Abstract
Using a validated tetracycline‐off‐inducible CD44 expression system in mouse model, we have previously demonstrated that the hyaluronan (HA) receptor CD44 promotes breast cancer (BC) metastasis to the liver. To unravel the mechanisms that underpin CD44‐promoted BC cell invasion, RNA samples were isolated from two cell models: (a) a tetracycline (Tet)‐Off‐regulated expression system of the CD44s in MCF‐7 cells and; (b) as a complementary approach, the highly metastatic BC cells, MDA‐MB‐231, were cultured in the presence and absence of 50 µg/mL of HA. Kynureninase (KYNU), identified by Microarray analysis, was up‐regulated by 3‐fold upon induction and activation of CD44 by HA; this finding suggests that KYNU is a potential novel transcriptional target of CD44‐downtstream signalling. KYNU is a pyridoxal phosphate (PLP) dependent enzyme involved in the biosynthesis of NAD cofactors from tryptophan that has been associated with the onset and development of BC. This review will attempt to identify and discuss the findings supporting this hypothesis and the mechanisms linking KYNU cell invasion via CD44.
Keywords: breast cancer, CD44, Hyaluronan, KYNU
Abbreviations
- 3‐HAA
anthranilic acid and 3‐hydroxyanthranilic acid
- 3HK
3‐hydroxy‐L‐kynurenine
- AKT
Protein kinase B
- BC
Breast cancer
- CAM
Cell adhesion molecule
- CD44
Cluster of differentiation 44
- cSCC
Cutaneous squamous cell carcinoma
- HA
Hyaluronan
- KMO
Kynurenine 3‐monooxygenase
- KYNU
Kynureninase
- NAD
Nicotinamide adenine dinucleotide
- NAH
N‐acetylhistidine
- NF‐κB
Nuclear factor‐kappa beta
- PI3K
phosphoinositide 3‐kinase
- PLP
pyridoxal‐5’‐phosphate
- QUIN
Quinolinic acid
- Ras
Rat sarcoma
- Tet
Tetracycline
- TGF‐β2
Transforming growth factor‐beta 2
- TNF
Tumour necrosis factor
- VCRL2
Vertebral, cardiac, renal and limb defects syndrome 2
- XA
xanthurenic acid
1. INTRODUCTION
Breast cancer (BC) is the most commonly diagnosed malignancy in women worldwide, including the Middle East region and Qatar, accounting for around 1/4th of all cancer cases. 1 BC cells frequently metastasize to the bone, liver, lung and brain, 2 and it is this ability of tumour cells to detach from the primary tumour, migrate and invade a new location in the body that is the most devastating aspect of cancer. 3 This process relies on cell adhesion molecules (CAM), located on cell surfaces for their essential role in cell‐to‐cell and cell‐extracellular adhesion. 4 CAMs form several protein families including cadherins, integrins, selectins and immunoglobins. 4 , 5
CD44 is an adhesion protein belonging to the CAM family and is the primary receptor for its ligand, Hyaluronic acid (HA) involved in regulating cellular proliferation, migration and invasion signalling. 6 In order to understand the function and signalling pathways involved in CD44‐mediated tumour cell invasion and metastasis, we developed a tetracycline (Tet)‐Off‐regulated expression system of the CD44s both in vitro, 7 and in vivo 8 and performed microarray analysis (12K CHIP from Affymetrix) to identify genes under the direct regulation of CD44‐downstream signalling. From the obtained list of targets, we have previously validated three genes (Cortactin, Survivin and TGF‐β2) as target genes that underpin CD44, along with their downstream signalling pathways. 7 , 8 , 9 Among these 200 genes, we have selected Kynureninase (KYNU) in order to provide and discuss lines of evidence from the literature, supporting our hypothesis that KYNU might be a novel transcriptional target of CD44‐downstream signalling.
