Abstract
Musculoskeletal pain (excluding bone cancer pain) affects more than 30% of the global population and imposes an enormous burden on patients, families, and caregivers related to functional limitation, emotional distress, effects on mood, loss of independence, and reduced quality of life. The pathogenic mechanisms of musculoskeletal pain relate to the differential sensory innervation of bones, joints, and muscles as opposed to skin and involve a number of peripheral and central nervous system cells and mediators. The interplay of neurons and non-neural cells (e.g. glial, mesenchymal, and immune cells) amplifies and sensitizes pain signals in a manner that leads to cortical remodeling. Moreover, sex, age, mood, and social factors, together with beliefs, thoughts, and pain behaviors influence the way in which musculoskeletal pain manifests and is understood and assessed. The aim of this narrative review is to summarize the different pathogenic mechanisms underlying musculoskeletal pain and how these mechanisms interact to promote the transition from acute to chronic pain.
Keywords: arthralgia, musculoskeletal pain, myalgia, neuroglia, physiology
Introduction
Chronic musculoskeletal pain is defined as a pain perceived in musculoskeletal tissues that lasts or recurs for more than 3 months, and is characterized by significant functional disability and emotional distress.1 Pain is categorized as primary chronic pain if it cannot be directly attributed to a known disease or damage process, or as secondary if it is caused by a disease or process that directly affects the bones, joints, muscles, and/or related soft tissues.2 The latter category describes a group of heterogeneous pain conditions caused by infection, crystal deposition, and auto-inflammatory processes that lead to persistent local or systemic inflammation and/or structural changes. Of note, central nervous system diseases associated with severe spasticity can also cause musculoskeletal pain. Despite their biological and physiological differences, these conditions share a common thread of persistent pain and repercussions that are felt in everyday life such as activity limitation and emotional distress.3 It follows that chronic musculoskeletal pain has a major social and emotional impact that can include decreased socialization, inability to work, loss of independence, anxiety, depression, and concern for the future.4
Chronic musculoskeletal pain is highly prevalent in the general population, affecting approximately 37% of the United States population, with an economic burden of $635 billion per year.5 Similar figures have been described for chronic musculoskeletal pain in the European Union.6 The overall prevalence of chronic musculoskeletal pain in the elderly ranges from 18.6% (Switzerland) to 45.6% (France).7 In a multicenter study conducted in Italy, 45% of the 1606 patients referred to pain clinics for the management of chronic non-oncological pain had chronic musculoskeletal pain.8
The aim of this narrative review is to focus on the different pathogenic mechanisms of musculoskeletal pain and how these mechanisms interact to promote the transition from acute to chronic pain.
Bone and muscle sensory innervation
Studies have outlined several differences between the sensory innervation of skin and bone.9,10 Human skin is innervated by a wide variety of sensory nerve fibers, including type II or Aß fibers (large myelinated sensory nerve fibers with conduction velocities >30 m/s), type III or Aδ fibers (thin myelinated sensory nerve fibers with conduction velocities between 2 and 30 m/s), and type IV or C fibers (unmyelinated nerve fibers with conduction velocity of <2 m/s). Almost 30% of sensory fibers in the skin are ‘peptide rich’ fibers that express tropomyosin receptor kinase A (TrkA) and release calcitonin gene-related peptide (CGRP), otherwise called TrkA+ fibers. This population is complemented by ‘peptide poor’ nerve fibers, which usually do not express TrkA (TrkA− fibers).
In contrast, adult bones are mainly innervated by Aδ fibers and TrkA+ C fibers (>80%), with little if any innervation by Aß fibers or TrkA− C fibers.9,10 The lack of innervation by Aß sensory nerve fibers is due to the fact that bone and joint structures are deep enough that fine touch, brushing, and light pressure are not relevant information, while the paucity of TrkA− C fibers is not completely understood. Most sensory nerve fibers in bone and joints are silent nociceptors that are only activated by high intensity, potentially dangerous stimuli.9 These primary sensory neurons are pseudo-unipolar neurons whose cell bodies are localized in the dorsal root ganglia (DRG) and project to the spinal cord, mainly in laminae I, II, and V. Bone is also innervated by adrenergic and cholinergic sympathetic nerve fibers, which serve several functions, including bone remodeling, vascular regulation, immune cell infiltration, and bone progenitor cell function.11
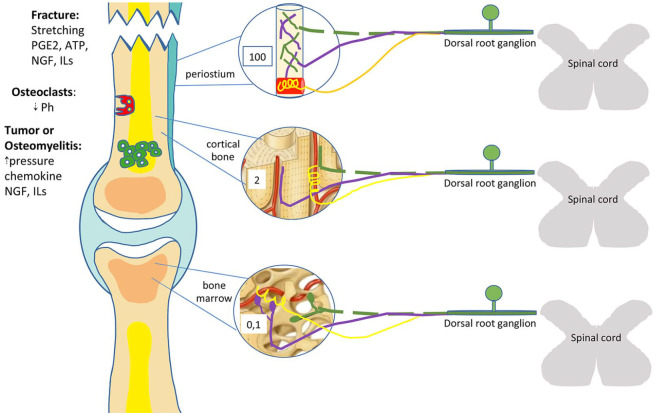
The innervation of bone and joints also varies in morphology, density, and the arrangement of nerve fibers. The periosteum has the densest sensory innervation of any bone compartment; Aδ and C-sensory nerve fibers are arranged like a fishnet for the purpose of detecting mechanical injury or the distortion of underlying cortical bone. In contrast, innervating sympathetic nerve fibers, usually associated with blood vessels, reproduce the morphology of a corkscrew.10 In cortical bone, sensory and sympathetic nerve fibers are predominantly confined to the vascularized Haversian canals. The relative densities of Aδ and C-sensory nerve fibers in the periosteum, cortical bone, and marrow are 100:2:0, respectively.12 In cortical bone, sensory nerves fibers are linear, while in bone marrow they branch out with varicose endings. Sympathetic fibers spiral around the vessels in bone marrow similarly to those in cortical bone10,13 (Figure 1).
