Abstract
Nonalcoholic fatty liver disease (NAFLD) is a broad-spectrum disease, ranging from simple hepatic steatosis to nonalcoholic steatohepatitis, which can progress to cirrhosis and liver cancer. Abnormal hepatic lipid accumulation is the major manifestation of this disease, and lipotoxicity promotes NAFLD progression. In addition, intermediate metabolites such as succinate can stimulate the activation of hepatic stellate cells to produce extracellular matrix proteins, resulting in progression of NAFLD to fibrosis and even cirrhosis. G protein-coupled receptors (GPCRs) have been shown to play essential roles in metabolic disorders, such as NAFLD and obesity, through their function as receptors for bile acids and free fatty acids. In addition, GPCRs link gut microbiota-mediated connections in a variety of diseases, such as intestinal diseases, hepatic steatosis, diabetes, and cardiovascular diseases. The latest findings show that gut microbiota-derived acetate contributes to liver lipogenesis by converting dietary fructose into hepatic acetyl-CoA and fatty acids. GPCR agonists, including peptides and natural products like docosahexaenoic acid, have been applied to investigate their role in liver diseases. Therapies such as probiotics and GPCR agonists may be applied to modulate GPCR function to ameliorate liver metabolism syndrome. This review summarizes the current findings regarding the role of GPCRs in the development and progression of NAFLD and describes some preclinical and clinical studies of GPCR-mediated treatment. Overall, understanding GPCR-mediated signaling in liver disease may provide new therapeutic options for NAFLD.
Keywords: Nonalcoholic fatty liver disease, G protein-coupled receptors, Metabolism, Bile acids, Short-chain fatty acids, Gut microbiota
Core Tip: Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease. Without effective treatment, NAFLD can progress to fibrosis, cirrhosis, and liver cancer. Currently, there is no effective treatment option. G protein-coupled receptors (GPCRs) have been shown to play essential roles in metabolic disorders, such as NAFLD, through their function as receptors for bile acids and free fatty acids. Therapies such as probiotics and GPCR agonists could be applied to modulate GPCR function to ameliorate liver metabolism syndrome. Herein, this review summarizes the current findings regarding the role of GPCRs in the development and progression of NAFLD.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a broad-spectrum disease characterized by pathological severity ranging from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH)[1]. NAFLD patients with progressive liver fibrosis have a high incidence of developing cirrhosis and hepatocellular carcinoma (HCC)[2]. NAFLD is often associated with other metabolic disorders, including obesity, diabetes, and insulin resistance[3]. Abnormal accumulation of hepatic lipids is the main manifestation of NAFLD. Lipotoxicity caused by toxic free fatty acids, such as palmitic acid, cholesterol, and ceramides, contributes to early-stage NAFLD progression to advanced NASH and advanced liver disease[4]. However, there is still no approved treatment for NAFLD[5]. G protein-coupled receptors (GPCRs) play important roles in metabolic disorders and can respond to various extracellular signals, including fatty acids (FAs)[6,7]. Data are increasingly showing that GPCRs and their signaling pathways are promising targets for NAFLD treatment.
Studies on the gut–liver axis are rapidly contributing to mounting evidence that dysbiosis of gut microbiota contributes to NAFLD progression via multiple mechanisms, including the secondary bile acid-induced senescence-associated secretory phenotype of hepatic stellate cells (HSCs)[8], bacterial product-induced proinflammatory responses through Toll-like receptors and toxic products[9,10]. In addition, bile acids (BAs), primarily produced in the liver and metabolized by gut microbiota, have pleiotropic roles in metabolism, including glucose homeostasis, digestion and absorption of dietary lipids, intestinal bacterial growth, and liver regeneration[11]. One of the molecular mechanisms for this BA interaction is with Takeda GPCR 5 and G protein-coupled bile acid receptor-1 (TGR5/GPBAR1) to regulate lipid and glucose metabolism[12,13].
In this review, we focus on the role of GPCRs in NAFLD development and progression by regulating nutrient metabolism. First, we introduce the role of GPCRs in liver metabolism and discuss how GPCR-mediated signaling impacts lipid synthesis, lipid and glucose metabolism, and production of extracellular matrix (ECM) proteins. Then, we summarize the role of GPCRs in NAFLD and advanced liver disease, followed by further discussion on current GPCR-targeted treatments in cellular and animal models for liver disease. Finally, we look to the therapeutic potential of GPCR-mediated signaling in liver disease and current preclinical and clinical trials.
EFFECT OF GPCRS ON LIVER METABOLISM
GPCRs, the largest family of membrane proteins, mediate cellular responses to various stimuli and play essential roles in most patho/physiological processes[14]. Some GPCRs can be activated by energy metabolites, such as FAs, saccharides, lactates, and ketone bodies[15]. Increasing evidence indicates that GPCRs play pivotal roles in liver metabolism by modulating diverse signaling pathways[16,17], including the Hedgehog, Wnt, Notch, and transforming growth factor-β pathways[18]. GPCRs are receptors of short-, medium-, and long-chain FAs and can regulate the secretion of gut hormones, lipid and glucose metabolism, and generation of ECM proteins (Figure 1). Herein, we discuss how GPCRs mediate liver metabolism via these ligands.
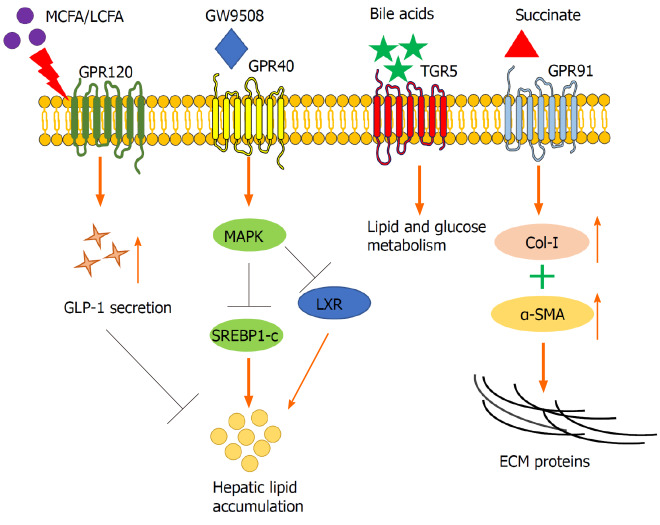
Figure 1.
The role of G protein-coupled receptors in liver metabolism and the generation of extracellular matrix proteins. G protein-coupled receptors are receptors of diverse molecules, such as fatty acids, bile acids, and other agonists (e.g., GW9508). They can regulate hepatic lipid and glucose metabolism and extracellular matrix (ECM) production via directly modulating hepatic cells (hepatocytes and hepatic stellate cells), and indirectly regulating gut hormones (e.g., glucagon-like peptide-1, GLP-1). α-SMA: α-smooth muscle actin; Col-I: Collagen type I; LCFA: Long-chain fatty acid; LXR: Liver X receptor; MAPK: Mitogen-activated protein kinase; MCFA: Medium-chain fatty acid; SREBP1-c: Sterol regulatory element-binding protein 1.
Medium-chain fatty acids and long-chain fatty acids
Long-chain fatty acids (LCFAs) with more than 16 carbons are the most common fatty acids in Western diets and are commonly associated with inflammation and lipid accumulation[19]. In contrast, consuming diets rich in medium-chain fatty acids with 8-12 carbons can increase energy expenditure and decrease fatty acid accumulation. Oleate, an unsaturated (18:1) LCFA, can stimulate glucagon-like peptide 1 (GLP-1) secretion from an immortalized murine enteroendocrine cell line of GLUTag cells through their receptors via upregulating GPR40 (FFAR1) and GPR41 (FFAR3)[20]. Another study showed that GPR120 (FFA4) functions as a receptor for LCFA to regulate the secretion of GLP-1 from the gastrointestinal tract[21]. GLP-1 shows anti-inflammatory activity, including suppression of inflammatory cytokine expression in macrophages[22]. Accumulating evidence indicates that GLP-1 receptor agonists, GLP-1, and glucagon receptor co-agonists are treatment options for NAFLD[23,24]. The GPR40 agonist GW9508 decreased oleic acid-induced lipid accumulation in liver cancer cell line HepG2 cells via activating mitogen-activated protein kinase signaling, to downregulate the expression of sterol regulatory element-binding protein 1[25]. The effect of GW9508 on sterol regulatory element-binding protein 1 was diminished in GPR40-knockdown HepG2 cells. Moreover, GW9508 attenuated liver X receptor-induced hepatic lipid accumulation via activating the mitogen-activated protein kinase signaling pathway[26]. Another report showed that GPR40-deficient mice were protected against conjugated linoleic acid-induced accumulation of triglycerides in the liver[27], which might be associated with the secretion of insulin from the pancreas[28]. GPR120, as a receptor for unsaturated LCFAs, plays an essential role in liver metabolism. Ichimura et al[29] reported that GPR120-deficient mice showed a marked increase in hepatic lipids with a 10% greater bodyweight increase than wild-type mice[29]. Gpr43−/− mice were obese compared to wild-type mice with an increase in short-chain fatty acid (SCFA)-producing bacteria in the gut and increased concentrations of fecal SCFA and plasma acetate[30]. In addition, a specific deficiency of GPR43 in the adipose tissue attenuated high-fat diet (HFD)-induced liver steatosis.
