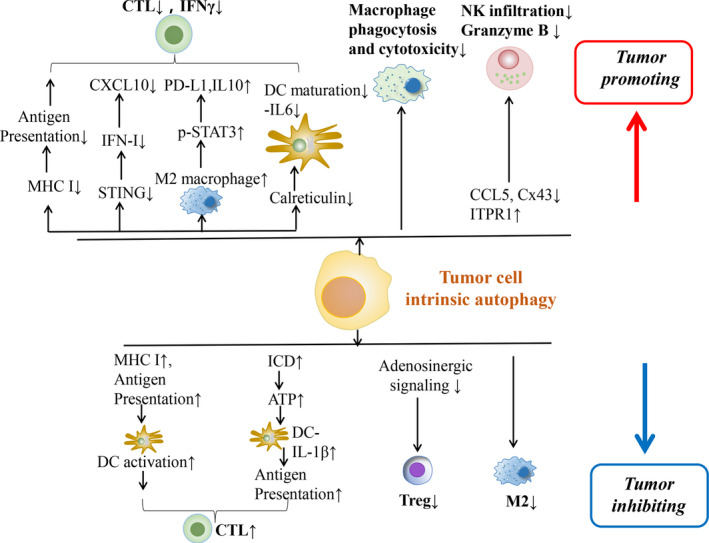
Schematic presentation regarding the potential mechanisms of tumour cell or immune cell instrinsic autophagy in modulating tumour‐immune interplay and the development of tumour. ATP, adenosine triphosphate; CCL5, chemokine (C‐C motif) ligand 5; CTL, cytotoxic T lymphocytes; Cx43, Connexin 43; DC, dendritic cell; ICD, immunogenic cell death; IFN‐I, type I interferon; ITPR1, inositol 1,4,5‐trisphosphate receptor, type 1; MHC, major histocompatibility class; NK, natural killer cells; PD‐L1, programmed death‐ligand 1; STAT3, signal transducer and activator of transcription 3; Treg, regulatory T cells
FIGURE 2.
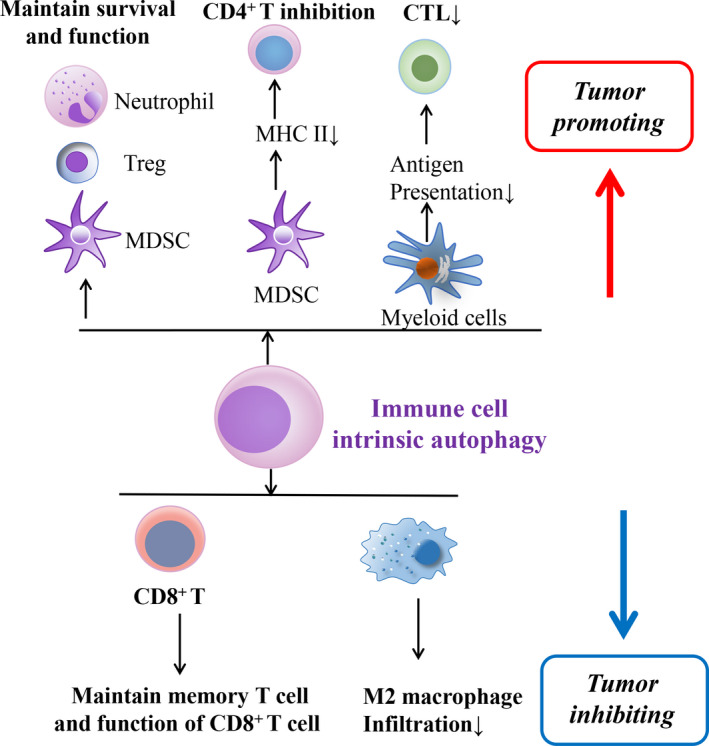
Schematic diagram indicating the possible mechanisms of immune cell instrinsic autophagy in regulating tumour‐immune interplay and the tumour outcome. CTL, cytotoxic T lymphocytes; MDSC, myeloid‐derived suppressor cells; MHC, major histocompatibility class; Treg, regulatory T cells
FIGURE 3.
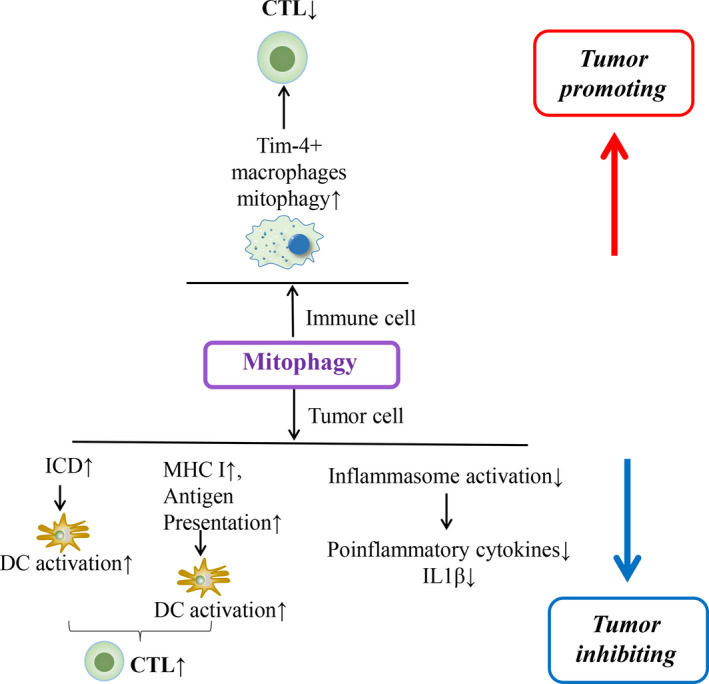
Schematic image demonstrating the potential role of mitophagy in regulating tumour immunity and the tumour outcome. CTL, cytotoxic T lymphocytes; DC, dendritic cell; ICD, immunogenic cell death; MHC, major histocompatibility class
TABLE 1.