Figure 1.
Organization of bone innervation of the periosteum, cortical bone, and marrow.
On the left side, main stimuli and mediators of bone pain are shown. Green dotted lines represent Aδ fibers, purple lines C-fibers, and yellow lines sympathetic fibers. Numbers shown refer to the relative density of nervous fibers in the periosteum, cortical bone, and marrow, respectively. See text for details.
ATP, adenosine triphosphate; ILs, interleukins; NGF, nerve growth factor; PGE2, prostaglandin E2.
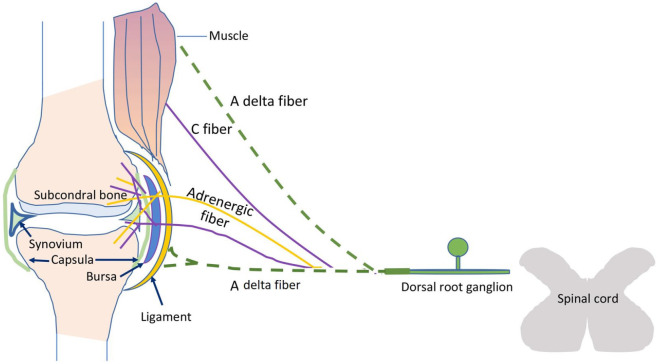
The ligaments, capsules, and menisci are also innervated by a rich network of Aδ and C-fibers. Polymodal C-fibers represent the most important type of joint receptor in all structures including the synovium, while the cartilage is aneural and avascular. This innervation pattern helps to reduce the occurrence of inflammation and prevents the sensation of pain during daily joint loading. Aδ fibers are present on the ligament surface where they act as high-threshold mechanoreceptors, responding to high intensity mechanical stimuli.14 Other innervated joint structures can also be a source of chronic pain: the infrapatellar fat pad, together with synovial membrane, is involved in the pathogenesis of osteoarthritis of the knee and associated pain.15 Finally, joint pain can also arise from adjacent or peri-articular structures including the bursae and tendons, or can be caused by extra-articular disorders such as polymyalgia rheumatica and fibromyalgia (Figure 2).
Figure 2.
Joint and muscle innervation.
Aδ fibers (green dotted lines) innervate ligaments while intraarticular structures are mostly innervated by C-sensory (violet lines) and sympathetic fibers (yellow lines). Cartilage (in light blue) is aneural and avascular under physiologic conditions.
A variety of nociceptors are present in skeletal muscle, including mechanic, mechano-heat, and polymodal nociceptors. Most of these nociceptors have a high stimulation threshold and are therefore not stimulated physiologic movement or muscle stretch. Some nociceptors respond when tonic muscle contractions become ischemic, exceeding the maximum muscle force (i.e. ischemic contractions). This subtype of muscle nociceptor is possibly involved in the pathogenesis of some tension-type headaches with increased electromyographic activity.16
In summary, bone and muscle are innervated by Aδ- and TrkA+-sensitive C-fibers, but also adrenergic and cholinergic sympathetic nerve fibers, arranged in several tridimensional patterns and in different densities among structures. All these fibers can contribute to pain transmission.
Neurobiology of bone/muscle pain
Normally, Aδ and C-sensory fibers in bone are silent and are only activated by noxious stimuli such as mechanical distortion, local acidosis, or an increase in intramedullary pressure (Figure 1). In the case of bone fracture, Aδ mechano-transducers that densely innervate the periosteum are activated, causing a sharp, stabbing pain. This initial pain usually subsides quickly and is replaced by a lower-intensity, dull, aching pain transmitted by the slower C-fibers of the periosteum, bone, and marrow.17
Local acidosis, caused by the increased release of protons from osteoclasts during bone resorption, can also induce bone pain. This is the case in bone cancer, some skeletal diseases, and synovial inflammation. Aδ and C-sensory nociceptive fibers in the bone and joints express acid-sensing ion channel (ASIC) 1, ASIC3, and transient receptor potential channel-vanilloid subfamily member 1 (TRPV1), another acid-sensing ion channel. These ion channels are activated when the local extracellular pH falls to nearly 4.17–19 Also in joint tissues, Aδ mechano-gated receptors responding to high-intensity stretch stimuli have been implicated together with ASICs in pain transmission.20
Intramedullary pressure and associated bone pain can be caused by neoplasms, intraosseous engorgement syndrome, or osteomyelitis. The discharge frequency of innervating Aδ and C-fibers increases proportionately with increasing intramedullary pressure.17
Lastly, a newly discovered mechanically gated ion channel, Piezo2, is expressed in 70% of the Aδ nociceptors that innervate bone marrow. Given the structure of this channel with between 25 and 30 trans-membrane repeats, it is thought that Piezo2 contributes to the mechanical sensitivity of Aδ mechano-nociceptors.10
Both purinergic (e.g. P2X3-R) and TRPV1 receptors have been identified as pain transducers in skeletal muscle. Adenosine triphosphate (ATP) released during trauma or other pathologic processes binds purinergic receptors to excite nociceptive fibers. For this reason, ATP is an important molecular signal of general tissue trauma.