SCFAs
SCFAs, including acetate, propionate, and butyrate, are the main metabolites of gut microbiota under an anaerobic microenvironment. SCFAs fuel intestinal cells and modulate the gut immune response via activating GPCRs (e.g., GPR41/FFAR3 and GPR43/FFAR2) and inhibiting histone deacetylase[31]. In addition, GPCRs modulate SCFA-mediated inflammatory responses. For example, an antagonist of GPR41 or GPR43 alone as well as in combination were able to recover the inhibiting effect of acetate on lipopolysaccharides/tumor necrosis factor alpha-induced interleukin (IL)-6 and IL-8 production in human umbilical vein endothelial cells[32]. GPR41/43 are also involved in the effect of butyrate and propionate on IL-6 production but not IL-8 production. A new finding showed that microbial acetate contributed to liver lipogenesis by converting dietary fructose into hepatic acetyl-CoA and fatty acids[33]. SCFAs are also associated with the ameliorating effect of fructo-oligosaccharides on steatohepatitis and chronic inflammation[34].
Bile acids
Toxic BAs can cause hepatocyte death by directly activating cell death receptors or inducing oxidative damage, resulting in mitochondrial dysfunction, endoplasmic reticulum stress, and cell death[35]. Conversely, BAs can activate nuclear farnesoid X receptor and GPCR signaling to protect against liver and gastrointestinal inflammation[36,37]. TGR5 is widely expressed in different nonparenchymal liver cells, altering expression in response to BAs like lithocholic acid. TGR5 deletion increased the sensitivity of cholic acid feeding and bile duct ligation-induced liver injury in the endothelin-1 associated signaling pathway[38]. Administration of parenteral nutrition increased liver weight, the infiltration of macrophages, and inflammatory cytokine IL-6 expression[39] in TGR5-/- mice compared to wild-type mice. Meanwhile, unconjugated primary BAs and secondary BAs were increased due to the elevated abundance of Bacteroides and Parabacteriodes in the gut. Another study showed that BA-activated Mas-related GPCR4 played a critical role in cholestatic itch[40].
Gut hormones
Gut hormones, such as peptide-YY and GLP-1, can be modulated through GPCRs to regulate insulin secretion in obese subjects[21,41]. For instance, SCFAs can stimulate incretin hormone GLP-1 secretion via GPR43 in the intestinal L cells to impact insulin sensitivity and appetite[42]. GLP-1 can regulate hepatic steatosis by preventing HFD-induced very-low-density lipoprotein overproduction and insulin resistance[43], accompanying the reduction in the mRNA and protein expression of sterol regulatory element-binding protein 1c, stearoyl-CoA desaturase-1, and fatty acid synthase.
Others
Conditional deletion of GPRC6a in hepatocytes by cross-breeding Alb-Cre and Gprc6aflox/flox mice resulted in abundant liver fat accumulation and glycogen depletion[44]. In addition, GPRC6a depletion altered the production of FGF-21 and its release, which controlled systemic energy homeostasis. Exposure of succinate upregulated the expression of GPR91 in primary and immortalized HSCs accompanying the increased expression of ECM proteins. Inhibiting GPR91 expression by lentivirus harboring shRNA reduced succinate-mediated HSC activation[45].
EFFECT OF GPCRS IN NAFLD, NASH, AND HCC
GPCRs
To date, some GPCRs have been shown to play critical roles in liver diseases (Table 1). Herein, we summarize some crucial GPCRs with potential clinical value at different stages of liver diseases, ranging from NAFLD to HCC. For instance, GPR120 is a functional receptor for ω-3 fatty acids that show strong anti-inflammatory and anti-insulin resistance effects. Oh et al[46] reported that GPR120 agonist cpdA treatment increased insulin sensitivity and glucose tolerance and decreased hepatic steatosis in HFD-induced obese mice[46].
Table 1.
The role of G protein-coupled receptors in nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and hepatocellular carcinoma
|
Liver disease
|
GPCRs
|
Expression
|
Ref.
|
| NAFLD/Steatosis | GPR120 | GPR120 agonist cpdA treatment increased insulin sensitivity and glucose tolerance and decreased hepatic steatosis in HFD-induced obese mice | [46] |
| HCC | GPR49 | GPR49 is highly expressed in human HCC cell lines PLC/PRF/5 and HepG2; overexpression of GPR49 in HCC tissue with a mutation of beta-catenin exon 3 was also shown | [47] |
| HCC | GPR137 | Knockdown of GPR137 in HepG2 cells induced cell cycle arrest and cell apoptosis. Additionally, low expression of GPR137 indicated the progression of human HCC and a low survival rate | [49] |
| NAFLD/Steatosis | GPR132 | GPR132 was involved in hepatic lipid metabolism and gallstone formation in mice because GPR132-deficient mice fed a lithogenic diet quickly developed gallstones and had a high cholesterol saturation index | [51] |
| NAFLD/Steatosis | GPR55 | GPR55-deficient (GPR55-/-) mice showed impaired insulin signaling and had a significant increase in total body fat and liver fatty acid synthase, resulting in the development of hepatic steatosis | [53] |
| NASH/Fibrosis | GPR91 | Succinate in the fatty liver can activate HSC via GPR91 receptor, resulting in NASH progression | [45] |
| Liver injury/Fibrosis | GPBAR1 | GPBAR1 is an upstream regulator of the axis expression of chemokine CCL2 and its receptor CCR2 in the interface of liver sinusoidal cells | [54] |
GPCRs: G protein-coupled receptors; HCC: Hepatocellular carcinoma; HFD: High-fat diet; HSC: Hepatic stellate cell; NAFLD: Nonalcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis.
GPR49, an orphan GPCR with unknown ligands, is highly expressed in human HCC cell lines PLC/PRF/5 and HepG2[47]. In addition, overexpression of GPR49 has been shown in HCC tissue with a mutation of beta-catenin exon three. Another orphan GPCR receptor, GPR137, is also broadly expressed in human liver cancer cell lines, such as HepG2 and Bel7404. The depletion of GPR137 by lentivirus-mediated RNA interference in these two cell lines remarkably inhibited the proliferation and colony formation capacity[48]. Knockdown of GPR137 in HepG2 cells resulted in cell cycle arrest and cell apoptosis, suggesting that targeting GPR137 can inhibit cancer growth. Moreover, low expression of GPR137 indicated the progression of human HCC and low survival rates[49].
GPR132 or G2A receptor is a proton-sensing GPCR and plays an important role in cell cycle and proliferation, oncogenesis, and the immune response[50]. GPR132 is also involved in hepatic lipid metabolism and gallstone formation in mice because GPR132-deficient mice fed a lithogenic diet quickly developed gallstones and had a high cholesterol saturation index[51].
Not all GPCRs have a protective effect against liver disease. Succinate was increased in fatty liver cells of high fat/calorie diet plus high fructose and glucose in drinking water-fed mice[45]. Exposure of succinate upregulated the expression of GPR91 in primary and immortalized HSCs and increased the expression of ECM proteins of these cells. Inhibiting GPR91 expression by lentivirus harboring shRNA reduced succinate-mediated HSC activation. Meanwhile, the expression of GPR91 was correlated with the severity of fibrosis in human NASH biopsy specimens[45]. GPR55 and its endogenous ligand, l-α-lysophosphatidylinositol are positively correlated with obesity and type 2 diabetes (T2D)[52]. Moreover, GPR55-deficient (GPR55-/-) mice showed impaired insulin signaling evidenced by reduced phosphorylation of protein kinase B and its downstream targets and had a significant increase in total body fat and liver fatty acid synthase, which can result in the development of hepatic steatosis[53]. In the same study, the author also found that lysophosphatidylinositol activated rat H4IIE liver cells and human HepG2 liver cells via GPR55 to enhance insulin-dependent protein kinase B phosphorylation. Deletion of GPBAR1, a GPCR for secondary BAs, accelerated the severity of liver injury caused by acetaminophen[54]. Further, GPBAR1 agonism mediated the axis expression of chemokine CCL2 and its receptor CCR2 in the interface of liver sinusoidal cells.
