Abstract
Obesity is a preventable risk factor for breast cancer following menopause. Regardless of menopausal status, obese women who develop breast cancer have a worsened prognosis. Breast tissue is comprised of mammary epithelial cells organized into ducts and lobules and surrounded by adipose-rich connective tissue. Studies utilizing multiple in vivo models of obesity as well as human breast tissue have contributed to our understanding of how obesity alters mammary tissue. Localized changes in mammary epithelial cell populations, elevated secretion of adipokines and angiogenic mediators, inflammation within mammary adipose tissue, and remodeling of the extracellular matrix may result in an environment conducive to breast cancer growth. Despite these significant alterations caused by obesity within breast tissue, studies have suggested that some, but not all, obesity-induced changes may be mitigated with weight loss. Here, we review our current understanding regarding the impact of obesity on the breast microenvironment, how obesity-induced changes may contribute to breast tumor progression, and the impact of weight loss on the breast microenvironment.
Keywords: obesity, breast cancer risk, inflammation, adipose, mammary epithelial cells
INTRODUCTION
Globally, obesity rates have tripled over the past 45 years [1]. In the United States alone, over 40% of the adult population is obese [2], and today, the majority of the world’s population live in countries where obesity is responsible for more deaths than malnutrition [1]. Clinically, body fatness is measured using body mass index (BMI), which is calculated by dividing a patient’s weight in kilograms by height in meters squared. BMI in the range of 18.5–24.9 kg/m2 is considered normal weight, while BMI in the range of 25.0–29.9 kg/m2 is defined as overweight and those greater than 30.0 kg/m2 are categorized as obese. Measurement of BMI can misidentify patients into BMI categories, particularly in patients with increased muscle mass [3], and discussions are ongoing to determine whether BMI categories should differ across regions or racial or ethnic groups [4]. While BMI is easily calculated and is clinically the most frequently used and reported measure of obesity, the relative contribution of diet, physical activity, body fat distribution, age and duration of obesity may all contribute to variations in obesity-related diseases.
Obesity has emerged as a risk factor for multiple types of cancer, including breast cancer [5]. However, obesity has divergent effects on breast cancer risk depending on whether a woman has transitioned through menopause [6–8]. Incremental increases in BMI are correlated with elevated risk for development of estrogen receptor alpha (ERα) and progesterone receptor-positive tumors following menopause [9–12]. In contrast, increased risk for development of triple negative breast cancer, characterized by a lack of expression of hormone receptors and HER2, is less clear for obese postmenopausal women [9, 11–15]. In addition to pathological classifications, advances in gene expression analysis has led to 5 major molecular classifications of breast tumors: Luminal A and B, which are distinguished by the expression of proliferation/cell cycle genes and luminal/hormonal regulation pathways; HER2 enriched, which has high expression of HER2 and proliferation-related genes, intermediate expression of luminal-related genes, and low expression of basal-related genes; Basal-like, which has high expression of proliferation-related genes and keratins associated with the basal layer of the skin; and Claudin-low [16–18]. Obese postmenopausal women are more likely to develop tumors of the Luminal B molecular subtype, which are associated with reduced relapse-free survival [19, 20]. Further, tumors of the Luminal A subtype from obese women demonstrate significant differences in gene expression associated with cell cycle regulation, p53 and mTOR signaling, DNA repair and transcription dysregulation compared to those from lean women [21]. Further studies are necessary to more clearly assess how obesity impacts the risk for the remaining molecular subtypes of breast cancer.
Intriguingly, obesity reduces the risk for premenopausal breast cancer within the general population when measured by BMI [7–9, 22, 23]. However, in younger women, alternate methods of measuring body fat distribution may be more important to assess risk. Multiple studies have demonstrated an increased risk for breast cancer in premenopausal women with increased waist circumference or waist-to-hip ratio [24–27], as well as hip circumference [28]. Racial differences may also influence how obesity influences breast cancer risk, as increased BMI is significantly correlated with elevated breast cancer risk in premenopausal Asian women [29]. Premenopausal women with a family history of breast cancer or genetically enhanced risk also have an additional risk for breast cancer associated with obesity [13, 25, 30–32].
Regardless of menopausal status, obese individuals diagnosed with breast cancer demonstrate significantly worse overall and breast cancer-specific survival compared to women with BMI in the normal range [11, 30, 33–37]. At the time of diagnosis, obese women present more frequently with advanced disease [10, 11, 30], large tumor size [10, 11, 35, 36, 38], lymph node involvement [10, 11, 30, 35, 36, 38], and increased stage [10, 11, 30]. Breast cancer patients with higher BMIs are also more likely to develop resistance to chemotherapy and endocrine therapies than people with lower BMIs [35, 39–45]. Understanding how obesity impacts normal breast tissue may provide mechanistic insight into how obesity enhances breast cancer risk and the development of aggressive breast tumors.
To investigate how obesity impacts non-cancerous tissue, numerous mouse models have been developed and characterized. Although a number of genetic models have been developed to examine obesity and feeding behaviors [46], the most commonly utilized genetic models to examine obesity in the mammary gland are those targeting leptin or its receptor. Leptin-deficient mice (ob/ob) or leptin receptor-deficient mice (db/db) lack food intake restraint, leading to obesity and obesity-associated diseases [47, 48]. Obesity is also modeled using diet-induced obesity, in which excess dietary calories, usually from a high fat diet (HFD), lead to an accumulation of body fat over a relatively long period of time. C57BL/6 mice are widely utilized for obesity and metabolic research, because when fed a HFD over time, C57BL/6 mice develop significant weight gain and metabolic abnormalities consistent with obesity in humans [49]. However, a significant drawback of this model is that C57BL/6 mice are resistant to mammary tumor development. Other mouse strains, which are susceptible to mammary tumor formation, are resistant to HFD-induced obesity [50–52], including BALB/c mice, in which limited numbers of mice gain significant weight when fed a HFD [53]. Obesity studies vary based on the percentage of fat in the diet used to induce obesity, feeding strategies for inducing obesity, as well as the composition of the diet to increase calories based on both sucrose and fat. High fat and high sucrose diets induce type II diabetes and have been frequently used to model metabolic syndrome, which is also associated with obesity [54]. While mice are a frequently used model for obesity, rat models of obesity have also been well characterized [55]. Outbred rat models, including Wistar and Sprague-Dawley rats, fed obesogenic diets can be identified as either obesity-prone or obesity-resistant [56, 57] providing useful models for the range of susceptibility to obesity observed in humans. Although diet-induced obesity models provide insight into obesity-induced changes within the mammary gland, challenges remain in separating diet-induced effects from obesity-specific effects.
Obesity induces multiple changes on divergent cell types within the mammary gland including mammary epithelial cells, which could become breast cancer-initiating cells, and cells of the surrounding microenvironment. The mammary epithelium is composed of a luminal layer of epithelial cells that are surrounded by an outer layer of myoepithelial cells, which contract to eject milk during lactation. The epithelium is organized into alveoli which are connected to ducts that transport milk to the nipple or teats during lactation. Surrounding the epithelium in the mammary gland is adipose tissue which contains mature adipocytes, as well as immune cells, fibroblasts, adipose stem cells, endothelial cells, and pericytes that aid in adipose tissue maintenance and homeostasis [58]. Obesity results in systemic changes in circulating hormones, including insulin and leptin, as well as localized pathological changes within mammary tissue. Systemic effects of obesity and their potential impact on breast cancer formation have been reviewed in other recent work [59–65]. Here we will focus on changes that occur within mammary tissue due to obesity that may contribute to breast cancer risk.
EPITHELIUM
The mammary gland is a unique organ in which the majority of epithelial development takes place after birth during puberty. During this time, rudimentary ducts grow into the adipose tissue and stroma of the mammary gland from the teat or nipple and form branches with variable amounts of alveoli. Epithelial cell growth and proliferation are coordinated by ovarian hormones and localized growth factors, which together regulate a compliment of stem/progenitor cells that are necessary for ductal growth, normal homeostasis as well as the development of alveoli during successive pregnancies. Current hypotheses suggest that these stem/progenitor cells are the cells-of-origin for the most common types of breast cancer [66–68]. Understanding how obesity disrupts the normal regulation of epithelial stem/progenitor cell populations may provide insight into how obesity enhances breast cancer risk.
Mammary epithelial stem/progenitor cells have been characterized and quantified using multiple methods. Using flow cytometry, luminal and basal/myoepithelial cell populations can be quantified using cell surface antibodies, including EpCAM, CD29, CD49f and CD24 [69–72]. Using a diet-induced obesity model, epithelial cells isolated from obese mice demonstrated increased EpCAMhiCD49flo luminal epithelial cells, while EpCAMloCD49fhi basal/myoepithelial cells were significantly decreased compared to controls [73]. Similarly, luminal epithelial cells, identified by markers EpCAM+CD24+CD49flo, were significantly increased in breast tissue isolated from reduction mammoplasty surgeries of women with an obese BMI compared to those with BMIs within the normal range [73]. Consistent with an enrichment in luminal epithelial cells quantified by flow cytometry, immunostaining of mammary tissue sections from obese mice demonstrated elevated numbers of ERα-positive luminal cells within ducts, while smooth muscle actin-expressing basal/myoepithelial cells were significantly decreased compared to those from lean mice [73, 74]. Loss of myoepithelial cells surrounding normal mammary ducts could enhance breast cancer risk due to their characterized role in suppression of breast cancer progression [75]. These results are in contrast to a study examining fine needle aspirates of breast epithelial cells from women at high risk for breast cancer. In this study, expression of vimentin, which is associated with mesenchymal traits, was significantly enhanced in breast epithelial cells of obese women [76]. It is possible that the results of this study reflect specific selection for patients with elevated breast cancer risk compared to the general population of women or differences in the ethnicity of the populations examined. Further studies will be necessary to identify the underlying cause of this difference in epithelial cell marker expression. In addition to examining cell-specific markers, stem/progenitor epithelial cells are frequently measured using in vitro assays to identify epithelial cell self-renewal when plated in limiting dilution. Epithelial cells with the ability to form colonies in suspension, called mammospheres, as well as form colonies on a feeder layer of NIH/3T3 cells were enriched in mammary glands from obese mice and women [73], however, the cell population enriched for stem/progenitor activity was not specifically identified. Through increased numbers of epithelial stem/progenitor cells, obesity may enhance targets for oncogenic transformation within breast tissue.
Within adipose tissue of the mammary gland, obesity alters the secretion of adipokines and growth factors. With increasing adiposity, local secretion of the adipokine leptin is significantly enhanced by adipocytes [77, 78], and leptin receptor is expressed within the mammary epithelium [79, 80]. In a diet-induced obesity model utilizing BALB/c mice, leptin expression was significantly increased within mammary tissue, and mammary epithelial cells demonstrated an altered distribution of apical polarity markers [81]. Treatment with exogenous leptin has been shown to enhance mammary epithelial cell self-renewal in culture [81, 82], as well as expression of stem cell marker ALDH1 within mammospheres [82]. Within the tumor microenvironment, leptin enhances cancer stem-like cells through regulation of genes critical for fatty acid β-oxidation, resulting in resistance to the chemotherapeutic agent, paclitaxel [83]. It is possible that leptin enhances lipid metabolism in mammary stem cells in a similar manner within mammary tissue in obesity. In addition to a role in enhancing mammary epithelial stem/progenitor cells, leptin has also been implicated in enhancing oxidative stress within mammary epithelial cells, which may elevate mutations in DNA and contribute to breast cancer risk [84]. Expression of insulin-like growth factor-1 (IGF-1) is also increased by cells within adipose tissue under conditions of obesity [85]. IGF-1 is critical for mammary epithelial growth during development [86], suggesting that elevated levels of IGF-1 may also impact mammary epithelial cells in obesity. Regulation of mammary epithelial stem/progenitor cells may be complex under conditions of obesity due to interactions among these factors.
