Abstract
BACKGROUND
There is an acute need to raise awareness of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH) among primary care physicians, endocrinologists and diabetologists to improve patient identification and address the current difficulties in NASH clinical trial enrollment. We examined the extent of knowledge and practice regarding NASH diagnosis and management guidelines. A randomized online convenience survey of 12869 physicians drawn from a national physician database of primary care physicians (PCPs), and gastroenterology and endocrinology specialists were queried via online survey. Our results, based on a cohort of 185 respondents, showed gaps in knowledge and practice between these three groups of practitioners, with primary care providers having the lowest adherence to published guidelines for diagnosis of NASH. Without clear knowledge and patient identification at the point of presentation - which is often in primary care or with specialties other than hepatology–many patients with NAFLD and NASH will remain undiagnosed and untreated, and clinical studies will continue to struggle with patient recruitment, hindering clinical development and optimal patient care.
AIM
To determine knowledge base concerning NASH diagnosis amongst gastroenterologists, endocrinologists and primary care physicians to improve referrals into clinical trials.
METHODS
A randomized online convenience survey of 12869 physicians drawn from a national physician database of PCPs, and gastroenterology and endocrinology specialists was conducted yielding a sample of 185 respondents.
RESULTS
The survey revealed that many physicians are either unaware of testing options other than biopsy, or do not use them in practice. Only 46% of endocrinologists and 42% of primary care physicians indicated they would refer a patient for specialist workup if they suspected NASH. Risk (25%) and inconvenience to patients (18%) are given as reasons for not referring those with suspected NASH for biopsy. For standard diagnostic algorithms such as Fibrosis-4 score, 18% of PCPs, 30% of endocrinologists and 65% gastroenterologists reported using these tests in clinical practice.
CONCLUSION
Substantial gaps in knowledge of the differences between NAFLD and NASH exist between these physician groups, with knowledge being particularly low among primary care doctors and endocrinologists. The use of a simple non-invasive screening algorithm may help to identify the right patients for clinical trials, which in turn will be vital to the development of effective and well-tolerated treatments for this increasingly ubiquitous condition.
Keywords: Non-alcoholic steatohepatitis, Non-alcoholic fatty liver disease, Enrollment, Screening, Diagnostics, Guidelines
Core Tip: Primary care physician knowledge of non-alcoholic steatohepatitis (NASH) diagnostics guidelines is key for appropriate patient management. We conducted a national online survey of physicians regarding their awareness of NASH guidelines. Endocrinologists and primary care physicians were significantly less likely than gastroenterologists to understand the differences between NASH and non-alcoholic fatty liver disease, as well as undertake diagnostic testing and necessary referrals for NASH. Only 18% of primary care physicians and 30% of endocrinologists were familiar with common indices such as the Fibrosis-4 score. Better education of primary care physicians about NASH could also serve as one way to identify candidates for important NASH clinical trials.
INTRODUCTION
Prevalence and challenges
Non-alcoholic fatty liver disease (NAFLD), defined as the presence of ≥ 5% steatosis in the absence of secondary causes of fat accumulation in the liver, is the most prevalent chronic liver disease worldwide, and is thought to affect about 25% of the adult population globally[1,2]. There is some variation regionally, from 13% in Africa to more than 30% in South America and the Middle East[3]. An increasing prevalence is being seen in the developed world; NAFLD is closely associated with metabolic syndrome with the conditions being found concurrently in a substantial proportion of patients. Indeed, both the NAFLD phenotype as well as its progression to more serious disease may be viewed as an outgrowth of metabolic alterations in the context of a genetic predisposition associated with higher energy intake[4]. Up to two-thirds of patients with type 2 diabetes, and more than 90% of patients undergoing bariatric (weight loss) surgery to treat obesity present with NAFLD. Similarly, approximately a third of patients with hypertension and half of patients with dyslipidemia show evidence of the condition[5]. In the United States, there also has been an increase in the prevalence of NAFLD in children, with estimated rates up to 17%. The condition is more common in boys and a higher prevalence is seen in Hispanic children compared with white, Asian or African-American children[6].