GPCR signaling and regulatory proteins
Generally, GPCRs are linked to distinct families of G proteins, including Gs, Gi, and Gq[55]. For example, the glucagon receptor most highly expressed in hepatocytes is linked to the stimulatory G proteins, Gs. Heterotrimeric G proteins are involved in the signaling of approximately 800 GPCR family members[56]. Some of these signaling pathways play an important role in liver metabolism. For instance, Gα12 protein (Gα12) ablation significantly increases fasting-induced fat accumulation in the liver of mice, and Gα12 expression is also decreased in liver biopsies of NAFLD patients[16]. A mechanistic study showed that Gα12 regulated mitochondrial respiration through modulating sirtuin 1 and peroxisome proliferator-activated receptor alpha expression. Moreover, the expression of Gα12 has been associated with the overall survival of HCC patients[57]. Understanding the role of G proteins in the liver also helps unlock the role of GPCRs in liver metabolism and disease progression.
Regulators of G protein signaling (RGS) proteins negatively regulate GPCR signaling. RGS5 can protect against NAFLD and NASH. In the liver, RGS5 is an essential molecule that protects against the progression of NAFLD. RGS5 directly binds to transforming growth factor beta-activated kinase 1 (TAK1) and inhibits its phosphorylation and the subsequent c-Jun N-terminal kinase/p38 pathways. RGS5 is a promising target molecule for fine-tuning the activity of transforming growth factor beta-activated kinase 1 and NAFLD treatment[58].
Activated HSCs are one of the major sources of myofibroblasts in many types of liver injury[59], which produce ECM proteins. GPCR-mediated signaling plays an important role in HSC contraction, migration, and activation. Bahrami et al[60] reported that RGS5 can regulate GPCR signaling in HSCs and modulate HSC activation and hepatic fibrogenesis[60]. RGS6−/− mice showed reduced alcohol consumption when given free access to alcohol. RGS6−/− mice were also protected from alcohol-induced hepatic steatosis, cardiac toxicity, dysfunction of the gut barrier, and endotoxemia when they were forced to consume alcohol[61]. Another study showed that overexpression of RGS16, specifically in the liver, displayed fatty liver after overnight fasting but low blood glucose levels compared with wild-type mice[62]. In contrast, RGS16-knockout mice showed a higher rate of fatty acid oxidation in liver extracts compared with wild-type mice, suggesting that RGS16 inhibits GPCR-mediated fatty acid oxidation. Therefore, RGS is a group of therapeutic candidates for modulating GPCRs to treat liver disease.
The signaling of most GPCRs via G proteins is regulated by GPCR kinases (GRKs)[63], which also function in the pathogenesis of liver injury. For instance, GRK2 hemizygous (GRK2+/-) mice showed a reduced level of triglycerides and a reduced liver-to-body weight ratio compared to wild-type mice when fed a methionine and choline-deficient diet[64]. Increased GRK2 protein and mRNA levels were also detected in human liver biopsies of steatosis and NASH patients. Moreover, high GRK2 expression exaggerated palmitic acid-triggered lipid accumulation in human hepatocytes[64].
Gut microbiota-mediated GPCR expression
The gut–liver axis plays a critical role in the development of liver diseases[65,66]. Gut microbiota-derived metabolites and their associated signaling pathways play important roles in NAFLD development[67]. Rau et al[68] reported that SCFA-producing bacteria were dominant in the fecal bacteria of NAFLD patients, accompanying high acetate and propionate in fecal metabolites[68]. These metabolites are associated with immunological features in NAFLD progression. Manipulation of gut microbiota is a promising preventive and therapeutic strategy for NAFLD. For instance, administration of a bacterial cocktail, consisting of three strains of Bifidobacterium adolescentis and three strains of Lactobacillus rhamnosus, alleviated a high-fat, high-cholesterol diet-induced NAFLD symptom in mice by increasing the concentration of intestinal SCFAs[69]. Similar findings have also been achieved in clinical trials for human patients[70,71]. Gut microbiota-derived metabolites contribute to the development of NAFLD, including SCFAs[68], endogenous alcohol[72,73], and BAs[74,75]. Gut microbiota in the colon is the main source of the production of SCFAs[76,77], which affect lipid and glucose metabolism. It has been demonstrated that both BAs[78] and SCFAs[79] can activate GPCRs to modulate immune responses. Hence, modulating gut microbiota is an attractive strategy to interfere with liver disease.
For example, farnesoid X receptor agonist fexaramine-induced lithocholic acid-producing bacteria Acetatifactor and Bacteroides impact liver bile acid synthesis, which in turn can enhance the expression of intestinal farnesoid X receptor targeted genes[80]. In hepatocytes, palmitate can be metabolized to sphingosine 1-phosphate (S1P), which binds to different types of S1P receptors (S1PRs) like S1PR1-3 to activate HSCs to myofibroblasts[81]. In addition, S1P can increase the recruitment of bone marrow mesenchymal stem cells via activating S1PRs to produce proinflammatory cytokines IL-1β, tumor necrosis factor alpha, and IL-6, resulting in acceleration of the pathophysiological process of liver disease[82]. The S1P-S1PR1 axis is also associated with chronic intestinal inflammation and colitis-associated cancer by modulating IL-6 and transcription factor STAT3[83].
In addition to liver disease, gut microbiota-associated products affect other diseases, such as inflammatory bowel disease, diabetes, autoimmune disease, and cardiovascular disease, through GPCRs[84-86]. For example, tryptamine, a tryptophan-derived monoamine produced by gut bacteria like Bacteroides thetaiotaomicron can activate the serotonin 5-HT4 receptor, which is uniquely expressed in colonic epithelial cells to increase ionic flux across colonic epithelium, altering the host gut transmit[87]. Increasing evidence shows that the microbiota plays a crucial role in influencing host appetite and eating-relative behavior[88]. Of note, the gut–liver axis is bidirectional[10,89] because the liver also impacts the components of gut microbiota (Figure 2) by primary BAs, which may result in a change of appetite[90].
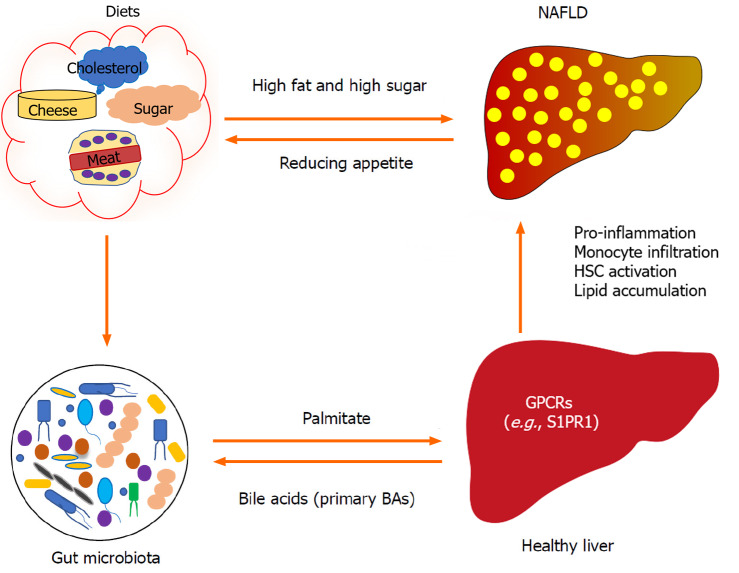
Figure 2.
G protein-coupled receptor-mediated interaction of the gut and liver. Gut microbiota-derived metabolites or molecules (e.g., palmitate) impact liver function by being metabolized to sphingosine 1-phosphate in hepatocytes, which can stimulate the activation of hepatic stellate cells (HSCs) and proinflammation via sphingosine 1-phosphate receptor 1 (S1PR1). In turn, primary bile acids (BAs) are synthesized in the liver, which can also influence the components of gut microbiota. A high fat and high sugar diet can induce nonalcoholic fatty liver disease (NAFLD) and change gut microbiota. Gut microbiota has been shown to impact appetite, and the progression of NAFLD may also impact the appetite. GPCRs: G protein-coupled receptors.
GPCR-BASED THERAPIES FOR LIVER DISEASES
GPCRs are a promising therapy for NAFLD treatment (Table 2). Recently, Pi et al[44] reported that Gprc6aLiver-cko mice (a strain with conditional depletion of GPRC6A in mouse hepatocytes) on a normal diet had excessive liver fat accumulation and impaired glucose and pyruvate tolerance without insulin resistance[44]. Intraperitoneal or oral administration of a peptide hormone metabolitin that binds to GPRC6A significantly ameliorated NAFLD symptoms and inhibited gut triglyceride and cholesterol absorption and insulin resistance via activating the 5’ AMP-activated protein kinase signaling pathway[91]. Metabolitin treatment was able to stimulate the expression of GLP-1, which further validated the metabolitin-GPRC6A interaction because the GPRC6A receptor functions as an amino acid sensor mediating GLP-1 secretion in the intestinal L cells[92].