Persistent activation of TRPV1 receptors can lead to their upregulation and, as a consequence, long-lasting hyperalgesia.16 In an acid-induced model of muscular pain, ASIC3 channels have also been shown to contribute to the development of mechanical hypersensitivity in this manner.21
Inflammatory pain and Aδ/C-fiber sensitization
Inflammation can cause allodynia (i.e. pain in response to innocuous stimuli) and/or hyperalgesia (i.e. an increased pain response to noxious stimuli) through the sensitization of sensory afferent fibers. During inflammation or tissue injury, damaged cells and immune cells release a variety of substances known as inflammatory mediators, such as bradykinin, nerve growth factor (NGF), prostaglandin E2 (PGE2), pro-inflammatory cytokines [e.g. interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α)] and chemokines (e.g. chemokine ligand 2)(22–24) (Figure 3). These inflammatory mediators act both directly on peripheral nociceptors, eliciting sensitization, and indirectly by promoting inflammation and the release of prostaglandins. Sensitization occurs via the activation of intracellular signaling pathways [e.g. mitogen-activated protein kinase (MAPK), protein kinase A (PKA), and protein kinase C (PKC)]. Key enzymes in these pathways enhance signaling efficiency in the nociceptor terminal through the phosphorylation of transducer receptors and voltage-gated ion channels. These changes occur rapidly to facilitate dynamic responses to injury and, as an end-result, increase or amplify nociceptive input to the central nervous system.23,24 Following acute inflammation, a significant percentage of unmyelinated C fibers, otherwise insensitive to mechanical stimulation in the normal joint, develop responsiveness to mechanical stimulation and exhibit increased activity. Accordingly, these ‘silent’ nociceptors contribute significantly to bone and joint pain under pathological conditions.20
Figure 3.

Schematic representation of the contribution of mast cells and microglia to degenerative joint diseases and neuro-inflammation.
At the articular level, mast cells are located mainly in the synovial membrane and joint capsule, and elsewhere, mostly along blood vessels and nerve endings of the joint. Peripheral and central mast cells are likely play a crucial role in the shift of acute to chronic pain by interacting with other immune cells and somatosensory nerve terminals. In the periphery, persistent mast-cell activation promotes the recruitment of other immune cells such as lymphocytes at the lesion site, amplifying inflammatory processes and causing a sensitization of peripheral nociceptors and spinal somatosensory neurons. In the CNS, mast cells amplify neuro-inflammatory processes by promoting glial-cell activation, which coordinates inflammation at the spinal level and central sensitization of central somatosensory neurons (courtesy of Wiley).21
CNS, central nervous system.
Mediators involved in pain sensitization
NGF is one of the best studied mediators of bone pain in both preclinical and clinical studies. NGF released in injured bone binds to TrkA on TrkA+ fibers, resulting in the downstream phosphorylation and sensitization of several receptors co-expressed on these nerve fibers, including TRPV1, ASIC3, prostaglandin receptors (EP2), bradykinin (B2) receptors, and mechano-transducers.23 As a result, nociceptors become excited by prostaglandins and bradykinin released by injured tissues or by small amounts of acid released by osteoclasts.25 The nociceptor threshold for action potential firing decreases while its firing rate increases.
In addition to eliciting the release of inflammatory mediators, articular microtrauma caused by injury or joint overuse that occurs with aging26 can also cause the release of damage-associated molecular pattern proteins (DAMPs). DAMPs are produced by stressed or dying cells and can include fibronectin and intracellular alarmins, as well as plasmatic exudate proteins like α2-macroglobulin. Sensory neurons express pattern recognition receptors, which interact with DAMPs to trigger an innate immune response and inflammation through the release of chemokines and inflammatory cytokines.26 The presence of inflammatory cells such as macrophages, T lymphocytes, and mast cells in the synovium contributes to joint pain and relates to pain severity. It was recently demonstrated that mast cells can both release and respond to NGF.26 Moreover, IL-18 secreted by neutrophils promotes mechanical hyperalgesia in a murine model of muscular pain.27
Finally, the binding of NGF to TrkA receptors results in the internalization of the NGF-receptor complex and drives the expression of various neurotransmitters, including substance P (SP), CGRP, and brain-derived neurotrophic factor (BDNF), as well as that of sodium and calcium channels. The cumulative effect is to increase nociceptor excitability.13 Recent evidence highlights the role of sodium ion channel subtype 1.8 and hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in painful neuropathy. HCNs are activated by guanosine 3’,5’-cyclic monophosphate (cGMP) and adenosine 3’,5’-cyclic monophosphate (cAMP) and act as weakly selective potassium channels.18
Ectopic nerve sprouting and the role of sympathetic/parasympathetic fibers
Bone pain can also be caused by the pathological sprouting of sensory and sympathetic nerve fibers surrounding sites of injury. Inflammatory and stromal cells release neurotrophic factors such as NGF and vascular endothelial growth factor to induce nerve sprouting and subsequent hyper-innervation of the periosteum, bone, and marrow.28 CGRP+ and SP+ sensory fibers located in the deep layers of the periosteum can emerge and penetrate the cartilage callus and newly formed bone tissue.11 Nerve sprouting is observed during normal bone healing, probably to discourage use of the injured bone until it completely heals. After healing has occurred, the newly sprouted nerve fibers are ‘pruned’ back so that the innervation of bone returns to its normal state.20 However, when normal bone healing does not occur, such as in the case of bone metastasis or osteoarthritis, the injured bone remains hyper-innervated and a state of abnormal pain sensitivity is preserved.13 Ectopic nerve sprouting has also been demonstrated in normally aneural and avascular areas of the human intervertebral disc.29 Similarly, sympathetic and sensory-nerve-fiber sprouting has been reported in the murine knee joint,30 and pharmacological suppression of sympathetic fiber activation significantly attenuates osteoarthritis-like pain-related behaviors.31 In osteoarthritis, sympathetic nerves can invade calcified cartilage, alter communication between cartilage and bone, and even sprout in the synovial membrane and upper dermis. Parasympathetic fibers can modulate local inflammation through acetylcholine binding of specific nicotinic receptors (i.e. alpha 7), resulting in a strong anti-inflammatory effect.32 Furthermore, sympathetic nerve fibers may play a role in osteoarthritis and complex regional pain syndrome by modulating the function of sensory nerve fibers.9,33 Catecholaminergic and cholinergic mediators produced in the bone or synovium can also directly affect cartilage due to the expression of their receptors on chondrocytes.
