Abstract
Bestrophinopathies are a group of clinically distinct inherited retinal dystrophies that typically affect the macular region, an area synonymous with central high acuity vision. This spectrum of disorders is caused by mutations in bestrophin1 (BEST1), a protein thought to act as a Ca2+-activated Cl- channel in the retinal pigment epithelium (RPE) of the eye. Although bestrophinopathies are rare, over 250 individual pathological mutations have been identified in the BEST1 gene, with many reported to have various clinical expressivity and incomplete penetrance. With no current clinical treatments available for patients with bestrophinopathies, understanding the role of BEST1 in cells and the pathological pathways underlying disease has become a priority. Induced pluripotent stem cell (iPSC) technology is helping to uncover disease mechanisms and develop treatments for RPE diseases, like bestrophinopathies. Here, we provide a comprehensive review of the pathophysiology of bestrophinopathies and highlight how patient-derived iPSC-RPE are being used to test new genomic therapies in vitro.
Keywords: BEST1, bestrophinopathies, CRISPR, gene editing, gene therapy, induced pluripotent stem cells
Introduction
Pathogenic mutations in BEST1 are known to cause a number of distinct autosomal dominant dystrophies including Best disease, Adult vitelliform macular degeneration (AVMD), autosomal dominant vitreoretinochoroidopathy (ADVIRC) and the recessive disease, autosomal recessive bestrophinopathy (ARB).1,2 This spectrum of diseases, collectively known as bestrophinopathies, affect the macular region, with patients typically presenting in clinic within the first two decades of life. The prevalence of individual bestrophinopathies is rare and varied, however, in a recent study of 3000 patient families with inherited disease, BEST1 was the 5th most mutated gene, accounting for 3.9% of affected families, and one of the highest causes of autosomal dominant macular dystrophy.3 Bestrophinopathies primarily affect the retinal pigment epithelium (RPE), a monolayer of cells that interacts with and sustains the light responsive retina. In the RPE, BEST1 is normally expressed on the basolateral membrane, where it acts as an ion channel.4–6 Dysfunction of RPE with age or as a result of inherited mutations, can lead to degeneration of the retina. Over the last 30 years, research has focused on investigating the biological function of BEST1 in the RPE and the mechanisms by which BEST1 mutations contribute to retinal disease. These advances have led to a better understanding of disease mechanisms and development of potential therapies to treat the unmet clinical needs for bestrophinopathies.
Clinical spectrum of bestrophinopathies
Best disease
Best disease is the most common bestrophinopathy, with an estimated prevalence ranging from 1:5,000 to 1:67,000 and an onset that usually occurs during childhood or early adulthood.7,8 The disease was first described in 1883 as a peculiar change in the macula9 and later identified in a large German family, by Dr Friedrich Best, who noted its dominant inheritance pattern.10 Best disease is characterised by the presence of vitelliform lesions that evolve over time, and progress through a number of stages, which may or may not follow chronologically for each patient. During the initial previtelliform stage, the fundus appearance and visual acuity (VA) are normal; however, there may be subtle changes of the RPE, appearing on the central retina as yellowish pigment changes, with some granularity defects.2,11 Best disease is most easily identified during the vitelliform stage, where a yellow demarcated ‘egg-yolk’ vitelliform lesion, 2–3 mm in diameter, is observed on the macula (Figure 1(a)). At the vitelliform stage there may be mild vision loss and a decrease in VA, although VA has been reported to be normal in some patients.2,11 Patients may also report photophobia, metamorphosis and night blindness at this stage.7,12
Figure 1.
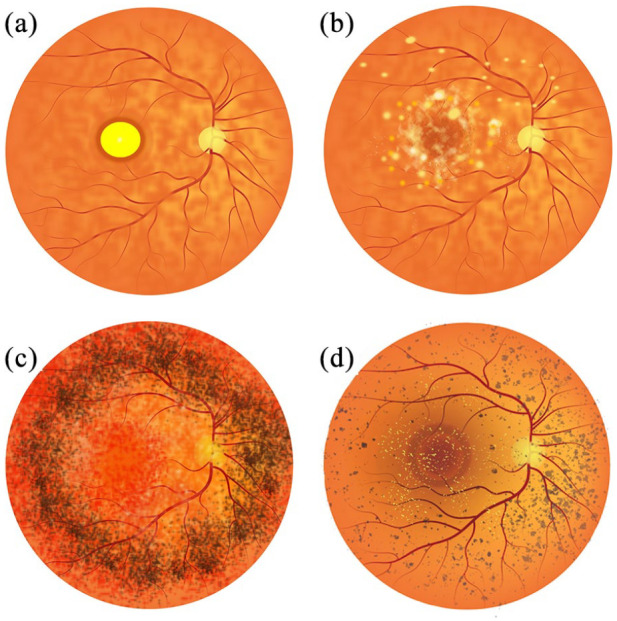
Representative illustrations of Bestrophinopathy fundus appearance. (a) Best disease, with the egg yolk-like vitelliform lesion observed at the macula. (b) Autosomal recessive bestrophinopathy, characterised by multifocal deposits and lesions around and beyond the macula. (c) Autosomal dominant vitreoretinochoroidopathy typified by presence of a hyperpigmented circumferential band of pigmentation in the peripheral retina. (d) Best-related retinitis pigmentosa characterised by the presence of peripheral pigment changes, bone spicules and foveal deposits.
Over time, the demarcated borders and yellow appearance of the vitelliform lesion can become more irregular and a partial resorption of fluid may lead to the appearance of a pseudohypopyon, where various inflammatory cells infiltrate the anterior chamber. Despite the dramatic appearance of a pseudohypopyon, VA may only be mildly affected.2,11 The disease can then progress to a vitelliruptive stage, where breakdown of the vitelliform lesion leads to a ‘scrambled-egg’ fundus appearance, with irregular yellow deposits present. At this stage, VA may be steady or may begin to decline as the disease progresses.2,13 Best disease can then progress to an atrophic stage, resulting in death of the RPE and loss of photoreceptors cells. Hyperpigmented fibrous scar tissue can be present in the macula, leading to widespread geographic atrophy, synonymous with progressive and irreversible retinal cell loss.11
During the atrophic stage VA can decline dramatically (from 6/12 to 6/60 or less) and loss is irreversible. The breakdown of the RPE barrier can also lead to choroid neovascularisation (CNV). The presence of newly created, weak blood vessels, prone to rupturing, can lead to subretinal bleeds, fluid accumulation and a sudden decrease in VA.14 Complications due to CNV have been reported to occur in 2%–9% of Best disease patients.7 Best disease is generally bilateral, with lesions displaying some relative symmetry, but cases of unilateral Best disease have also been described.15
For the most part, vision loss is gradual with 75% of affected individuals retaining a VA of 6/12 or better into their fifties in at least one eye;13 however, the presence of CNV can lead to a steep decline in VA. Patients can also present with hyperopia, astigmatism16 and anterior segment abnormalities, such as shallow anterior chambers, putting them in the risk group for having a narrow anterior angle and suffering from acute angle closure glaucoma (AACG). Best disease can also be multifocal, with numerous vitelliform lesions varying in size clustered around the macular.17 Patients can also be prone to subretinal haemorrhages, the formation of macular holes, and retinal detachment in response to modest trauma.7,18
Adult vitelliform macular degeneration
Adult vitelliform macular degeneration is a dominantly inherited bestrophinopathy. AVMD typically has an onset of between 30 and 50 years of age, with more females seeming to be affected.12 AVMD patients present with vitelliform lesions (similar to Figure 1(a)) that can be smaller than those observed in Best disease patients. Generally, AVMD has a less dramatic effect on vision and patients may not progress through all the stages described for Best disease, however, although CNV is rare, atrophy can occur7 with complications, such as CNV or pigment epithelial detachment.19,20 In general, AVMD is clinically indistinguishable from Best disease and has been described as a milder form of Best disease, owing to its later onset and slower progression. The resemblance between the two conditions has led to the suggestion that AVMD should be reclassified as Best disease.2,7