Table 2.
G protein-coupled receptor-mediated treatment in nonalcoholic fatty liver disease
|
GPCRs
|
Treatment
|
Study
|
Effect
|
Ref.
|
| GPRC6A | Metabolitin, a peptide hormone | Mice | Specifically deleting Gprc6a in mouse hepatocytes caused hepatic fat accumulation. Metabolitin can significantly ameliorate NAFLD symptoms and inhibit gut triglyceride and cholesterol absorption and insulin resistance via GPRC6A-mediated activation of the 5’ AMP-activated protein kinase signaling pathway | [44,91] |
| GPR39 | Agonist TC-G1008 | Mice | Oral administration of TC-G1008 inhibited hepatic cell necrosis in concanavalin A-induced hepatitis liver in mice. In addition, acute administration of TC-G1008 reduced ethanol intake | [93,94] |
| GPR40 | Agonist SCO-267 | Mice | GPR40 deficiency was associated with hepatic inflammation and steatosis in low-fat diet-fed mice. Oral administration of SCO-267 reduced HFD-induced increase in liver weight, triglyceride and collagen production, and serum alanine aminotransferase | [95,96] |
| GPR40 | Docosahexaenoic acid | Primary hepatocytes, HFD-fed mice | Treatment with DHA, an omega-3 fatty acid, inhibited lipid droplets by interacting with GPR40 in primary hepatocytes via reduced expression of lipogenic enzymes. In addition, it significantly reduced the HFD-induced liver steatosis score in mice | [99] |
| GPR43 | Compound probiotics | Rats | Overexpressing GPR43 in adipose tissue kept mice lean on a HFD diet. Compound probiotics can modulate gut microbiota dysbiosis, SCFAs, and their receptors, like GPR43, in NAFLD rats | [30,102] |
| GPR84 | Antagonist PBI-4547GPR84 Antagonists CpdA and CpdB | Gpr84-/- mice; Wild-type mice | PBI-4547 treatment ameliorated NAFLD-associated metabolic dysregulation, hepatic steatosis and ballooning, which was depleted in Gpr84-/- mice. Inhibition of GPR84 with antagonists CpdA and CpdB significantly reduced myeloid cell infiltration and ameliorated inflammation and fibrosis in acute liver injury | [100,101] |
| GPR120 | TUG-891Agonist III | Hepatocytes; Mice | Agonist TUG-891 inhibited lipid accumulation in hepatocytes. Agonist III significantly suppressed macrophage infiltration, ROS production, hepatic inflammation, ER stress, and steatohepatitis | [97,98] |
DHA: Docosahexaenoic acid; ER: endoplasmic reticulum; GPCRs: G protein-coupled receptors; HFD: High-fat diet; NAFLD: Nonalcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis; ROS: Reactive oxygen species; SCFA: Short-chain fatty acid.
Oral administration of a GPR39 agonist, TC-G1008, inhibited liver injury marker glutamic-pyruvic transaminase expression and reduced hepatic cell necrosis in concanavalin A-induced hepatitis liver in mice[93]. Another study showed that an acute dose of TC-G1008 reduced ethanol intake in mice without affecting total fluid intake[94]. GPR40 deficiency was associated with hepatic inflammation and steatosis in low-fat diet-fed mice[95]. Oral administration of a GPR40 full agonist, SCO-267, reduced liver weight, triglyceride and collagen production, and serum alanine amino-transferase without affecting food intake or glucose levels in choline-deficient, L-amino acid-defined, high-fat diet-fed mice[96]. Furthermore, SCO-267 improved mitochondrial function and beta-oxidation, while inhibiting lipogenesis, inflammation, and generation of reactive oxygen species in the liver.
GPR120 agonist III significantly suppressed macrophage infiltration and reactive oxygen species production and reversed hepatic inflammation, endoplasmic reticulum stress, and apoptosis in high-fat, high-cholesterol diet or methionine and choline-deficient-induced steatohepatitis[97]. In addition, GPR120 agonist TUG-891 inhibited lipid accumulation in hepatocytes[98].
Treatment with docosahexaenoic acid, an omega-3 fatty acid, inhibited lipid droplets by interacting with GPR40 in primary hepatocytes via reduced expression of lipogenic enzymes, such as fatty acid synthase, acetyl-CoA carboxylase, and stearoyl-CoA desaturase-1[99]. PBI-4547, a fatty acid mimetic, is a GPR84 antagonist. In a mouse model of diet-induced obesity, PBI-4547 treatment ameliorated NAFLD-associated metabolic dysregulation and hepatic steatosis and ballooning, which was depleted in GPR84-/- mice[100]. PBI-4547 increased liver fatty acid oxidation and gene expression of mitochondrial uncoupling proteins. Another study showed that inhibition of GPR84 with antagonists CpdA and CpdB significantly reduced myeloid cell infiltration and ameliorated inflammation and fibrosis in acute liver injury[101]. GPR43-deficient mice became obese when fed a normal diet. In contrast, mice with GPR43 overexpressed specifically in adipose tissue remained lean even on a high-fat diet[30]. Oral administration of compound probiotics ameliorated HFD-induced gut microbe dysbiosis and chronic metabolic inflammation in NAFLD rats via GPR43[102].
URGENT NEED FOR CLINICAL TRIALS
GPCRs, as the largest group of transmembrane receptors[103], play important roles in various diseases, including inflammatory bowel diseases[104,105], kidney diseases[106], liver diseases[107], bone disease[6], central nervous system disorders[108], heart diseases[109], and respiratory diseases[110]. To date, about fifty GPCR targeting peptides have been approved to treat metabolic diseases and tumors[111]. With the analysis of public databases, Sriram and Insel[112] reported that about 35% of approved drugs target GPCRs[112]. GPCRs and GPCR-associated proteins consist of about 17% of all protein targets for approved drugs. The application of GPCRs has been tested in clinical trials for metabolic disorders[113,114], including obesity and diabetes.
For example, T2D patients aged 20 or older orally received 75 mg of GPR119 agonist DS-8500a daily for 4 wk, resulting in enhanced insulin secretory capacity compared to placebo treatment[114]. In addition, DS-8500a significantly reduced total cholesterol, low-density lipoprotein cholesterol, and triglyceride concentrations and significantly increased high-density lipoprotein cholesterol concentrations compared to placebo treatment. No significant treatment-associated adverse events occurred in this trial. Another phase 2 clinical trial showed that daily treatment of GPR40 agonist Fasiglifam for 12 wk significantly improved glycemic control in T2D patients who were not responsible to diet or metformin treatment compared to placebo treatment, evidenced by the reduction of hemoglobin A(1c) from baseline[115]. In addition, Fasiglifam did not cause a higher risk of hypoglycemic events in patients.
However, our current knowledge about the function of GPCRs in liver metabolism disorders is still limited. When considering potential drugs for treatments, even fewer candidates have been tested in clinical trials. Some experimental trials in rodents have been investigated to explore the role of GPCRs in liver diseases, such as GPR40[99] and GPR43[102]. Some of the GPCRs, such as prostaglandin E2 receptors[116] and beta-2 adrenergic receptor[117], have been investigated in preclinical studies using tissue biopsies of human patients. Meanwhile, the side effects of GPCR-targeted molecules need to be considered when designing new therapeutic agents. For example, Fasiglifam increased the ratio of liver enzymes aspartate aminotransferase/alanine transaminase in T2D patients compared to placebo treatment while being evaluated for cardiovascular safety in a phase 3 trial[118].
CONCLUSION
Many GPCRs have critical roles in metabolic disorders, including NAFLD, through their function as receptors for metabolites, such as SCFAs and BAs. GPCR-mediated signaling pathways are involved in hepatic lipid accumulation and fibrogenesis and can also modulate the secretion of gut hormones (e.g., GLP-1), which further impacts liver function, suggesting that GPCRs play a pivotal role in the gut–liver axis. Furthermore, GPCRs and their associated molecules are candidates as biomarkers for NAFLD diagnosis. Overall, GPCRs and their regulating factors provide potential pharmacological targets for NAFLD treatment.