The natural history of NAFLD is such that the majority of patients will eventually succumb to closed volume-related mortality. However, it is estimated that up to 20% of patients with NAFLD will, during the clinical course of their disease, progress to non-alcoholic steatohepatitis (NASH), which is associated with liver inflammation and hepatocyte injury[7]. NASH also is associated with significant liver-related outcomes including fibrosis, cirrhosis, hepatocellular carcinoma, liver failure and liver death in 15%-25% of patients[8-11]. The prevalence of NASH is difficult to determine, as an unambiguous diagnosis requires a liver biopsy. In 2016 Younossi et al[12] reported rates of NASH among patients with NAFLD ranging from almost 7% for those without an indication for biopsy to 59% in biopsied patients[12]. Similarly, the rates of further progression of NASH are unclear, but it is thought that 10%-20% of patients will develop higher-grade fibrosis and < 5% will progress to cirrhosis[10]. NAFLD is also the most rapidly increasing indication for liver transplant[11]. The substantial prevalence of NAFLD, with an estimated 65 million patients in the United States. And 52 million in Europe (Germany, France, Italy and United Kingdom), is associated with a significant economic burden from direct medical costs estimated at $103 billion and $37 billion, respectively. The burden is significantly higher when indirect and societal costs are included[12].
In spite of its ubiquity, knowledge of NAFLD and NASH is suboptimal in clinical practice. Patients frequently present late in the NAFLD spectrum, as the condition is often silent and asymptomatic. Thus, NASH diagnosis and referral remain low. Although many potential treatment options are in clinical development, it follows that recruitment for clinical trials is extremely challenging. In April of this year, 35 clinical trials of products to treat NASH at Phase II or III were listed as recruiting globally, and requiring at least 13000 patients. However, enrollment rates are typically less than one patient per clinical research site per month, with less than 25% of recent trials achieving > 0.5 patients per site per month. Clearly, this dearth of patient enrollment will severely hamper the development and approval of new treatment options.
Knowledge of diagnostics guidelines
Several guidelines for the diagnosis and management of NAFLD and NASH have been published, including by EASL[13] and National Institute for Health and Care Excellence[14]. These guidelines have been reviewed and compared elsewhere[15]. Updated guidelines were published in 2018[1] and a clinical guidelines synopsis followed some months later[2]. However, anecdotal evidence suggests knowledge of the guidelines is poor outside of specialist physicians, and that guidelines are not being followed to the same extent that is seen in other chronic disease settings such as diabetes. The impact of this is far-reaching. Without clear knowledge and patient identification at the point of presentation – which is often in primary care or with specialties other than hepatology – many patients with NAFLD and NASH will remain undiagnosed and untreated, and clinical studies will continue to struggle with patient recruitment, hindering clinical development and optimal patient care.
MATERIALS AND METHODS
To investigate this further, a recent survey carried out by Accelerated Enrollment Solutions (AES) examined the extent of knowledge and practice regarding NASH diagnosis and management guidelines. A randomized online convenience survey of 12869 physicians drawn from a national physician database of primary care physicians (PCPs), and gastroenterology and endocrinology specialists was undertaken, yielding a cohort of 185 (response rate of 1.13%) primary care physicians and medical specialists across a number of disciplines in the United States (Table 1). Respondent physicians in the survey came from 34 states and were generally representative of the population as a whole. When asked how many years the respondents were in practice, 0.5% were in practice 0-5 years, 13.5% for 6-10 years, 38.4% for 11-20 years, 28.1% for 21-30 years and 19.5% for greater than 30 years.
Table 1.