Autosomal recessive bestrophinopathy
ARB is an autosomal recessive disease resulting from bi-allelic homozygous or compound heterozygous mutations within the BEST1 locus. Parents of ARB patients generally have normal vision, suggesting a tolerance of BEST1 haploinsufficiency.7,21 ARB is estimated to have a prevalence of 1:1,000,000, with an onset range between 4 and 40 years of age, although a juvenile onset is typical.7,22 ARB presents distinctively, with an alteration in the RPE leading to the formation of multiple subretinal deposit at the macula and midperipheral retina21 (Figure 1(b)). The distinctive vitelliform macular lesions seen in Best disease are rare but may be present in some ARB patients at late stage.23 The fundus of patients with ARB has a speckled appearance with multiple yellow/white, round, demarcated, and partially confluent lesions located towards the fovea and the vascular arcades, at the posterior pole and around the optic nerve.21,24 ARB also appears to affect the periphery, indicated by presence of peripheral drusen and RPE atrophy.23
Patients also typically present with accumulation of subretinal fluid, culminating in some patients having cystoid macular edema with retinal fibrosis.7,22,25,26 Some developmental anomalies may also be present in ARB, and patients may be hyperopic and have shallow anterior chambers, increasing their risk of AACG.7,21 Vision loss is slow and progressive,23 but in some cases central vision may eventually stabilise; however, complications such as CNV and AACG can lead to a rapid deterioration of vision.7,21,24,27
Autosomal dominant vitreoretinochoroidopathy
ADVIRC follows a dominant mode of inheritance and seems to differ from the other bestrophinopathies as it does not present with distinctive lesions of the macula. Instead, ADVIRC patients present with a strongly demarcated 360-degree circumferential hyperpigmented band in the peripheral retina, from the equator to the ora serrata28,29 (Figure 1(c)). ADVIRC is a very rare condition with a prevalence of 1;1,000,000, with only five BEST1 mutations currently known to cause the disease.28,30 The typical age of onset is during early childhood, with the hyperpigmented band generally seen in early stage patients. For older patients, the hyperpigmented band is considered a hallmark for ADVIRC; however, a recent reports have described its absence in some patients, indicating a high phenotypic variability.2,31,32
During early stage disease, ADVIRC affects the peripheral retina, with very little change in the appearance of the central retina. As ADVIRC progresses, it can encroach onto the macular region, causing central vision loss.33 VA can range from 6/6, to absence of light perception in some cases; however, the majority of patients are able to maintain good vision of at least 6/12 throughout life.31,33,34 Patients can also present with punctate white retinal opacities, a pale optic disc and attenuated and narrow blood vessels.33,34 Further complications include retinal neovascularisation (leading to retinal bleeding and macula edema), retinal fibrosis, atrophy of the underlying choroid, retinal detachment and vitreous haemorrhage.7,28,33,35 Interestingly, ADVIRC patients may also have a wide range of eye development issues, including: microcornea, nanopthalmos, discrete rotatory nystagmus, hyperopia, presenile cataracts, iris dysgenesis, optic nerve dysplasia and a shallow anterior chamber leading to risk of AACG.7,28,33–35 These findings suggest that bestrophinopathy mutations may also contribute to broader ocular defects. Microcornea, rod-cone dystrophy and staphyloma (MRCS), a disease linked to BEST1 mutations, shares many ADVIRC-associated clinical features and the phenotype could be on the spectrum of ADVIRC disease expressivity.28,36
Retinitis pigmentosa
A number of patients have also been diagnosed with a bestrophinopathy-related form of retinitis pigmentosa,37 classified by dense pigmentary changes in the peripheral retina. This can be accompanied by retinal gliosis, vascular attenuation, peripheral bone spicules, pale optic discs, yellow and foveal deposits and macular edema (Figure 1(d)), leading to patients typically having vision loss and night blindness. However, these may represent misdiagnosed cases of ADVIRC or ARB.1,2,38 Targeted next-generation sequencing has retrospectively diagnosed cases of bestrophinopathy-related RP as ADVIRC, despite patients lacking the hallmark ADVIRC hyperpigmented circumferential band on clinical presentation.32 Alternatively, BEST1-related RP may be the result of multigenic mutations.39,40
Diagnosis of bestrophinopathies
Although the clinically distinct features of the bestrophinopathies may be sufficient for diagnosis, a number of additional tests can be used for confirmation. The electrooculogram (EOG) measures the standing potential, the electrical difference between the front and the back of the eye, and is used to assess the potential across the RPE, an indicator of its health.41 The EOG measures the potential during exposure to the dark and following exposure to light, to calculate a light peak (LP): dark trough ratio, termed the Arden ratio.41 According to the International Society for Clinical Electrophysiology of Vision (https://iscev.wildapricot.org/standards), a standard Arden ratio is between 1.7 and 4.3; this typically decreases below 1.5 in patients with bestrophinopathies.21,23,28,33,35,41 However, in some patients, the Arden ratio may be normal or only slightly reduced,11,12,33,42 this has been observed in 8% of Best disease cases,31 highlighting the need for genetic testing to correctly distinguish and diagnose a bestrophinopathy. The electroretinogram (ERG) is typically found to be normal in patients, although delayed cone and rod response may be observed in ADVIRC patients28,31,34 and full-field, pattern and multifocal ERG’s can be affected in ARB patients.2,24,27,43
Disease progression can be monitored using optical coherence tomography (OCT), measuring foveal thickness, retinal degeneration and RPE atrophy, and the presence of retinal edema or subretinal fluid.7,16,23,35 Fluorescein angiography and fundus autofluorescence, can also be used to examine blood vessel structure and accumulation of lipofuscin respectively in patients.7 Hyperfluorescence is observed during early stage Best disease, but can decline as the disease progresses. In ADVIRC patients, autofluorescence appears normal in the central retina, but is often blocked in the periphery by the characteristic peripheral hyperpigmented ring.34 Patchy areas of hyperautofluorescence, corresponding to fluid accumulation and small confluent lesions, are typically observed in ARB patients.21 CNV, a serious complication in bestrophinopathy, can be confirmed using OCT and fluorescein angiography. Despite these modern imaging techniques, genetic testing is generally used to confirm clinically suspected bestrophinopathy and identify novel BEST1 mutations.1,21,28,35,44
Clinical management of bestrophinopathies