There are several advantages to targeting GPCRs to treat NAFLD compared with other NAFLD therapeutics. First, therapeutic candidates or drugs can be easily found. The data from public databases (ChEMBL, Guide to PHARMACOLOGY/GtoPdb, and DrugBank) show that about 35% of approved drugs target GPCRs[112]. Second, functional selection of GPCR ligands helps minimize the potential side effects of selected treatment[119]. Third, GPCRs are implicated in the development and progression of NAFLD, including lipid metabolism, proinflammation, and fibrosis. Therefore, targeting GPCR can be applied to different stages of NAFLD therapy, ranging from simple steatosis to NASH. However, current GPCR-mediated treatments in hepatic steatosis, liver fibrosis, and liver cancer are mainly performed either in cells or animals. Few preclinical and clinical trials in humans have been carried out so far. More work is needed to unmask the role of GPCRs in the clinic.
The structures of GPCRs are critically important for de novo design of GPCR targeting drugs. However, only about 60 GPCR structures have been resolved with the advanced technologies like X-ray crystallography and cryo-electron microscopy[120]. A new protocol has optimized the precrystallization process for resolving GPCR structures via X-ray crystallography[121]. Some technologies such as cell-based electrical impedance also help identify GPCR-targeting molecules[122]. In addition, computer-based design of GPCR allosteric receptors helps reveal the unknown GPCR signaling pathways and the relative molecular mechanism[123]. In conclusion, advanced technologies help unravel the clear role of each GPCR in both physiological and pathological environment to accelerate GPCR-mediated therapy.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: November 11, 2020
First decision: December 17, 2020
Article in press: January 21, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen Y S-Editor: Zhang L L-Editor: Filipodia P-Editor: Ma YJ
Contributor Information
Ming Yang, Department of Surgery, University of Missouri, Columbia, MO 65212, United States. yangmin@health.missouri.edu.
Chun-Ye Zhang, Department of Veterinary Pathobiology, University of Missouri, Columbia, MO 65212, United States.
References
- 1.Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, Nieuwdorp M, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279–297. doi: 10.1038/s41575-020-0269-9. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindenmeyer CC, McCullough AJ. The Natural History of Nonalcoholic Fatty Liver Disease-An Evolving View. Clin Liver Dis. 2018;22:11–21. doi: 10.1016/j.cld.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb A, Canbay A. Why Bile Acids Are So Important in Non-Alcoholic Fatty Liver Disease (NAFLD) Progression. Cells. 2019;8 doi: 10.3390/cells8111358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J, Sun P, Siwko S, Liu M, Xiao J. The role of GPCRs in bone diseases and dysfunctions. Bone Res. 2019;7:19. doi: 10.1038/s41413-019-0059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng WT, Sun WY, Li XR, Sun JC, Du JJ, Wei W. Emerging Roles of G Protein-Coupled Receptors in Hepatocellular Carcinoma. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19051366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Yang M, Ericsson AC. Antimicrobial Peptides: Potential Application in Liver Cancer. Front Microbiol. 2019;10:1257. doi: 10.3389/fmicb.2019.01257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol. 2020;318:G554–G573. doi: 10.1152/ajpgi.00223.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donepudi AC, Boehme S, Li F, Chiang JY. G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice. Hepatology. 2017;65:813–827. doi: 10.1002/hep.28707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov. 2012;11:603–619. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- 16.Kim TH, Yang YM, Han CY, Koo JH, Oh H, Kim SS, You BH, Choi YH, Park TS, Lee CH, Kurose H, Noureddin M, Seki E, Wan YY, Choi CS, Kim SG. Gα12 ablation exacerbates liver steatosis and obesity by suppressing USP22/SIRT1-regulated mitochondrial respiration. J Clin Invest. 2018;128:5587–5602. doi: 10.1172/JCI97831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Millar JS, Brownell N, Briand F, Rader DJ. Modulation of HDL metabolism by the niacin receptor GPR109A in mouse hepatocytes. Biochem Pharmacol. 2010;80:1450–1457. doi: 10.1016/j.bcp.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu YJ, Sun WY, Zhang S, Wu JJ, Wei W. The emerging roles of β-arrestins in fibrotic diseases. Acta Pharmacol Sin. 2015;36:1277–1287. doi: 10.1038/aps.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery MK, Osborne B, Brown SH, Small L, Mitchell TW, Cooney GJ, Turner N. Contrasting metabolic effects of medium- versus long-chain fatty acids in skeletal muscle. J Lipid Res. 2013;54:3322–3333. doi: 10.1194/jlr.M040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thombare K, Ntika S, Wang X, Krizhanovskii C. Long chain saturated and unsaturated fatty acids exert opposing effects on viability and function of GLP-1-producing cells: Mechanisms of lipotoxicity. PLoS One. 2017;12:e0177605. doi: 10.1371/journal.pone.0177605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 22.Guo C, Huang T, Chen A, Chen X, Wang L, Shen F, Gu X. Glucagon-like peptide 1 improves insulin resistance in vitro through anti-inflammation of macrophages. Braz J Med Biol Res. 2016;49:e5826. doi: 10.1590/1414-431X20165826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel V, Joharapurkar A, Kshirsagar S, Sutariya B, Patel M, Patel H, Pandey D, Patel D, Ranvir R, Kadam S, Bahekar R, Jain M. Coagonist of GLP-1 and Glucagon Receptor Ameliorates Development of Non-Alcoholic Fatty Liver Disease. Cardiovasc Hematol Agents Med Chem. 2018;16:35–43. doi: 10.2174/1871525716666180118152158. [DOI] [PubMed] [Google Scholar]
- 24.Valdecantos MP, Pardo V, Ruiz L, Castro-Sánchez L, Lanzón B, Fernández-Millán E, García-Monzón C, Arroba AI, González-Rodríguez Á, Escrivá F, Álvarez C, Rupérez FJ, Barbas C, Konkar A, Naylor J, Hornigold D, Santos AD, Bednarek M, Grimsby J, Rondinone CM, Valverde ÁM. A novel glucagon-like peptide 1/glucagon receptor dual agonist improves steatohepatitis and liver regeneration in mice. Hepatology. 2017;65:950–968. doi: 10.1002/hep.28962. [DOI] [PubMed] [Google Scholar]
- 25.Ou HY, Wu HT, Lu FH, Su YC, Hung HC, Wu JS, Yang YC, Wu CL, Chang CJ. Activation of free fatty acid receptor 1 improves hepatic steatosis through a p38-dependent pathway. J Mol Endocrinol. 2014;53:165–174. doi: 10.1530/JME-14-0003. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Meng X, Xu J, Huang X, Li H, Li G, Wang S, Man Y, Tang W, Li J. GPR40 agonist ameliorates liver X receptor-induced lipid accumulation in liver by activating AMPK pathway. Sci Rep. 2016;6:25237. doi: 10.1038/srep25237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sartorius T, Drescher A, Panse M, Lastovicka P, Peter A, Weigert C, Kostenis E, Ullrich S, Häring HU. Mice Lacking Free Fatty Acid Receptor 1 (GPR40/FFAR1) are Protected Against Conjugated Linoleic Acid-Induced Fatty Liver but Develop Inflammation and Insulin Resistance in the Brain. Cell Physiol Biochem. 2015;35:2272–2284. doi: 10.1159/000374031. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt J, Liebscher K, Merten N, Grundmann M, Mielenz M, Sauerwein H, Christiansen E, Due-Hansen ME, Ulven T, Ullrich S, Gomeza J, Drewke C, Kostenis E. Conjugated linoleic acids mediate insulin release through islet G protein-coupled receptor FFA1/GPR40. J Biol Chem. 2011;286:11890–11894. doi: 10.1074/jbc.C110.200477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, Choquet H, Besnard P, Lecoeur C, Vivequin S, Ayukawa K, Takeuchi M, Ozawa K, Tauber M, Maffeis C, Morandi A, Buzzetti R, Elliott P, Pouta A, Jarvelin MR, Körner A, Kiess W, Pigeyre M, Caiazzo R, Van Hul W, Van Gaal L, Horber F, Balkau B, Lévy-Marchal C, Rouskas K, Kouvatsi A, Hebebrand J, Hinney A, Scherag A, Pattou F, Meyre D, Koshimizu TA, Wolowczuk I, Tsujimoto G, Froguel P. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483:350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 30.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, van Esch BCAM, Henricks PAJ, Folkerts G, Garssen J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front Pharmacol. 2018;9:533. doi: 10.3389/fphar.2018.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao S, Jang C, Liu J, Uehara K, Gilbert M, Izzo L, Zeng X, Trefely S, Fernandez S, Carrer A, Miller KD, Schug ZT, Snyder NW, Gade TP, Titchenell PM, Rabinowitz JD, Wellen KE. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579:586–591. doi: 10.1038/s41586-020-2101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takai A, Kikuchi K, Ichimura M, Tsuneyama K, Moritoki Y, Matsumoto K, Tsunashima H, Onda T, Kuniyoshi N, Nariyama T, Ohyatsu S, Kubota J, Nagumo K, Sato S, Hara M, Miyakawa H. Fructo-oligosaccharides ameliorate steatohepatitis, visceral adiposity, and associated chronic inflammation via increased production of short-chain fatty acids in a mouse model of non-alcoholic steatohepatitis. BMC Gastroenterol. 2020;20:46. doi: 10.1186/s12876-020-01194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191–1212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111–128. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klindt C, Reich M, Hellwig B, Stindt J, Rahnenführer J, Hengstler JG, Köhrer K, Schoonjans K, Häussinger D, Keitel V. The G Protein-Coupled Bile Acid Receptor TGR5 (Gpbar1) Modulates Endothelin-1 Signaling in Liver. Cells. 2019;8 doi: 10.3390/cells8111467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willis KA, Gomes CK, Rao P, Micic D, Moran ER 3rd, Stephenson E, Puchowicz M, Al Abdallah Q, Mims TS, Gosain A, Yin D, Talati AJ, Chang EB, Han JC, Pierre JF. TGR5 signaling mitigates parenteral nutrition-associated liver disease. Am J Physiol Gastrointest Liver Physiol. 2020;318:G322–G335. doi: 10.1152/ajpgi.00216.2019. [DOI] [PubMed] [Google Scholar]
- 40.Meixiong J, Vasavda C, Snyder SH, Dong X. MRGPRX4 is a G protein-coupled receptor activated by bile acids that may contribute to cholestatic pruritus. Proc Natl Acad Sci U S A. 2019;116:10525–10530. doi: 10.1073/pnas.1903316116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larraufie P, Martin-Gallausiaux C, Lapaque N, Dore J, Gribble FM, Reimann F, Blottiere HM. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8:74. doi: 10.1038/s41598-017-18259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khound R, Taher J, Baker C, Adeli K, Su Q. GLP-1 Elicits an Intrinsic Gut-Liver Metabolic Signal to Ameliorate Diet-Induced VLDL Overproduction and Insulin Resistance. Arterioscler Thromb Vasc Biol. 2017;37:2252–2259. doi: 10.1161/ATVBAHA.117.310251. [DOI] [PubMed] [Google Scholar]
- 44.Pi M, Xu F, Ye R, Nishimoto SK, Williams RW, Lu L, Darryl Quarles L. Role of GPRC6A in Regulating Hepatic Energy Metabolism in Mice. Sci Rep. 2020;10:7216. doi: 10.1038/s41598-020-64384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu XJ, Xie L, Du K, Liu C, Zhang NP, Gu CJ, Wang Y, Abdelmalek MF, Dong WY, Liu XP, Niu C, Yang C, Diehl AM, Wu J. Succinate-GPR-91 receptor signalling is responsible for nonalcoholic steatohepatitis-associated fibrosis: Effects of DHA supplementation. Liver Int. 2020;40:830–843. doi: 10.1111/liv.14370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh DY, Walenta E, Akiyama TE, Lagakos WS, Lackey D, Pessentheiner AR, Sasik R, Hah N, Chi TJ, Cox JM, Powels MA, Di Salvo J, Sinz C, Watkins SM, Armando AM, Chung H, Evans RM, Quehenberger O, McNelis J, Bogner-Strauss JG, Olefsky JM. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med. 2014;20:942–947. doi: 10.1038/nm.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto Y, Sakamoto M, Fujii G, Tsuiji H, Kenetaka K, Asaka M, Hirohashi S. Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology. 2003;37:528–533. doi: 10.1053/jhep.2003.50029. [DOI] [PubMed] [Google Scholar]
- 48.Shao X, Liu Y, Huang H, Zhuang L, Luo T, Huang H, Ge X. Down-regulation of G protein-coupled receptor 137 by RNA interference inhibits cell growth of two hepatoma cell lines. Cell Biol Int. 2015;39:418–426. doi: 10.1002/cbin.10412. [DOI] [PubMed] [Google Scholar]
- 49.Liu F, Zhu C, Huang X, Cai J, Wang H, Wang X, He S, Liu C, Yang X, Zhang Y, Zhang T. A low level of GPR37 is associated with human hepatocellular carcinoma progression and poor patient survival. Pathol Res Pract. 2014;210:885–892. doi: 10.1016/j.prp.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Murakami N, Yokomizo T, Okuno T, Shimizu T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J Biol Chem. 2004;279:42484–42491. doi: 10.1074/jbc.M406561200. [DOI] [PubMed] [Google Scholar]
- 51.Johnson LE, Elias MS, Bolick DT, Skaflen MD, Green RM, Hedrick CC. The G protein-coupled receptor G2A: involvement in hepatic lipid metabolism and gallstone formation in mice. Hepatology. 2008;48:1138–1148. doi: 10.1002/hep.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreno-Navarrete JM, Catalán V, Whyte L, Díaz-Arteaga A, Vázquez-Martínez R, Rotellar F, Guzmán R, Gómez-Ambrosi J, Pulido MR, Russell WR, Imbernón M, Ross RA, Malagón MM, Dieguez C, Fernández-Real JM, Frühbeck G, Nogueiras R. The L-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes. 2012;61:281–291. doi: 10.2337/db11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipina C, Walsh SK, Mitchell SE, Speakman JR, Wainwright CL, Hundal HS. GPR55 deficiency is associated with increased adiposity and impaired insulin signaling in peripheral metabolic tissues. FASEB J. 2019;33:1299–1312. doi: 10.1096/fj.201800171R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biagioli M, Carino A, Fiorucci C, Marchianò S, Di Giorgio C, Bordoni M, Roselli R, Baldoni M, Distrutti E, Zampella A, Fiorucci S. The Bile Acid Receptor GPBAR1 Modulates CCL2/CCR2 Signaling at the Liver Sinusoidal/Macrophage Interface and Reverses Acetaminophen-Induced Liver Toxicity. J Immunol. 2020;204:2535–2551. doi: 10.4049/jimmunol.1901427. [DOI] [PubMed] [Google Scholar]
- 55.Rossi M, Zhu L, McMillin SM, Pydi SP, Jain S, Wang L, Cui Y, Lee RJ, Cohen AH, Kaneto H, Birnbaum MJ, Ma Y, Rotman Y, Liu J, Cyphert TJ, Finkel T, McGuinness OP, Wess J. Hepatic Gi signaling regulates whole-body glucose homeostasis. J Clin Invest. 2018;128:746–759. doi: 10.1172/JCI94505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang YM, Kuen DS, Chung Y, Kurose H, Kim SG. Gα12/13 signaling in metabolic diseases. Exp Mol Med. 2020;52:896–910. doi: 10.1038/s12276-020-0454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang YM, Lee CG, Koo JH, Kim TH, Lee JM, An J, Kim KM, Kim SG. Gα12 overexpressed in hepatocellular carcinoma reduces microRNA-122 expression via HNF4α inactivation, which causes c-Met induction. Oncotarget. 2015;6:19055–19069. doi: 10.18632/oncotarget.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Ma J, Nie H, Zhang XJ, Zhang P, She ZG, Li H, Ji YX, Cai J. Hepatic Regulator of G Protein Signaling 5 Ameliorates Nonalcoholic Fatty Liver Disease by Suppressing Transforming Growth Factor Beta-Activated Kinase 1-c-Jun-N-Terminal Kinase/p38 Signaling. Hepatology. 2020 doi: 10.1002/hep.31242. [DOI] [PubMed] [Google Scholar]