KYNU is a hydrolase involved in Tryptophan metabolism, contributing to the synthesis of NAD+ cofactors via the Kynurenine pathway; a vital pathway of L‐tryptophan catabolism in both bacteria as well as eukaryotes. 10 In the pathway, KYNU catalyses L‐kynurenine (bacteria) and 3‐hydroxy‐L‐kynurenine (3HK) (eukaryotes) through a pyridoxal‐5′‐phosphate (PLP) dependent mechanism, to produce anthranilic acid and 3‐hydroxyanthranilic acid (3‐HAA), respectively. 10 KYNU is expressed in almost all body organs, and in higher levels in the liver, the urinary bladder and the appendix. 11 KYNU is involved in various inflammatory and cardiovascular diseases, in addition to several types of cancers, acting via different pathways (Figure 1). 12 , 13 , 14 , 15
FIGURE 1.
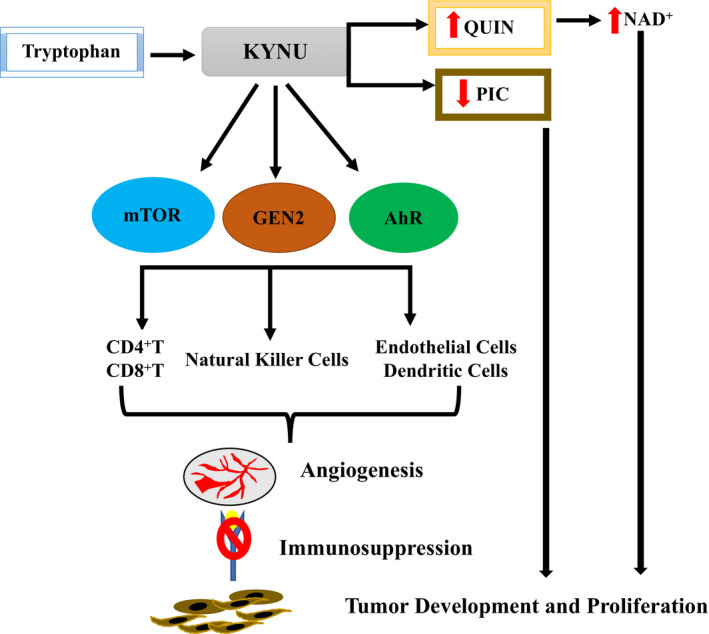
KYNU pathway involvement in mechanisms associated with tumour development
Here we discuss the findings, from the literature, supporting the hypothesis that KYNU is a transcriptional target of CD44 as well as known signalling pathways linking the activation of CD44 by HA to the transactivation of KYNU in promoting breast tumour cell invasion.
2. STRUCTURE OF KYNU
KYNU, a member of the aminotransferase superfamily is located on the long arm of chromosome 2 (2q22; from base pairs 143 506 498 to base pairs 143 799 892), encompassing 293.39 kb of DNA, including 44 introns and 21 exons. 16 KYNU, normally confined within the cytoplasm of the cells of various body tissues, requires the cofactor pyridoxal‐5′‐phosphate for its activity. 17
KYNU protein consists of 465 amino acids and exists as a homodimer structurally homologous to other members of the PLP‐dependent aspartate aminotransferase family. 18 Each monomer is composed of two regions: a small and a large domain, with a sizeable opening, containing the active site, formed at the junction between these domains in the dimerized form. 18 Like other members of this family, KYNU’s active site features a conserved lysine, which forms the PLP‐enzyme Schiff base, with a variety of nearby amino acids, maintaining the cofactor's proper orientation through hydrogen bonding. 18 Similarly, a conserved arginine appears to be critical to binding and orienting the substrate within the active site. 16 This binding results in a conformational change that puts strain on the substrate's bonds, which would be released upon hydrolysis. 16
3. FUNCTIONS OF KYNU
KYNU is involved in the biosynthesis of NAD + from tryptophan via the kynurenine pathway. 19 Specifically, it degrades kynurenine, a catabolite in tryptophan metabolism, into anthranilic acid.