Lastly, nerve sprouting has also been demonstrated in muscles. When long-lasting pathological alterations occur in muscle tissue, the density of neuropeptide-containing nerve endings increases. In the rat gastrocnemius–soleus muscle, chronic inflammation is associated with a twofold increase in the number of SP+ fibers.16
Central spinal plasticity and cortical remodeling
While primary hyperalgesia coincides with the site of damage and is sustained by peripheral sensitization, secondary hypersensitivity that develops distant from the injury site is explained by central spinal plasticity.34 C fiber activation drives transcriptional changes through increased glutamatergic and neuropeptide release in spinal neurons; neurotransmitter binding increases intracellular calcium, induces kinase activation (e.g. PKA, PKC, calmodulin protein kinase II , extracellular regulated kinase), and ultimately causes rapid post-translational as well as long-term translational and transcriptional changes. The cumulative effect of these changes is to increase local excitatory activity and alter local and descending inhibitory control. A persistent peripheral nociceptive stimulus can cause long-term potentiation (LTP) at the spinal level, that is, a long-lasting increase in synaptic transmission. LTP is thought to be a critical mechanism underlying central hyperexcitability. Recent evidence suggests that greater homosynaptic and heterosynaptic LTP develop in a context of muscle pain than in cutaneous pain.35 Consistent with these observations, afferent sensory fibers express increased numbers of alfa-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors after repetitive stimulation.36
Several mechanisms have been proposed to explain central neuronal hyperexcitability. An increase in the release of SP, CGRP, and inflammatory cytokines in the spinal dorsal horn occurs 4 weeks after a bone fracture.37 Increased spinal FOS expression (a marker of injury-induced spinal hyperexcitability) and altered inhibition from the rostral ventromedial medulla (RVM) have been demonstrated in a model of knee osteoarthritis.38 Moreover, increased expression of the endogenous opioid peptide dynorphin (a molecule implicated in chronic pain) promotes the activation of spinal bradykinin receptors.39 Modifications in neural morphology, function, and distribution have been demonstrated in subcortical and cortical regions such as the thalamus, primary somatosensory cortex, and primary motor cortex in patients with chronic musculoskeletal pain.40 Tonic muscle pain recruits a number of brain areas including the cingulate cortex, amygdala, insula, nucleus accumbens, medial prefrontal cortex, and dorsolateral prefrontal cortex.41 These mesolimbic–prefrontal structures participate in the cognitive affective aspects of pain including behavioral reactions, fear, aversive conditioning, and carefulness. Moreover, these regions modulate descending pain inhibition [i.e. via the periaqueductal grey (PAG)-RVM pathway], which, as described previously, has an important role in abnormal pain processing.40
Crosstalk between the immune and nervous systems
Persistent injurious stimuli can sustain prolonged inflammation in the nervous system at both the peripheral and central levels.22 Combined and coordinated immune and neuronal responses occur to establish a crosstalk wherein the two systems regulate one other. This process can involve non-neuronal cells such as glial, epithelial cells, mast cells, and mesenchymal cells. Once activated, these cells can discharge pro-inflammatory mediators like PGE2, TNF-α, IL-1β, granulocyte-macrophage colony-stimulating factor, and NGF in proximity to nociceptors and, in turn, drive antidromic action potentials and induce neurogenic inflammation by eliciting the release of other factors such as CGRP and SP. CGRP and SP promote vascular permeability and the extravasation of immune cells.23 Therefore, neurons together with glia, mesenchymal cells, and immune cells constitute a coordinated network that responds to injurious stimuli. In a pathological scenario, this network elicits an immune response that amplifies inflammation and tissue damage, produces allodynia and hyperalgesia, and modifies pain processing in a way that may favor its chronification.42 Immune–neuronal crosstalk is bidirectional and must be kept in mind when considering potential therapeutic targets for alleviating pain.22,43 Indeed, new therapies that target neurotrophin release and immune-cell activation or migration are currently under study.43
Glial contribution and endocannabinoids
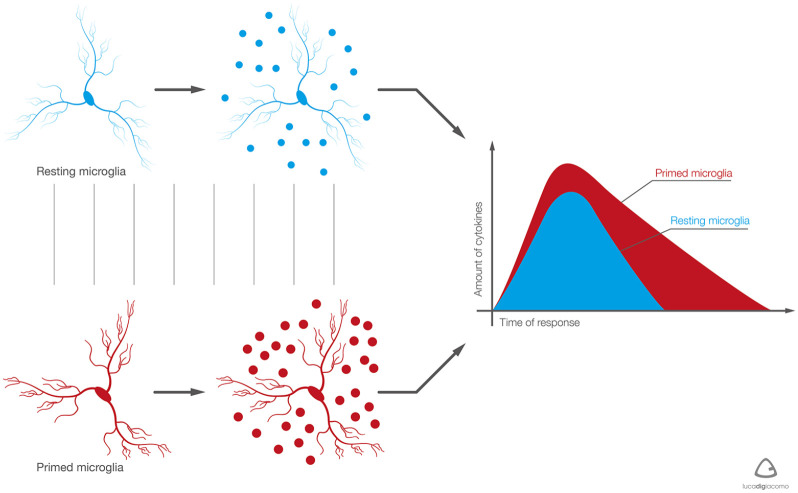
Glial cells account for almost 50% of the nervous system and consist of astrocytes, microglia, and oligodendrocytes. Astrocytes are the most abundant glial cells and have a complex and heterogeneous morphology.44 Microglia have been defined the scavengers of the nervous system as they behave much like macrophages, protecting neurons from infected or damaged cells. Astrocytes and microglia are quiescent under normal physiological conditions but can be activated by tissue damage and during pain. This reactive state is characterized by an increase in both the number and morphology of cells. Morphological changes in so-called ‘primed microglia’ include hypertrophy of the soma, the retraction of processes, and changes in surface receptor expression45 (Figure 4). In discogenic pain, neuro-inflammatory markers such as NGF, interferon gamma, and IL-8 stimulate microglial activation, proliferation, and chemotaxis.45,46
Figure 4.