- 59.Kisseleva T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology. 2017;65:1039–1043. doi: 10.1002/hep.28948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bahrami AJ, Gunaje JJ, Hayes BJ, Riehle KJ, Kenerson HL, Yeung RS, Stempien-Otero AS, Campbell JS, Mahoney WM Jr. Regulator of G-protein signaling-5 is a marker of hepatic stellate cells and expression mediates response to liver injury. PLoS One. 2014;9:e108505. doi: 10.1371/journal.pone.0108505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart A, Maity B, Anderegg SP, Allamargot C, Yang J, Fisher RA. Regulator of G protein signaling 6 is a critical mediator of both reward-related behavioral and pathological responses to alcohol. Proc Natl Acad Sci U S A. 2015;112:E786–E795. doi: 10.1073/pnas.1418795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pashkov V, Huang J, Parameswara VK, Kedzierski W, Kurrasch DM, Tall GG, Esser V, Gerard RD, Uyeda K, Towle HC, Wilkie TM. Regulator of G protein signaling (RGS16) inhibits hepatic fatty acid oxidation in a carbohydrate response element-binding protein (ChREBP)-dependent manner. J Biol Chem. 2011;286:15116–15125. doi: 10.1074/jbc.M110.216234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gurevich VV, Gurevich EV. GPCR Signaling Regulation: The Role of GRKs and Arrestins. Front Pharmacol. 2019;10:125. doi: 10.3389/fphar.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cruces-Sande M, Vila-Bedmar R, Arcones AC, González-Rodríguez Á, Rada P, Gutiérrez-de-Juan V, Vargas-Castrillón J, Iruzubieta P, Sánchez-González C, Formentini L, Crespo J, García-Monzón C, Martínez-Chantar ML, Valverde ÁM, Mayor F Jr, Murga C. Involvement of G protein-coupled receptor kinase 2 (GRK2) in the development of non-alcoholic steatosis and steatohepatitis in mice and humans. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3655–3667. doi: 10.1016/j.bbadis.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 65.Ohtani N, Kawada N. Role of the Gut-Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship. Hepatol Commun. 2019;3:456–470. doi: 10.1002/hep4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quesada-Vázquez S, Aragonès G, Del Bas JM, Escoté X. Diet, Gut Microbiota and Non-Alcoholic Fatty Liver Disease: Three Parts of the Same Axis. Cells. 2020;9 doi: 10.3390/cells9010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68:359–370. doi: 10.1136/gutjnl-2018-316307. [DOI] [PubMed] [Google Scholar]
- 68.Rau M, Rehman A, Dittrich M, Groen AK, Hermanns HM, Seyfried F, Beyersdorf N, Dandekar T, Rosenstiel P, Geier A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United European Gastroenterol J. 2018;6:1496–1507. doi: 10.1177/2050640618804444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang G, Jiao T, Xu Y, Li D, Si Q, Hao J, Zhao J, Zhang H, Chen W. Bifidobacterium adolescentis and Lactobacillus rhamnosus alleviate non-alcoholic fatty liver disease induced by a high-fat, high-cholesterol diet through modulation of different gut microbiota-dependent pathways. Food Funct. 2020;11:6115–6127. doi: 10.1039/c9fo02905b. [DOI] [PubMed] [Google Scholar]
- 70.Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Li Volti G, Galvano F. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545–553. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 71.Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276–1285. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 73.Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, Zhao X, Li N, Li S, Xue G, Cheng W, Li B, Li H, Lin W, Tian C, Zhao J, Han J, An D, Zhang Q, Wei H, Zheng M, Ma X, Li W, Chen X, Zhang Z, Zeng H, Ying S, Wu J, Yang R, Liu D. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab 2019; 30: 675-688. :e7. doi: 10.1016/j.cmet.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 74.Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, Luketic VA, Siddiqui MS, Boyett S, Min HK, Kumar DP, Kohli R, Zhou H, Hylemon PB, Contos MJ, Idowu M, Sanyal AJ. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534–548. doi: 10.1002/hep.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janssen AWF, Houben T, Katiraei S, Dijk W, Boutens L, van der Bolt N, Wang Z, Brown JM, Hazen SL, Mandard S, Shiri-Sverdlov R, Kuipers F, Willems van Dijk K, Vervoort J, Stienstra R, Hooiveld GJEJ, Kersten S. Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: a potential role for bile acids. J Lipid Res. 2017;58:1399–1416. doi: 10.1194/jlr.M075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holmes ZC, Silverman JD, Dressman HK, Wei Z, Dallow EP, Armstrong SC, Seed PC, Rawls JF, David LA. Short-Chain Fatty Acid Production by Gut Microbiota from Children with Obesity Differs According to Prebiotic Choice and Bacterial Community Composition. mBio. 2020;11 doi: 10.1128/mBio.00914-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fiorucci S, Biagioli M, Zampella A, Distrutti E. Bile Acids Activated Receptors Regulate Innate Immunity. Front Immunol. 2018;9:1853. doi: 10.3389/fimmu.2018.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhan K, Gong X, Chen Y, Jiang M, Yang T, Zhao G. Short-Chain Fatty Acids Regulate the Immune Responses via G Protein-Coupled Receptor 41 in Bovine Rumen Epithelial Cells. Front Immunol. 2019;10:2042. doi: 10.3389/fimmu.2019.02042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, Patterson AD, Gonzalez FJ, Chiang JYL. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. 2018;68:1574–1588. doi: 10.1002/hep.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kleuser B. Divergent Role of Sphingosine 1-Phosphate in Liver Health and Disease. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19030722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rohrbach T, Maceyka M, Spiegel S. Sphingosine kinase and sphingosine-1-phosphate in liver pathobiology. Crit Rev Biochem Mol Biol. 2017;52:543–553. doi: 10.1080/10409238.2017.1337706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, Hait NC, Allegood JC, Price MM, Avni D, Takabe K, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23:107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Melhem H, Kaya B, Ayata CK, Hruz P, Niess JH. Metabolite-Sensing G Protein-Coupled Receptors Connect the Diet-Microbiota-Metabolites Axis to Inflammatory Bowel Disease. Cells. 2019;8 doi: 10.3390/cells8050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang C, Yang M. The Role and Potential Application of Antimicrobial Peptides in Autoimmune Diseases. Front Immunol. 2020;11:859. doi: 10.3389/fimmu.2020.00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bhattarai Y, Williams BB, Battaglioli EJ, Whitaker WR, Till L, Grover M, Linden DR, Akiba Y, Kandimalla KK, Zachos NC, Kaunitz JD, Sonnenburg JL, Fischbach MA, Farrugia G, Kashyap PC. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe 2018; 23: 775-785. :e5. doi: 10.1016/j.chom.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van de Wouw M, Schellekens H, Dinan TG, Cryan JF. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J Nutr. 2017;147:727–745. doi: 10.3945/jn.116.240481. [DOI] [PubMed] [Google Scholar]
- 89.Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, Carling D, Swann JR, Gibson G, Viardot A, Morrison D, Louise Thomas E, Bell JD. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Teng B, Huang C, Cheng CL, Udduttula A, Yu XF, Liu C, Li J, Yao ZY, Long J, Miao LF, Zou C, Chu J, Zhang JV, Ren PG. Newly identified peptide hormone inhibits intestinal fat absorption and improves NAFLD through its receptor GPRC6A. J Hepatol. 2020;73:383–393. doi: 10.1016/j.jhep.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 92.Oya M, Kitaguchi T, Pais R, Reimann F, Gribble F, Tsuboi T. The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J Biol Chem. 2013;288:4513–4521. doi: 10.1074/jbc.M112.402677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muneoka S, Goto M, Nishimura T, Enomoto K, Kadoshima-Yamaoka K, Tomimori Y. G Protein-Coupled Receptor 39 Agonist Improves Concanavalin A-Induced Hepatitis in Mice. Biol Pharm Bull. 2019;42:1415–1418. doi: 10.1248/bpb.b18-00982. [DOI] [PubMed] [Google Scholar]
- 94.Cuzon Carlson VC, Ford MM, Carlson TL, Lomniczi A, Grant KA, Ferguson B, Cervera-Juanes RP. Modulation of Gpr39, a G-protein coupled receptor associated with alcohol use in non-human primates, curbs ethanol intake in mice. Neuropsychopharmacology. 2019;44:1103–1113. doi: 10.1038/s41386-018-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu Z, Li Y, Syn WK, Li AJ, Ritter S, Wank SA, Lopes-Virella MF, Huang Y. GPR40-Deficiency Is Associated with Hepatic FAT/CD36 Upregulation, Steatosis, Inflammation and Cell Injury in C57BL/6 Mice. Am J Physiol Endocrinol Metab. 2020 doi: 10.1152/ajpendo.00257.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ookawara M, Matsuda K, Watanabe M, Moritoh Y. The GPR40 Full Agonist SCO-267 Improves Liver Parameters in a Mouse Model of Nonalcoholic Fatty Liver Disease without Affecting Glucose or Body Weight. J Pharmacol Exp Ther. 2020;375:21–27. doi: 10.1124/jpet.120.000046. [DOI] [PubMed] [Google Scholar]