Summary of literatures regarding tumour or immune cell‐intrinsic autophagy in the modulation of tumour immunity
| Mechanisms involved in regulating tumour immunity | Tumour type | Source of autophagy | Authors | Year | The impact of autophagy on tumours |
|---|---|---|---|---|---|
|
HPV16 E7‐STING and IFN‐I↓‐CTLs suppression MHC‐I degradation‐antigen presentation↓‐ CTL↓ Mitophagy‐CTL inhibition Mitophagy‐ICD‐ CTL and DC activation↑ |
HNSCC Pancreatic cancer Ovarian cancer Hepatocellular carcinoma |
HNSCC Pancreatic cancer Tim‐4+ macrophages Hepatocellular carcinoma |
Luo et al 31 Yamamoto et al 42 Xia et al 10 Yu et al 74 |
2020 2020 2020 2020 |
Tumour‐promoting Tumour‐promoting Tumour‐promoting Tumour‐inhibiting |
|
p‐STAT3↑‐PD‐L1↑‐CTL inhibition MHC‐I and antigen presentation↑‐DC activation Mitophagy‐inflammasome activation↓ |
Lung adenocarcinoma Glioblastoma Hepatocellular carcinoma |
Lung adenocarcinoma Glioblastoma Hepatocellular carcinoma |
Liu et al 46 Li et al 67 Li et al 73 |
2019 2019 2019 |
Tumour‐promoting Tumour‐inhibiting Tumour‐inhibiting |
|
M2 macrophage‐TLR4‐mediated MyD88↑‐p38↑‐STAT3↑‐PD‐L1, IL10↑‐CTL↓ SOX2‐STING↓‐IFN‐I↓‐CD8+ T cells↓ Macrophage phagocytosis and cytotoxicity↓ Macrophage phagocytosis and cytotoxicity↓ and CD8+ T cells↓ Maintain function of MDSC and MHC‐II↓,CD4 T cells inhibition Mitophagy‐MHC‐I and antigen presentation by DCs↑ |
Melanoma HNSCC Glioblastoma Glioblastoma Melanoma Colorectal cancer |
TRAPs HNSCC Glioblastoma Glioblastoma MDSC Colorectal cancer |
Wen et al 24 Tan et al 30 Zhang et al 21 Zhang et al 23 Alissafi et al 58 Ziegler et al 68 |
2018 2018 2018 2018 2018 2018 |
Tumour‐promoting Tumour‐promoting Tumour‐promoting Tumour‐promoting Tumour‐promoting Tumour‐inhibiting |
|
Macrophage phagocytosis and cytotoxicity↓ Tumour‐derived CCL5↓‐NK cell infiltration↓ Maintaining memory T cell‐CD8+ T cells↑ upon stimulation |
NSCLC Melanoma Breast cancer |
NSCLC Melanoma CD8+ T cells |
Zhang et al 22 Mgrditchian et al 18 Curry et al 71 |
2017 2017 2017 |
Tumour‐promoting Tumour‐promoting Tumour‐inhibiting |
|
Inhibiting mTORC1 and c‐Myc function and glycolytic metabolism‐maintaining Treg function Maintain survival and function of MDSCs |
Colon adenocarcinoma Breast cancer |
Treg MDSC |
Wei et al 56 Parker et al 57 |
2016 2016 |
Tumour‐promoting Tumour‐promoting |
|
Cx43 in tumour cells↓‐NK cells↓ Maintain survival and function of neutrophils |
Melanoma Hepatocellular carcinoma |
Melanoma Neutrophils |
Tittarelli et al 15 Li et al 60 |
2015 2015 |
Tumour‐promoting Tumour‐promoting |
|
Autophagy sensor ITPR1↑‐NK‐derived granzyme B degradation Adenosinergic signalling↓‐Treg↓ in early phase of tumorigenesis |
Renal cancer NSCLC |
Renal cancer NSCLC |
Messai et al 17 Rao et all 61 |
2014 2014 |
Tumour‐promoting Tumour‐inhibiting |
|
Granzyme B released into tumour cells by NK cells↓ CTL cytotoxicity↓ Calreticulin ↓‐maturation of IL‐6 secreting DCs, IFNγ released by CTLs↓ Antigen presentation of myeloid cells↓‐CTL↓ Infiltrated M2 macrophage↓ |
Breast cancer Breast cancer Bladder cancer, cervical cancer and melanoma Colon cancer, melanoma Hepatocellular carcinoma |
Breast cancer Breast cancer Tumour cells Myeloid cells Macrophage |
Baginska et al 12 Akalay et al 47 Garg et al 50 Baghdadi et al 59 Lin et al 70 |
2013 2013 2013 2013 2013 |
Tumour‐promoting Tumour‐promoting Tumour‐promoting Tumour‐promoting Tumour‐inhibiting |
| Antigen presentation by DCs↑‐CD8+ T cells↑ | Breast cancer and lung cancer | TRAPs | Li et al 65 | 2012 | Tumour‐inhibiting |
|
p‐STAT3↑‐tumour susceptibility to CTL‐mediated lysis↑ IFN‐I↓‐CD8+ T cells↓, CXCL10↓ ICD↑‐ATP release into TME↑‐IL‐1β from DCs↑‐Tumour lysis‐DC phagocytosis and antigen presentation↑‐CTL activation↑ |
Lung cancer and melanoma Breast cancer Colon cancer |
Lung cancer and melanoma Breast cancer Colon cancer |
Noman et al 45 Wei et al 41 Michaud et al 53 |
2011 2011 2011 |
Tumour‐promoting Tumour‐promoting Tumour‐inhibiting |
| p‐STAT3↑‐DC maturation↓‐CTL cytotoxicity↓ | Melanoma and lung cancer | Melanoma and lung cancer | Yu et al 43 | 2007 | Tumour‐promoting |
Abbreviations: ATP, adenosine triphosphate; CCL5, chemokine (C‐C motif) ligand 5; CTL, cytotoxic T lymphocytes; Cx43, Connexin 43; DC, dendritic cell; HNSCC, head and neck squamous cell carcinoma; ICD, immunogenic cell death; IFN‐I, type I interferon; ITPR1, inositol 1,4,5‐trisphosphate receptor, type 1; MDSC, myeloid‐derived suppressor cells; MHC, major histocompatibility class; mTORC1, mammalian target of rapamycin complex 1; NK, natural killer cells; NSCLC, non–small cell lung cancer; PD‐L1, programmed death‐ligand 1; STAT3, signal transducer and activator of transcription 3; TLR4, Toll‐like receptor 4; TME, tumour microenvironment; TRAPs, tumour cell‐released autophagosomes; Treg, regulatory T cells.