There are currently no treatments for the bestrophinopathies; however, complications experienced as a result of disease, can be managed. One common complication is neovascularisation, which, although problematic, has been treated using photodynamic therapy (with the use of verteporfin),45 photocoagulation or through anti-vascular endothelial growth factor (VEGF) injections, such as Avastin or Bevacizumab.7,46 Interestingly, sufficient VA can be retained in some Best disease patients displaying CNV without any treatment.14 Complications such as macula edema have been treated using oral acetazolamide with a nepafenac suspension39 (p. 129), while vitrectomy surgery has been used to repair macular holes.18 In patients at risk of AACG, monitoring intra-ocular pressure and irido-corneal angle with goniocsopy is recommended. Intraocular pressure can be used to assess the development of glaucoma, which can be controlled with topical drops or treated with prophylactic yttrium aluminium garnet (YAG)-laser iridotomy.35 Bestrophinopathy patients are at risk of subretinal haemorrhages, they may therefore be advised to avoid contact sport and wear safety glasses at times to circumvent this complication.47
Bestrophin-1
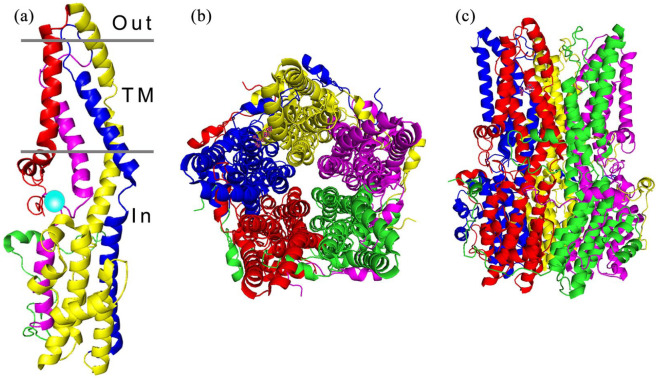
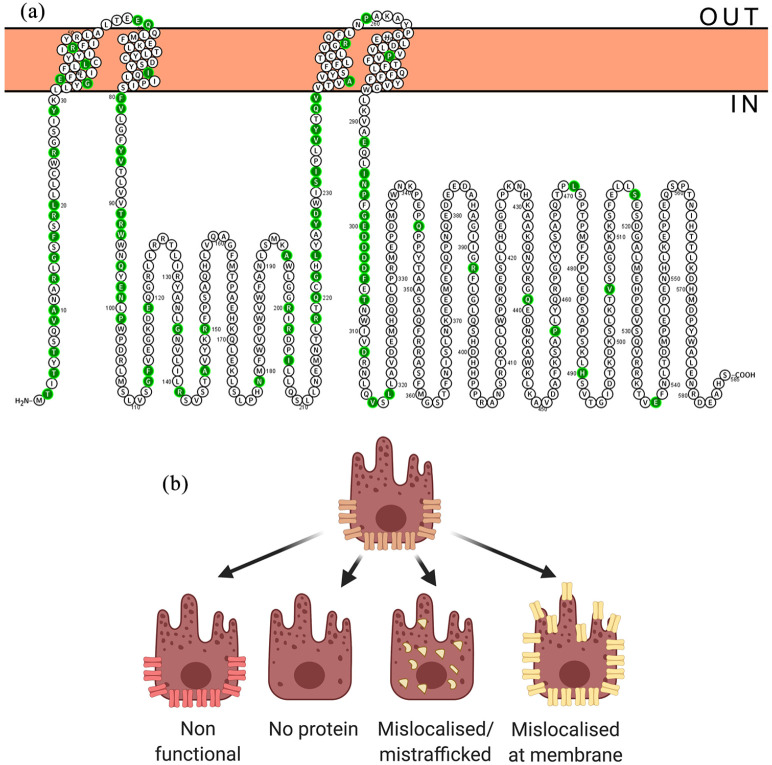
Bestrophinopathies are caused by mutations in the Bestrophin-1 (BEST1) gene, which maps to a 16 kB region of chromosome 11 (11q13), where it spans 11 exons, producing a 1758 bp transcript expressed exclusively in the RPE, of the adult eye.7,48–50 BEST1 is also expressed in extra-ocular tissues including the trachea, lung, kidney, sperm, colon, and testes, and within astrocytes, neurons, and epithelia of the central nervous system.7,51–53 In addition BEST1 is also expressed in the retina during foetal development.54 BEST1 is a member of the Bestrophin family, an ancient group of membrane proteins, and has been identified in most metazoan organisms.55 The coding region, which starts in the second exon, encodes a 68 kD protein, consisting of 585 amino acids4 with a highly conserved intracellular N-terminal domain containing 4 transmembrane spanning domains and a long diverse cystolic C-terminal domain tail.56–59 Chicken and bacterial proteins have been used to analyse the crystal structure of BEST1, revealing a homo-pentameric organisation comprised of five BEST1 protomers58,59 arranged around a central axis to produce a barrel-shaped pore (Figure 2). The channel protrudes just outside the cell membrane, with the majority of the protein located in the cytosol. In the pentameric structure, Ca2+ clasps within each protomer come together as a belt around the central section of the channel, forming a hydrophobic neck, which is dilated by the binding of cytosolic calcium, allowing the flux of Cl- ions.58,59 Over 250 distinct pathogenic BEST1 mutations have been discovered thus far,48,60 these are thought to affect channel formation, channel function, or protein/channel localisation (Figure 3).
Figure 2.
Architecture of the BEST1 channel. (a) The structure of a BEST1 protein unit is divided into four segments, composed of alpha helices represented as S1a-c (red), S2a-h (yellow), S3a-b (blue) and S4a-b (magenta), the transmembrane regions (TM) are indicated. The calcium clasp is represented by a turquoise sphere and the start of the C-terminal tail is coloured green. (b) The BEST1 channel, viewed from the extracellular side, is formed from five BEST1 proteins arranged in a pentameric structure, forming a barrel shaped ion pore (c).
Figure 3.
BEST1 disease causing mutations. (a) Annotated BEST1 protein sequence with ClinVar benign/likely benign mutations indicated in green. (b) Potential effects of mutation on BEST1 channel.
The retinal pigment epithelium
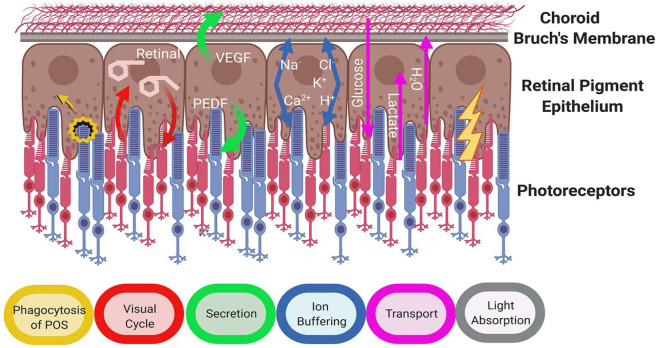
Bestrophinopathies primarily affect the RPE, a monolayer of pigmented cells that lies between the neural retina and the choriocapillaris, directly below the cone and rod photoreceptors. RPE cells form tight connections with each other acting as a physical barrier between the retina and the underlying choroid and forming an essential component of the blood-retinal barrier.61 The RPE apical membrane is scattered with microvilli, which project between the outer segments of the photoreceptors. Despite its simple structure, the RPE performs a number of crucial roles that are needed for photoreceptors to detect light and keep the retina healthy (Figure 4). Disruption in these functions can lead to retinal degeneration and loss of vision.
Figure 4.
Crucial roles of retinal pigment epithelium (Adapted from Strauss, 200561).
The RPE is involved in the phagocytosis and degradation of photoreceptor outer segments (POS). The removal and processing of POS waste prevents the build-up of photo-oxidative by-products, and is crucial for RPE and photoreceptor cell health.62 Furthermore, essential substances contained within the POS, such as retinal, can be recycled by the RPE, as part of the visual cycle, and returned to photoreceptor cells to enable phototransduction.61 The RPE is a highly active phagocytic monolayer, engulfing and ingesting around 25,000 outer segment discs per day, with each RPE cell in the macular fovea phagocytosing around 20 POS daily.63 Changes in cell pH, Ca2+ and ion balance can impact on RPE phagocytosis of POS and affect lysosomal function,61,64 therefore disruption of cell homeostasis can affect the removal of waste, resulting in the build-up of toxic debris within and around cells, ultimately leading to cell atrophy.65
The RPE maintains a healthy retinal environment and structural integrity by secreting signalling molecules, growth factors, neuroprotective factors and immunosuppressive factors, for example, pigment epithelium-derived factor (PEDF) and VEGF, allowing the RPE to communicate with other tissues.61 As a crucial component of the blood: retina barrier the RPE is involved in the transepithelial transport of molecules between the retina and the choroid61 and maintaining the ionic homeostasis of the subretinal environment, transporting water and metabolites (e.g. glucose and lactate), controlling pH and removing waste products. The RPE regulates the buffering of ions in the subretinal space in response to the fast paced, light responsive activity of photoreceptors, maintaining ionic balance and pH. To do this, cells express a number of key pumps, transporter and ion channels at the apical and basal surfaces, including ligand-gated/voltage-gated/Ca2+-activated potassium channels, Na+/K+–ATPase, voltage-dependent/ligand-gated Ca2+ channels, volume-regulated anion channels and Ca2+-activated chloride channels.66
Role of BEST1
Bestrophinopathies are classed as channelopathies, due to the effects of mutations on the conductance of currents through the cell membrane. BEST1 was first identified as a chloride channel in 2002 after overexpression of exogenous BEST1 in HEK293 cells induced chloride currents that were calcium sensitive.67 Similarly, studies in human foetal RPE suggest it is an Ca2+ responsive channel, which is required to maintain the RPE transepithelial potential.68 In addition, structural studies support the hypothesis that BEST1 is a Ca2+ -dependent Cl- channel, controlling the flux of Cl- ions in cells.58,59 However, BEST1 may be involved in a number of processes within epithelial cells. BEST1 interacts with the CaV1.3α1D and CaVβ subunits of L-type voltage-dependent calcium channels,69,70 participating in intracellular Ca2+ signalling and potentially contributing to the generation of the LP.70–72 BEST1 may also regulate intracellular Ca2+, by modulating the release of Ca2+ from endoplasmic reticulum stores, this hypothesis is aided by the finding that some protein can localise away from the membrane.71,73,74 There is also evidence to suggest that BEST1 can act directly as a volume-regulated anion channel, regulating cell volume and homeostasis in RPE cells,75 a role which would be essential for maintaining cell homeostasis.