- 97.Chen X, Liu C, Ruan L. G-Protein-Coupled Receptors 120 Agonist III Improves Hepatic Inflammation and ER Stress in Steatohepatitis. Dig Dis Sci. 2020 doi: 10.1007/s10620-020-06280-9. [DOI] [PubMed] [Google Scholar]
- 98.Park S, Kang S, Im D. TUG-891, an agonist of GPR120, inhibits lipid accumulation in hepatocytes. FASEB J . 2017;31:992–995. [Google Scholar]
- 99.On S, Kim HY, Kim HS, Park J, Kang KW. Involvement of G-Protein-Coupled Receptor 40 in the Inhibitory Effects of Docosahexaenoic Acid on SREBP1-Mediated Lipogenic Enzyme Expression in Primary Hepatocytes. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20112625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simard JC, Thibodeau JF, Leduc M, Tremblay M, Laverdure A, Sarra-Bournet F, Gagnon W, Ouboudinar J, Gervais L, Felton A, Letourneau S, Geerts L, Cloutier MP, Hince K, Corpuz R, Blais A, Quintela VM, Duceppe JS, Abbott SD, Blais A, Zacharie B, Laurin P, Laplante SR, Kennedy CRJ, Hébert RL, Leblond FA, Grouix B, Gagnon L. Fatty acid mimetic PBI-4547 restores metabolic homeostasis via GPR84 in mice with non-alcoholic fatty liver disease. Sci Rep. 2020;10:12778. doi: 10.1038/s41598-020-69675-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Puengel T, De Vos S, Hundertmark J, Kohlhepp M, Guldiken N, Pujuguet P, Auberval M, Marsais F, Shoji KF, Saniere L, Trautwein C, Luedde T, Strnad P, Brys R, Clément-Lacroix P, Tacke F. The Medium-Chain Fatty Acid Receptor GPR84 Mediates Myeloid Cell Infiltration Promoting Steatohepatitis and Fibrosis. J Clin Med. 2020;9 doi: 10.3390/jcm9041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liang Y, Liang S, Zhang Y, Deng Y, He Y, Chen Y, Liu C, Lin C, Yang Q. Oral Administration of Compound Probiotics Ameliorates HFD-Induced Gut Microbe Dysbiosis and Chronic Metabolic Inflammation via the G Protein-Coupled Receptor 43 in Non-alcoholic Fatty Liver Disease Rats. Probiotics Antimicrob Proteins. 2019;11:175–185. doi: 10.1007/s12602-017-9378-3. [DOI] [PubMed] [Google Scholar]
- 103.Bebelman MP, Crudden C, Pegtel DM, Smit MJ. The Convergence of Extracellular Vesicle and GPCR Biology. Trends Pharmacol Sci. 2020;41:627–640. doi: 10.1016/j.tips.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 104.Zeng Z, Mukherjee A, Varghese AP, Yang XL, Chen S, Zhang H. Roles of G protein-coupled receptors in inflammatory bowel disease. World J Gastroenterol. 2020;26:1242–1261. doi: 10.3748/wjg.v26.i12.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Włodarczyk M, Sobolewska-Włodarczyk A, Cygankiewicz AI, Jacenik D, Krajewska WM, Stec-Michalska K, Piechota-Polańczyk A, Wiśniewska-Jarosińska M, Fichna J. G protein-coupled receptor 55 (GPR55) expresses differently in patients with Crohn's disease and ulcerative colitis. Scand J Gastroenterol. 2017;52:711–715. doi: 10.1080/00365521.2017.1298834. [DOI] [PubMed] [Google Scholar]
- 106.Asico LD, Rozyyev S, Crusan AM, Jose PA, Villar VAM. Elucidating the Role of Lipid Rafts on G Protein-Coupled Receptor Function in the Mouse Kidney: An In Vivo Approach. Methods Mol Biol. 2021;2187:187–206. doi: 10.1007/978-1-0716-0814-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Keitel V, Häussinger D. Role of TGR5 (GPBAR1) in Liver Disease. Semin Liver Dis. 2018;38:333–339. doi: 10.1055/s-0038-1669940. [DOI] [PubMed] [Google Scholar]
- 108.Chang J, Mancuso MR, Maier C, Liang X, Yuki K, Yang L, Kwong JW, Wang J, Rao V, Vallon M, Kosinski C, Zhang JJ, Mah AT, Xu L, Li L, Gholamin S, Reyes TF, Li R, Kuhnert F, Han X, Yuan J, Chiou SH, Brettman AD, Daly L, Corney DC, Cheshier SH, Shortliffe LD, Wu X, Snyder M, Chan P, Giffard RG, Chang HY, Andreasson K, Kuo CJ. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat Med. 2017;23:450–460. doi: 10.1038/nm.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J, Gareri C, Rockman HA. G-Protein-Coupled Receptors in Heart Disease. Circ Res. 2018;123:716–735. doi: 10.1161/CIRCRESAHA.118.311403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dhyani V, Gare S, Gupta RK, Swain S, Venkatesh KV, Giri L. GPCR mediated control of calcium dynamics: A systems perspective. Cell Signal. 2020;74:109717. doi: 10.1016/j.cellsig.2020.109717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davenport AP, Scully CCG, de Graaf C, Brown AJH, Maguire JJ. Advances in therapeutic peptides targeting G protein-coupled receptors. Nat Rev Drug Discov. 2020;19:389–413. doi: 10.1038/s41573-020-0062-z. [DOI] [PubMed] [Google Scholar]
- 112.Sriram K, Insel PA. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol Pharmacol. 2018;93:251–258. doi: 10.1124/mol.117.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Terauchi Y, Yamada Y, Watada H, Nakatsuka Y, Shiosakai K, Washio T, Taguchi T. Efficacy and safety of the G protein-coupled receptor 119 agonist DS-8500a in Japanese type 2 diabetes mellitus patients with inadequate glycemic control on sitagliptin: A phase 2 randomized placebo-controlled study. J Diabetes Investig. 2018;9:1333–1341. doi: 10.1111/jdi.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Watada H, Shiramoto M, Irie S, Terauchi Y, Yamada Y, Shiosakai K, Myobatake Y, Taguchi T. G protein-coupled receptor 119 agonist DS-8500a effects on pancreatic β-cells in Japanese type 2 diabetes mellitus patients. J Diabetes Investig. 2019;10:84–93. doi: 10.1111/jdi.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burant CF, Viswanathan P, Marcinak J, Cao C, Vakilynejad M, Xie B, Leifke E. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:1403–1411. doi: 10.1016/S0140-6736(11)61879-5. [DOI] [PubMed] [Google Scholar]
- 116.Cusimano A, Foderà D, Lampiasi N, Azzolina A, Notarbartolo M, Giannitrapani L, D'Alessandro N, Montalto G, Cervello M. Prostaglandin E2 receptors and COX enzymes in human hepatocellular carcinoma: role in the regulation of cell growth. Ann N Y Acad Sci. 2009;1155:300–308. doi: 10.1111/j.1749-6632.2009.03701.x. [DOI] [PubMed] [Google Scholar]
- 117.Wu FQ, Fang T, Yu LX, Lv GS, Lv HW, Liang D, Li T, Wang CZ, Tan YX, Ding J, Chen Y, Tang L, Guo LN, Tang SH, Yang W, Wang HY. ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1α. J Hepatol. 2016;65:314–324. doi: 10.1016/j.jhep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 118.Menon V, Lincoff AM, Nicholls SJ, Jasper S, Wolski K, McGuire DK, Mehta CR, Rosenstock J, Lopez C, Marcinak J, Cao C, Nissen SE GRAND 306 Investigators. Fasiglifam-Induced Liver Injury in Patients With Type 2 Diabetes: Results of a Randomized Controlled Cardiovascular Outcomes Safety Trial. Diabetes Care. 2018;41:2603–2609. doi: 10.2337/dc18-0755. [DOI] [PubMed] [Google Scholar]
- 119.Bosier B, Hermans E. Versatility of GPCR recognition by drugs: from biological implications to therapeutic relevance. Trends Pharmacol Sci. 2007;28:438–446. doi: 10.1016/j.tips.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 120.Li Y, Sun Y, Song Y, Dai D, Zhao Z, Zhang Q, Zhong W, Hu LA, Ma Y, Li X, Wang R. Fragment-Based Computational Method for Designing GPCR Ligands. J Chem Inf Model. 2020;60:4339–4349. doi: 10.1021/acs.jcim.9b00699. [DOI] [PubMed] [Google Scholar]
- 121.Audet M, Villers K, Velasquez J, Chu M, Hanson C, Stevens RC. Small-scale approach for precrystallization screening in GPCR X-ray crystallography. Nat Protoc. 2020;15:144–160. doi: 10.1038/s41596-019-0259-y. [DOI] [PubMed] [Google Scholar]
- 122.Doijen J, Van Loy T, Landuyt B, Luyten W, Schols D, Schoofs L. Advantages and shortcomings of cell-based electrical impedance measurements as a GPCR drug discovery tool. Biosens Bioelectron. 2019;137:33–44. doi: 10.1016/j.bios.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 123.Chen KM, Keri D, Barth P. Computational design of G Protein-Coupled Receptor allosteric signal transductions. Nat Chem Biol. 2020;16:77–86. doi: 10.1038/s41589-019-0407-2. [DOI] [PubMed] [Google Scholar]