Number and proportion of participants by specialty
|
Specialty
|
n
(%)
|
| Gastroenterology | 64 (35) |
| Endocrinology | 60 (32) |
| Primary care | |
| Family practice | 39 (21) |
| Internal medicine | 6 (3) |
| General practice | 2 (1) |
| Other | 14 (8) |
The survey aimed to shed light on medical specialists’ and primary care physicians’ knowledge and practice regarding NASH diagnosis and management guidelines, and also to identify whether any recommendations could be made to improve adherence to guidelines in clinical practice. To determine “best practice” baseline for comparison purposes, and to identify practices of greatest importance for clinicians, we utilized practice guidelines developed in 2018 by the American Association for the Study of Liver Diseases. Results are presented here for the three largest groups – gastroenterologists, endocrinologists and primary care physicians. Statistical work was done in Statistical Analysis Software, with significance determined by chi-squared tests.
RESULTS
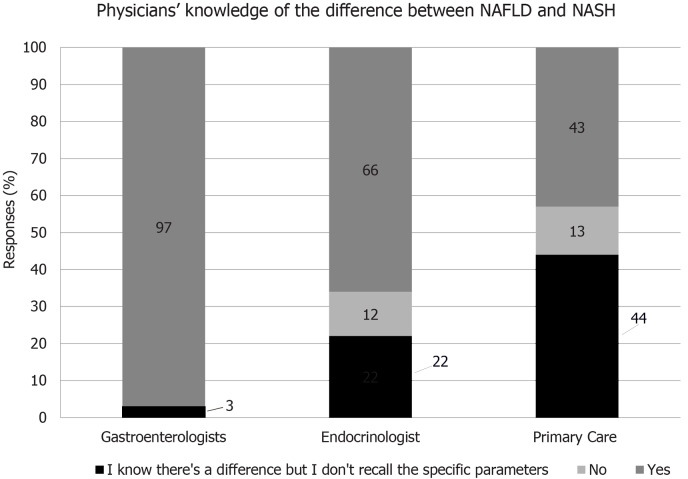
Appreciation of disease pathology and progression is important in the identification of at-risk and existing patients. However, the survey revealed substantial gaps in knowledge of the differences between NAFLD and NASH in these physician groups, with knowledge being particularly low with primary care doctors and endocrinologists (Figure 1).
Figure 1.
Knowledge of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis among physicians. NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis.
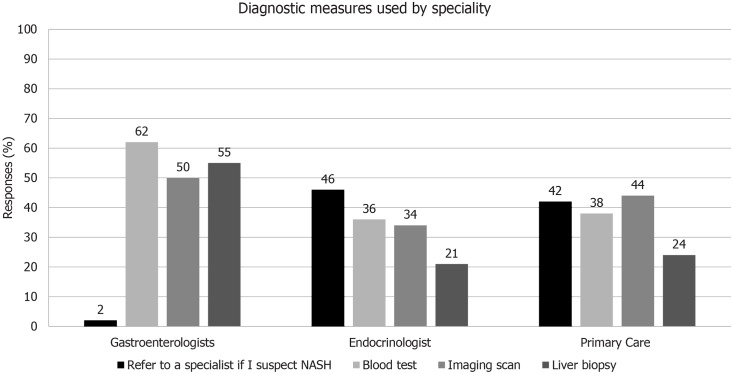
Gastroenterologists were generally well informed, and therefore, it was not surprising that physicians in this group were most likely to undertake diagnostic tests firsthand (blood tests, imaging or liver biopsy) and least likely to refer the patient to another specialist (Figure 2). However, the likelihood of referral was relatively low for other groups, with only 46% of endocrinologists and 42% of primary care physicians indicating they would refer a patient for specialist workup if they suspected NASH. The lack of referral is worrying, considering the low levels of confidence in differentiating NAFLD and NASH, as well as suboptimal disease awareness among these specialties, particularly given the risk that many patients may remain undiagnosed.
Figure 2.
Diagnosis measures for non-alcoholic fatty liver disease/non-alcoholic steatohepatitis by physician specialty. NASH: Non-alcoholic steatohepatitis.
Although liver biopsy is the gold standard for diagnosis of NAFLD and NASH, its invasive nature means it is rarely used outside specialist care. Risk (25%) and inconvenience to patients (18%) are given as reasons for not referring those with suspected NASH for biopsy. However, most frequently, physicians in all disciplines fail to recommend biopsy because they believe the outcome will not affect any subsequent treatment plan (34%). With the current lack of treatment availability, this is true, but with many products in the development pipeline, unambiguous identification of NASH patients is fundamental for the clinical trials that ultimately will lead to approval of new, effective and well-tolerated treatments.