Resting and primed microglia: the main differences.
With aging and/or pain stimulus, the phenotype of microglia becomes predominantly primed. It responds in a more intense manner, producing a greater amount of pro-inflammatory mediators and for a longer time. Primed microglia induce persistent neuro-inflammatory response, capable of damaging tissue integrity and neuron function. (Courtesy of the Paolo Procacci Foundation.)
Glial stimulation enhances the secretion of IL-1, IL-6, and TNFα; this upregulation of pro-inflammatory mediators in the spinal cord has been linked to mechanical allodynia and spontaneous pain in models of bone cancer pain.47 After activation, spinal glia exhibit an increased expression of P2X-Rs. The stimulation of P2X4Rs leads to calcium influx, p38-MAPK activation, and consequent synaptic release of BDNF.48 The binding of BDNF to TrkB receptors in the dorsal horn further increases the concentration of intracellular chloride and reduces gamma-aminobutyric-acid-mediated inhibition in a mechanism thought to underlie allodynia and hyperalgesia.49
A growing body of evidence underscores the importance of cannabinoid receptors (CB1 and CB2) expressed both on glia and neurons at peripheral, spinal, and supraspinal sites.50 These receptors bind several endogenous lipid ligands (so-called endocannabinoids, such as arachidonyl ethanolamide and 2-arachidonyl-glycerol) and modulate pain, mood, appetite, and emesis. While CB1 is expressed on nociceptors, both CB1 and CB2 are present in the DRG. At the spinal level, CB1 is present in the dorsolateral funiculus, around the central canal, and in the superficial dorsal horn, while CB2 is distributed on lumbar glial cells and activated microglia. In the brain, CB1 is detected in the thalamus, amygdala, parabrachial nucleus, PAG, and RVM, structures that participate together in pain transmission, modulation, and perception. The mechanism underlying the antinociceptive action of cannabinoids includes the inhibition of presynaptic neurotransmitter release, reduction of neuronal excitability at the postsynaptic level, and activation of descending inhibitory pathways.50 The roles of different cannabinoid receptors are still under study: in a preclinical study of muscle pain, both CB1 and CB2 were involved in nociceptive modulation.51
Sex and musculoskeletal pain
In recent years, clinical research has identified some sex-related clinical manifestations of painful musculoskeletal conditions.34,52 Studies suggest that compared with men, women experience higher pain intensity, greater pain-related interference with function, and more disability. Women also report worse depression, anxiety, and self-efficacy.53 Several biological and biochemical sex differences have been characterized in a context of musculoskeletal pain. Female muscle has a higher density of mechanically sensitive Aδ and C afferents that increase their activity in response to mechanical distortion or metabolites such as ATP, lactate, and protons.52 At the level of the DRG, female tissues display the specific upregulation of TRPV1, while male tissues appear to distinctly upregulate ASIC3.52
Women also appear to respond to tissue damage with higher cytokine production than men and in a manner that corresponds with a stronger inflammatory response and higher level of pain.34 Moreover, it has been suggested that morphine is more effective in men than in women, and that the incidence of opioid-related adverse events is higher in women than in men. At the glial level, morphine can stimulate the production of pro-inflammatory cytokines by binding to TLR4, causing a reduction in the analgesic effect of morphine. This action is more pronounced in women than in men.34
The exclusive use of male animals in preclinical experiments has generated an important sex bias on our understanding of pain neurobiology,49 since it is now recognized that glial cells behave differently in male versus female tissues. Whereas pain-associated glial proliferation in the DRG is quite similar between the sexes, females show a relatively weaker upregulation of P2X4Rs and the activation of a microglia-independent pathway. Moreover, microglial BDNF does not appear to be involved in the maintenance of neuropathic pain in female mice. Instead, preclinical data suggest that T lymphocytes play an important role in pain hypersensitivity in females.49 Other evidence suggests that the ability of testosterone to elicit microglial-mediated sensitization is independent of sex.48 The exact nature of sex differences in pain modulation is still not fully understood.