Although obesity leads to alterations both in the microenvironment of the breast and in circulating hormones, the effect of individual fatty acids on the mammary gland are not well-understood. Feeding rodents diets enriched in specific fatty acids following weaning results in developmental changes in ductal growth, which occur prior to the onset of diet-induced obesity. However, divergent effects on mammary development have been observed based on the type of fatty acids administered and the strain of mice examined. C57BL/6 female mice weaned onto a diet high in saturated fat from lard demonstrated reduced ductal branching, slower ductal elongation, and diminished proliferation of mammary epithelial cells [53, 73, 74]. This change in ductal growth may be due to reduced secretion of the growth factor amphiregulin by mammary stromal cells [53]. These changes within the mammary epithelium were strain-specific, as BALB/c female mice fed the same diet during this developmental window demonstrated increased epithelial cell proliferation [53]. BALB/c female mice weaned onto a diet enriched in corn oil, containing primarily omega (ω)-6 polyunsaturated fatty acids, demonstrated increased epithelial cell proliferation and enhanced responsiveness to estrogen and progesterone supplementation [87, 88]. Consistent with this study, BALB/c mice fed ω−6 polyunsaturated fatty acids demonstrated a significant increase in ERα binding sites within the mammary gland [89], however whether this increased ERα expression was present in epithelial cells or mammary stromal cells, including adipocytes and macrophages, was not examined. In contrast, BALB/c female and male mice fed the trans-10, cis-12 isomer of conjugated linoleic acid at weaning demonstrated ovary-independent ductal growth [90] and enhanced formation of terminal end buds [91]. This ductal growth was enhanced indirectly through increased secretion of IGF-1 from mammary stromal cells [90]. Understanding how timing of obesity during mammary gland growth and development could provide insight into differing breast cancer risk in premenopausal and postmenopausal women.
Although studies in BALB/c mice examining the effect of fatty acids have observed a growth promoting effect during the pubertal developmental window, different effects may occur in mature animals. Adult BALB/c female mice fed ω−3 polyunsaturated fatty acids demonstrated reduced ductal branching, smaller ductal size, and diminished stroma surrounding ducts compared to those fed isocaloric ω−6 polyunsaturated fatty acids [92]. Further studies are necessary to understand how fatty acids impact epithelial cell growth and development, particularly as a result of their effects on multiple cell types within the mammary stroma, including adipocytes and inflammatory cells [90, 92]. Dietary consumption of animal fat has been implicated in increased risk for breast cancer after adjustment for height, weight, family history of breast cancer, and oral contraceptive use [93], and understanding how individual fatty acids impact the mammary epithelium may provide insight into the complicated relationship between obesity and dietary influences on breast cancer risk.
ADIPOCYTES & ADIPOSE-DERIVED STROMAL CELLS
Adipocytes are the main energy storage cells in the body and secrete various adipokines that regulate energy intake and inflammation. Compared with other fat depots within the body, the adipocytes in the mammary gland have a unique relationship with the surrounding epithelial cells. Within the resting mammary gland, adipocytes are a major component of the stroma. However, during pregnancy, while the epithelium proliferates to form alveoli for lactation, adipocytes disappear, and few adipocytes are visible in the mammary gland during lactation. Following lactation, the mammary epithelium undergoes apoptosis during involution, and adipocytes rapidly reappear in the mammary gland. Using elegant lineage tracing studies, Wang et al. demonstrated that mammary adipocytes are plastic and undergo a de-differentiation program during pregnancy that is reversed during involution [94]. Adipocytes play a key role in involution by transferring milk lipid into the central lipid droplet and supporting epithelial cell regression [95]. Studies utilizing mice fed a high fat diet during pregnancy suggest that obesity may enhance inflammation within mammary fat resulting in reduced ability for lactation [96, 97], however the underlying mechanism is not well-understood.
With increasing weight gain, adipocytes undergo hypertrophy and eventually die, triggering the release of cellular contents [98, 99]. Adipocyte death activates an innate immune response, instigating a proinflammatory state of adipose tissue under obesity. Through release of proinflammatory cytokines, macrophages are recruited to adipose tissue and surround dying adipocytes in what have been termed crown-like structures (CLS) [98]. While adipocyte death appears to initiate the inflammatory mammary microenvironment, inflammation is sustained by the resulting tissue remodeling. Under conditions of obesity, adipocytes may enhance breast cancer risk directly through secretion of adipokines and growth factors that enhance the proliferation of epithelial cells, as well as indirectly by creating an environment conducive to tumor growth through localized inflammation and tissue remodeling (Fig. 1).
Fig. 1. Obesity promotes cellular remodeling within the mammary gland.
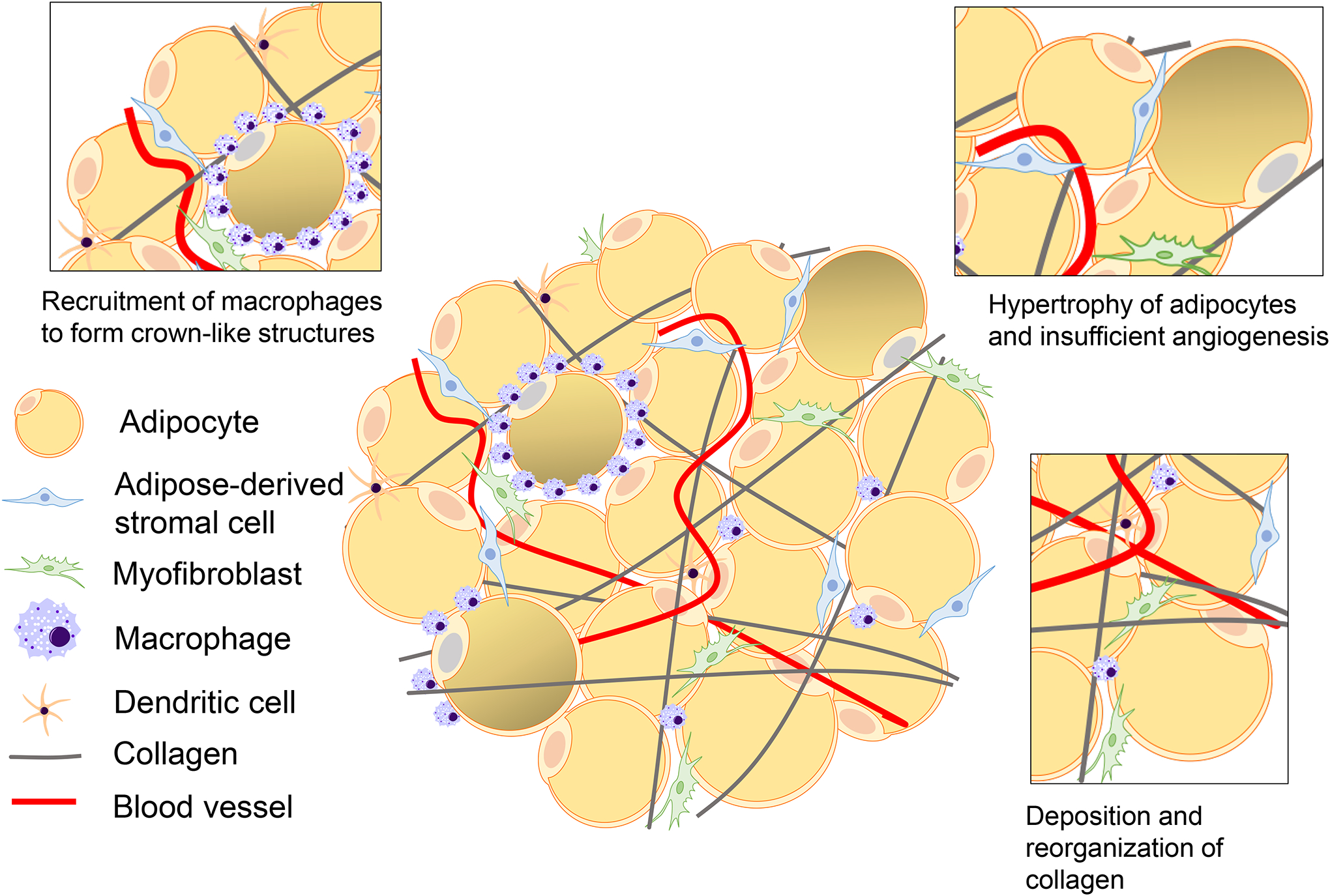
With the onset of obesity, adipocytes enlarge, leading to tissue hypoxia, adipocyte death, and enhanced secretion of adipokines, including leptin. Macrophages are recruited into adipose tissue in obesity by CCL2 and dendritic cells and form crown-like structures around necrotic adipocytes. Tissue hypoxia and inflammatory cytokines increase angiogenesis. Myofibroblasts and adipose-derived stromal cells increase production and reorganization of collagen, leading to adipose tissue fibrosis
In addition to adipocytes, adipose tissue contains a reservoir of multipoint, heterogenous cells that function to maintain adipose tissue homeostasis through differentiation into adipocytes and secretion of paracrine factors. In culture, adipose stem cells have the ability to differentiate along multiple lineages including adipocytes, osteoblasts, and fibroblasts in vitro [100, 101]. However, challenges exist in identifying and isolating adipose stem cells from other stromal cells present within adipose tissue, including fibroblasts, endothelial cells, pericytes, as well as adipocytes, which when cultured in vitro lose their stored lipid and resemble fibroblasts. Isolation of human and mouse mammary adipose stem cells can be performed through enzymatic digestion of mammary tissue and density separation of the stromal vascular fraction from the denser epithelial organoids and the lighter mature adipocytes [73, 102], followed by fluorescence-activated cell sorting to isolate cells based on cell surface expression of markers CD90, CD73, CD105 and CD44 [103]. Frequently, studies examining adipose stem cells within the mammary gland isolate the whole mixture of stromal cells. When grown in culture, this collection of cells is termed adipose-derived stromal cells and are utilized together to examine the functional capabilities of the adipose stem cells. Adipose stem cells have been shown to incorporate into the mammary tumor microenvironment to promote cancer growth [104], suggesting that changes in these cells within the obese breast microenvironment could promote early tumor progression.