Although BEST1 is primarily thought to be a Cl- channel, it is highly permeable to other molecules, such as HCO3-,76 glutamate77 and gamma aminobutyric acid (GABA),78 implying that the channel could potentially serve as a pH sensor/regulator and be involved in neurotransmitter release. In addition, the presence of developmental ocular defects, for example, microcornea and nanophthalmos, in some bestrophinopathy patients, suggests that BEST1 may play a role in normal eye development.16,35 Although protein is only observed in the adult RPE, BEST1 is expressed in human retinal cells during early development.54 Given that eye development is reliant on correct spatial signalling from neighbouring cells, the expression of mutant BEST1 outside the RPE may affect cell: cell interactions, interfering with normal ocular developmental pathways.79,80
It is widely accepted that the BEST1 channel is activated by the binding of Ca2+, however the protein also contains an ATP binding motif, which may be important in modulating channel function. The binding of ATP can modulate channel activity, resulting in increased currents in the presence of Ca2+. This response is disrupted in iPSC-RPE cells from patients with a mutation in the binding motif, suggesting a relevance to physiological BEST1 function in human cells.72 ATP is the primary candidate for the substance released during the light peak in electrophysiological recordings, such as the EOG test. The EOG is the defining diagnostic test for bestrophinopathies, with patients generally recording a decrease in the recorded light peak: dark trough ratio. The light peak reflects the increased conductance of Cl- across the RPE basolateral membrane. This is thought to occur in response to an unknown ‘light peak substance’ (LPS), released by photoreceptors after exposure to light, which binds to a receptor on the RPE cells, initiating a cascade that results in depolarisation of the RPE basolateral membrane. Previously, ATP was thought to increase intracellular calcium through purinergic receptors, driving the conductance of chloride Cl- across the basal membrane.81 However, the direct interaction of ATP with BEST1 within the RPE provides another means to drive depolarisation in response to light.
Model systems for bestrophinopathies
A number of animal models have been used to investigate BEST1 in RPE cells, including rats, mice, and dogs. The rat model of Best disease, created by overexpressing mutated forms of BEST1, localises the protein in the RPE basolateral membrane and displays electrophysiological findings typical of human disease, yet no ocular disease phenotype was observed.82 Similarly in a BEST1-/- knock out mouse model,5,83 although a reduced light peak, reminiscent of Best disease, was observed, no ocular phenotypes were reported. Knock-in mice expressing mutant forms of BEST1 may represent better rodent models as the reduced electrophysiological responses are also accompanied by lipid accumulation, and retinal detachments.11,84 There may be a limit to how much rodent models can contribute to our knowledge of bestrophinopathies as these animals do not possess a macula and therefore might not develop the equivalent retinal lesions seen in human macular diseases.85
Although dogs do not have a macula, there is a region in the retina called the area centralis, populated with a higher density of cones and free from large blood vessels. Within this region is a foveal-like region, susceptible to a canine form of recessive bestrophinopathy,86 termed canine multifocal retinopathy (CMR). CMR affects a number of canine breeds and is caused by mutations in cBEST1, making the dog a naturally occurring animal model of bestrophinopathy.87,88 In these animals, the area centralis is affected by vitelliform lesions typical of Best disease, with focal detachment between the RPE and the neural retina also noted89 or, more typically, multiple retinal lesions, reminiscent of ARB.21,90 The CMR model is aiding current knowledge of disease progression through lesions, pseudohypopyon and atrophy stages. Loss of RPE apical microvilli leading to microdetachment of the retina is thought to be the earliest features of CMR, indicating an RPE-photoreceptor disease interface for bestrophinopathy.91,92
Much of the early work examining BEST1 in human cells involved overexpression of the protein in the kidney epithelial cell lines, HEK-293 or MDCKII.67,93 Yet, RPE cells have a unique polarisation signature, independent of E-Cadherin, where sorting of proteins to the apical and basement membrane for example, Na+ /K+ ATPase and monocarboxylate transporter 1, is reversed compared to other epithelial cells.94 Wild type (WT) BEST1 does not localise to the membrane of HEK-29337 and MDCKII already express endogenous ion channels that could impact on electrophysiological recordings. In addition, overexpression studies in foetal RPE have provided valuable insights into the oligomerisation of BEST1, its role as an anion channel and its involvement in regulation of transepithelial resistance.68,95 However, the availability of foetal RPE tissue limits its use as a common model system.
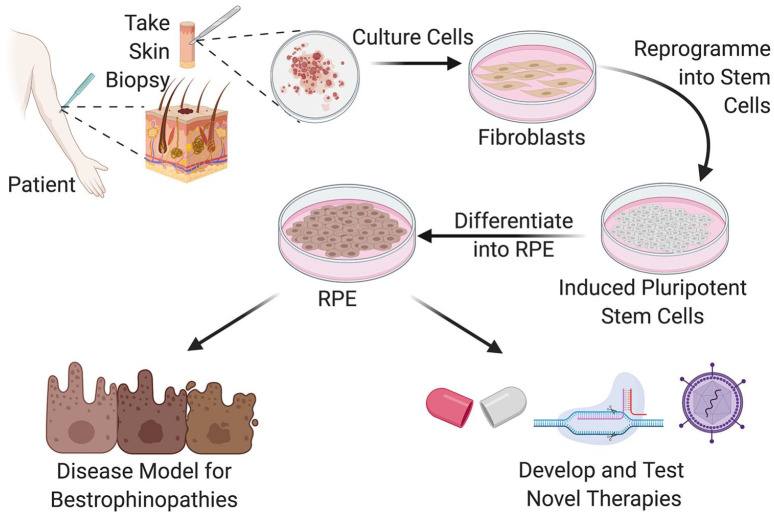
The advent of induced pluripotent stem cell (iPSC) technology has transformed the modelling of bestrophinopathies in vitro (Figure 5). Reprogramming of human somatic cells to a state of pluripotency using embryonic transcription factors has enabled researchers to derive RPE from patients skin or blood sample, providing a cell model which contains an individual patients unique genetic makeup.96–98 iPSC-derived RPE have a pigmented, cobblestone-like epithelial morphology, replicate many of the functions of RPE and express BEST1 in the basolateral membrane, allowing the investigation of inherited ophthalmic disorders within a disease-in-a-cell system.
Figure 5.
Generation of patient derived iPSC-RPE as a human disease model system for the study of bestrophinopathies and development novel forms of treatments for patients.
Disease modelling using iPSC-RPE has replicated many of the features of bestrophinopathies, including reduced channel activity, defects in POS phagocytosis lysosome defects, accumulation of lipofuscin and reduced net fluid transport.40,54,99–102 iPSC-RPE studies have also provided more evidence suggesting BEST1 can function as a voltage gated anion channel,5 regulate calcium signalling6 and be regulated by ATP.72 Cellular studies have identified distinct pathological features that can be used to distinguish between the effects of different mutations102 and different bestrophinopathies for example, anion transport is increased in ADVIRC cells and decreased in Best disease cells compared to controls.100 Disease-in-a-dish modelling using iPSC-RPE may yet reveal more about the nature of BEST1 mutations. However, the power of these cells is the ability to investigate new therapeutics by performing drug/compound screens and developing the next generation of personalised genomic medicines.
Therapies for bestrophinopathies
Ophthalmology has been at the forefront of developing the new era of genomic and regenerative medicine approaches, including gene therapy, CRISPR genome editing, antisense oligonucleotide and stem cell therapies. Rapid clinical advances for retinal degeneration have been enabled by the accessibility and small size (6 mm) of the macula, ocular immune privilege, real-time ocular imaging, visual function testing, and the availability of two eyes in a patient–allowing one to serve as an untreated. Currently there are no curative therapies for bestrophinopathies, therefore research is focusing on developing a number of clinical treatments.