Interestingly, neuronal activation of N-methyl D-aspartate (NMDA) in a pain context is consistent between the sexes,48 and similarly, no sex differences have been observed in the expression of IL-10 or macrophages in muscle pain.54
Mood, demographic and social factors
Depressive mood, tendency for somatization, and pessimistic beliefs about health are important factors influencing the occurrence and persistence of primary musculoskeletal pain, as well as related disability.55 There is a complex interplay between emotional status, cognition, and pain. Chronic pain can negatively affect emotions, attention, and memory. On the other hand, emotions and cognition can either increase or decrease the perception of pain.56 Negative treatment expectation dampens the analgesic effects of drugs,57 whereas the expectation of pain relief is an important component of placebo analgesia.58
An association between common musculoskeletal painful conditions and psychosocial aspects of work activity (e.g. perceived job dissatisfaction, job strain, and boredom with actual work) has been reported in observational studies.59
Education and socioeconomic status may contribute to the experience and chronification of pain, especially in musculoskeletal diseases.60 For example, individuals with low levels of education and those living in areas of poverty are at a higher risk of developing pain and disability related to knee osteoarthritis.61
Psychosocial factors also influence gene expression in chronic musculoskeletal pain: for example, higher levels of BDNF expression are related to higher biopsychosocial complexity and can be linked to an increased risk of central plasticity.62 Other research suggests that different micro-ribonucleic acid (micro-RNA) fragments have higher expression in neuropathic versus nociceptive musculoskeletal pain.63
In summary, the assessment of and therapeutic development for chronic musculoskeletal pain must be global, focusing not only on molecular targets but also on demographic, psychological, and contributing social factors.55
Discussion
In recent years, there has been a remarkable interest in the pathogenesis of chronic musculoskeletal pain. Research indicates that musculoskeletal pain can be driven by the stimulation of Aδ and C-fibers through mechanical distortion, local acidosis around nociceptors, and increased bone medullary pressure. These events in turn promote the release of inflammatory mediators, immune and nervous system interactions, and glial stimulation at both peripheral and central sites. Moreover, musculoskeletal pain is modulated through other mechanisms such as changes in transcriptional activity, the ectopic sprouting of sensory nerve fibers, and crosstalk between sympathetic and sensory nerve fibers. A full knowledge of the mechanisms involved in the chronification of musculoskeletal pain can help pain physicians offer the most appropriate treatment. For example, NGF has a pivotal role in musculoskeletal pain and NGF blockade might prevent or attenuate peripheral sensitization and nerve sprouting to reduce bone pain. Unfortunately, the clinical utility of anti-NGF antibodies (e.g. tanezumab) is limited by an unfavorable side-effect profile.9 Other interesting potential therapies include NMDA receptor and P2X-R antagonists, anti-IL-6 receptor antibodies (e.g. tocilizumab), and anti-TNF antibodies (e.g. adalimumab).19 Neurolytic approaches such as radiofrequency ablation have also been proposed to reduce articular pain, showing initial efficacy in osteoarthritis. The long-term effects of these strategies are still unclear. Recently, epigenetic strategies (e.g. the use of micro-RNA fragments) have been proposed as an adjuvant approach to improve diagnostic accuracy and for therapeutic purposes.63
Finally, sex-specific pain mechanisms as well as mood and socioeconomic factors require better clarification in a context of musculoskeletal pain in order to allow physicians to provide a more targeted and tailored management approach.
Limitations
Even if significant steps have been made in understanding some mechanisms that drive musculoskeletal pain, we are only beginning to elucidate how nerve, bone, muscle, and joint interact with one other. The pathophysiological mechanisms described in the present review provide only a partial picture and are likely interlinked. Several additional molecular mechanisms potentially involved in pain generation have been described in the literature but have not yet been validated and are therefore not reported in this review. In reality, the generation and persistence of pain is a dynamic process that is far from being fully understood.
Conclusion
The high socioeconomic impact of musculoskeletal pain warrants an appropriate therapeutic strategy based on a thorough knowledge of its pathogenic mechanisms. In the same manner, musculoskeletal pain must be understood through the multidisciplinary assessment of all pertinent aspects, including psychological, demographic, cognitive, emotional, and socioeconomical factors. All these factors must be considered in future efforts identifying potential therapeutic targets and strategies for improving quality of life among patients who suffer from chronic musculoskeletal pain.
Acknowledgments
The authors are grateful to Dr Ashley Symons for her revision of the English language and proofreading. Her professional support was kindly sponsored by the Paolo Procacci Foundation.
Footnotes
Author contributions: All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. F Puntillo carried out most of the research and drafted the initial manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this research has been possible thanks to an unconditional grant provided by the ‘Paolo Procacci Foundation-ONLUS,’ Via Tacito 7, 00193 Roma, Italy.
Conflict of interest statement: Giustino Varrassi is a member of the journal’s editorial board. The other authors do not have potential conflict of interest to declare.
Compliance with ethics guidelines: This article is based on previously conducted studies and does not contain any studies with human participants or animal performed by the authors without a previous Ethics Committee approval.
Open access: This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any non-commercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original authors and the source, provide a link to the Creative Commons license, and indicate if changes were made.
ORCID iDs: Ivan Urits  https://orcid.org/0000-0002-3652-6085
https://orcid.org/0000-0002-3652-6085
Giustino Varrassi  https://orcid.org/0000-0002-3822-2923
https://orcid.org/0000-0002-3822-2923
Contributor Information
Filomena Puntillo, Department of Interdisciplinary Medicine, ‘Aldo Moro’ University of Bari, Piazza G. Cesare 11, Bari 70124, Italy; Anesthesia, Intensive Care and Pain Unit, Policlinico Hospital, Bari, Italy.
Mariateresa Giglio, Anesthesia, Intensive Care and Pain Unit, Policlinico Hospital, Bari, Italy.
Antonella Paladini, Department of MESVA, University of L’Aquila, L’Aquila, Italy.
Gaetano Perchiazzi, Department of Surgical Science, Hedenstierna Laboratory, Uppsala University, Uppsala, Sweden.
Omar Viswanath, Department of Anesthesiology, Creighton University School of Medicine, Omaha, NE, USA.
Ivan Urits, Department of Anesthesia, Beth Israel Deaconess Med Center, Harvard Medical School, Boston, MA, USA.
Carlo Sabbà, Department of Interdisciplinary Medicine, ‘Aldo Moro’ University of Bari, Bari, Italy.
Giustino Varrassi, Paolo Procacci Foundation, Roma, Italy.