Adipose-derived stromal cells isolated from subcutaneous, visceral, and mammary adipose tissue in both obese mice and humans have reduced ability to differentiate in vitro into adipocytes [85, 105–109], osteoblasts [110, 85, 106] and cartilage [111] than those from lean individuals, suggesting that obesity reduces either adipose stem cell numbers or plasticity. Expression of the nuclear transcription factor PPARγ is downregulated in adipose-derived stromal cells from obese tissue, which inhibits adipose stem cell differentiation toward adipocytes and potentially leads to reduced adipocyte renewal and repair [112, 113]. Concurrent with the loss of adipose stem cell multipotency in culture, human and mouse obese adipose tissue demonstrates an increased population of myofibroblasts as observed by elevated expression of α-smooth muscle actin, a myofibroblast marker, within adipose tissue [85, 114, 115]. This increase toward myofibroblast differentiation of adipose-derived stromal cells from obese mammary glands may result from downregulation of microRNA 140 [115]. This myofibroblast phenotype has similarities to cancer associated fibroblasts within the tumor microenvironment. In addition to decreased differentiation ability, adipose-derived stromal cells from obese mammary fat appear to have increased proliferative capacity. In a diet-induced obesity model, adipose-derived stromal cells isolated from obese mice had increased proliferation rates compared to those isolated from mammary glands of control mice [85]. However, isolation of adipose-derived stromal cells from obese patients to examine proliferative ability resulted in divergent responses [105, 109], and further studies are necessary to determine how obesity impacts the proliferative ability of human adipose-derived stromal cells. Adipose-derived stromal cells from obese adipose tissue also appear to have increased motility. When isolated from subcutaneous and visceral adipose tissue of obese human subjects, adipose-derived stromal cells showed increased migration on Transwell membranes than those from lean patients [105, 116, 117]. Adipose stem cells are significantly increased in number in circulation in obese mice, which may enhance trafficking of these cells to distant tissue sites [104]. Increased migration of adipose-derived stromal cells could enhance stroma formation within developing tumors or promote the growth of metastases at distant sites. Accompanying these obesity-induced physiological changes, adipose-derived stromal cells from obese humans expressed an inflammatory gene signature with upregulation of IL-1β, IL-6, TNF-α, and CCL2/MCP-1 [116]. Together, these data suggest that obesity significantly alters the functional behavior of adipose-derived stromal cells, which may enhance the growth of oncogenically transformed epithelial cells.
With the change in functional behavior of adipose-derived stromal cells, changes in the composition of the extracellular matrix in adipose tissue are also observed. Structurally, the obese mammary gland contains increased fibrotic components such as collagen I, collagen VI, fibronectin, and hyaluronic acid [114, 118–120], and the orientation of these fibrous components are altered in response to obesity [114]. In mammary glands of diet-induced obesity models and ob/ob mice, collagen is more linear than in lean controls, and collagen fiber length in human obese breast tissue is significantly longer compared to those observed in women in the normal BMI range [114]. Adipose-derived stromal cells from obese mice partially unfold fibronectin, leading to matrix rigidity [114]. These changes in the rigidity of the extracellular matrix also impact the stromal cells within the tissue. Culturing adipose-derived stromal cells isolated from control mice on decellularized extracellular matrix isolated from ob/ob mice in culture resulted in increased myofibroblast differentiation compared to those cultured on extracellular matrix isolated from control mice [114], suggesting that obesity-induced changes to the extracellular matrix may alter the differentiation of adipose-derived stromal cells within the adipose tissue. Endotropin, a cleavage product of collagen VI alpha 3 chain, is enhanced under conditions of obesity and enhances the recruitment of macrophages and endothelial cells [120]. Further, endotrophin expression promoted progression of aggressive, metastatic tumors in the MMTV-PyMT model of mammary tumorigenesis [120]. Obesity is positively correlated with increased collagen and extracellular matrix deposition within breast tumors [114], suggesting that changes that occur within the extracellular matrix of tumor-free mammary tissue may be propagated within the tumor microenvironment.
Adipose tissue within the mammary gland is a site of extragonadal estrogen synthesis through activity of aromatase (CYP19A1), the enzyme responsible for estrogen biosynthesis. Both aromatase expression and estrogen synthesis increase in adipose tissue with aging [121]. In postmenopausal women, adipose tissue inflammation due to obesity is associated with elevated aromatase expression and increased circulating estrogen levels [122–124], as the promoter that regulates CYP19A1 expression in adipose tissue is regulated by class 1 cytokines and TNF-α [125–128]. In addition to adipose-derived stromal cells, macrophages have been shown to express aromatase in the mammary gland [129]. Since macrophages are recruited by dying adipocytes to form CLSs under conditions of obesity, it is possible that obesity-induced inflammation as well as increased macrophage recruitment enhance local estrogen concentrations in breast tissue of obese postmenopausal women. Increased estrogen within the mammary gland may significantly impact epithelial cell stem/progenitor populations within normal mammary tissue as well as other estrogen-responsive cells within the microenvironment. Clinically, aromatase is an important therapeutic target for treatment of breast cancer. However, obese patients diagnosed with ERα-positive breast cancers demonstrated less treatment efficacy from aromatase inhibitors than lean breast cancer patients [42–45], and it has been hypothesized that this decreased treatment response could be due to inadequate suppression of aromatase within the obese microenvironment [130].
IMMUNE CELLS
Mammary adipose tissue contains resident macrophages which originate from yolk sac-derived or fetal liver-derived macrophages and have the ability to self-renew in adult mice [131–133]. This population appears to be robustly identified by the expression of CD206 [133]. Resident macrophages often reside between adipocytes and along vasculature, and perform homeostatic functions such as regulating adipocyte metabolism, resolving inflammation, and removing apoptotic cells [134]. Within the mammary gland, resident macrophages also interact with both luminal and basal epithelial cells [135] and play a role in maintaining epithelial stem/progenitor cell niches [136, 137]. Critical to mammary gland function following lactation, resident macrophages remodel the mammary gland during the process of involution [138].
While resident macrophages play a role in early changes in adipose tissue associated with obesity [139], bone marrow-derived macrophages are recruited into adipose tissue in response to secretion of the chemokine CCL2/MCP-1 by adipose-derived stromal cells and adipocytes. [140–143]. The CCL2/CCR2 axis plays an important role in macrophage recruitment during obesity, as genetic deletion of either CCL2 or CCR2 in mouse models results in a significant reduction of macrophage recruitment into adipose tissue [144, 145]. Gene expression analyses of resident and bone marrow-derived macrophages in obesity suggest that there are significant functional differences between the two macrophage types [142]. In obese mice, macrophages comprise 40% of the cell populations of visceral fat, compared to 10% in lean mice [146]. In obese mammary adipose tissue, macrophages form CLSs to clear dead adipocytes and adipocyte debris [98]. The presence of CLSs in mammary adipose tissue is considered to be a hallmark of obesity [122, 146, 147]. Bone marrow-derived macrophages within CLSs secrete inflammatory cytokines such as TNFα, IL-1β and IL-6 and express marker CD11c [146, 147], but recent transcriptomic and proteomic analyses have suggested that using a binary M1/M2 classification in obesity is an oversimplification [148]. Macrophages in mammary adipose tissue of obese mice appear to have unique metabolic activation, which may be driven by release of fatty acids by adipocytes, leading to a distinct proinflammatory phenotype [149, 150]. It is currently unclear whether these inflammatory macrophage populations interact with epithelial cell populations in the mammary gland or remain associated with adipocytes. However, a recent study suggests that these metabolically activated macrophages may act within the tumor microenvironment to promote proliferation of aggressive cells within tumors [151], suggesting that these macrophages may directly interact with carcinoma cells.
In addition to secreting inflammatory cytokines, macrophages in an obese microenvironment enhance extracellular matrix remodeling. Transcriptional profiling of human macrophages from subcutaneous adipose tissue of obese subjects demonstrated elevated levels of extracellular matrix components, including type I collagen, than macrophages from lean counterparts [152]. Expression of macrophage-inducible C-type leptin (Mincle) by macrophages in CLSs promoted interstitial fibrosis in mice fed a high fat diet, and reduced Mincle expression in macrophages resulted in significantly reduced adipose tissue fibrosis [153]. Within mammary tissue of obese mice, extracellular matrix protein, decorin, was significantly increased surrounding mammary ducts, and depletion of macrophages in obese mice led to reduced decorin deposition [154], suggesting that macrophages actively contribute to the deposition of extracellular matrix surrounding epithelial cells in obesity. Macrophages also influence gene expression in adjacent stromal cells. Co-culture of human preadipocytes with macrophages from obese subjects promoted secretion of extracellular matrix components by preadipocytes [155, 156]. Macrophage secreted factors reduced the ability of adipose-derived stromal cells to differentiate into adipocytes [155], suggesting that macrophage-driven inflammation may also impact the differentiation potential of adipose stem cells. Together these data suggest macrophages play direct and indirect roles in adipose tissue fibrosis in obesity.
In addition to macrophages, other myeloid lineage cell populations are increased within obese adipose tissue. In normal weight mice, neutrophils are observed at low levels compared to other immune cells [157]. With the onset of obesity in mice, the neutrophil population significantly expands in visceral adipose tissue; however, whether this is a transient or stable event remains unclear for the duration of obesity [158, 159]. Myeloid-derived suppressor cells (MDSCs) are known to increase in number in adipose tissue under conditions of obesity [160]. MDSCs, which function to suppress inflammation, reduce proliferation, and promote apoptosis of T cells, may also contribute to an exhaustion phenotype of CD8+ T cells [160, 161]. Antigen-presenting dendritic cells are also increased in number in subcutaneous and visceral obese adipose tissue of both humans and mice [162–165], and dendritic cells may contribute to obesity-induced inflammation [162, 164]. Dendritic cells isolated from diet-induced obesity mice demonstrated an increased proinflammatory phenotype with elevated secretion of IL-1β [162]. Together these data suggest that obesity promotes an influx of immune cells into adipose tissue of the mammary gland, leading to increased inflammatory cytokines within the microenvironment.
While cells of the innate immune system play a significant role in obesity-induced inflammation, obesity also alters adaptive immune cells in mammary adipose tissue. In both high fat diet-fed mice and ob/ob mice, CD4+ regulatory T cells were significantly reduced, while CD8+ T cells were increased in visceral adipose tissue compared to lean controls [166, 167]. These changes in T cell populations preceded macrophage recruitment into adipose tissue [168, 167, 169], suggesting that CD8+ T cells may play a role in the initiation of adipose tissue inflammation [167]. Further, conditioned media collected from adipose-derived stromal cells from obese mice had an increased ability to promote T cell proliferation in culture than conditioned media from control mice [116], which may contribute to obesity-induced T cell exhaustion [161]. Additionally, leptin produced by adipocytes in obesity has been shown to increase fatty acid oxidation within CD8+ T cells leading to downregulation of CD8+ T cell functions and increased immunosuppression [170]. However, in humans, changes in T cell populations are less consistent than in mice. In both visceral and subcutaneous fat, regulatory T cells were significantly increased in patients with higher BMIs [168, 171–173], and the effect of obesity on CD8+ T cell recruitment to human adipose tissue was less clear [168, 172]. Understanding how obesity alters T cell populations and functions in normal breast tissue may provide insight into breast cancer risk, as decreased immunosurveillance may be a component of the earliest stages of breast cancer [174].