2. AUTOPHAGY IN ESTABLISHED TUMOURS PROMOTES EVASION FROM INNATE AND ADAPTIVE IMMUNE SURVEILLANCE
Autophagy could directly or indirectly exert its effect on the innate immunity mediated by natural killer (NK) cells, dendritic cells (DCs) and macrophage population. First, autophagy of tumour cells promotes adaptive resistance to NK‐induced tumour lysis. NK cells, which are considered as the first‐line defence against tumours, releasing perforin, and granzyme B for the lysis of tumour cells. 11 , 12 Its anti‐cancer role has been validated in malignancies, such as gastric cancer 13 and lung cancer. 14 By exploiting the in vivo and in vitro breast cancer models, Baginska et al observed that the autophagy provoked by the hypoxic TME is involved in the degradation of granzyme B originated from NK into cancer cells, thus counteracting the apoptotic cell death effect induced by NK cells. 12 Besides, several studies also suggest additional mechanisms that contribute to the low tumour immunosurveillance and cytotoxicity of NK cells for cancers. Gap junctions (GJs) are interacting channels that mediate the exchange of the small molecules between cells composed by connexin subunits, among which Connexin 43 (Cx43) is uncovered as the major GJ protein located at the immunological synapse and bridging the interplay between immune cells and cancer cells. 15 Hypoxia‐induced autophagic flux results in the degradation of Cx43 in melanoma cells and impairs the cytotoxic effects of NK cells upon cancer cells. In agreement, elevated Cx43 expression levels in tumour cells are beneficial to enhance the efficacy of NK‐based immunotherapy. 15 Inositol 1,4,5‐trisphosphate receptor, type 1 (ITPR1), as one ligand‐gated channel of ion for managing calcium release from the endoplasmic reticulum, is reported to be able to induce autophagy. 16 Messai's study regarding clear cell renal cell carcinomas (CCRCC) indicated that the elevated expression of ITPR1 evoked by HIF‐2α initiated the autophagic degradation of granzyme B and abolished the NK‐induced killing effect on tumour cells. In agreement with that, they implanted the tumours in mice and observed reduced tumour growth by inhibiting ITPR, while the depletion of NK cells reverted the tumour suppression. 17 As another line of evidence of autophagy‐mediated immune resistance, depletion of autophagy‐promoting Beclin 1 (BECN1) leads to increased intensity of chemokine (C‐C motif) ligand 5 (CCL5) expression within melanoma cells and redirects massive NK cells into the tumour microenvironment, thus leading to tumour suppression. 18
Macrophages may also exert innate immune surveillance in the TME through their phagocytic functions. 19 , 20 , 21 A glioblastoma study employing a combinatorial treatment to target both VEGF and CD47, the latter of which inhibited the phagocytic effect of macrophages, revealed that it could trigger autophagy of cancer cells which attenuated the phagocytosis and cytotoxicity of macrophage population. Inhibition of various signalling pathways, including Akt/mTOR and Erk, was responsible for the enhanced autophagy. 21 The same group also demonstrated that the combination of anti‐CD47 therapy with autophagy inhibitor would robustly improve the therapeutic efficacy against non–small cell lung cancer (NSCLC).These results suggest that autophagy originated from tumour cells could impede the phagocytic function of macrophages. 22 Zhang et al found that autophagy occurring in glioblastoma cells could mitigate the immunotherapeutic efficacy of anti‐CD47‐SIRPα treatment, displaying as the reduced macrophage‐derived phagocytosis and subsequent attenuation of CD8+ T‐cell cytotoxicity. 23 Notably, macrophages may be regulated by tumour cell‐released autophagosomes (TRAPs) and affect the cytotoxic T lymphocytes (CTLs). 24 TRAPs are a type of double‐membrane vesicles released into the TME by tumour cells, which escape from the lysosome fusion stage of classical autophagy. 25 Wen and colleagues uncovered that within several tumour models, the TRAPs could skew macrophages into M2‐phenotype with higher levels of PD‐L1 and IL‐10 via Toll‐like receptor 4 (TLR4)‐MyD88‐p38‐STAT3 pathway, therefore resulting in suppression of CTL function and reduced IFN‐γ secretion. 24
Moreover, tumour‐associated autophagy also contributes to evasion from adaptive immunity. For example, the response rate of head and neck squamous cell carcinoma (HNSCC) to immunotherapy remains less than 15%, for which low immunogenicity and a poor infiltration of CTLs were indicated as the possible reason. 26 , 27 Type I interferon (IFN‐I) signalling promotes anti‐tumour effects by mediating the recruitment and maturation of antigen‐presenting cells (APCs). Stimulator of IFN genes (STING) is a pivotal adaptor protein that could activate the IFN‐I pathway. 28 , 29 Nonetheless, STING is frequently inhibited in TME, contributing to tumour escape from innate immune sensing. Recent studies identified previously unknown functions of oncogenes in suppressing the STING‐IFN‐I innate immune sensing pathway. 30 , 31 Specifically, SOX2, previously known as a cancer stemness gene, was correlated with immunosuppression. 30 , 32 , 33 SOX2 amplification in tumour cells leads to an increased autophagic influx, which promoted the turnover of STING in HNSCC cells. Inhibition of autophagy could rescue SOX2‐potentiated suppression of STING. In addition, the results of in vivo experiment suggested that SOX2‐expressing tumours contained lower numbers of CD8+ CTLs and that those infiltrating T cells expressed higher levels of PD‐1 than SOX2‐negative tumours. 