3.1. Physiological functions of KYNU in normal cells
In most mammalian cells, the KYNU pathway is the primary path of tryptophan metabolism, producing metabolites, such as kynurenic acid (KYNA), xanthurenic acid (XA), and 3‐hydroxyanthranilic acid (3‐HAA). 20 Of these three, 3‐HAA is the main product of this pathway and is eventually converted to NAD+ (21), while KYNA and XA appear to only be produced when KYNU is fully saturated. 21
3.2. Functions of KYNU in vertebral, cardiac, renal and limb defects syndrome 2 (VCRL2)
KYNU is linked with tryptophan utilization and metabolic diseases, including vertebral, cardiac, renal and limb defects syndrome 2 (VCRL2), 19 which is an autosomal recessive congenital malformation syndrome; This association was made through a study of individuals with truncated KYNU genes; in vitro functional assays demonstrated that these mutations inhibited KYNU enzymatic activity, which resulted in enhanced 3HK levels and loss of NAD and NAH(H). 19
Further, in vivo study demonstrated that heterozygous KYNU ± mice with a niacin‐free diet during the early embryonic stages was able to sustain normal embryonic development due to the sufficient supply of the de novo NAD+. 19 On the other hand, homozygous null KYNU −/− mice with a niacin‐free diet were inviable and all embryos died even when niacin was only limited from embryonic day 0.5 to 5. Further research in mutant KYNU −/− mice born to mothers on a niacin‐free diet demonstrated lack of NAD due to KYNU loss; mice developed several congenital abnormalities including cleft palate, club foot, syndactyly, and caudal regression syndrome. 19 It was found that the elevated levels of niacin in mice were plausibly transferred from their mothers, thus, providing a protective effect on genetic‐based NAD paucity. 19 To sum up, the kynurenine pathway synthesis of NAD + is essential and mutations in KYNU leads to congenital malfunctions and inviable embryos. 19
3.3. Functions of KYNU in breast cancer and its association to CD44‐signalling
Although KYNU is often associated with metabolic diseases, its role in cancer lies nascent and only a few studies have investigated the link between KYNU and CD44, and its association with cancer. One of the key pathways dysregulated in cancer is the PI3K/AKT pathway; this pathway regulates various physiological functions, including cellular migration, invasion and cell survival. 22 , 23 CD44 activates PI3K/AKT and Ras pathways through its interaction with HER‐2 and c‐Met, respectively. 7 , 24 The activation of this pathway has, however, been shown to be impacted by the knockdown of KYNU, which reduced levels of phosphorylated metabolites p‐PI3K/p‐AKT, 12 and indicated a role for KYNU in triggering the PI3K/AKT pathway. It could be speculated that KYNU may be a plausible target of CD44 through which it interacts with HER‐2 and activates the downstream pathway. Thus, KYNU can be a candidate therapeutic target as the PI3K/AKT pathway can be modulated by silencing KYNU.
Another study analysing inflammation‐associated mechanisms, which are regulating and blocking the TNF‐induced signal transduction in primary human monocytes, identified KYNU as one of the proteins linked to nuclear factor κB (NF‐κB) pathway. 25 Moreover, NF‐κB plays a crucial role in tumour invasion and metastasis, 26 thus suggesting a role of KYNU in promoting tumour cell invasion. The study also reported that long‐term incubation of cells in TNF correlated with increased expression of KYNU and increased phosphorylation of CD44. 25 Similarly, to the dual controversial role of many other genes (eg p53), while, some studies support its oncogenic role, 27 , 28 , 29 , 30 , 31 , 32 , 33 a few studies have also shown its role as a tumour suppressor. 14 , 34
The first study by Rose et al, (1967) reported increased activity of the kynurenine pathway, along with increased KYNU, KMO and kynurenine aminotransferase‐II activity in BC patients, 33 indicating an oncogenic role of KYNU. Further, microarray data from invasive BC patients showed differential expression of KYNU in the BC subtypes. 28 , 30 While, no change in KYNU expression was observed in the luminal subtypes, KYNU expression was enhanced in the HER2‐positive, claudin low and aggressive basal BC subtypes, 28 , 30 , 31 thus linking KYNU overexpression with higher rates of lymph node metastasis and promotion of BC progression and metastasis. Interestingly, a study in cutaneous squamous cell carcinoma (cSCC) analysed the expression of KYNU, using Gene Expression Omnibus and the Oncomine databases, and further validated the results using structural and functional assays. 12 The results showed that KYNU expression was up‐regulated in cSCC, and blocking KYNU significantly inhibited cSCC cell proliferation, migration and invasion, thus pointing to KYNU as a plausible target for therapeutic interventions for cSCC. 12
In addition to our previous study showing CD44 activation of NF‐kB, another study revealed that NF‐kB induced significant expression of KYNU in triple‐negative BC cell lines, further supporting our hypothesis that CD44‐activated NF‐kB might transactivate KYNU. 29 Such enhanced KYNU expression has been correlated with BC metastasis to the lung and brain. 27 , 32 On the other hand, RAS, the upstream pathway for PI3K/AKT pathway is associated with BC metastasis 35 ; stimulating the RAS signalling pathway correlated with up‐regulation of the expression of KYNU, 36 thus indicating an oncogenic role of KYNU in BC metastasis.