ENDOTHELIAL CELLS
As adipocytes undergo hypertrophy in response to elevated fat storage under conditions of obesity, adipose tissue is thought to become a hypoxic microenvironment. It has been suggested that the diameter of adipocytes can exceed the normal diffusion distance of oxygen across tissues, resulting in a barrier to oxygen diffusion in the mammary gland with obesity [175]. Consistent with larger sized adipocytes, capillary density is reduced in obese human subjects compared to lean subjects in both visceral and subcutaneous adipose depots [176, 177]. Despite the increase in adipose tissue mass, blood flow is not increased within adipose tissue of obese patients [178–180]. In response to reduced oxygen levels, hypoxia-inducible factor 1 alpha (HIFα) expression is induced [181]. The development of hypoxia within adipose tissue is thought to be a driver in the establishment of the inflammatory response in obesity [182]. The inflammatory response, leading to a release of cytokines, chemokines, and angiogenic factors, serves to stimulate angiogenesis.
Angiogenesis is essential for support of the growing adipose tissue. During development, angiogenesis and adipogenesis are spatially and temporally coupled as adipose tissue expands [183]. Angiogenesis is triggered by tissue hypoxia [184], and obesity-induced tissue hypoxia leads to increased secretion of CCL2/MCP-1 by adipose-derived stromal cells and adipocytes to aid in novel blood vessel formation [185]. Angiogenesis results from an imbalanced production of overlapping angiogenic factors and inhibitors, including vascular endothelial growth factor A (VEGFA), fibroblast growth factor-2 (FGF2), adiponectin, and thrombospondin-1 [186–188]. Conditioned media collected from adipocytes or adipose-derived stromal cells of obese women increased endothelial cell proliferation, migration, and sprouting compared to adipocytes isolated from lean women [189, 190], suggesting that angiogenic factors are elevated in response to the adipose tissue hypoxia.
Several adipokines, including leptin, resistin, and visfatin modulate angiogenesis and vascular survival [191, 192]. Elevated leptin production by obese adipocytes promotes angiogenesis by directly activating leptin receptors on endothelial cells [193]. Leptin receptor activation promotes upregulation of FGF2 and VEGFA and stimulates vascular permeability [191]. Leptin may also enhance capillary stability by maintaining the fate of pericytes, which are multifunctional cells that surround and support capillaries, through increased Hedgehog signaling [194]. In culture, adipocytes isolated from high fat diet-fed mice and ob/ob mice upregulated angiopoietin-like 4 and other proangiogenic factors after being stimulated by IL-1β or PPARγ [195, 196], suggesting that signaling from inflammatory macrophages within the tissue may also enhance angiogenesis. In addition, adipocyte lipids, such as monobutyrin, induce angiogenesis [197]. Increased localized concentrations of angiogenic factors within the hypoxic adipose tissue of the obese breast could contribute to breast cancer risk through increasing angiogenesis in early neoplastic lesions.
WEIGHT LOSS
The effects of weight loss on the risk for breast cancer recurrence is currently under investigation in long-term, clinical trials [198], as little is known about how weight loss impacts breast cancer risk for obese women. For humans, weight loss is often achieved by reducing caloric intake, increasing physical activity, or through surgical intervention. Large observational studies suggest that risk of breast cancer is reduced after intentional weight loss [199]. Bariatric surgery frequently results in more sustained weight loss in morbidly obese patients [200, 201]. Retrospective studies examining patients that have undergone surgical weight loss interventions suggest that weight loss has beneficial effects on reducing breast cancer risk [202], potentially for both pre and postmenopausal women [203]. Weight reduction through bariatric surgery has been linked to reduced risk for HER2-positive and ERα-positive breast cancers [203–205].
As obesity rates around the globe continue to rise, researchers have begun to address whether the effects of obesity on cell populations within the mammary gland are reversible with weight loss. In a murine diet-induced obesity model, weight loss can be accomplished by replacing a high fat diet with a low-fat diet, resulting in significant weight loss [73, 85, 206]. Formerly obese mice demonstrate reduced mammary gland weights [53, 73], decreased adipocyte diameters [53, 207] and diminished numbers of CLSs in adipose tissue [206, 207] compared to obese mice. However, methylation changes that occur in the mammary gland with obesity may not be reversible. Mammary glands of formerly obese mice demonstrated epigenetic programing more similar to adipose tissue from obese mice than lean controls [206], suggesting that weight loss is insufficient to revert hypermethylation of genes in the mammary gland, and epigenetic changes that occur due to obesity may persist after weight stabilization.
However, some of the major changes induced by obesity appear to be reversible with weight loss. Following weight loss, obesity-induced mammary gland inflammation was reduced, resulting in decreased expression of inflammatory cytokines CCL2/MCP-1, IL-1β, TNF-α, and Cox2 within adipose tissue [207, 208]. Similarly, reduction of BMI in humans through both surgical and intentional weight loss resulted in decreased inflammatory macrophage infiltration into adipose tissue [208–212]. These changes are observed in conjunction with increased expression of the anti-inflammatory markers IL-10 and IL-1 receptor antagonist by macrophages and adipose-derived stromal cells [208, 211]. Changes in mammary epithelial cell populations may also be reversible with weight loss. Weight loss in obese pubertal mice restored terminal end bud formation and ductal elongation within the mammary gland, reestablishing normal mammary gland development and epithelial function [53]. Further, weight loss in adult mice resulted in epithelial cell expression of ERα and smooth muscle actin that was similar to lean mice, as well as epithelial cell stem/progenitor activity that was reduced compared to obese mice [73]. Together, these results suggest that weight loss results in reduced inflammatory signaling and phenotypically normal epithelial cells.
While weight loss appears to reduce inflammation within adipose tissue, other obesity-induced changes may be more resistant to change. Adipose-derived stromal cells in subcutaneous adipose tissue from both humans and mice that lost weight through both intentional and surgical interventions demonstrated restored adipogenic differentiation capacities compared to adipose-derived stromal cells isolated from obese individuals and mice [85, 201, 213, 214]. However, adipose-derived stromal cells from mice after weight loss demonstrated intermediate myofibroblast differentiation compared to adipose-derived stromal cells from either lean or obese mice [85], suggesting that myofibroblast differentiation within the population of adipose-derived stromal cells was not entirely reversible following weight loss. With a reduction of adipose tissue mass and inflammation upon weight loss, it would be hypothesized that adipose tissue extracellular matrix would also undergo significant remodeling. Consistent with this hypothesis, following weight stabilization after non-surgical weight reduction, expression of extracellular matrix proteins was significantly decreased in subcutaneous adipose tissue of women [215]. Studies examining human adipose tissue one year after bariatric surgery reported significant loss of fibrosis in subcutaneous adipose tissue [216–218]. However, patients exhibiting greater total fibrosis prior to surgery lost less fat mass and demonstrated higher total fibrosis after surgery than patients who began with less adipose tissue fibrosis [216–218]. Further work is necessary to understand if the changes in adipose tissue fibrosis resolve over time with weight loss, or if there are more permanent structural differences that persist.
CONCLUSIONS
Obesity is a complex disorder, resulting in changes in circulating hormones, inflammatory cytokines, and metabolites as well as local changes within the breast microenvironment. Within breast tissue, epithelial stem/progenitor cells are increased, which may increase populations of cells susceptible to acquire mutations leading to breast tumor formation. Within the surrounding adipose tissue, obesity induces chronic inflammation, adipose tissue hypoxia, and extracellular matrix deposition and fibrosis. Localized growth factors and adipokines may impact both the epithelial cells as well as the surrounding stromal cells within the obese breast microenvironment (Fig. 2). These sequelae of obesity within the breast microenvironment have been linked with breast tumor growth and progression. This suggests that obese breast tissue, prior to tumor formation, is an environment conducive for tumor development and may significantly contribute to breast cancer risk.
Fig. 2. Local and systemic changes due to obesity that may contribute to breast cancer risk.
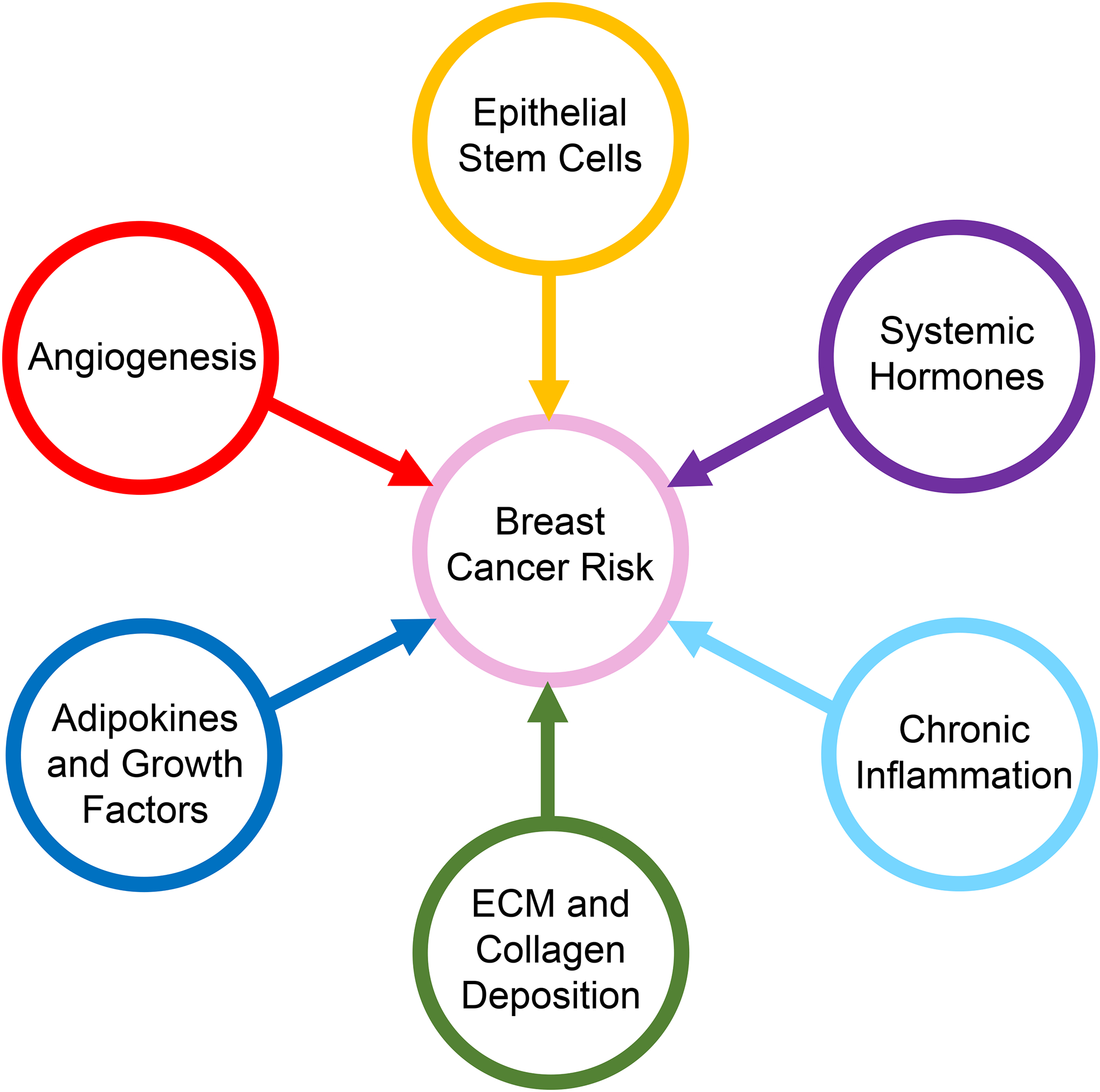
Obesity leads to increased systemic hormones including insulin and leptin. Within the mammary gland, mammary epithelial stem/progenitor cells are enriched, which may increase the cells of origin for breast cancer. Within the mammary gland, chronic inflammation, increased local adipokines and growth factors, elevated angiogenesis, and increased extracellular matrix (ECM) production create an environment conducive for breast cancer growth
With these changes in breast tissue under conditions of obesity, it is not clear why the risk factors for breast cancer are clearly strongest in postmenopausal women. In a mouse model examining the impact of timing of diet-induced obesity on mammary cancer risk following treatment with carcinogen dimethylbenz[a]anthracene, mice were exposed to a high fat/high sugar diet during pregnancy, after weaning or after puberty [219]. Timing of exposure to the high fat/high sugar diet was a critical determinant of mammary tumor incidence [219]. Data from multiple large epidemiological studies have demonstrated that postmenopausal women have an increased risk for developing ERα-positive luminal breast cancers [38, 220, 221]. Although a body of work has examined the origins of ERα-positive breast tumors, the process of ERα-positive tumorigenesis is not well understood. Obesity may promote the growth of epithelial cells that are vulnerable to oncogenic transformation during a window of susceptibility following menopause [222]. Since epigenetic changes have been observed in mammary tissue as a consequence of obesity [206], it is also possible that obesity impacts the genetics of epithelial cells before and after menopause in ways that could modify breast cancer risk. While the majority of work to understand the effects of obesity on breast tissue has been performed in nulliparous, cycling mice or premenopausal patients, it is unclear how loss of ovarian hormones further alters breast tissue in obesity. Additional changes in localized estrogen production, inflammation, fibrosis and/or metabolism could significantly enhance breast cancer risk during this period, and further studies are necessary to identify how these changes might impact breast tissue following menopause.