30 HPV+ HNSCC is driven by a distinct aetiology, with different immune infiltration patterns from HPV‐ tumours. Interestingly, HPV+ HNSCCs contain less T‐cell receptor richness, in contrast to its usually heavy immune infiltration. 34 IFN‐I is essential for tumour‐specific CTL expansion. A recent study showed that HPV16 E7 could contribute to the autophagic degradation of STING by binding to NLRX1, which was shown to promote autophagosome formation. 5 , 31 , 35 , 36 NLRX1 deficiency in the tumour cells promoted CD8+ CTL expansion located in the tumour‐draining lymph nodes and reduces CTL exhaustion in the TME. 31 In agreement, additional studies also found that high‐risk HPV subtypes utilize a number of strategies to antagonize IFN‐I induction 37 , 38 , 39 ; Gariglio and colleagues observed that HPV E7 could attenuate the IFN‐I activation in HPV‐transformed cells via epigenetic silencing of sensor genes including RIG‐I, cGAS and STING in an SUV39H1‐dependent manner. 37 NLRX1 has an LC3‐interacting region and can directly interact with LC3. Such interaction underpins an NLRX1‐mediated mitophagy process. Depletion of NLRX1 promotes mitochondria‐derived reactive oxygen species, which arguably amplifies the production of Th1 cytokines. 40 The role of autophagy in inhibiting IFN‐I was also corroborated using a transgenic FIP200 (FAK family‐interacting protein of 200 kD)‐deficient mouse model. FIP200 is an essential autophagy gene, in the absence of which mammary tumorigenesis is suppressed. The study found that inhibition of autophagy promoted the activation of IFN‐I signalling as well as its downstream chemokines such as CXCL10, subsequently inducing CD8+ CTL expansion in the TME. 41 Of interest, Yamamoto and colleagues recently reported that autophagy is responsible for the degradation of MHC‐I in pancreatic ductal adenocarcinoma by employing the autophagy cargo receptor NBR1, resulting in the tumour immune evasion. 42 Thus, modulating selective autophagy represents a non‐tapped approach to fine‐tune host immune responses.
Additional evidence implies that autophagy is also responsible for tumour immune escape by stimulating signal transducer and activator of transcription 3 (STAT3) signalling, an oncogenic pathway. The STAT3 pathway has been an important link between tumour and immune cells. 7 , 43 Wang et al reported that STAT3 activation occurring in tumour cells could significantly reduce the production of pro‐inflammatory cytokines and chemokines critical for APC maturation and its recruitment to the tumour bed. 44 Autophagy has been shown to increase STAT3 phosphorylation in multiple tumour models. 45 , 46 , 47 Autophagy may also inhibit adaptive immunity by dampening the immunogenic cell death (ICD)‐induced immune killing. ICD can be triggered by several anti‐cancer treatments such as chemotherapy, radiotherapy 48 , 49 and hypericin‐based photodynamic therapy (Hyp‐PDT). 50 This phenomenon is predominantly represented as the calreticulin (CRT) exposure on the cellular surface, the secretion of high mobility group box 1 (HMGB1) along with adenosine triphosphate (ATP). 51 , 52 , 53 These are pivotal to the proper processing of antigen by APCs, and these molecules, including CRT, HMGB1 and ATP were defined as damage‐associated molecular patterns (DAMPs). 54 Garg et al reported that by genetically blocking autophagy in the tumour model under Hyp‐PDT, an increase in CRT and ICD‐caused immune reaction was detected. This was elucidated as the up‐regulation of IL6‐producing mature DCs and CTLs along with IFN‐γ. 50
In addition to tumour‐intrinsic autophagy, immune cell–inherent autophagy may also deliver resistance to immune killing. Myeloid‐derived suppressor cells (MDSCs) and regulatory T cells (Tregs) are the dominant subsets in the TME to promote tumour immune escape. 55 , 56 HMGB1‐induced autophagy was found essential for maintaining the survival of MDSCs in the TME. 57 Autophagy could also induce the lysosomal breakdown of MHC‐II and repress the anti‐tumour effect of CD4+ T cells. 58 As a critical adaptive mechanism in a nutrient‐poor environment, autophagy in the MDSCs and Tregs is essential to maintain their survival and sustained production of transforming growth factor‐β (TGF‐β), which dampens the activation of CTLs. 56 , 59 , 60
3. THE PROTECTIVE ROLE OF AUTOPHAGY IN PROMOTING NEOANTIGEN PRESENTATION
Compelling evidence demonstrates that the functions of autophagy in tumour initiation and established tumour response to therapy are different. One of the examples is that the genetic deletion of BECN1 enhances spontaneous tumour formation. 61 A recent study suggests that such autophagy‐mediated protection depends on immune surveillance. Autophagy promotes the processing and presentation of neoantigens from transforming cells to CTLs, leading to the elimination of target cells. 62 Under the circumstances of compromised proteasomal function, autophagy is central for the assembly of neoantigens with MHC‐I complex in APCs to facilitate its cross‐presentation to CD8+ T cells. 4 , 63 , 64 In addition, autophagy in transforming cells facilitates antigen presentation to CD8+ T cells. 65 , 66 Here, we summarize some evidence for the above notion and other potential mechanisms of autophagy that contribute to anti‐tumour immunity.