On the other hand, a recent study reported a negative correlation of KYNU expression and BC histological grades tumour stages; KYNU expression was lost in poorly differentiated BC cells and stage 3 BC. 14 KYNU was negatively associated with HER‐2 expression and Ki‐67. 14 Moreover, both in vitro and in vivo experiments revealed that loss of KYNU activated BC cell proliferation, differentiation, colony formation and xenograft tumour growth. 14
4. POTENTIAL INHIBITORS OF KYNU
KYNU may play a role in underlying mechanisms resulting in the production of the excitotoxin moiety quinolinic acid (QUIN), which is a metabolite of tryptophan that has been shown to be a neurotoxin. 37 Several studies have been carried out to develop suitable inhibitors targeting KYNU in order to guide the design of appropriate therapeutic strategies in bacteria as well as mammals. In fact, few of the bacterial KYNU inhibitors that mimic the molecular transition state of a range of substrates have been developed including (4S)‐ and (4R)‐dihydro‐L‐kynurenine, 38 a series of S‐aryl‐L‐cysteine S,S‐dioxides 39 and a phosphinic acid 40 analogue of kynurenine. On the other hand, in human macrophages, S‐aryl‐L‐cysteine S,S‐dioxides produced a molecule (2‐amino‐5‐methyl‐S‐phenyl cysteine S,S‐dioxide), which significantly blocked INF‐γ‐induced QUIN synthesis. 37 Another kynurenine derivative (2‐amino‐4‐[3’‐hydroxy‐phenyl]‐4‐hydroxybutanoic acid) was developed in order to incorporate a hydroxy‐l moiety at C7 and C3 to resemble the substrates conversion state and enhance specificity towards human KYNU. 41
5. CONCLUSION
KYNU appears to play a major role in the development and dissemination of breast tumours, but its underlying mechanisms are still poorly understood. KYNU interacts with several signalling pathways that promote breast tumour cell invasion and metastasis. In particular, findings from our own work and others support our hypothesis that CD44‐HA interaction might activate NF‐kB, which in turn transactivate KYNU, ultimately leading to BC cell invasion (Figure 2).
FIGURE 2.