Although obesity induces multiple changes within breast tissue that could promote cancer growth, women can have multiple risk factors for breast cancer, and it is not clear how these risk factors interact. Obesity significantly enhances underlying genetic risk factors within epithelial cells, resulting in further increased breast cancer risk in women with known genetic mutations and familial risk [13, 25, 30–32]. Obesity-induced changes within the breast stroma could support early tumor progression in these women, and weight loss interventions may be particularly beneficial to reduce this underlying risk. Understanding how obesity impacts breast cancer risk, specifically in high-risk premenopausal women, could lead to novel chemoprevention strategies. Mammographic breast density is another significant risk factor for breast cancer, and obesity and breast density together enhance breast cancer risk [223, 224]. Breast density is also associated with increased immune cells within breast tissue [225], although little is known how these two risk factors modify inflammation within breast tissue. Future studies to investigate how risk factors interact together to modify the breast microenvironment would significantly enhance our understanding of breast cancer risk and identify possible points of intervention for cancer prevention and treatment.
Acknowledgements:
The authors would like to thank Abbey Williams, Brenna Walton, and Genevra Kuziel for helpful discussions and suggestions.
Funding: This work was supported by National Institutes of Health R01 CA227542 (L.M.A.) and T32 GM007215 (L.E.H.).
Footnotes
Conflicts of Interest/Competing Interests: The authors have no conflicts of interests to report.
REFERENCES
- 1.World Health Organization. Obesity and overweight. World Health Organization. 2017. doi:/entity/mediacentre/factsheets/fs311/en/index.html. [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics, 2020. [PubMed] [Google Scholar]
- 3.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32(6):959–66. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet. 2014;384(9945):755–65. doi: 10.1016/s0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer--viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8). doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6). doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 8.Lahmann PH, Hoffmann K, Allen N, van Gils CH, Khaw KT, Tehard B et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. 2004;111(5). doi: 10.1002/ijc.20315. [DOI] [PubMed] [Google Scholar]
- 9.White AJ, Nichols HB, Bradshaw PT, Sandler DP. Overall and central adiposity and breast cancer risk in the Sister Study. Cancer. 2015;121(20). doi: 10.1002/cncr.29552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sebastiani F, Cortesi L, Sant M, Lucarini V, Cirilli C, De Matteis E et al. Increased incidence of breast cancer in postmenopausal women with high body mass index at the Modena Screening Program. J Breast Cancer. 2016;19(3). doi: 10.4048/jbc.2016.19.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015. doi:2319235 [pii]; 10.1001/jamaoncol.2015.1546 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int J Cancer. 2009;124(3). doi: 10.1002/ijc.23943. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Cook LS, Tang MT, Porter PL, Hill DA, Wiggins CL et al. Body mass index and risk of luminal, HER2-overexpressing, and triple negative breast cancer. Breast Cancer Res Treat. 2016;157(3). doi: 10.1007/s10549-016-3825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritte R, Lukanova A, Berrino F, Dossus L, Tjønneland A, Olsen A et al. Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: a large prospective cohort study. Breast Cancer Res. 2012;14(3). doi: 10.1186/bcr3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Stefanick ML, Wactawski-Wende J et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(3). doi: 10.1158/1055-9965.EPI-10-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26(8):1533–46. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24 Suppl 2:S26–35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–7. doi:JCO.2008.18.1370 [pii]; 10.1200/JCO.2008.18.1370 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaudet MM, Press MF, Haile RW, Lynch CF, Glaser SL, Schildkraut J et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130(2):587–97. doi: 10.1007/s10549-011-1616-x [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toro AL, Costantino NS, Shriver CD, Ellsworth DL, Ellsworth RE. Effect of obesity on molecular characteristics of invasive breast tumors: gene expression analysis in a large cohort of female patients. BMC Obes. 2016;3:22. doi: 10.1186/s40608-016-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vatten LJ, Kvinnsland S. Prospective study of height, body mass index and risk of breast cancer. Acta Oncol. 1992;31(2). doi: 10.3109/02841869209088902. [DOI] [PubMed] [Google Scholar]
- 23.Ursin G, Longnecker MP, Haile RW, Greenland S. A Meta-analysis of body mass index and risk of premenopausal breast cancer. Epidemiol. 1995;6(2). doi: 10.1097/00001648-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Bandera EV, Chandran U, Hong CC, Troester MA, Bethea TN, Adams-Campbell LL et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat. 2015;150(3):655–66. doi: 10.1007/s10549-015-3353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris HR, Willett WC, Terry KL, Michels KB. Body fat distribution and risk of premenopausal breast cancer in the Nurses’ Health Study II. J Natl Cancer Inst. 2011;103(3). doi: 10.1093/jnci/djq500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen GC, Chen SJ, Zhang R, Hidayat K, Qin JB, Zhang YS et al. Central obesity and risks of pre- and postmenopausal breast cancer: a dose-response meta-analysis of prospective studies. Obes Rev. 2016;17(11):1167–77. doi: 10.1111/obr.12443. [DOI] [PubMed] [Google Scholar]
- 27.Sonnenschein E, Toniolo P, Terry MB, Bruning PF, Kato I, Koenig KL et al. Body fat distribution and obesity in pre- and postmenopausal breast cancer. Int J Epidemiol. 1999;28(6):1026–31. doi: 10.1093/ije/28.6.1026. [DOI] [PubMed] [Google Scholar]
- 28.Fagherazzi G, Chabbert-Buffet N, Fabre A, Guillas G, Boutron-Ruault MC, Mesrine S et al. Hip circumference is associated with the risk of premenopausal ER-/PR- breast cancer. Int J Obes. 2012;36(3):431–9. doi: 10.1038/ijo.2011.66. [DOI] [PubMed] [Google Scholar]
- 29.Amadou A, Hainaut P, Romieu I. Role of obesity in the risk of breast cancer: lessons from anthropometry. J Oncol. 2013;2013:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahin S, Erdem GU, Karatas F, Aytekin A, Sever AR, Ozisik Y et al. The association between body mass index and immunohistochemical subtypes in breast cancer. Breast. 2017;32. doi: 10.1016/j.breast.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137(1). doi: 10.1007/s10549-012-2339-3. [DOI] [PubMed] [Google Scholar]
- 32.Cecchini RS, Costantino JP, Cauley JA, Cronin WM, Wickerham DL, Land SR et al. Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP P-1 and STAR breast cancer prevention trials. Cancer Prev Res. 2012;5(4):583–92. doi: 10.1158/1940-6207.Capr-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–14. doi:mdu042 [pii]; 10.1093/annonc/mdu042 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123(3). doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 35.Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29(1):25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 36.Loi S, Milne RL, Friedlander ML, McCredie MR, Giles GG, Hopper JL et al. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1686–91. doi: 10.1158/1055-9965.epi-05-0042. [DOI] [PubMed] [Google Scholar]
- 37.Ewertz M, Gray KP, Regan MM, Ejlertsen B, Price KN, Thürlimann B et al. Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the Breast International Group 1–98 Trial. J Clin Oncol. 2012;30(32). doi: 10.1200/JCO.2011.40.8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biglia N, Peano E, Sgandurra P, Moggio G, Pecchio S, Maggiorotto F et al. Body mass index (BMI) and breast cancer: impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol Endocrinol. 2013;29(3):263–7. doi: 10.3109/09513590.2012.736559 [doi]. [DOI] [PubMed] [Google Scholar]
- 39.Karatas F, Erdem GU, Sahin S, Aytekin A, Yuce D, Sever AR et al. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast. 2017;32:237–44. doi: 10.1016/j.breast.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Ioannides SJ, Barlow PL, Elwood JM, Porter D. Effect of obesity on aromatase inhibitor efficacy in postmenopausal, hormone receptor-positive breast cancer: a systematic review. Breast Cancer Res Treat. 2014;147(2):237–48. doi: 10.1007/s10549-014-3091-7. [DOI] [PubMed] [Google Scholar]
- 41.Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118(23):5937–46. doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28(21):3411–5. doi: 10.1200/jco.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- 43.Gnant M, Pfeiler G, Stoger H, Mlineritsch B, Fitzal F, Balic M et al. The predictive impact of body mass index on the efficacy of extended adjuvant endocrine treatment with anastrozole in postmenopausal patients with breast cancer: an analysis of the randomised ABCSG-6a trial. Br J Cancer. 2013;109(3):589–96. doi: 10.1038/bjc.2013.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolters R, Schwentner L, Regierer A, Wischnewsky M, Kreienberg R, Wockel A. Endocrine therapy in obese patients with primary breast cancer: another piece of evidence in an unfinished puzzle. Breast Cancer Res Treat. 2012;131(3):925–31. doi: 10.1007/s10549-011-1874-7. [DOI] [PubMed] [Google Scholar]
- 45.Pfeiler G, Konigsberg R, Fesl C, Mlineritsch B, Stoeger H, Singer CF et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Clin Oncol. 2011;29(19):2653–9. doi: 10.1200/jco.2010.33.2585. [DOI] [PubMed] [Google Scholar]
- 46.Barrett P, Mercer JG, Morgan PJ. Preclinical models for obesity research. Dis Model Mech. 2016;9(11):1245–55. doi: 10.1242/dmm.026443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14(3):141–8. [DOI] [PubMed] [Google Scholar]
- 48.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317–8. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 49.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81(2):243–8. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 50.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262(6 Pt 2):R1025–32. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 51.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37(9):1163–7. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 52.Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ et al. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia. 2013;56(5):1129–39. doi: 10.1007/s00125-013-2846-8 [doi]. [DOI] [PubMed] [Google Scholar]