The efficient uptake and presentation of tumour antigen is essential to subvert the immunosuppressant TME. Li and colleagues showed that tumour cell autophagy triggered by the synthetic Nano‐DOX contributed to the increased immunogenicity of glioblastoma. These are presented as the elevated expression of MHC‐I complex and antigen presentation on tumour cells, the activation of DCs, and the transmission of DAMPs into extracellular TME. 67 Michaud et al observed that autophagy of colon cancer cells could promote ICD, including the ATP release followed by IL1‐β released from activated DCs, and the latter cytokine might enhance DC functions. 53 Additionally, the autophagosome extruded by tumour cells, called TRAPs could also implicate in this process. One study indicated that TRAPs produced by alpha‐tocopheryloxyacetic acid (a‐TEA) treatment in breast and lung cancer models might boost the potential of DCs to intake and present antigens, then inducing the activation of CD8+ T cells. 65 Autophagy‐mediated reduction of lysosomal integrity could potentiate MHC‐I presentation and augment the cross‐dressing of MHC‐antigen complexes to DCs, contributing to significant CD8+ T‐cell activation. 68 To address the tumour stage–dependent dichotomous roles of autophagy, genetically engineered mouse models offer a robust tool. For example, in the early stage of carcinogenesis of KRasG12D murine lung cancer, autophagy inhibited Treg infiltration through suppressing adenosinergic signalling and repressed tumour growth. 61 However, the autophagy at later stage potentiated tumour progression via dampening oxidative stress as well as inhibiting the DNA damage response. 61
Similar to the observation in tumour cells, autophagy in macrophages was shown to promote the surface expression of MHC‐II. 69 In a diethylnitrosamine‐induced hepatocellular carcinoma model, autophagy in macrophages was essential for their intratumoral infiltration. 70 Another study reported that autophagy of T cells induced by metformin in a breast cancer model of mice could substantially enhance the functional CD8+ T‐cell response by maintaining T‐cell function; meanwhile, the autophagy of CD8+ memory T cells is considered indispensable to maintain their survival and sustain tumour immunosurveillance after tumour resection. 71
4. THE CRUCIAL ROLE OF MITOPHAGY IN REGULATING TUMOUR IMMUNE RESPONSE
Autophagy‐mediated turnover of aged and/or damaged mitochondria is known as mitophagy. 72 , 73 The role of mitophagy in modulating the tumour immunity is emerging. On one side, Ziegler and colleagues show that mitophagy promotes anti‐tumour immunity. Increased mitophagy in intestinal epithelial cells triggers iron accumulation–induced lysosomal membrane permeabilization, which promotes the release of proteases into the cytosol and augments of MHC class I presentation. 68 Besides, in the hepatocellular carcinoma (HCC) model, mitophagy could be induced upon the icaritin treatment, which subsequently triggers ICD and augments anti‐tumour immunity. 74 On the other hand, mitophagy can also suppress inflammation. FUN14 domain‐containing 1 (FUNDC1), one mitophagy receptor that initiates the mitophagy, suppresses inflammasome activation and related immune responses. 73 In addition, Xia and colleagues uncovered that in mice ovarian cancer models with peritoneal metastasis, the infiltrating Tim4+ tumour‐associated macrophages (TAMs) exhibited higher mitophagy activity, thereby inhibiting the T cell–mediated anti‐tumour immunity and facilitating tumour progression. 10 Thus, mitophagy may regulate different inflammatory pathways where mitochondria maintains their homeostasis. 75 Its role in tumour cells and immune cells likely impose different impacts on anti‐tumour immunity (Figure 3). Future different studies using genetically engineered models, syngeneic models and human material are needed to better refine the role of mitophagy of different cell types in regulating tumour immunogenecity.
5. UPSTREAM REGULATORS OF AUTOPHAGY INVOLVED IN THE TUMOUR IMMUNE RESPONSE
In TME, autophagy can be induced by several stress factors, including hypoxia, endoplasmic reticulum (ER) stress, nutrient deprivation, extracellular matrix (ECM) disassociation and DAMPs. 57 , 76 , 77 , 78 , 79 Hypoxia is revealed in approximately 50%‐60% tumours, and several hypoxia‐mediated pathways are reported to induce autophagy. 80 , 81 HIF1α translocates into nucleus under hypoxic conditions, resulting in increased adenovirus E1B 19 kD‐interacting protein 3 (BNIP3) and its interacting partner BNIP3L. The BNIP3‐BNIP3L complex promotes autophagy in a BECN1‐dependent fashion. 82 In relation to that, another study found that NANOG could transcriptionally improve the level of BNIP3L, thereby inducing autophagy and abolishing the CTL‐mediated tumour lysis. 80 With the increased ratio of ADP:ATP within the hypoxic TME, adenosine monophosphate–activated protein kinase (AMPK) could be activated to stimulate autophagy via attenuation of the mammalian target of rapamycin (mTOR) pathway. 83 , 84
Another process closely associated with hypoxia, epithelial to mesenchymal transition (EMT) is another inducer of autophagy in TME, which confers tumour resistance to CTL killing. EMT of cancer cells accompanied with Snail homolog 1 (SNAI1) overexpression up‐regulates BECN1, leading to increased autophagy. 85 , 86 EMT could activate autophagy through regulating genes of DAPK1, PTEN and CDKN2A, enabling the cancer evasion from CTL cytotoxicity. 47
HMGB1, as an inducer of ICD, can trigger autophagy in TME. A co‐culture study revealed that HMGB1 could induce autophagy in colon cancer cells in an ER stress‐JNK phosphorylation‐dependent manner. 78 Another study implied that HMGB1, similar to BNIP3, dissociated Bcl2 from BECN1, which in turn triggered autophagy. 79
Mitophagy in tumours may be modulated by other upstream modulators. For instance, the STAT3 status, the FUNDC1 expression and the icaritin treatment implicate in regulating mitophagy and tumour immunity. 68 , 73 , 74 In addition, high expression levels of arginase‐1 suppress mTORC1 activation, which then contributes to enhanced mitophagy level in TAMs. 10
6. CONCLUSIONS
In summary, despite the dichotomous functions of autophagy in regulating anti‐tumour immune responses, its predominant function is likely dependent on cancer stages, cancer types, immune infiltration profiles and modelling methods. Autophagy in immune cells is an essential protective mechanism by facilitating tumour neoantigen presentation. However, autophagy in cancer cells may promote adaptive resistance to immune killing by dampening IFN‐I‐mediated immune sensing and rapid turnover of cytotoxic effector molecules. Global inhibition of autophagy may not yield the maximal benefits due to its interference with the antigen presentation machinery in the APCs; even such inhibition may sensitize tumours to immune killing. Thus, the characterization of specific genes regulating tumour immunogenicity and innovation in targeted delivery of autophagy inhibitors into tumour cells are among the most urgent tasks to sensitize cold cancers to immunotherapy.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Xiaobo Luo: Conceptualization (lead); Funding acquisition (lead); Writing‐original draft (lead); Writing‐review & editing (lead). Yan Qiu: Conceptualization (equal); Writing‐original draft (lead); Writing‐review & editing (equal). Palani Dinesh: Writing‐original draft (equal); Writing‐review & editing (equal). Wang Gong: Writing‐original draft (equal). Lu Jiang: Writing‐review & editing (equal). Xiaodong Feng: Writing‐review & editing (equal). Jing Li: Writing‐review & editing (equal). Yuchen Jiang: Writing‐review & editing (supporting). Yu L. Lei: Conceptualization (equal); Funding acquisition (equal); Supervision (lead); Writing‐review & editing (lead). Qianming Chen: Conceptualization (equal); Funding acquisition (equal); Supervision (lead); Writing‐review & editing (lead).