Gene therapy
Gene therapy involves introducing exogenous genetic material, that is, a working copy of the BEST1 gene, into the cells of a host in an attempt to treat the underlying cause of a disease. This approach is predicted to be optimal in loss of function mutations, where levels of functional protein are low or absent (Figure 6). A number of research groups have provided proof-of-concept approaches treating bestrophinopathy with a gene augmentation approach. Guziewicz and colleagues, have used subretinal injections of BEST1 adeno-associated virus (AAV)-mediated augmentation gene therapy to reverse the early retinal microdetachments and lesions seen in canine models of ARB (CMR). Improvements were maintained for at least 23 months and no reports of retinal toxicity noted up to 6 weeks post-injection. This approach also preserved the cytoarchitecture of the RPE–Photoreceptor interface and normalised the otherwise diseased hyperthick outer nuclear layer, providing proof of principle that gene therapy may be a viable treatment for patients with CMR-like ARB.88
Figure 6.
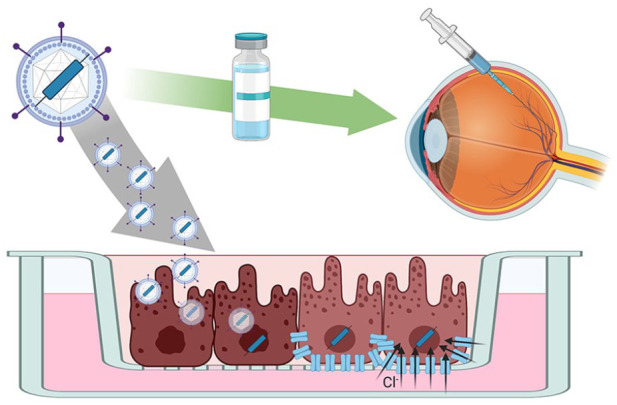
Gene therapy approaches for bestrophinopathies can be tested in patient iPSC-RPE cells in a trial in a dish scenario. This approach could be used as a screen to identify mutations responsive to the treatment prior to clinical application in the patient.
IPSC-RPE are also being used to assess the feasibility of BEST1 gene therapy in human cells (Figure 6). Initial studies, using iPSC-RPE from patients with ARB show that gene therapy delivered using baculovirus rescues the Ca2+ -dependent Cl- channel deficiencies observed in cells from patients with recessive disease.103 Surprisingly, supplemental gene therapy may also be a potential treatment for dominant bestrophinopathies. Ji and colleagues examined iPSC-RPE cells derived from six patients with Best disease, where dominant BEST1 mutations affected the subcellular localisation or function of BEST1. Here they found that treatment of patient iPSC-RPE with BEST1-AAV restored deficiencies in Ca2+ -dependent chloride channel activity, with the viral BEST1 protein localising to the plasma membrane.102 Despite these successes and exciting proof-of-concepts for recessive and dominant disease, gene therapy may not be a suitable treatment for all BEST1 mutations. Gene augmentation using lentiviral BEST1 was able to restore Ca2+-activated Cl- channel in activity and phagocytosis of outer segments in 3 of 4 patient lines tested, but one cell line, with the Ala146Lys mutation, did not respond to the treatment. This may be due to dominant effect of the mutated protein on the supplemental WT BEST1 protein.104 Although gene therapy for BEST1 is still in its infancy, early indications in these preclinical models suggest it may be a viable option for recessive disease and, in some cases, dominant disease with responsive mutations, highlighting the need for careful screening of potential gene therapy candidates.
Genome editing
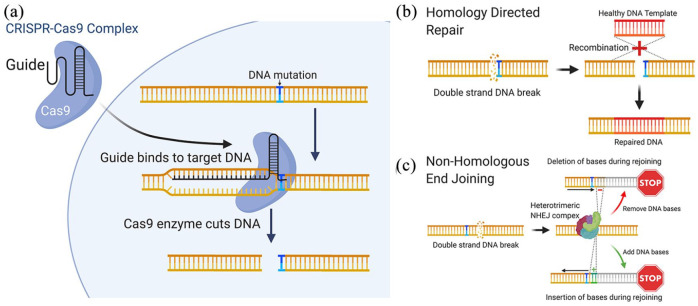
Individual mutations within the BEST1 gene can have different consequences on the protein, affecting its localisation, the formation of the BEST1 pentametric channel formation or channel function. The interaction of the dominant protein with the supplemental protein may still affect individual patients’ responses to gene therapy. In these cases, alternative options need to be investigated. Genome editing provides a therapeutic means to get to the heart of the problem, changing the mutated DNA sequence or switching off a faulty gene. The CRISPR-Cas9 (Clustered Regularly Interspaced short palindromic repeats with CRISPR associated protein 9) system is currently the most popular, efficient and adaptable method to induce permanent changes in cellular DNA.105
Initially described as a bacterial defence mechanism to identify and inactivate invading viral DNA, CRISPR-Cas9 is now one of the most promising means to treat inherited diseases (31). CRISPR-Cas9 is an RNA guided endonuclease that can be directed to cut double stranded DNA at a specific site upstream of a short protospacer adjacent motif (PAM). The cut site can be determined by providing a complementary single guide RNA (sgRNA), which directs Cas9 to produce a double-strand break at a precise sequence (Figure 7(a)). At this point the cell activates mechanisms to repair the DNA using either homology directed repair (HDR) or non-homologous end joining (NHEJ), which provides opportunities to edit the gene.106
Figure 7.
CRISPR genome editing can be used to treat patient mutations at the molecular level. (a) The CRISPR-Cas9 complex is used to create a double strand DNA break in close proximity to the patient. (b) A corrected DNA template can be incorporated into the sequence using homology directed repair, alternatively and (c) non-homologous end joining can be used to create insertions/deletions, resulting in frame-shifts that disrupt the target gene.
Homology-directed repair (HDR) is a critical DNA repair mechanism, commonly active during meiosis, requiring the presence of a homologous DNA for example, a sister chromatid. For genome editing, an sgRNA targeting a mutation site can be introduced into the cell alongside a homologous WT DNA template, which is then incorporated into the gene during the repair, replacing and correcting the mutated DNA region (Figure 7(b)). However due to the practical limitations of HDR, the probability of donor DNA being used as a template in non-dividing somatic cells, like the RPE, is low, therefore HDR repair editing may not be efficient in an RPE cell in situ. An alternative and simpler method of editing uses NHEJ, a common cellular mechanism that joins the two ends of double-stranded DNA breaks back together. This method is highly prone to errors, resulting in indel creation and subsequent transcriptional frameshifts, which lead to degradation of the transcript (Figure 7(c)). NHEJ can therefore be a useful way of switching off a gene in somatic cells, and could be used to target autosomal dominant diseases by directing Cas9 to target specific gene mutation sites on the dominant mutated allele.
Recently, Sinha and colleagues104 demonstrated the first proof-of-concept approach for gene editing in Best disease using NHEJ, with an average frame shift efficiency of 96%. CRISPR/Cas9 treatment of patient derived iPSC-RPE improved BEST1 channel activity and rhodopsin degradation. However, a major concern was the presence of off-target editing effects, which could affect the expression of other key genes in edited cells. A number of challenges remain before gene editing can be widely used in clinic, including the delivery of the gene editing system to target cells, efficiency of the therapy, utilisation of repair mechanisms in RPE and retinal cells, and prevention of off-target effects.107,108
Pharmacological approaches
Although a great deal of attention is being placed on using genomic medicine to treat inherited diseases, pharmacological approaches may be valid for bestrophinopathies.109,110 Singh and colleagues,111 investigated whether modulating proteolytic machinery using valproic acid, a histone deacetylase inhibitor, in combination with rapamycin, an inducer of autophagy, could rescue POS processing defects observed in bestrophinopathies. This combinatory treatment increased the rate of POS degradation and reduced the build-up of autofluorescence in patient derived iPSC-RPE, suggesting a link between POS handling and proteolysis in RPE. Furthermore, this treatment also delayed disease progression in a canine model of ARB.