- 53.Olson LK, Tan Y, Zhao Y, Aupperlee MD, Haslam SZ. Pubertal exposure to high fat diet causes mouse strain-dependent alterations in mammary gland development and estrogen responsiveness. Int J Obes. 2010;34(9):1415–26. doi: 10.1038/ijo.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panchal SK, Brown L. Rodent models for metabolic syndrome research. J Biomed Biotechnol. 2011;2011:351982. doi: 10.1155/2011/351982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol. 2012;Chapter 5:Unit5.61. doi: 10.1002/0471141755.ph0561s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giles ED, Jackman MR, MacLean PS. Modeling diet-induced obesity with obesity-prone rats: implications for studies in females. Front Nutr. 2016;3:50. doi: 10.3389/fnut.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imaoka T, Nishimura M, Daino K, Morioka T, Nishimura Y, Uemura H et al. A rat model to study the effects of diet-induced obesity on radiation-induced mammary carcinogenesis. Radiat Res. 2016;185(5):505–15. doi: 10.1667/rr14309.1. [DOI] [PubMed] [Google Scholar]
- 58.Gimble JM, Bunnell BA, Frazier T, Rowan B, Shah F, Thomas-Porch C et al. Adipose-derived stromal/stem cells: a primer. Organogenesis. 2013;9(1):3–10. doi: 10.4161/org.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bowers LW, Rossi EL, O’Flanagan CH, deGraffenried LA, Hursting SD. The role of the insulin/IGF system in cancer: lessons learned from clinical trials and the energy balance-cancer link. Front Endocrinol. 2015;6:77. doi: 10.3389/fendo.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson AR, Makowski L. Nutrition and metabolic correlates of obesity and inflammation: clinical considerations. J Nutr. 2015;145(5). doi: 10.3945/jn.114.200758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95(3). doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang C, LeRoith D, Gallagher EJ. Diabetes, obesity, and breast cancer. Endocrinol. 2018;159(11):3801–12. doi: 10.1210/en.2018-00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hursting SD. Obesity, energy balance, and cancer: a mechanistic perspective. Cancer Treat Res. 2014;159. doi: 10.1007/978-3-642-38007-5_2. [DOI] [PubMed] [Google Scholar]
- 64.Matthews SB, Thompson HJ. The obesity-breast cancer conundrum: an analysis of the issues. Int J Mol Sci. 2016;17(6). doi: 10.3390/ijms17060989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sánchez-Jiménez F, Pérez-Pérez A, de la Cruz-Merino L, Sánchez-Margalet V. Obesity and breast cancer: role of leptin. Front Oncol. 2019;9. doi: 10.3389/fonc.2019.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oakes SR, Gallego-Ortega D, Ormandy CJ. The mammary cellular hierarchy and breast cancer. Cell Mol Life Sci. 2014;71(22):4301–24. doi: 10.1007/s00018-014-1674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Visvader JE. Cells of origin in cancer. Nature. 2011;469(7330):314–22. doi:nature09781 [pii]; 10.1038/nature09781 [doi]. [DOI] [PubMed] [Google Scholar]
- 68.Tharmapalan P, Mahendralingam M, Berman HK, Khokha R. Mammary stem cells and progenitors: targeting the roots of breast cancer for prevention. EMBO J. 2019;38(14):e100852. doi: 10.15252/embj.2018100852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shehata M, Teschendorff A, Sharp G, Novcic N, Russell IA, Avril S et al. Phenotypic and functional characterisation of the luminal cell hierarchy of the mammary gland. Breast Cancer Res. 2012;14(5):R134. doi: 10.1186/bcr3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8(1):R7. doi: 10.1186/bcr1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 72.Stingl J, Eaves CJ, Zandieh I, Emerman JT. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat. 2001;67(2):93–109. doi: 10.1023/a:1010615124301. [DOI] [PubMed] [Google Scholar]
- 73.Chamberlin T, D’Amato JV, Arendt LM. Obesity reversibly depletes the basal cell population and enhances mammary epithelial cell estrogen receptor alpha expression and progenitor activity. Breast Cancer Res. 2017;19(1):128. doi: 10.1186/s13058-017-0921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamikawa A, Ichii O, Yamaji D, Imao T, Suzuki C, Okamatsu-Ogura Y et al. Diet-induced obesity disrupts ductal development in the mammary glands of nonpregnant mice. Dev Dyn. 2009;238(5):1092–9. doi: 10.1002/dvdy.21947. [DOI] [PubMed] [Google Scholar]
- 75.Barsky SH, Karlin NJ. Myoepithelial cells: autocrine and paracrine suppressors of breast cancer progression. J Mammary Gland Biol Neoplasia. 2005;10(3):249–60. doi: 10.1007/s10911-005-9585-5. [DOI] [PubMed] [Google Scholar]
- 76.Pilie PG, Ibarra-Drendall C, Troch MM, Broadwater G, Barry WT, Petricoin EF 3rd et al. Protein microarray analysis of mammary epithelial cells from obese and nonobese women at high risk for breast cancer: feasibility data. Cancer Epidemiol Biomarkers Prev. 2011;20(3):476–82. doi: 10.1158/1055-9965.EPI-10-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sainz N, Barrenetxe J, Moreno-Aliaga MJ, Martinez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64(1):35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 78.Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity. 2006;14 Suppl 5:254s–8s. doi: 10.1038/oby.2006.319. [DOI] [PubMed] [Google Scholar]
- 79.Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12(5):1447–53. doi: 10.1158/1078-0432.Ccr-05-1913. [DOI] [PubMed] [Google Scholar]
- 80.Miyoshi Y, Funahashi T, Tanaka S, Taguchi T, Tamaki Y, Shimomura I et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118(6):1414–9. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- 81.Tenvooren I, Jenks MZ, Rashid H, Cook KL, Muhlemann JK, Sistrunk C et al. Elevated leptin disrupts epithelial polarity and promotes premalignant alterations in the mammary gland. Oncogene. 2019;38(20):3855–70. doi: 10.1038/s41388-019-0687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Esper RM, Dame M, McClintock S, Holt PR, Dannenberg AJ, Wicha MS et al. Leptin and adiponectin modulate the self-renewal of normal human breast epithelial stem cells. Cancer Prev Res. 2015;8(12):1174–83. doi: 10.1158/1940-6207.capr-14-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C et al. JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018;27(1):136–50.e5. doi: 10.1016/j.cmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mahbouli S, Der Vartanian A, Ortega S, Rouge S, Vasson MP, Rossary A. Leptin induces ROS via NOX5 in healthy and neoplastic mammary epithelial cells. Oncol Rep. 2017;38(5):3254–64. doi: 10.3892/or.2017.6009. [DOI] [PubMed] [Google Scholar]
- 85.Hillers LE, D’Amato JV, Chamberlin T, Paderta G, Arendt LM. Obesity-activated adipose-derived stromal cells promote breast cancer growth and invasion. Neoplasia. 2018;20(11):1161–74. doi: 10.1016/j.neo.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kleinberg DL, Feldman M, Ruan W. IGF-I: an essential factor in terminal end bud formation and ductal morphogenesis. J Mammary Gland Biol Neoplasia. 2000;5(1):11. doi: 10.1023/a:1009507030633. [DOI] [PubMed] [Google Scholar]
- 87.Welsch CW, O’Connor DH. Influence of the type of dietary fat on developmental growth of the mammary gland in immature and mature female BALB/c mice. Cancer Res. 1989;49(21):5999–6007. [PubMed] [Google Scholar]
- 88.Welsch CW, DeHoog JV, O’Connor DH, Sheffield LG. Influence of dietary fat levels on development and hormone responsiveness of the mouse mammary gland. Cancer Res. 1985;45(12 Pt 1):6147–54. [PubMed] [Google Scholar]
- 89.Hilakivi-Clarke L, Stoica A, Raygada M, Martin M. Consumption of a high-fat diet alters estrogen receptor content, protein kinase C activity, and mammary gland morphology in virgin and pregnant mice and female offspring. Cancer Res. 1998;58(4):654–60. [PubMed] [Google Scholar]
- 90.Berryhill GE, Gloviczki JM, Trott JF, Aimo L, Kraft J, Cardiff RD et al. Diet-induced metabolic change induces estrogen-independent allometric mammary growth. Proc Natl Acad Sci U S A. 2012;109(40):16294–9. doi: 10.1073/pnas.1210527109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ip MM, McGee SO, Masso-Welch PA, Ip C, Meng X, Ou L et al. The t10,c12 isomer of conjugated linoleic acid stimulates mammary tumorigenesis in transgenic mice over-expressing erbB2 in the mammary epithelium. Carcinogenesis. 2007;28(6):1269–76. doi: 10.1093/carcin/bgm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khadge S, Thiele GM, Sharp JG, McGuire TR, Klassen LW, Black PN et al. Long-chain omega-3 polyunsaturated fatty acids modulate mammary gland composition and inflammation. J Mammary Gland Biol Neoplasia. 2018;23(1–2):43–58. doi: 10.1007/s10911-018-9391-5. [DOI] [PubMed] [Google Scholar]
- 93.Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Premenopausal dietary fat in relation to pre- and post-menopausal breast cancer. Breast Cancer Res Treat. 2014;145(1):255–65. doi: 10.1007/s10549-014-2895-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang QA, Song A, Chen W, Schwalie PC, Zhang F, Vishvanath L et al. Reversible de-differentiation of mature white adipocytes into preadipocyte-like precursors during lactation. Cell Metab. 2018;28(2):282–8.e3. doi: 10.1016/j.cmet.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zwick RK, Rudolph MC, Shook BA, Holtrup B, Roth E, Lei V et al. Adipocyte hypertrophy and lipid dynamics underlie mammary gland remodeling after lactation. Nature Commun. 2018;9(1):3592. doi: 10.1038/s41467-018-05911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weaver SR, Bohrer JC, Prichard AS, Perez PK, Streckenbach LJ, Olson JM et al. Serotonin deficiency rescues lactation on day 1 in mice fed a high fat diet. PLoS One. 2016;11(9):e0162432. doi: 10.1371/journal.pone.0162432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flint DJ, Travers MT, Barber MC, Binart N, Kelly PA. Diet-induced obesity impairs mammary development and lactogenesis in murine mammary gland. Am J Physiol Endocrinol Metab. 2005;288(6):E1179–87. doi: 10.1152/ajpendo.00433.2004. [DOI] [PubMed] [Google Scholar]
- 98.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 99.Giordano A, Murano I, Mondini E, Perugini J, Smorlesi A, Severi I et al. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res. 2013;54(9). doi: 10.1194/jlr.M038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95. doi: 10.1091/mbc.E02-02-0105 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 102.Kilroy G, Dietrich M, Wu X, Gimble JM, Floyd ZE. Isolation of murine adipose-derived stromal/stem cells for adipogenic differentiation or flow cytometry-based analysis. Methods Mol Biol. 2018;1773:137–46. doi: 10.1007/978-1-4939-7799-4_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641–8. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, Daquinag AC, Amaya-Manzanares F, Sirin O, Tseng C, Kolonin MG. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72(20):5198–208. doi: 10.1158/0008-5472.can-12-0294. [DOI] [PubMed] [Google Scholar]
- 105.Pachon-Pena G, Serena C, Ejarque M, Petriz J, Duran X, Oliva-Olivera W et al. Obesity determines the immunophenotypic profile and functional characteristics of human mesenchymal stem cells from adipose tissue. Stem Cells Transl Med. 2016;5(4):464–75. doi: 10.5966/sctm.2015-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frazier TP, Gimble JM, Devay JW, Tucker HA, Chiu ES, Rowan BG. Body mass index affects proliferation and osteogenic differentiation of human subcutaneous adipose tissue-derived stem cells. BMC Cell Biol. 2013;14:34. doi: 10.1186/1471-2121-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58(7):1550–7. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Permana PA, Nair S, Lee YH, Luczy-Bachman G, Vozarova De Courten B, Tataranni PA. Subcutaneous abdominal preadipocyte differentiation in vitro inversely correlates with central obesity. Am J Physiol Endocrinol Metab. 2004;286(6):E958–62. doi: 10.1152/ajpendo.00544.2003. [DOI] [PubMed] [Google Scholar]