Guidelines recommend that patients who are at increased risk of having steatohepatitis and/or advanced fibrosis should routinely be referred for further investigation by biopsy. Many of these patients will be those with concurrent metabolic syndrome–i.e., those presenting at primary care or in endocrinology clinics. The low referral rate for biopsy suggests either a deeper lack of willingness to recommend this procedure on the part of the physician, or a suboptimal knowledge of the guidelines for diagnosis and management of NAFLD and NASH.
The survey also revealed that many physicians are either unaware of testing options other than biopsy, or do not use them in practice. NAFLD is generally recognized through abnormal liver chemistries–most commonly patients have a mildly elevated aspartate transaminase (AST) and/or alanine transaminase (ALT), with an AST:ALT ratio < 1, which in later stages may reverse. Thus, AST:ALT > 1[16,17], although a normal or near normal ALT level, does not preclude NASH.
DISCUSSION
How to identify the NASH patient?
The fibrosis-4 (FIB-4) index is a biomarker test that uses outcomes from standard and easily available blood serum tests to generate a score that is correlated with the degree of liver damage in people with a variety of liver diseases. A score can be derived from age, AST and ALT, and platelet counts, and can be used as an indicator of NASH. However, only 36% of PCPs had knowledge of either this or the NAFLD Fibrosis Score (NFS), a non-invasive scoring system that takes into consideration age, hyperglycemia, body mass index, platelet count, albumin and AST/ALT ratio, as diagnostic determinants of NAFLD. In endocrinologists and gastroenterologists, these tests were familiar to 58% and 82%, respectively. However, only 18% of PCPs, 30% of endocrinologists and 65% gastroenterologists reported using these tests in clinical practice. There were significant differences between physician groups (P < 0.0001) in both of these cases. Given that many physicians do not opt for liver biopsy, these non-invasive tests could be crucial to more widespread patient identification. Both of these tests are recommended in the 2018 guidelines[1] as clinically useful tools and decision aids that should be used to differentiate patients at higher risk of advanced fibrosis or cirrhosis. Thus, the lack of awareness in this area is of real concern.
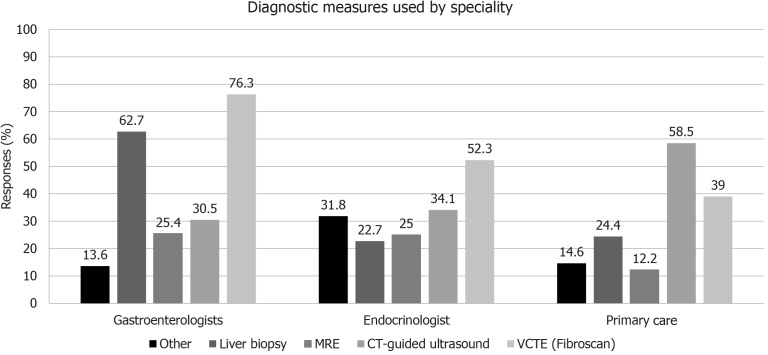
Imaging studies are also an important part of the workup for NAFLD, and while imaging is used by 98% of gastroenterologists, only 83% and 82% of endocrinologists and PCPs, respectively, use the technique. Again, a significant difference (P < 0.0004) was seen between physician groups. Outside of liver biopsy in the gastroenterology cohort, vibration-controlled transient elastography [VCTE (FibroScan®)] and computed tomography-guided ultrasound were the techniques most commonly employed (Figure 3).
Figure 3.
Diagnostic techniques used in non-alcoholic steatohepatitis physicians of different specialties. MRE: Magnetic resonance elastography; VCTE: Vibration-controlled transient elastography; CT: Computed tomography.