ACKNOWLEDGMENTS
We would like to thank all grant supports, including the National Natural Science Foundation of China (81902782, 81520108009, 81621062, 81730030), the 111 MOE project of China (B14038), NIH R01 DE026728 and U01 DE029255.
Luo X, Qiu Y, Dinesh P, et al. The functions of autophagy at the tumour‐immune interface. J Cell Mol Med. 2021;25:2333–2341. 10.1111/jcmm.16331
Contributor Information
Yu L. Lei, Email: leiyuleo@umich.edu.
Qianming Chen, Email: qmchen@scu.edu.cn.
REFERENCES
- 1. Jiang G‐M, Tan Y, Wang H, et al. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol Cancer. 2019;18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Münz C. Enhancing immunity through autophagy. Annu Rev Immunol. 2009;27:423‐449. [DOI] [PubMed] [Google Scholar]
- 4. You L, Jin S, Zhu L, Qian W. Autophagy, autophagy‐associated adaptive immune responses and its role in hematologic malignancies. Oncotarget. 2017;8:12374‐12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lei Y, Kansy BA, Li J, et al. EGFR‐targeted mAb therapy modulates autophagy in head and neck squamous cell carcinoma through NLRX1‐TUFM protein complex. Oncogene. 2016;35:4698‐4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White E. The role for autophagy in cancer. J Clin Invest. 2015;125:42‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viry E, Noman MZ, Arakelian T, et al. Hijacker of the antitumor immune response: autophagy is showing its worst facet. Front Oncol. 2016;6:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qu X, Yu J, Bhagat G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. White E. Deconvoluting the context‐dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xia H, Li S, Li X, et al. Autophagic adaptation to oxidative stress alters peritoneal residential macrophage survival and ovarian cancer metastasis. JCI Insight. 2020;5:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baginska J, Viry E, Berchem G, et al. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer‐mediated lysis under hypoxia. Proc Natl Acad Sci U S A. 2013;110:17450‐17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577‐583. [PubMed] [Google Scholar]
- 14. Villegas FR, Coca S, Villarrubia VG, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23‐28. [DOI] [PubMed] [Google Scholar]
- 15. Tittarelli A, Janji B, Van Moer K, Noman MZ, Chouaib S. The Selective degradation of synaptic connexin 43 protein by hypoxia‐induced autophagy impairs natural killer cell‐mediated tumor cell killing. J Biol Chem. 2015;290:23670‐23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Engedal N, Torgersen ML, Guldvik IJ, et al. Modulation of intracellular calcium homeostasis blocks autophagosome formation. Autophagy. 2013;9:1475‐1490. [DOI] [PubMed] [Google Scholar]
- 17. Messai Y, Noman MZ, Hasmim M, et al. ITPR1 protects renal cancer cells against natural killer cells by inducing autophagy. Cancer Res. 2014;74:6820‐6832. [DOI] [PubMed] [Google Scholar]
- 18. Mgrditchian T, Arakelian T, Paggetti J, et al. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5‐dependent manner. Proc Natl Acad Sci U S A. 2017;114(44):E9271‐E9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gordon SR, Maute RL, Dulken BW, et al. PD‐1 expression by tumour‐associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Willingham SB, Volkmer J‐P, Gentles AJ, et al. The CD47‐signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662‐6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang X, Wang S, Nan Y, et al. Inhibition of autophagy potentiated the anti‐tumor effects of VEGF and CD47 bispecific therapy in glioblastoma. Appl Microbiol Biotechnol. 2018;102:6503‐6513. [DOI] [PubMed] [Google Scholar]
- 22. Zhang X, Fan J, Wang S, et al. Targeting CD47 and autophagy elicited enhanced antitumor effects in non‐small cell lung cancer. Cancer Immunol Res. 2017;5:363‐375. [DOI] [PubMed] [Google Scholar]
- 23. Zhang X, Chen W, Fan J, et al. Disrupting CD47‐SIRPα axis alone or combined with autophagy depletion for the therapy of glioblastoma. Carcinogenesis. 2018;39:689‐699. [DOI] [PubMed] [Google Scholar]
- 24. Wen Z‐F, Liu H, Gao R, et al. Tumor cell‐released autophagosomes (TRAPs) promote immunosuppression through induction of M2‐like macrophages with increased expression of PD‐L1. J Immunother Cancer. 2018;6:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gärtner K, Battke C, Dünzkofer J, et al. Tumor‐derived extracellular vesicles activate primary monocytes. Cancer Med. 2018;7:2013‐2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan YS, Sansanaphongpricha K, Prince M, et al. Engineering vaccines to reprogram immunity against head and neck cancer. J Dent Res. 2018;97:627‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polverini PJ, D'Silva NJ, Lei YL. Precision therapy of head and neck squamous cell carcinoma. J Dent Res. 2018;97:614‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP‐AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tan YS, Sansanaphongpricha K, Xie Y, et al. Mitigating SOX2‐potentiated immune escape of head and neck squamous cell carcinoma with a STING‐inducing nanosatellite vaccine. Clin Cancer Res. 2018;24:4242‐4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luo X, Donnelly CR, Gong W, et al. HPV16 drives cancer immune escape via NLRX1‐mediated degradation of STING. J Clin Invest. 