- 109.Onate B, Vilahur G, Ferrer-Lorente R, Ybarra J, Diez-Caballero A, Ballesta-Lopez C et al. The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB J. 2012;26(10):4327–36. doi: 10.1096/fj.12-207217. [DOI] [PubMed] [Google Scholar]
- 110.Strong AL, Hunter RS, Jones RB, Bowles AC, Dutreil MF, Gaupp D et al. Obesity inhibits the osteogenic differentiation of human adipose-derived stem cells. J Transl Med. 2016;14:27. doi: 10.1186/s12967-016-0776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu CL, Diekman BO, Jain D, Guilak F. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: the effects of free fatty acids. Int J Obes. 2013;37(8):1079–87. doi: 10.1038/ijo.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim KH, Song MJ, Chung J, Park H, Kim JB. Hypoxia inhibits adipocyte differentiation in a HDAC-independent manner. Biochem Biophys Res Commun. 2005;333(4):1178–84. doi: 10.1016/j.bbrc.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 113.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2(3):331–41. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 114.Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7(301):301ra130. doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wolfson B, Zhang Y, Gernapudi R, Duru N, Yao Y, Lo PK et al. A high-fat diet promotes mammary gland myofibroblast differentiation through MicroRNA 140 downregulation. Mol Cell Biol. 2017;37(4). doi: 10.1128/mcb.00461-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Serena C, Keiran N, Ceperuelo-Mallafre V, Ejarque M, Fradera R, Roche K et al. Obesity and type 2 diabetes alters the immune properties of human adipose derived stem cells. Stem Cells. 2016;34(10):2559–73. doi: 10.1002/stem.2429. [DOI] [PubMed] [Google Scholar]
- 117.Strong AL, Semon JA, Strong TA, Santoke TT, Zhang S, McFerrin HE et al. Obesity-associated dysregulation of calpastatin and MMP-15 in adipose-derived stromal cells results in their enhanced invasion. Stem Cells. 2012;30(12):2774–83. doi: 10.1002/stem.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Druso JE, Fischbach C. Biophysical properties of extracellular matrix: linking obesity and cancer. Trends Cancer. 2018;4(4):271–3. doi: 10.1016/j.trecan.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18(4):470–7. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Invest. 2012;122(11):4243–56. doi: 10.1172/jci63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bulun SE, Simpson ER. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab. 1994;78(2):428–32. doi: 10.1210/jcem.78.2.8106632. [DOI] [PubMed] [Google Scholar]
- 122.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res. 2011;4(7):1021–9. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brown KA, Iyengar NM, Zhou XK, Gucalp A, Subbaramaiah K, Wang H et al. Menopause is a determinant of breast aromatase expression and its associations with BMI, inflammation, and systemic markers. J Clin Endocrinol Metab. 2017;102(5). doi: 10.1210/jc.2016-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wake DJ, Strand M, Rask E, Westerbacka J, Livingstone DE, Soderberg S et al. Intra-adipose sex steroid metabolism and body fat distribution in idiopathic human obesity. Clin Endocrinol. 2007;66(3):440–6. doi: 10.1111/j.1365-2265.2007.02755.x. [DOI] [PubMed] [Google Scholar]
- 125.Irahara N, Miyoshi Y, Taguchi T, Tamaki Y, Noguchi S. Quantitative analysis of aromatase mRNA expression derived from various promoters (I.4, I.3, PII and I.7) and its association with expression of TNF-alpha, IL-6 and COX-2 mRNAs in human breast cancer. Int J Cancer. 2006;118(8):1915–21. doi: 10.1002/ijc.21562. [DOI] [PubMed] [Google Scholar]
- 126.Salama SA, Kamel MW, Diaz-Arrastia CR, Xu X, Veenstra TD, Salih S et al. Effect of tumor necrosis factor-alpha on estrogen metabolism and endometrial cells: potential physiological and pathological relevance. J Clin Endocrinol Metab. 2009;94(1):285–93. doi: 10.1210/jc.2008-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res. 2002;4(2):65–9. doi: 10.1186/bcr425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reed MJ, Coldham NG, Patel SR, Ghilchik MW, James VH. Interleukin-1 and interleukin-6 in breast cyst fluid: their role in regulating aromatase activity in breast cancer cells. J Endocrinol. 1992;132(3):R5–8. doi: 10.1677/joe.0.132r005. [DOI] [PubMed] [Google Scholar]
- 129.Brady NJ, Farrar MA, Schwertfeger KL. STAT5 deletion in macrophages alters ductal elongation and branching during mammary gland development. Dev Biol. 2017;428(1). doi: 10.1016/j.ydbio.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Folkerd EJ, Dixon JM, Renshaw L, A’Hern RP, Dowsett M. Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J Clin Oncol. 2012;30(24):2977–80. doi: 10.1200/jco.2012.42.0273. [DOI] [PubMed] [Google Scholar]
- 131.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K et al. A lineage of myeloid cells independent of myb and hematopoietic stem cells. Science. 2012;336(6077). doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 132.Hassnain Waqas SF, Noble A, Hoang AC, Ampem G, Popp M, Strauß S et al. Adipose tissue macrophages develop from bone marrow-independent progenitors in Xenopus laevis and mouse. J Leukoc Biol. 2017;102(3). doi: 10.1189/jlb.1A0317-082RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jappinen N, Felix I, Lokka E, Tyystjarvi S, Pynttari A, Lahtela T et al. Fetal-derived macrophages dominate in adult mammary glands. Nature Commun. 2019;10(1):281. doi: 10.1038/s41467-018-08065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunol. 2018;155(4):407–17. doi: 10.1111/imm.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stewart TA, Hughes K, Hume DA, Davis FM. Developmental stage-specific distribution of macrophages in mouse mammary gland. Front Cell Dev Biol. 2019;7:250. doi: 10.3389/fcell.2019.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gyorki DE, Asselin-Labat ML, van Rooijen N, Lindeman GJ, Visvader JE. Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Res. 2009;11(4):R62. doi: 10.1186/bcr2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chakrabarti R, Celia-Terrassa T, Kumar S, Hang X, Wei Y, Choudhury A et al. Notch ligand Dll1 mediates cross-talk between mammary stem cells and the macrophageal niche. Science. 2018;360(6396). doi: 10.1126/science.aan4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O’Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176(3):1241–55. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muir LA, Kiridena S, Griffin C, DelProposto JB, Geletka L, Martinez-Santibanez G et al. Frontline Science: Rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages. J Leukoc Biol. 2018;103(4):615–28. doi: 10.1002/jlb.3hi1017-422r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61(2):346–54. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56(1):16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 143.Kaplan JL, Marshall MA, C CM, Harmon DB, Garmey JC, Oldham SN et al. Adipocyte progenitor cells initiate monocyte chemoattractant protein-1-mediated macrophage accumulation in visceral adipose tissue. Mol Metab. 2015;4(11):779–94. doi: 10.1016/j.molmet.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim J, Chung K, Choi C, Beloor J, Ullah I, Kim N et al. Silencing CCR2 in macrophages alleviates adipose tissue inflammation and the associated metabolic syndrome in dietary obese mice. Mol Ther Nucleic Acids. 2016;5:e280. doi: 10.1038/mtna.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/jci200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/jci200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kraakman MJ, Murphy AJ, Jandeleit-Dahm K, Kammoun HL. Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front Immunol. 2014;5:470. doi: 10.3389/fimmu.2014.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20(4):614–25. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boutens L, Hooiveld GJ, Dhingra S, Cramer RA, Netea MG, Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia. 2018;61(4):942–53. doi: 10.1007/s00125-017-4526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tiwari P, Blank A, Cui C, Schoenfelt KQ, Zhou G, Xu Y et al. Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer. J Exp Med. 2019;216(6):1345–58. doi: 10.1084/jem.20181616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008;9(1):R14. doi: 10.1186/gb-2008-9-1-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tanaka M, Ikeda K, Suganami T, Komiya C, Ochi K, Shirakawa I et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nature Commun. 2014;5:4982. doi: 10.1038/ncomms5982. [DOI] [PubMed] [Google Scholar]
- 154.Chamberlin T, Thompson V, Hillers-Ziemer LE, Walton BN, Arendt LM. Obesity reduces mammary epithelial cell TGFβ1 activity through macrophage-mediated extracellular matrix remodeling. FASEB J. 2020. doi: 10.1096/fj.202000228RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinol. 2007;148(2):868–77. doi: 10.1210/en.2006-0687. [DOI] [PubMed] [Google Scholar]
- 156.Keophiphath M, Achard V, Henegar C, Rouault C, Clement K, Lacasa D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol. 2009;23(1):11–24. doi: 10.1210/me.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ferrante AW. The immune cells in adipose tissue. Diabetes Obes Metab. 2013;15(0 3):34–8. doi: 10.1111/dom.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49(9). doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 159.Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nature Med. 2012;18(9). doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286(26):23591–9. doi: 10.1074/jbc.M111.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kado T, Nawaz A, Takikawa A, Usui I, Tobe K. Linkage of CD8(+) T cell exhaustion with high-fat diet-induced tumourigenesis. Sci Rep. 2019;9(1):12284. doi: 10.1038/s41598-019-48678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reynolds CM, McGillicuddy FC, Harford KA, Finucane OM, Mills KH, Roche HM. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells-implications for diet-induced insulin resistance. Mol Nutr Food Res. 2012;56(8):1212–22. doi: 10.1002/mnfr.201200058. [DOI] [PubMed] [Google Scholar]
- 163.Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61(9):2238–47. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stefanovic-Racic M, Yang X, Turner MS, Mantell BS, Stolz DB, Sumpter TL et al. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes. 2012;61(9):2330–9. doi: 10.2337/db11-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB et al. Adipose tissue dendritic cells are independent contributors to obesity-induced inflammation and insulin resistance. J Immunol. 2016;197(9):3650–61. doi: 10.4049/jimmunol.1600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 168.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185(3):1836–45. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes. 2008;32(3):451–63. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 170.Zhang C, Yue C, Herrmann A, Song J, Egelston C, Wang T et al. STAT3 activation-induced fatty acid oxidation in CD8(+) T effector cells is critical for obesity-promoted breast tumor growth. Cell Metab. 2020;31(1):148–61.e5. doi: 10.1016/j.cmet.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zeyda M, Huber J, Prager G, Stulnig TM. Inflammation correlates with markers of T-cell subsets including regulatory T cells in adipose tissue from obese patients. Obesity. 2011;19(4). doi: 10.1038/oby.2010.123. [DOI] [PubMed] [Google Scholar]