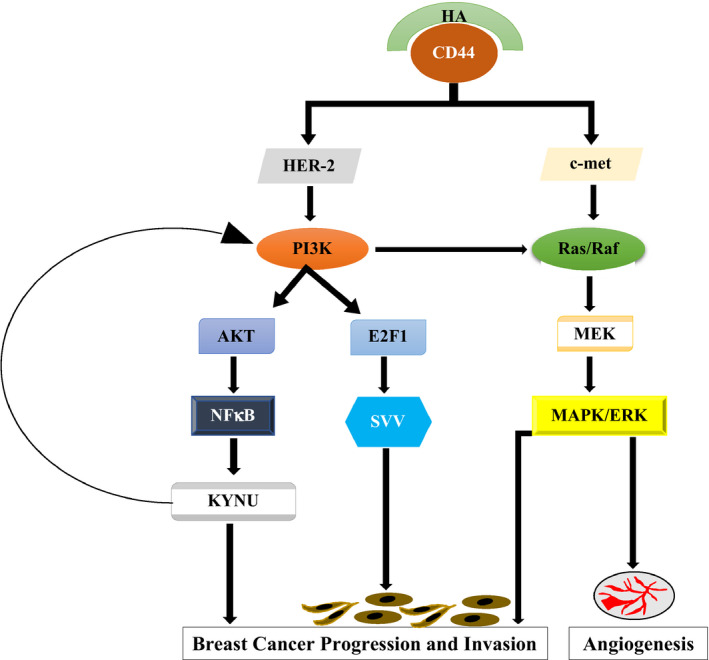
Suggested mechanisms linking HA‐CD44 interaction to KYNU transcription
On the other hand, another finding from our own previous study identified the PI3K pathway as a potential molecular link between HA/CD44 activation and survivin transcription, 42 and PI3K/AKT increased KYNU expression. 12 , 35 , 36 Therefore, CD44‐HA interaction might activate KYNU via the PI3K pathway. In conclusion, this review provides several lines of evidence supporting our hypothesis that KYNU might be a novel transcriptional target of CD44‐signalling involved in promoting BC cell invasion via NF‐κB and/or PI3K pathways. Ongoing in vitro and in vivo experiments aim to validate KYNU as a novel target of CD44‐promoted BC cell invasion and metastasis.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTION
Maryam Al‐Mansoob: Writing‐original draft (lead). Ishita Gupta: Writing‐review & editing (equal). Allal Ouhtit: Conceptualization (lead); Funding acquisition (lead); Writing‐review & editing (equal). Radoslaw Stefan Rusyniak: Editing (equal).
AUTHOR CONTRIBUTIONS
‘AO: Conceptualization. MAM: writing – original draft. IG and AO: Writing – review and editing. RSR: Editing. AO: funding acquisition. All authors have read and agreed to the published version of the manuscript.’
ACKNOWLEDGEMENTS
This research was funded by Qatar University Internal grant number: QUST‐1‐CAS2019‐22, Qatar Foundation grant number: UREP24‐117‐1‐027 and APC. Open Access funding was provided by the Qatar National Library.
Al‐Mansoob M, Gupta I, Stefan Rusyniak R, Ouhtit A. KYNU, a novel potential target that underpins CD44‐promoted breast tumor cell invasion. J Cell Mol Med. 2021;25:2309–2314. 10.1111/jcmm.16296
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data or datasets were created, generated or analysed in this study.
REFERENCES
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941‐1953. 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 2. Weigelt B, Peterse JL, van't Veer LJ.Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591‐602. 10.1038/nrc1670 [DOI] [PubMed] [Google Scholar]
- 3. McSherry EA, Donatello S, Hopkins AM, McDonnell S. Molecular basis of invasion in breast cancer. Cell Mol Life Sci. 2007;64(24):3201‐3218. 10.1007/s00018-007-7388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bendas G, Borsig L. Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. Int J Cell Biol. 2012;2012:676731. 10.1155/2012/676731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig‐CAMs in cancer. Nat Rev Cancer. 2004;4(2):118‐132. 10.1038/nrc1276 [DOI] [PubMed] [Google Scholar]
- 6. Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61(7):1303‐1313. 10.1016/0092-8674(90)90694-a [DOI] [PubMed] [Google Scholar]
- 7. Hill A, McFarlane S, Mulligan K, et al. Cortactin underpins CD44‐promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene. 2006;25(45):6079‐6091. 10.1038/sj.onc.1209628 [DOI] [PubMed] [Google Scholar]
- 8. Ouhtit A, Abd Elmageed ZY, Abdraboh ME, Lioe TF, Raj MHG. In vivo evidence for the role of CD44s in promoting breast cancer metastasis to the liver. Am J Pathol. 2007;171(6):2033‐2039. 10.2353/ajpath.2007.070535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ouhtit A, Madani S, Gupta I, et al. TGF‐beta2: a novel target of CD44‐promoted breast cancer invasion. Journal of Cancer. 2013;4(7):566‐572. 10.7150/jca.6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phillips RS. Structure and mechanism of kynureninase. Arch Biochem Biophys. 2014;544:69‐74. 10.1016/j.abb.2013.10.020 [DOI] [PubMed] [Google Scholar]