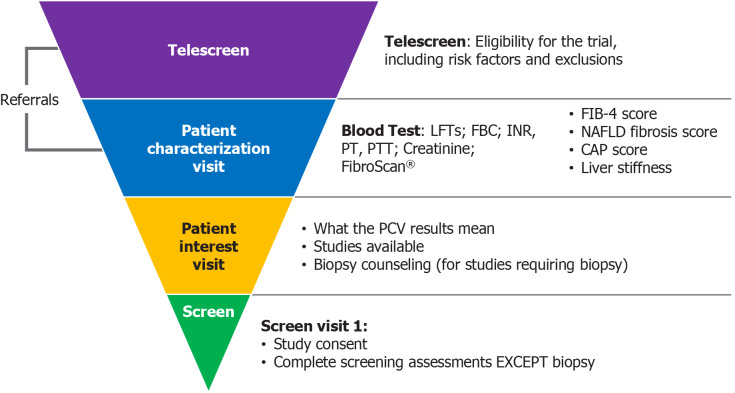
While the survey sample was small, some clear trends emerged. Although the prevalence of NAFLD and NASH is high in the general population, there are no widely accepted screening processes, even in high-risk patients[7]. As well as exacerbating under-diagnosis and under-treatment, the absence of a standardized screening system contributes to the inadequacy of the numbers of patients available for clinical trials. Currently, trials in NAFLD and NASH tend to recruit and enroll patients who already have, or are very likely to have, a diagnosis. Therefore, to optimize enrollment, an improved process for patient identification would be of great value. This need not involve invasive procedures; rather, the focus should be on identifying those individuals who are most likely to meet clinical trial eligibility criteria. This key subset of patients then can be referred for biopsy to obtain a definitive histological diagnosis. As up to 25% of patients with NAFLD are expected to show evidence of NASH on biopsy, such a screening algorithm is perhaps the most efficient and cost-effective way to identify appropriate patients. It is estimated this process would allow the screening of up to 120000 patients annually, which would greatly aid drug developers and researchers in populating clinical studies in the coming years (Figure 4).
Figure 4.
The non-alcoholic steatohepatitis patient recruitment screening pathway. LFT: Liver function test; FBC: Full blood count; INR: International normalized ratio; PT: Prothrobmin time; PTT: Partial thromboplastin time; CAP: Controlled attenuation parameter; PCV: Porcine circovirus; NAFLD: Non-alcoholic fatty liver disease; FIB: Fibrosis.
CONCLUSION
Guidelines exist for the diagnosis of NAFLD and NASH. However, disease awareness is low, and therefore, patients are not coming through the referral pathway into the clinical studies required to push forward the development of new treatment options. This will be essential given the rise in the prevalence of NASH and the lack of approved treatment options. The clear association between NAFLD/NASH and metabolic disorders is well known, and reflected in guidance statements. Although routine screening is not recommended, the guidelines indicate physicians should have a high index of suspicion when dealing with patients presenting with these conditions. Furthermore, physicians are advised to use clinical decision aids such as NFS, FIB-4 or VCTE to identify patients who are at risk, and who would benefit from a further referral or more conclusive diagnostic testing[1]. The results from this survey suggest these recommendations are not being implemented in clinical practice, with many physicians having a poor understanding of the stages of disease and the available diagnostic techniques.
The majority of patients with NASH will present at primary care, or specialties other than hepatology. For example, endocrinologists or diabetologists are likely to see a substantial number of high-risk patients. Although it is important to raise awareness across all specialties, there is an acute need to raise awareness and improve the knowledge of NAFLD/NASH among primary care physicians, endocrinologists and diabetologists to improve patient identification and address the current difficulties in NASH clinical trial enrollment. The use of a simple non-invasive screening algorithm may help to identify the right patients for clinical trials, which in turn will be vital to the development of effective and well-tolerated treatments for this increasingly ubiquitous condition.
ARTICLE HIGHLIGHTS
Research background
Medical specialist and primary care physician knowledge of non-alcoholic steatohepatitis (NASH) treatments, especially those contained in international guidelines, is important to standardize for the benefit of patient care.