- 172.Travers RL, Motta AC, Betts JA, Bouloumié A, Thompson D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int J Obesity. 2015;39(5). doi: 10.1038/ijo.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29(10). doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 174.Adhikary S, Hoskin TL, Stallings-Mann ML, Arshad M, Frost MH, Winham SJ et al. Cytotoxic T cell depletion with increasing epithelial abnormality in women with benign breast disease. Breast Cancer Res Treat. 2020;180(1):55–61. doi: 10.1007/s10549-019-05493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brahimi-Horn MC, Pouyssegur J. Oxygen, a source of life and stress. FEBS Lett. 2007;581(19):3582–91. doi: 10.1016/j.febslet.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 176.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58(3):718–25. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE Jr., Peterson CA et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab. 2011;96(12):E1990–8. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Blaak EE, van Baak MA, Kemerink GJ, Pakbiers MT, Heidendal GA, Saris WH. Beta-adrenergic stimulation and abdominal subcutaneous fat blood flow in lean, obese, and reduced-obese subjects. Metabolism. 1995;44(2):183–7. doi: 10.1016/0026-0495(95)90262-7. [DOI] [PubMed] [Google Scholar]
- 179.Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI et al. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100(2):274–80. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Virtanen KA, Lonnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T et al. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab. 2002;87(8):3902–10. doi: 10.1210/jcem.87.8.8761. [DOI] [PubMed] [Google Scholar]
- 181.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 182.Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr. 2008;100(2):227–35. doi: 10.1017/s0007114508971282. [DOI] [PubMed] [Google Scholar]
- 183.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56(6):1517–26. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 184.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 185.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100(12):7265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–64. [DOI] [PubMed] [Google Scholar]
- 187.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65(10):3967–79. doi: 10.1158/0008-5472.can-04-2427. [DOI] [PubMed] [Google Scholar]
- 188.Cao Y Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9(2):107–15. [DOI] [PubMed] [Google Scholar]
- 189.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–8. doi: 10.1161/01.cir.0000121425.42966.f1. [DOI] [PubMed] [Google Scholar]
- 190.Bougaret L, Delort L, Billard H, Lequeux C, Goncalves-Mendes N, Mojallal A et al. Supernatants of adipocytes from obese versus normal weight women and breast cancer cells: in vitro impact on angiogenesis. J Cell Physiol. 2017;232(7):1808–16. doi: 10.1002/jcp.25701. [DOI] [PubMed] [Google Scholar]
- 191.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A. 2001;98(11):6390–5. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC et al. Biological action of leptin as an angiogenic factor. Science. 1998;281(5383):1683–6. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 193.Adya R, Tan BK, Randeva HS. Differential effects of leptin and adiponectin in endothelial angiogenesis. J Diabetes Res. 2015;2015:648239. doi: 10.1155/2015/648239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xie G, Swiderska-Syn M, Jewell ML, Machado MV, Michelotti GA, Premont RT et al. Loss of pericyte smoothened activity in mice with genetic deficiency of leptin. BMC Cell Biol. 2017;18(1):20. doi: 10.1186/s12860-017-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kolb R, Kluz P, Tan ZW, Borcherding N, Bormann N, Vishwakarma A et al. Obesity-associated inflammation promotes angiogenesis and breast cancer via angiopoietin-like 4. Oncogene. 2019;38(13):2351–63. doi: 10.1038/s41388-018-0592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPARγ activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab. 2008;295(5):E1056–64. doi: 10.1152/ajpendo.90345.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dobson DE, Kambe A, Block E, Dion T, Lu H, Castellot JJ Jr. et al. 1-Butyryl-glycerol: a novel angiogenesis factor secreted by differentiating adipocytes. Cell. 1990;61(2):223–30. doi: 10.1016/0092-8674(90)90803-m. [DOI] [PubMed] [Google Scholar]
- 198.Weight McTiernan A., physical activity and breast cancer survival. Proc Nutr Soc. 2018:1–9. doi: 10.1017/s0029665118000010. [DOI] [PubMed] [Google Scholar]
- 199.Byers T, Sedjo RL. Does intentional weight loss reduce cancer risk? Diabetes Obes Metab. 2011;13(12):1063–72. doi: 10.1111/j.1463-1326.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- 200.Sjöström L, Gummesson A, Sjöström CD, Narbro K, Peltonen M, Wedel H et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10(7):653–62. doi: 10.1016/s1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 201.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 202.Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of breast cancer development? A systematic review. Obes Surg 2017;27(11):3014–20. doi: 10.1007/s11695-017-2901-5. [DOI] [PubMed] [Google Scholar]
- 203.Feigelson HS, Caan B, Weinmann S, Leonard AC, Powers JD, Yenumula PR et al. Bariatric surgery is associated with reduced risk of breast cancer in both premenopausal and postmenopausal women. Ann Surg. 2019. doi: 10.1097/sla.0000000000003331. [DOI] [PubMed] [Google Scholar]
- 204.Heshmati K, Harris DA, Rosner B, Pranckevicius E, Ardestani A, Cho N et al. Association of bariatric surgery status with reduced HER2+ breast cancers: a retrospective cohort study. Obes Surg. 2019;29(4):1092–8. doi: 10.1007/s11695-018-03701-7. [DOI] [PubMed] [Google Scholar]
- 205.Hassinger TE, Mehaffey JH, Hawkins RB, Schirmer BD, Hallowell PT, Schroen AT et al. Overall and estrogen receptor-positive breast cancer incidences are decreased following bariatric surgery. Obes Surg. 2019;29(3):776–81. doi: 10.1007/s11695-018-3598-9. [DOI] [PubMed] [Google Scholar]
- 206.Rossi EL, de Angel RE, Bowers LW, Khatib SA, Smith LA, Van Buren E et al. Obesity-associated alterations in inflammation, epigenetics, and mammary tumor growth persist in formerly obese mice. Cancer Prev Res. 2016;9(5):339–48. doi: 10.1158/1940-6207.capr-15-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bhardwaj P, Du B, Zhou XK, Sue E, Harbus MD, Falcone DJ et al. Caloric restriction reverses obesity-induced mammary gland inflammation in mice. Cancer Prev Res. 2013;6(4):282–9. doi: 10.1158/1940-6207.CAPR-12-0467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 208.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–86. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 209.Kovacikova M, Sengenes C, Kovacova Z, Siklova-Vitkova M, Klimcakova E, Polak J et al. Dietary intervention-induced weight loss decreases macrophage content in adipose tissue of obese women. Int J Obes. 2011;35(1):91–8. doi: 10.1038/ijo.2010.112. [DOI] [PubMed] [Google Scholar]
- 210.Silva KR, Liechocki S, Carneiro JR, Claudio-da-Silva C, Maya-Monteiro CM, Borojevic R et al. Stromal-vascular fraction content and adipose stem cell behavior are altered in morbid obese and post bariatric surgery ex-obese women. Stem Cell Res Ther. 2015;6:72. doi: 10.1186/s13287-015-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18(14):1657–69. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 212.Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A et al. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94(11):4619–23. doi: 10.1210/jc.2009-0925. [DOI] [PubMed] [Google Scholar]
- 213.Mitterberger MC, Mattesich M, Zwerschke W. Bariatric surgery and diet-induced long-term caloric restriction protect subcutaneous adipose-derived stromal/progenitor cells and prolong their life span in formerly obese humans. Exp Gerontol. 2014;56:106–13. doi: 10.1016/j.exger.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 214.Rossmeislova L, Malisova L, Kracmerova J, Tencerova M, Kovacova Z, Koc M et al. Weight loss improves the adipogenic capacity of human preadipocytes and modulates their secretory profile. Diabetes. 2013;62(6):1990–5. doi: 10.2337/db12-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kolehmainen M, Salopuro T, Schwab US, Kekalainen J, Kallio P, Laaksonen DE et al. Weight reduction modulates expression of genes involved in extracellular matrix and cell death: the GENOBIN study. Int J Obes. 2008;32(2):292–303. doi: 10.1038/sj.ijo.0803718. [DOI] [PubMed] [Google Scholar]
- 216.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59(11):2817–25. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liu Y, Aron-Wisnewsky J, Marcelin G, Genser L, Le Naour G, Torcivia A et al. Accumulation and changes in composition of collagens in subcutaneous adipose tissue after bariatric surgery. J Clin Endocrinol Metab. 2016;101(1):293–304. doi: 10.1210/jc.2015-3348. [DOI] [PubMed] [Google Scholar]
- 218.Abdennour M, Reggio S, Le Naour G, Liu Y, Poitou C, Aron-Wisnewsky J et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J Clin Endocrinol Metab. 2014;99(3):898–907. doi: 10.1210/jc.2013-3253. [DOI] [PubMed] [Google Scholar]
- 219.Lambertz IU, Luo L, Berton TR, Schwartz SL, Hursting SD, Conti CJ et al. Early exposure to a high fat/high sugar diet increases the mammary stem cell compartment and mammary tumor risk in female mice. Cancer Prev Res. 2017;10(10):553–62. doi: 10.1158/1940-6207.Capr-17-0131. [DOI] [PubMed] [Google Scholar]
- 220.Phipps AI, Buist DS, Malone KE, Barlow WE, Porter PL, Kerlikowske K et al. Breast density, body mass index, and risk of tumor marker-defined subtypes of breast cancer. Ann Epidemiol. 2012;22(5):340–8. doi: 10.1016/j.annepidem.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Body size and risk of luminal, HER2-overexpressing, and triple-negative breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2078–86. doi: 10.1158/1055-9965.EPI-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Terry MB, Michels KB, Brody JG, Byrne C, Chen S, Jerry DJ et al. Environmental exposures during windows of susceptibility for breast cancer: a framework for prevention research. Breast Cancer Res. 2019;21(1):96. doi: 10.1186/s13058-019-1168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shieh Y, Scott CG, Jensen MR, Norman AD, Bertrand KA, Pankratz VS et al. Body mass index, mammographic density, and breast cancer risk by estrogen receptor subtype. Breast Cancer Res. 2019;21(1):48. doi: 10.1186/s13058-019-1129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Engmann NJ, Scott CG, Jensen MR, Winham S, Miglioretti DL, Ma L et al. Combined effect of volumetric breast density and body mass index on breast cancer risk. Breast Cancer Res Treat. 2019;177(1):165–73. doi: 10.1007/s10549-019-05283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Huo CW, Chew G, Hill P, Huang D, Ingman W, Hodson L et al. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 2015;17:79. doi: 10.1186/s13058-015-0592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