- 11. Larkin PB, Sathyasaikumar KV, Notarangelo FM, et al. Tryptophan 2,3‐dioxygenase and indoleamine 2,3‐dioxygenase 1 make separate, tissue‐specific contributions to basal and inflammation‐induced kynurenine pathway metabolism in mice. Biochim Biophys Acta. 2016;1860(11):2345–2354. 10.1016/j.bbagen.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ci C, Wu C, Lyu D, et al. Downregulation of kynureninase restrains cutaneous squamous cell carcinoma proliferation and represses the PI3K/AKT pathway. Clin Exp Dermatol. 2020;45(2):194‐201. 10.1111/ced.14072 [DOI] [PubMed] [Google Scholar]
- 13. Harden JL, Lewis SM, Lish SR, et al. The tryptophan metabolism enzyme L‐kynureninase is a novel inflammatory factor in psoriasis and other inflammatory diseases. J Allergy Clin Immunol. 2016;137(6):1830‐1840. 10.1016/j.jaci.2015.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Feng X, Lai J, et al. A novel role of kynureninase in the growth control of breast cancer cells and its relationships with breast cancer. J Cell Mol Med. 2019;23(10):6700‐6707. 10.1111/jcmm.14547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sirka OK, Shamir ER, Ewald AJ. Myoepithelial cells are a dynamic barrier to epithelial dissemination. J Cell Biol. 2018;217(10):3368‐3381. 10.1083/jcb.201802144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehta PK, Hale TI, Christen P. Aminotransferases: demonstration of homology and division into evolutionary subgroups. Eur J Biochem. 1993;214(2):549‐561. 10.1111/j.1432-1033.1993.tb17953.x [DOI] [PubMed] [Google Scholar]
- 17. Phillips RS, Iradukunda EC, Hughes T, Bowen JP. Modulation of enzyme activity in the kynurenine pathway by kynurenine monooxygenase inhibition. Frontiers in Molecular Biosciences. 2019;6: 10.3389/fmolb.2019.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lima S, Khristoforov R, Momany C, Phillips RS. Crystal structure of Homo sapiens kynureninase. Biochemistry. 2007;46(10):2735‐2744. 10.1021/bi0616697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi H, Enriquez A, Rapadas M, et al. NAD deficiency, congenital malformations, and niacin supplementation. N Engl J Med. 2017;377(6):544‐552. 10.1056/NEJMoa1616361 [DOI] [PubMed] [Google Scholar]
- 20. Michelhaugh SK, Guastella AR, Mittal S. Overview of the kynurenine pathway of tryptophan metabolism. In: Mittal S, ed. Targeting the Broadly Pathogenic Kynurenine Pathway. Cham: Springer International Publishing; 2015:3‐9. [Google Scholar]
- 21. Bender DA. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983;6(2):101‐197. 10.1016/0098-2997(83)90005-5 [DOI] [PubMed] [Google Scholar]
- 22. Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3‐kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606‐619. 10.1038/nrg1879 [DOI] [PubMed] [Google Scholar]
- 23. Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3‐kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615‐675. 10.1146/annurev.cellbio.17.1.615 [DOI] [PubMed] [Google Scholar]
- 24. Granito A, Guidetti E, Gramantieri L. c‐MET receptor tyrosine kinase as a molecular target in advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2015;2:29‐38. 10.2147/jhc.S77038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Welz B, Bikker R, Junemann J, et al. Proteome and phosphoproteome analysis in TNF long term‐exposed primary human monocytes. Int J Mol Sci. 2019;20(5):1241. 10.3390/ijms20051241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu C, Qin M, Tan L, Liu S, Huang J. NIBP impacts on the expression of E‐cadherin, CD44 and vimentin in colon cancer via the NF‐κB pathway. Mol Med Rep. 2016;13(6):5379‐5385. [DOI] [PubMed] [Google Scholar]