2020;130:1635‐1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ji J, Yu Y, Li Z‐L, et al. XIAP limits autophagic degradation of Sox2 and is a therapeutic target in nasopharyngeal carcinoma stem cells. Theranostics. 2018;8:1494‐1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee Y, Shin JH, Longmire M, et al. CD44+ cells in head and neck squamous cell carcinoma suppress T‐Cell‐mediated immunity by selective constitutive and inducible expression of PD‐L1. Clin Cancer Res. 2016;22:3571‐3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saloura V, Fatima A, Zewde M, et al. Characterization of the T‐Cell receptor repertoire and immune microenvironment in patients with locoregionally advanced squamous cell carcinoma of the head and neck. Clin Cancer Res. 2017;23:4897‐4907. [DOI] [PubMed] [Google Scholar]
- 35. Lei Y, Wen H, Ting JP. The NLR protein, NLRX1, and its partner, TUFM, reduce type I interferon, and enhance autophagy. Autophagy. 2013;9:432‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lei YU, Wen H, Yu Y, et al. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity. 2012;36:933‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lo Cigno I, Calati F, Borgogna C, et al. Human papillomavirus E7 oncoprotein subverts host innate immunity via SUV39H1‐mediated epigenetic silencing of immune sensor genes. J Virol. 2020;94:e01812‐e1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lo CI, Calati F, Albertini S, Gariglio M. Subversion of host innate immunity by human papillomavirus oncoproteins. Pathogens. 2020;9:e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shaikh MH, Bortnik V, McMillan NA, Idris A. cGAS‐STING responses are dampened in high‐risk HPV type 16 positive head and neck squamous cell carcinoma cells. Microb Pathog. 2019;132:162‐165. [DOI] [PubMed] [Google Scholar]
- 40. Zhang Y, Yao Y, Qiu X, et al. Listeria hijacks host mitophagy through a novel mitophagy receptor to evade killing. Nat Immunol. 2019;20:433‐446. [DOI] [PubMed] [Google Scholar]
- 41. Wei H, Wei S, Gan B, Peng X, Zou W, Guan JL. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev. 2011;25:1510‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamamoto K, Venida A, Yano J, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC‐I. Nature. 2020;581:100‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41‐51. [DOI] [PubMed] [Google Scholar]
- 44. Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat‐3 signaling in tumor cells. Nat Med. 2004;10:48‐54. [DOI] [PubMed] [Google Scholar]
- 45. Noman MZ, Janji B, Kaminska B, et al. Blocking hypoxia‐induced autophagy in tumors restores cytotoxic T‐cell activity and promotes regression. Cancer Res. 2011;71:5976‐5986. [DOI] [PubMed] [Google Scholar]
- 46. Liu Y, Zhang H, Wang Z, Wu P, Gong W. 5‐Hydroxytryptamine1a receptors on tumour cells induce immune evasion in lung adenocarcinoma patients with depression via autophagy/pSTAT3. Eur J Cancer. 2019;114:8‐24. [DOI] [PubMed] [Google Scholar]
- 47. Akalay I, Janji B, Hasmim M, et al. Epithelial‐to‐mesenchymal transition and autophagy induction in breast carcinoma promote escape from T‐cell‐mediated lysis. Cancer Res. 2013;73:2418‐2427. [DOI] [PubMed] [Google Scholar]
- 48. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51‐72. [DOI] [PubMed] [Google Scholar]
- 49. Dudek AM, Garg AD, Krysko DV, De Ruysscher D, Agostinis P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev. 2013;24:319‐333. [DOI] [PubMed] [Google Scholar]
- 50. Garg AD, Dudek AM, Ferreira GB, et al. ROS‐induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy. 2013;9:1292‐1307. [DOI] [PubMed] [Google Scholar]
- 51. Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL‐1beta‐dependent adaptive immunity against tumors. Nat Med. 2009;15:1170‐1178. [DOI] [PubMed] [Google Scholar]
- 52. Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54‐61. [DOI] [PubMed] [Google Scholar]
- 53. Michaud M, Martins I, Sukkurwala AQ, et al. Autophagy‐dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573‐1577. [DOI] [PubMed] [Google Scholar]
- 54. Garg AD, Dudek AM, Agostinis P. Autophagy‐dependent suppression of cancer immunogenicity and effector mechanisms of innate and adaptive immunity. Oncoimmunology. 2013;2:e26260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Talmadge JE, Gabrilovich DI. History of myeloid‐derived suppressor cells. Nat Rev Cancer. 2013;13:739‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wei J, Long L, Yang K, et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol. 2016;17:277‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Parker KH, Horn LA, Ostrand‐Rosenberg S. High‐mobility group box protein 1 promotes the survival of myeloid‐derived suppressor cells by inducing autophagy. J Leukoc Biol. 2016;100:463‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alissafi T, Hatzioannou A, Mintzas K, et al. Autophagy orchestrates the regulatory program of tumor‐associated myeloid‐derived suppressor cells. J Clin Invest. 2018;128:3840‐3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baghdadi M, Yoneda A, Yamashina T, et al. TIM‐4 glycoprotein‐mediated degradation of dying tumor cells by autophagy leads to reduced antigen presentation and increased immune tolerance. Immunity. 2013;39:1070‐1081. [DOI] [PubMed] [Google Scholar]