Recent interest has also turned to the use of molecular chaperones and proteasome inhibitors as therapeutics for bestrophinopathies. The proteasome inhibitor, bortezomib and chemical chaperone, 4-phenylbutyrate (4PBA) have been used in combination to guide correct trafficking of exogenous mutant BEST1 to the plasma membrane in MDCKII cells. This combination can also rescue channel activity defects in HEK293 cells expressing inducible forms of mutant BEST1. Similarly, 4PBA and its analogue, 2-naphthoxyacetic acid, were able to increase BEST1 protein expression in iPSC-RPE from Best disease and ARB patients and restore channel function in HEK293 cells expressing mutated forms of BEST1.112
Conclusion
Bestrophinopathies are distinct retinal dystrophies with varying clinical heterogeneity and penetrance that typically lead to central vision loss at an early age and have a huge impact on the daily lives of patients. The gene responsible for these diseases, BEST1, is a highly conserved anion channel, yet there are few animal models available to fully understand its role in the development of disease, and although BEST1 expression is widespread throughout the body, it is still unclear why BEST1 mutations manifest in vision loss only. Patient derived iPSC-RPE are a crucial disease-in-a-dish model system that have enabled a greater understanding of BEST1 function and its role in human disease. These cells will be important in revealing the role of individual mutations in the development of distinct bestrophinopathies and could help to interrogate the heterogeneity of disease, by identifying potential genetic modifiers of disease in families. In the future, iPSC may also help gain valuable insight into the importance of the RPE: retinal interface in bestrophinopathies, through the culture of retinal organoids. The importance of iPSC-RPE can be fully appreciated in their use to develop and test potential genomic therapies for bestrophinopathies. Current research suggests that, although there may not be a common approach to treat all bestrophinopathies, a range of options could be available for patients in the future, providing permanent treatments for these inherited diseases.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors gratefully acknowledge funding the Macular Society, the Uren Foundation and the London Project to Cure Blindness.
ORCID iDs: Simranjeet Singh Grewal  https://orcid.org/0000-0002-5461-0790
https://orcid.org/0000-0002-5461-0790
Amanda-Jayne F. Carr  https://orcid.org/0000-0002-5469-0030
https://orcid.org/0000-0002-5469-0030
Contributor Information
Simranjeet Singh Grewal, UCL Institute of Ophthalmology, University College London, London, UK.
Joseph J. Smith, UCL Institute of Ophthalmology, University College London, London, UK
Amanda-Jayne F. Carr, UCL Institute of Ophthalmology, University College London, 11-43 Bath Street, London EC1V 9EL, UK.
References
- 1. Shah M, Shah M, Broadgate S, et al. Association of clinical and genetic heterogeneity with BEST1 sequence variations. JAMA Ophthalmol 2020; 138: 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leroy BP. Bestrophinopathies. In: Traboulsi EI. (ed.) Genetic diseases of the eye. 2nd ed. Oxford: Oxford University Press, 2012, pp. 426–436. [Google Scholar]
- 3. Pontikos N, Arno G, Jurkute N, et al. Genetic basis of inherited retinal disease in a molecularly characterized cohort of more than 3000 families from the United Kingdom. Ophthalmology 2020; 127: 1384–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marmorstein AD, Marmorstein LY, Rayborn M, et al. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci USA 2000; 97: 12758–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Milenkovic A, Brandl C, Milenkovic VM, et al. Bestrophin 1 is indispensable for volume regulation in human retinal pigment epithelium cells. Proc Natl Acad Sci USA 2015; 112: E2630–E2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh R, Shen W, Kuai D, et al. IPS cell modeling of best disease: insights into the pathophysiology of an inherited macular degeneration. Hum Mol Genet 2013; 22: 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boon CJF, Klevering BJ, Leroy BP, et al. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog Retin Eye Res 2009; 28: 187–205. [DOI] [PubMed] [Google Scholar]
- 8. Mullins RF, Oh KT, Heffron E, et al. Late development of vitelliform lesions and flecks in a patient with best disease: clinicopathologic correlation. Arch Ophthalmol 2005; 123: 1588–1594. [DOI] [PubMed] [Google Scholar]
- 9. Adams JE. Case showing peculiar changes in the macula. Trans Ophthalmol Soc UK 1883; 3: 113–114. [Google Scholar]
- 10. Best F. Ueber eine hereditare Maculaaffektion: Beitrage zur Vererbungslehre. Z Augenheilkd 1905; 13: 199–212. [Google Scholar]
- 11. Marmorstein AD, Cross HE, Peachey NS. Functional roles of bestrophins in ocular epithelia. Prog Retin Eye Res 2009; 28: 206–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Renner AB, Tillack H, Kraus H, et al. Morphology and functional characteristics in adult vitelliform macular dystrophy. Retina 2004; 24: 929–939. [DOI] [PubMed] [Google Scholar]
- 13. Fishman GA, Baca W, Alexander KR, et al. Visual acuity in patients with best vitelliform macular dystrophy. Ophthalmology 1993; 100: 1665–1670. [DOI] [PubMed] [Google Scholar]
- 14. Chung MM, Oh KT, Streb LM, et al. Visual outcome following subretinal hemorrhage in best disease. Retina 2001; 21: 575–580. [DOI] [PubMed] [Google Scholar]
- 15. Arora R, Khan K, Kasilian ML, et al. Unilateral BEST1-associated retinopathy. Am J Ophthalmol 2016; 169: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wittstrom E, Ekvall S, Schatz P, et al. Morphological and functional changes in multifocal vitelliform retinopathy and biallelic mutations in BEST1. Ophthalmic Genet 2011; 32: 83–96. [DOI] [PubMed] [Google Scholar]
- 17. Lacassagne E, Dhuez A, Rigaudière F, et al. Phenotypic variability in a French family with a novel mutation in the BEST1 gene causing multifocal best vitelliform macular dystrophy. Mol Vis 2011; 17: 309–322. [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Xuan Y, Zhang Y, et al. Bilateral macular holes and a new onset vitelliform lesion in Best disease. Ophthalmic Genet 2017; 38: 79–82. [DOI] [PubMed] [Google Scholar]
- 19. Krämer F, White K, Pauleikhoff D, et al. Mutations in the VMD2 gene are associated with juvenile-onset vitelliform macular dystrophy (Best disease) and adult vitelliform macular dystrophy but not age-related macular degeneration. Eur J Hum Genet 2000; 8: 286–292. [DOI] [PubMed] [Google Scholar]
- 20. Battaglia Parodi M, Di Crecchio L, Ravalico G. Vascularized pigment epithelial detachment in adult-onset foveomacular vitelliform dystrophy. Eur J Ophthalmol 2000; 10: 266–269. [DOI] [PubMed] [Google Scholar]
- 21. Burgess R, Millar ID, Leroy BP, et al. Biallelic mutation of BEST1 causes a distinct retinopathy in humans. Am J Hum Genet 2008; 82: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sheng X, Chen X, Zhao K, et al. A novel homozygous BEST1 mutation correlates with complex ocular phenotypes. Ophthalmology 2013; 120: 1511–1512. [DOI] [PubMed] [Google Scholar]
- 23. Casalino G, Khan KN, Armengol M, et al. Autosomal recessive bestrophinopathy: clinical features, natural history, and genetic findings in preparation for clinical trials. Ophthalmology. Epub ahead of print 8 October 2020. DOI: 10.1016/j.ophtha.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gerth C, Zawadzki RJ, Werner JS, et al. Detailed analysis of retinal function and morphology in a patient with autosomal recessive bestrophinopathy (ARB). Doc Ophthalmol 2009; 118: 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. MacDonald IM, Gudiseva HV, Villanueva A, et al. Phenotype and genotype of patients with autosomal recessive bestrophinopathy. Ophthalmic Genet 2012; 33: 123–129. [DOI] [PubMed] [Google Scholar]
- 26. Sharon D, Al-Hamdani S, Engelsberg K, et al. Ocular phenotype analysis of a family with biallelic mutations in the BEST1 gene. Am J Ophthalmol 2014; 157: 697–709. [DOI] [PubMed] [Google Scholar]
- 27. McMurray RJ, Gadegaard N, Tsimbouri PM, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater 2011; 10: 637–644. [DOI] [PubMed] [Google Scholar]
- 28. Yardley J, Leroy BP, Hart-Holden N, et al. Mutations of VMD2 splicing regulators cause nanophthalmos and autosomal dominant vitreoretinochoroidopathy (ADVIRC). Invest Ophthalmol Vis Sci 2004; 45: 3683–3689. [DOI] [PubMed] [Google Scholar]