Nicola Brienza, Department of Interdisciplinary Medicine, ‘Aldo Moro’ University of Bari, Bari, Italy.
References
- 1. Treede R, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain 2019; 160: 19–27. [DOI] [PubMed] [Google Scholar]
- 2. Perrot S, Cohen M, Barke A, et al. The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain 2019; 160: 77–82. [DOI] [PubMed] [Google Scholar]
- 3. Blyth FM, Briggs AM, Schneider CH, et al. The global burden of musculoskeletal pain—where to from here? Am J Public Health 2019; 109: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wallis J, Taylor N, Bunzli S, et al. Experience of living with knee osteoarthritis: a systematic review of qualitative studies. BMJ Open 2019; 9: e030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grace PM, Hutchinson M, Maier S, et al. Pathological pain and the neuroimmune interface. Nat Rev Immunol 2014; 14: 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langley P, Müller-Schwefe G, Nicolaou A, et al. The impact of pain on labor force participation, absenteeism and presenteeism in the European Union. J Med Econ 2010; 13: 662–672. [DOI] [PubMed] [Google Scholar]
- 7. Camilloni A, Nati G, Maggiolini P, et al. Chronic non-cancer pain in primary care: an Italian cross-sectional study. Signa Vitae 2020; 1: 9. [Google Scholar]
- 8. Latina R, De Marinis MG, Giordano F, et al. Epidemiology of chronic pain in the Latium region, Italy: a cross-sectional study on the clinical characteristics of patients attending pain clinics. Pain Manag Nurs 2019; 20: 373–381. [DOI] [PubMed] [Google Scholar]
- 9. Mantyh PW. The neurobiology of skeletal pain. Eur J Neurosci 2014; 39: 508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ivanusic JJ. Molecular mechanisms that contribute to bone marrow pain. Front Neurol 2017; 11: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brazill J, Beeve AT, Craft CS, et al. Nerves in bone: evolving concepts in pain and anabolism. J Bone Miner Res 2019; 34: 1393–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castañeda-Corral G, Jimenez-Andrade JM, Bloom AP, et al. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience 2011; 31: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mantyh PW. Mechanisms that drive bone pain across the lifespan. Br J Clin Pharmacol 2019; 85: 1103–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belluzzi E, Stocco E, Pozzuoli A, et al. Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. Biomed Res Int 2019; 2019: 6390182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perrot S. Osteoarthritis pain. Best Pract Res Clin Rheumatol 2015; 29: 90–97 [DOI] [PubMed] [Google Scholar]
- 16. Mense S. The pathogenesis of muscle pain. Curr Pain Headache Rep 2003; 7: 419–425. [DOI] [PubMed] [Google Scholar]
- 17. Oostinga D, Steverink JG, Van Wijck AJM, et al. An understanding of bone pain: a narrative review. Bone 2020; 134: 115272 [DOI] [PubMed] [Google Scholar]
- 18. Morgan M, Nencini S, Thai J, et al. Activation alters the function of Aδ and C fiber sensory neurons that innervate bone. Bone 2019; 123: 168–175. [DOI] [PubMed] [Google Scholar]
- 19. Peters CM, Munoz-Islas E, Ramirez-Rosas MB, et al. Mechanisms underlying non-malignant skeletal pain. Curr Opin Physiol 2019; 11: 103–108. [Google Scholar]
- 20. Staunton CA, Lewis R, Barrett-Jolley R. Ion channels and osteoarthritic pain: potential for novel analgesics. Curr Pain Headache Rep 2013; 17: 378. [DOI] [PubMed] [Google Scholar]
- 21. Karczewski J, Spencer RH, Garsky VM, et al. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol 2010; 161: 950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fusco M, Skaper SD, Coaccioli S, et al. Degenerative joint diseases and neuroinflammation. Pain Pract 2017; 17: 522–532. [DOI] [PubMed] [Google Scholar]
- 23. Torsney C. Inflammatory pain neural plasticity. Curr Opin Physiol 2019; 11: 51–57. [Google Scholar]
- 24. Scanzello CR. Chemokines and inflammation in osteoarthritis: insights from patients and animal models. J Orthop Res 2017; 35: 735–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nencini S, Ringuet M, Kim D-H, et al. Mechanisms of nerve growth factor signaling in bone nociceptors and in an animal model of inflammatory bone pain. Mol Pain 2017; 13: 1744806917697011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Syx D, Tran PB, Miller RE, et al. Peripheral mechanisms contributing to osteoarthritis pain. Curr Rheumatol Rep 2018; 20: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshida S, Hagiwara Y, Tsuchiya M, et al. Involvement of neutrophils and interleukin-18 in nociception in a mouse model of muscle pain. Mol Pain 2018; 14: 1744806918757286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chartier SR, Thompson ML, Longo G, et al. Exuberant sprouting of sensory and sympathetic nerve fibers in nonhealed bone fractures and the generation and maintenance of chronic skeletal pain. Pain 2014; 155: 2323–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Binch AL, Cole AA, Breakwell LM, et al. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res Ther 2014; 16: 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jimenez-Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther 2012; 14: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Courties A, Sellam J, Berenbaum F. Role of the autonomic nervous system in osteoarthritis. Best Pract Res Clin Rheumatol 2017; 31: 661–675. [DOI] [PubMed] [Google Scholar]
- 32. Longo G, Osikowicz M, Ribeiro-da-Silva A. Sympathetic fiber sprouting in inflamed joints and adjacent skin contributes to pain-related behavior in arthritis. J Neurosci 2013; 33: 10066–10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pepper A, Li W, Kingery WS, et al. Changes resembling complex regional pain syndrome following surgery and immobilization. J Pain 2013; 14: 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hansson P. Translational aspects of central sensitization induced by primary afferent activity: what it is and what it is not. Pain 2014; 155: 1932–1934. [DOI] [PubMed] [Google Scholar]