- 60. Li X‐F, Chen D‐P, Ouyang F‐Z, et al. Increased autophagy sustains the survival and pro‐tumourigenic effects of neutrophils in human hepatocellular carcinoma. J Hepatol. 2015;62:131‐139. [DOI] [PubMed] [Google Scholar]
- 61. Rao S, Tortola L, Perlot T, et al. A dual role for autophagy in a murine model of lung cancer. Nat Commun. 2014;5:3056. [DOI] [PubMed] [Google Scholar]
- 62. Zhong Z, Sanchez‐Lopez E, Karin M. Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell. 2016;166:288‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity. 2013;39:211‐227. [DOI] [PubMed] [Google Scholar]
- 64. Shibutani ST, Saitoh T, Nowag H, Münz C, Yoshimori T. Autophagy and autophagy‐related proteins in the immune system. Nat Immunol. 2015;16:1014‐1024. [DOI] [PubMed] [Google Scholar]
- 65. Li Y, Hahn T, Garrison K, et al. The vitamin E analogue α‐TEA stimulates tumor autophagy and enhances antigen cross‐presentation. Cancer Res. 2012;72:3535‐3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li Y, Wang L‐X, Pang P, et al. Cross‐presentation of tumor associated antigens through tumor‐derived autophagosomes. Autophagy. 2009;5:576‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li T‐F, Xu Y‐H, Li KE, et al. Doxorubicin‐polyglycerol‐nanodiamond composites stimulate glioblastoma cell immunogenicity through activation of autophagy. Acta Biomater. 2019;86:381‐394. [DOI] [PubMed] [Google Scholar]
- 68. Ziegler PK, Bollrath J, Pallangyo CK, et al. Mitophagy in intestinal epithelial cells triggers adaptive immunity during tumorigenesis. Cell. 2018;174:88‐101.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang Z, Song P, Li Y, et al. Recombinant human arginase I elicited immunosuppression in activated macrophages through inhibiting autophagy. Appl Microbiol Biotechnol. 2019;103:4825‐4838. [DOI] [PubMed] [Google Scholar]
- 70. Lin H, Yan J, Wang Z, et al. Loss of immunity‐supported senescence enhances susceptibility to hepatocellular carcinogenesis and progression in Toll‐like receptor 2‐deficient mice. Hepatology. 2013;57:171‐182. [DOI] [PubMed] [Google Scholar]
- 71. Curry A, Khatri I, Kos O, Zhu F, Gorczynski R. Importance of CD200 expression by tumor or host cells to regulation of immunotherapy in a mouse breast cancer model. PLoS One. 2017;12:e0171586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xu Y, Shen J, Ran Z. Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy. 2020;16:3‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li W, Li Y, Siraj S, et al. FUN14 domain‐containing 1‐mediated mitophagy suppresses hepatocarcinogenesis by inhibition of inflammasome activation in mice. Hepatology. 2019;69:604‐621. [DOI] [PubMed] [Google Scholar]
- 74. Yu Z, Guo J, Hu M, Gao Y, Huang L. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma. ACS Nano. 2020;14:4816‐4828. [DOI] [PubMed] [Google Scholar]
- 75. Dagvadorj J, Mikulska‐Ruminska K, Tumurkhuu G, et al. Recruitment of pro‐IL‐1α to mitochondrial cardiolipin, via shared LC3 binding domain, inhibits mitophagy and drives maximal NLRP3 activation. Proc Natl Acad Sci U S A. 2021;118:e2015632118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rosenfeldt MT, Ryan KM. The role of autophagy in tumour development and cancer therapy. Expert Rev Mol Med. 2009;11:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zheng Y, Zhu G. HMGB1 suppresses colon carcinoma cell apoptosis triggered by co‐culture with dendritic cells via an ER stress‐associated autophagy pathway. Mol Med Rep. 2018;17:3123‐3132. [DOI] [PubMed] [Google Scholar]
- 79. Kang R, Livesey KM, Zeh HJ, Loze MT, Tang D. HMGB1: a novel Beclin 1‐binding protein active in autophagy. Autophagy. 2010;6:1209‐1211. [DOI] [PubMed] [Google Scholar]
- 80. Janji B, Berchem G, Chouaib S. Targeting autophagy in the tumor microenvironment: new challenges and opportunities for regulating tumor immunity. Front Immunol. 2018;9:887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vaupel P, Briest S, Höckel M. Hypoxia in breast cancer: pathogenesis, characterization and biological/therapeutic implications. Wien Med Wochenschr. 2002;152:334‐342. [DOI] [PubMed] [Google Scholar]
- 82. Bellot Grégory, Garcia‐Medina R, Gounon P, et al. Hypoxia‐induced autophagy is mediated through hypoxia‐inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570‐2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP‐activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF‐1, BNIP3, and BNIP3L. Cell Death Differ. 2008;15:1572‐1581. [DOI] [PubMed] [Google Scholar]
- 85. Siemens H, Jackstadt R, Hünten S, et al. miR‐34 and SNAIL form a double‐negative feedback loop to regulate epithelial‐mesenchymal transitions. Cell Cycle. 2011;10:4256‐4271. [DOI] [PubMed] [Google Scholar]
- 86. Yu Y, Yang L, Zhao M, et al. Targeting microRNA‐30a‐mediated autophagy enhances imatinib activity against human chronic myeloid leukemia cells. Leukemia. 2012;26:1752‐1760. [DOI] [PubMed] [Google Scholar]