- 29. Kellner U, Jandeck C, Kraus H, et al. Autosomal dominant vitreoretinochoroidopathy with normal electrooculogram in a German family. Graefes Arch Clin Exp Ophthalmol 1998; 236: 109–114. [DOI] [PubMed] [Google Scholar]
- 30. Chen CJ, Kaufman S, Packo K, et al. Long-term macular changes in the first proband of autosomal dominant vitreoretinochoroidopathy (ADVIRC) due to a newly identified mutation in BEST1. Ophthalmic Genet 2016; 37: 102–108. [DOI] [PubMed] [Google Scholar]
- 31. Pasquay C, Wang LF, Lorenz B, et al. Bestrophin 1 – phenotypes and functional aspects in bestrophinopathies. Ophthalmic Genet 2015; 36: 193–212. [DOI] [PubMed] [Google Scholar]
- 32. Boulanger-Scemama E, Sahel JA, Mohand-Said S, et al. AUTOSOMAL DOMINANT VITREORETINOCHOROIDOPATHY: when molecular genetic testing helps clinical diagnosis. Retina 2019; 39: 867–878. [DOI] [PubMed] [Google Scholar]
- 33. Lafaut BA, Loeys B, Leroy BP, et al. Clinical and electrophysiological findings in autosomal dominant vitreoretinochoroidopathy: report of a new pedigree. Graefes Arch Clin Exp Ophthalmol 2001; 239: 575–582. [DOI] [PubMed] [Google Scholar]
- 34. Vincent A, McAlister C, Vandenhoven C, et al. BEST1-related autosomal dominant vitreoretinochoroidopathy: a degenerative disease with a range of developmental ocular anomalies. Eye 2011; 25: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burgess R, MacLaren RE, Davidson AE, et al. ADVIRC is caused by distinct mutations in BEST1 that alter pre-mRNA splicing. J Med Genet 2009; 46: 620–625. [DOI] [PubMed] [Google Scholar]
- 36. Reddy MA, Francis PJ, Berry V, et al. A clinical and molecular genetic study of a rare dominantly inherited syndrome (MRCS) comprising of microcornea, rod-cone dystrophy, cataract, and posterior staphyloma. Br J Ophthalmol 2003; 87: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davidson AE, Millar ID, Urquhart JE, et al. Missense mutations in a retinal pigment epithelium protein, Bestrophin-1, cause retinitis pigmentosa. Am J Hum Genet 2009; 85: 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brodsky MC, Schimmenti L, Iezzi R. The Best retinitis pigmentosa masquerade. Ophthalmology 2019; 126: 1694. [DOI] [PubMed] [Google Scholar]
- 39. Dalvin LA, Abou Chehade JE, Chiang J, et al. Retinitis pigmentosa associated with a mutation in BEST1. Am J Ophthalmol Case Rep 2016; 2: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson AA, Guziewicz KE, Lee CJ, et al. Bestrophin 1 and retinal disease. Prog Retin Eye Res 2017; 58: 45–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Constable PA, Bach M, Frishman LJ, et al. ISCEV standard for clinical electro-oculography (2017 update). Doc Ophthalmol 2017; 134: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Testa F, Rossi S, Passerini I, et al. A normal electro-oculography in a family affected by best disease with a novel spontaneous mutation of the BEST1 gene. Br J Ophthalmol 2008; 92: 1467–1470. [DOI] [PubMed] [Google Scholar]
- 43. Pomares E, Burés-Jelstrup A, Ruiz-Nogales S, et al. Nonsense-mediated decay as the molecular cause for autosomal recessive bestrophinopathy in two unrelated families. Invest Ophthalmol Vis Sci 2012; 53: 532–537. [DOI] [PubMed] [Google Scholar]
- 44. Nguyen TT, Poornachandra B, Verma A, et al. Next generation sequencing identifies novel disease-associated BEST1 mutations in bestrophinopathy patients. Sci Rep 2018; 8: 10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Andrade RE, Farah ME, Costa RA. Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization in best disease. Am J Ophthalmol 2003; 136: 1179–1181. [DOI] [PubMed] [Google Scholar]
- 46. Hussain RN, Shahid FL, Empeslidis T, et al. Use of intravitreal bevacizumab in a 9-year-old child with choroidal neovascularization associated with autosomal recessive bestrophinopathy. Ophthalmic Genet 2015; 36: 265–269. [DOI] [PubMed] [Google Scholar]
- 47. Chowers I, Zamir E, Banin E, et al. Blunt trauma in Best’s vitelliform macular dystrophy. Br J Ophthalmol 2000; 84: 1330–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Petrukhin K, Koisti MJ, Bakall B, et al. Identification of the gene responsible for Best macular dystrophy. Nat Genet 1998; 19: 241–247. [DOI] [PubMed] [Google Scholar]
- 49. Stone EM, Nichols BE, Streb LM, et al. Genetic linkage of vitelliform macular degeneration (Best’s disease) to chromosome 11q13. Nat Genet 1992; 1: 246–250. [DOI] [PubMed] [Google Scholar]
- 50. Marquardt A, Stöhr H, Passmore LA, et al. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best’s disease). Hum Mol Genet 1998; 7: 1517–1525. [DOI] [PubMed] [Google Scholar]
- 51. Oh SJ, Woo J, Lee YS, et al. Direct interaction with 14-3-3γ promotes surface expression of Best1 channel in astrocyte. Mol Brain 2017; 10: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soria RB, Spitzner M, Schreiber R, et al. Bestrophin-1 enables Ca2+-activated Cl- conductance in epithelia. J Biol Chem 2009; 284: 29405–29412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Milenkovic A, Schmied D, Tanimoto N, et al. The Y227N mutation affects bestrophin-1 protein stability and impairs sperm function in a mouse model of Best vitelliform macular dystrophy. Biol Open 2019; 8: bio041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carter DA, Smart MJK, Letton WVG, et al. Mislocalisation of BEST1 in iPSC-derived retinal pigment epithelial cells from a family with autosomal dominant vitreoretinochoroidopathy (ADVIRC). Sci Rep 2016; 6: 33792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Milenkovic VM, Langmann T, Schreiber R, et al. Molecular evolution and functional divergence of the bestrophin protein family. BMC Evol Biol 2008; 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Milenkovic VM, Rivera A, Horling F, et al. Insertion and topology of normal and mutant bestrophin-1 in the endoplasmic reticulum membrane. J Biol Chem 2007; 282: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 57. Tsunenari T, Sun H, Williams J, et al. Structure-function analysis of the bestrophin family of anion channels. J Biol Chem 2003; 278: 41114–41125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kane Dickson V, Pedi L, Long SB. Structure and insights into the function of a Ca2+-activated Cl− channel. Nature 2014; 516: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang T, Liu Q, Kloss B, et al. Structure and selectivity in bestrophin ion channels. Science 2014; 346: 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smith JJ, Nommiste B, Carr AJF. Bestrophin1: a gene that causes many diseases. Adv Exp Med Biol 2019; 1185: 419–423. [DOI] [PubMed] [Google Scholar]
- 61. Strauss O. The retinal pigment epithelium in visual function. Physiol Rev 2005; 85: 845–881. [DOI] [PubMed] [Google Scholar]
- 62. Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology 2010; 25: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hartzell C. Looking chloride channels straight in the eye: bestrophins, lipofuscinosis, and retinal degeneration. Physiology 2005; 20: 292–302. [DOI] [PubMed] [Google Scholar]
- 64. Reichhart N, Strauß O. Ion channels and transporters of the retinal pigment epithelium. Exp Eye Res 2014; 126: 27–37. [DOI] [PubMed] [Google Scholar]
- 65. Strick DJ, Vollrath D. Focus on molecules: MERTK. Exp Eye Res 2010; 91: 786–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wimmers S, Karl MO, Strauss O. Ion channels in the RPE. Prog Retin Eye Res 2007; 26: 263–301. [DOI] [PubMed] [Google Scholar]
- 67. Sun H, Tsunenari T, Yau K-W, et al. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci USA 2002; 99: 4008–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Marmorstein AD, Kinnick TR, Stanton JB, et al. Bestrophin-1 influences transepithelial electrical properties and Ca 2 + signaling in human retinal pigment epithelium. Mol Vis 2015; 21: 347–359. [PMC free article] [PubMed] [Google Scholar]
- 69. Strauss O, Milenkovic V, Striessnig J, et al. Direct interaction of Bestrophin-1 and beta-subunits of voltage-dependent calcium channels. Investig Ophthalmol Vis Sci 2008; 49: 5182. [Google Scholar]
- 70. Wu J, Marmorstein AD, Striessnig J, et al. Voltage-dependent calcium channel CaV1.3 subunits regulate the light peak of the electroretinogram. J Neurophysiol 2007; 97: 3731–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rosenthal R, Bakall B, Kinnick T, et al. Expression of bestrophin-1, the product of the VMD2 gene, modulates voltage-dependent Ca2+ channels in retinal pigment epithelial cells. FASEB J 2006; 20: 178–180. [DOI] [PubMed] [Google Scholar]