- 35. Zhang J, Hoheisel U, Klein T, et al. High-frequency modulation of rat spinal field potentials: effects of slowly conducting muscle vs. skin afferents. J Neurophysiol 2016; 115: 692–700. [DOI] [PubMed] [Google Scholar]
- 36. Li J, Baccei ML. Functional organization of cutaneous and muscle afferent synapses onto immature spinal lamina I projection neurons. J Neurosci 2017; 37: 1505–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi X, Guoa TZ, Weia T, et al. Facilitated spinal neuropeptide signaling and upregulated inflammatory mediator expression contribute to post fracture nociceptive sensitization. Pain 2015; 156: 1852–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Havelin J, Imbert I, Cormier J, et al. Central sensitization and neuropathic features of ongoing pain in a rat model of advanced osteoarthritis. J Pain 2016; 17: 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luo MC, Chen Q, Ossipov MH, et al. Spinal dynorphin and bradykinin receptors maintain inflammatory hyperalgesia. J Pain 2008; 9: 1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pelletier R, Higgins J, Bourbonnais D. Is neuroplasticity in the central nervous system the missing link to our understanding of chronic musculoskeletal disorders? BMC Musculoskelet Disord 2015; 16: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Niddam DM, Hsieh JC. Neuroimaging of muscle pain in humans. J Chin Med Assoc 2009; 72: 285–292. [DOI] [PubMed] [Google Scholar]
- 42. Varrassi G, Alon E, Bagnasco M, et al. Toward an effective and safe treatment of inflammatory pain: a Delphi-guided expert consensus. Adv Ther 2019; 36: 2618–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Conaghan PG, Cook AD, Hamilton JA, et al. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat Rev Rheumatol 2019; 15: 355–363. [DOI] [PubMed] [Google Scholar]
- 44. Zhang D, Hu X, Qian L, et al. Astrogliosis in CNS pathologies: is there a role for microglia? Mol Neurobiol 2010; 41: 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Varrassi G, Fusco M, Skaper S, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther 2018; 7: 59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Navone SE, Peroglio M, Guarnaccia L, et al. Mechanical loading of intervertebral disc modulates microglia proliferation, activation, and chemotaxis. Osteoarthritis Cartilage 2018; 26: 978–987. [DOI] [PubMed] [Google Scholar]
- 47. Zhou Y-Q, Liu Z, Liu H-Q, et al. Targeting glia for bone cancer pain. Expert Opin Ther Targets 2016; 20: 1365–1374. [DOI] [PubMed] [Google Scholar]
- 48. Mapplebeck JC, Beggs S, Salter MW. Molecules in pain and sex: a developing story. Mol Brain 2017; 10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mapplebeck JC, Beggs S, Salter MW. Sex differences in pain: a tale of two immune cells. Pain 2016; 157(Suppl. 1): S2–S6. [DOI] [PubMed] [Google Scholar]
- 50. Starowicz K, Finn DP. Cannabinoids and pain sites and mechanisms of action. Adv Pharmacol 2017; 80: 437–475. [DOI] [PubMed] [Google Scholar]
- 51. Bagüés A, Martín MI, Sánchez-Robles EM. Involvement of central and peripheral cannabinoid receptors on antinociceptive effect of tetrahydrocannabinol in muscle pain. Eur J Pharmacol 2014; 745: 69–75. [DOI] [PubMed] [Google Scholar]
- 52. Queme LF, Jankowski MP. Sex differences and mechanisms of muscle pain. Curr Opin Physiol 2019; 11: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stubbs D, Krebs E, Bair M, et al. Sex differences in pain and pain-related disability among primary care patients with chronic musculoskeletal pain. Pain Med 2010; 11: 232–239. [DOI] [PubMed] [Google Scholar]
- 54. Price T, Ray P. Recent advances toward understanding the mysteries of the acute to chronic pain transition. Curr Opin Physiol 2019; 11: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vargas-Prada S, Coggon D. Psychological and psychosocial determinants of musculoskeletal pain and associated disability. Best Pract Res Clin Rheumatol 2015; 29: 374–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bushnell MC, Cˇeko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013; 14: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bingel U, Wanigasekera V, Wiech K, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med 2011; 3: 70ra14. [DOI] [PubMed] [Google Scholar]
- 58. Benedetti F, Mayberg HS, Wager TD, et al. Neurobiological mechanisms of the placebo effect. J Neurosci 2005; 25: 10390–10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Macfarlane GJ, Pallewate N, Paudyal P, et al. Evaluation of work-related psychosocial factors and regional musculoskeletal pain: results from a EULAR task force. Ann Rheum Dis 2009; 68: 885–891. [DOI] [PubMed] [Google Scholar]
- 60. Borsook D, Youssef AM, Simons L, et al. When pain gets stuck: the evolution of pain chronification and treatment resistance. Pain 2018; 159: 2421–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cleveland RJ, Luong ML, Knight JB, et al. Independent associations of socioeconomic factors with disability and pain in adults with knee osteoarthritis. BMC Musculoskelet Disord 2013; 14: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Giacobino P, Luthi F, Stenz L, et al. Altered BDNF methylation in patients with chronic musculoskeletal pain and high biopsychosocial complexity. J Pain Res 2020; 13: 1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dayer CF, Luthi F, Le Carre’ J, et al. Differences in the miRNA signatures of chronic musculoskeletal pain patients from neuropathic or nociceptive origins. PLoS One 2019; 14: e0219311. [DOI] [PMC free article] [PubMed] [Google Scholar]