Research motivation
We sought to document to what degree knowledge of NASH diagnostics, as recommended in United States guidelines, varied among United States specialists and primary care providers.
Research objectives
We sought to document to what degree knowledge of NASH diagnostics, as recommended in United States guidelines, varied among United States specialists and primary care providers.
Research methods
We utilized a randomized, online national convenience survey sample of gastroenterologists, endocrinologists, and primary care physicians to inquire about their knowledge and practice regarding NASH.
Research results
While gastroenterologists were relatively well informed, endocrinologists and primary care physicians were less likely to understand the differences between NASH and non-alcoholic fatty liver disease (NAFLD), as well as undertake diagnostic testing and necessary referrals for NASH. Only 18% of primary care physicians and 30% of gastroenterologists were familiar with common indices such as the Fibrosis-4 score by which suspect NASH patients might be identified. Only 46% of endocrinologists and 42% of primary care physicians would refer a patient with a NASH profile for a NASH work-up by a specialist. Risk (25%) and inconvenience to patients (18%) were given as reasons for not referring those with suspected NASH for biopsy.
Research conclusions
Suboptimal knowledge of NASH and NAFLD by primary care physicians and by endocrinologists, both groups to which many NASH patients would be likely to present, may impair the definitive diagnosis of NASH and actions to minimize its effects. Reversing this knowledge gap can help in identification of additional and appropriate patients for enrollment into important NASH clinical trials.
Research perspectives
It is important to raise awareness of NASH among physicians of all kinds. Improved patient identification can not only improve care for the individual patient, but is also necessary to assure sufficient participation of confirmed NASH patients into randomized, placebo-controlled clinical trials for new treatment modalities.
ACKNOWLEDGEMENTS
The authors would like to thank Lesley Jacques of Watermeadow Medical, an Ashfield Company, part of UDG Healthcare plc for editorial assistance.
Footnotes
Conflict-of-interest statement: Dr. Wessels is an employee of AES, a clinical trials site management organization conducting clinical studies on NASH, which provided funding for this work. No other conflicts are reported.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Manuscript source: Unsolicited manuscript
Peer-review started: November 23, 2020
First decision: December 7, 2020
Article in press: December 28, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Jin SY, Kamiya A, Smolic M S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
Contributor Information
David Hermanus Wessels, Medical Office, A.E.S., Chorley PR7 1NY, Lancashire, United Kingdom. dawie.wessels@globalaes.com.
Zeil Rosenberg, Chief Medical Office, Accelerated Enrollment Solutions, Horsham, PA 19044, United States.
References
- 1.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 2.Paul S, Davis AM. Diagnosis and Management of Nonalcoholic Fatty Liver Disease. JAMA. 2018;320:2474–2475. doi: 10.1001/jama.2018.17365. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Abenavoli L, Milic N, Di Renzo L, Preveden T, Medić-Stojanoska M, De Lorenzo A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J Gastroenterol. 2016;22:7006–7016. doi: 10.3748/wjg.v22.i31.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: Current and emerging. J Hepatol. 2018;68:362–375. doi: 10.1016/j.jhep.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 7.Spengler EK, Loomba R. Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Mayo Clin Proc. 2015;90:1233–1246. doi: 10.1016/j.mayocp.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65:1017–1025. doi: 10.1016/j.metabol.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin Liver Dis. 2016;20:205–214. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Bataller R, Rombouts K, Altamirano J, Marra F. Fibrosis in alcoholic and nonalcoholic steatohepatitis. Best Pract Res Clin Gastroenterol. 2011;25:231–244. doi: 10.1016/j.bpg.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 13.European Association for the Study of the Liver (EASL) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence (NICE) Published July 2016. Available from: https://www.nice.org.uk/guidance/ng49/resources .
- 15.Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J Gastroenterol. 2018;24:3361–3373. doi: 10.3748/wjg.v24.i30.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowman JK, Tomlinson JW, Newsome PN. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011;33:525–540. doi: 10.1111/j.1365-2036.2010.04556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342:1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]