- 72. Zhang Y, Kittredge A, Ward N, et al. ATP activates bestrophin ion channels through direct interaction. Nat Commun 2018; 9: 3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Strauß O, Müller C, Reichhart N, et al. The role of bestrophin-1 in intracellular Ca2+ signaling. Adv Exp Med Biol 2014; 801: 113–119. [DOI] [PubMed] [Google Scholar]
- 74. Gómez NM, Tamm ER, Strauβ O. Role of bestrophin-1 in store-operated calcium entry in retinal pigment epithelium. Pflugers Arch 2013; 465: 481–495. [DOI] [PubMed] [Google Scholar]
- 75. Fischmeister R, Hartzell HC. Volume sensitivity of the bestrophin family of chloride channels. J Physiol 2005; 562: 477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Qu Z, Hartzell HC. Bestrophin Cl- channels are highly permeable to HCO3-. Am J Physiol Cell Physiol 2008; 294: C1371–C1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Woo DH, Han K-SS, Shim JW, et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 2012; 151: 25–40. [DOI] [PubMed] [Google Scholar]
- 78. Lee S, Yoon BE, Berglund K, et al. Channel-mediated tonic GABA release from glia. Science 2010; 330: 790–796. [DOI] [PubMed] [Google Scholar]
- 79. Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development 2000; 127: 3581–3591. [DOI] [PubMed] [Google Scholar]
- 80. Carricondo PC, Andrade T, Prasov L, et al. Nanophthalmos: a review of the clinical spectrum and genetics. J Ophthalmol 2018; 2018: 2735465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Peterson WM, Meggyesy C, Yu K, et al. Extracellular ATP activates calcium signaling, ion, and fluid transport in retinal pigment epithelium. J Neurosci 1997; 17: 2324–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Marmorstein AD, Stanton JB, Yocom J, et al. A model of best vitelliform macular dystrophy in rats. Invest Ophthalmol Vis Sci 2004; 45: 3733–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Marmorstein LY, Wu J, McLaughlin P, et al. The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by Bestrophin (Best-1). J Gen Physiol 2006; 127: 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang Y, Stanton JB, Wu J, et al. Suppression of Ca2+ signaling in a mouse model of Best disease. Hum Mol Genet 2010; 19: 1108–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Huber G, Heynen S, Imsand C, et al. Novel rodent models for macular research. PLoS ONE 2010; 5: e13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Beltran WA, Cideciyan AV, Guziewicz KE, et al. Canine retina has a primate fovea-like bouquet of cone photoreceptors which is affected by inherited macular degenerations. PLoS ONE 2014; 9: e90390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zangerl B, Wickström K, Slavik J, et al. Assessment of canine BEST1 variations identifies new mutations and establishes an independent bestrophinopathy model (cmr3). Mol Vis 2010; 16: 2791–2804. [PMC free article] [PubMed] [Google Scholar]
- 88. Guziewicz KE, Cideciyan AV, Beltran WA, et al. BEST1 gene therapy corrects a diffuse retina-wide microdetachment modulated by light exposure. Proc Natl Acad Sci USA 2018; 115: E2839–E2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Guziewicz KE, Zangerl B, Lindauer SJ, et al. Bestrophin gene mutations cause canine multifocal retinopathy: a novel animal model for best disease. Invest Ophthalmol Vis Sci 2007; 48: 1959–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Guziewicz KE, Slavik J, Lindauer SJP, et al. Molecular consequences of BEST1 gene mutations in canine multifocal retinopathy predict functional implications for human bestrophinopathies. Investig Ophthalmol Vis Sci 2011; 52: 4497–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Guziewicz KE, Sinha D, Gómez NM, et al. Bestrophinopathy: an RPE-photoreceptor interface disease. Prog Retin Eye Res 2017; 58: 70–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guziewicz KE, McTish E, Dufour VL, et al. Underdeveloped RPE apical domain underlies lesion formation in canine bestrophinopathies. Adv Exp Med Biol 2018; 1074: 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Johnson AA, Lee Y-S, Chadburn AJ, et al. Disease-causing mutations associated with four bestrophinopathies exhibit disparate effects on the localization, but not the oligomerization, of Bestrophin-1. Exp Eye Res 2014; 121: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marmorstein AD. The polarity of the retinal pigment epithelium. Traffic 2001; 2: 867–872. [DOI] [PubMed] [Google Scholar]
- 95. Johnson AA, Lee YS, Brett Stanton J, et al. Differential effects of best disease causing missense mutations on bestrophin-1 trafficking. Hum Mol Genet 2013; 22: 4688–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Carr A-JF, Vugler AA, Hikita S, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS ONE 2009; 4: e8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brandl C, Zimmermann SJ, Milenkovic VM, et al. In-depth characterisation of retinal pigment epithelium (RPE) cells derived from human induced pluripotent stem cells (hiPSC). NeuroMol Med 2014; 16: 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Carter DA, Nommiste B, Coffey PJ, et al. Spontaneous generation of patient-specific retinal pigment epithelial cells using induced pluripotent stem cell technology. In: Davidson Negraes P, Ulrich H. (eds) Working with stem cells. Cham: Springer, 2016, pp. 143–162. [Google Scholar]
- 99. Marmorstein AD, Johnson AA, Bachman LA, et al. Mutant Best1 expression and impaired phagocytosis in an iPSC model of autosomal recessive bestrophinopathy. Sci Rep 2018; 8: 4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nachtigal AL, Milenkovic A, Brandl C, et al. Mutation-dependent pathomechanisms determine the phenotype in the bestrophinopathies. Int J Mol Sci 2020; 21: 1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Moshfegh Y, Velez G, Li Y, et al. BESTROPHIN1 mutations cause defective chloride conductance in patient stem cell-derived RPE. Hum Mol Genet 2016; 25: 2672–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ji C, Li Y, Kittredge A, et al. Investigation and restoration of BEST1 activity in patient-derived RPEs with dominant mutations. Sci Rep 2019; 9: 19026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Li Y, Zhang Y, Xu Y, et al. Patient-specific mutations impair BESTROPHIN1’s essential role in mediating Ca2+-dependent Cl-currents in human RPE. Elife 2017; 6: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sinha D, Steyer B, Shahi PK, et al. Human iPSC modeling reveals mutation-specific responses to gene therapy in a genotypically diverse dominant maculopathy. Am J Hum Genet 2020; 107: 278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jinek M, Chylinski K, Fonfara I, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012; 337: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ryu SM, Hur JW, Kim K. Evolution of CRISPR towards accurate and efficient mammal genome engineering. BMB Rep 2019; 52: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lino CA, Harper JC, Carney JP, et al. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv 2018; 25: 1234–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang XH, Tee LY, Wang XG, et al. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol Ther Nucleic Acids 2015; 4: e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yang T, Justus S, Li Y, et al. BEST1: the best target for gene and cell therapies. Mol Ther 2015; 23: 1805–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Uggenti C, Briant K, Streit AK, et al. Restoration of mutant bestrophin-1 expression, localisation and function in a polarised epithelial cell model. Dis Model Mech 2016; 9: 1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Singh R, Kuai D, Guziewicz KE, et al. Pharmacological modulation of photoreceptor outer segment degradation in a human iPS cell model of inherited macular degeneration. Mol Ther 2015; 23: 1700–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Liu J, Taylor RL, Baines RA, et al. Small molecules restore Bestrophin 1 expression and function of both dominant and recessive bestrophinopathies in patient-derived retinal pigment epithelium. Investig Ophthalmol Vis Sci 2020; 61: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]