- 27. Bos PD, Zhang XH, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005‐1009. 10.1038/nature08021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401‐404. 10.1158/2159-8290.Cd-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D'Amato NC, Rogers TJ, Gordon MA, et al. A TDO2‐AhR signaling axis facilitates anoikis resistance and metastasis in triple‐negative breast cancer. Cancer Res. 2015;75(21):4651‐4664. 10.1158/0008-5472.CAN-15-2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Q, Zhai J, Kong X, et al. Comprehensive analysis of the expression and prognosis for TDO2 in breast cancer. Mol Ther Oncolyt. 2020;17:153‐168. 10.1016/j.omto.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Minn AJ, Gupta GP, Siegel PM, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436(7050):518‐524. 10.1038/nature03799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rose DP. The influence of sex, age and breast cancer on tryptophan metabolism. Clin Chim Acta. 1967;18(2):221‐225. 10.1016/0009-8981(67)90161-1 [DOI] [PubMed] [Google Scholar]
- 34. Goode G, Pratap S, Eltom SE. Depletion of the aryl hydrocarbon receptor in MDA‐MB‐231 human breast cancer cells altered the expression of genes in key regulatory pathways of cancer. PLoS One. 2014;9(6):e100103‐e100103. 10.1371/journal.pone.0100103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carné Trécesson SD, Souazé F, Basseville A, et al. BCL‐XL directly modulates RAS signalling to favour cancer cell stemness. Nature Commun. 2017; 8(1): 1123. 10.1038/s41467-017-01079-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palanichamy K, Thirumoorthy K, Kanji S, et al. Methionine and kynurenine activate oncogenic kinases in glioblastoma, and methionine deprivation compromises proliferation. Clin Cancer Res. 2016;22(14):3513‐3523. 10.1158/1078-0432.Ccr-15-2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Drysdale MJ, Reinhard JF. S‐aryl cysteine S, S‐dioxides as inhibitors of mammalian kynureninase. Bioorg Med Chem Lett. 1998;8(2):133‐138. 10.1016/s0960-894x(97)10209-8 [DOI] [PubMed] [Google Scholar]
- 38. Phillips RS, Dua RK. Stereochemistry and mechanism of aldol reactions catalyzed by kynureninase. J Am Chem Soc. 1991;113(19):7385‐7388. 10.1021/ja00019a039 [DOI] [Google Scholar]
- 39. Dua RK, Taylor EW, Phillips RS. S‐Aryl‐L‐cysteine S, S‐dioxides: design, synthesis, and evaluation of a new class of inhibitors of kynureninase. J Am Chem Soc. 1993;115(4):1264‐1270. 10.1021/ja00057a007 [DOI] [Google Scholar]
- 40. Ross FC, Botting NP, Leeson PD. Synthesis of phosphinic acid transition state analogues for the reaction catalysed by kynureninase. Bioorg Med Chem Lett. 1996;6(22):2643‐2646. [Google Scholar]
- 41. Walsh HA, Leslie PL, O'Shea KC, Botting NP. 2‐Amino‐4‐[3'‐hydroxyphenyl]‐4‐hydroxybutanoic acid; a potent inhibitor of rat and recombinant human kynureninase. Bioorg Med Chem Lett. 2002;12(3):361‐363. 10.1016/s0960-894x(01)00758-2 [DOI] [PubMed] [Google Scholar]
- 42. Abdraboh ME, Gaur RL, Hollenbach AD, Sandquist D, Raj MH, Ouhtit A. Survivin is a novel target of CD44‐promoted breast tumor invasion. Am J Pathol. 2011;179(2):555‐563. 10.1016/j.ajpath.2011.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data or datasets were created, generated or analysed in this study.


