Abstract
Background:
Axillary node status is used in clinical practice to guide the selection of axillary surgery in breast cancer patients. However, to date, the optimal axillary management following neoadjuvant therapy (NAT) for breast cancer remains controversial. Our study aimed to investigate the association of molecular subtype, clinical stage, and ypN status after NAT in breast cancer patients, especially those achieving breast pathological complete remission (pCR).
Patients and methods:
Patients receiving ⩾4 cycles of NAT were retrospectively included between January 2009 and January 2020. ypN status was compared among patients with different breast pCR statuses, clinical stages, and molecular subtypes in univariate and multivariate analyses.
Results:
A total of 1999 patients were included: 457 (22.86%), 884 (44.22%), and 658 (32.92%) patients with cT1-2N0, cT1-2N1, and locally advanced breast cancer (LABC), respectively. Altogether, 435 (21.8%) patients achieved breast pCR: 331 with ypN– and 104 with ypN+ status. Patients achieving breast pCR had a significantly lower ypN+ rate than those without pCR [23.9% versus 62.5%, odds ratio (OR) = 0.14, 95% confidence interval (CI) = 0.09–0.21]. For patients with breast pCR, the ypN+ rate was 6.4%, 25.7%, and 33.9% in cT1-2N0, cT1-2N1, and LABC patients, respectively (p < 0.001). Furthermore, the ypN+ rate was 30.8%, 16.8%, 17.5%, 29.6%, and 27.6% in breast pCR patients with the Luminal A, Luminal B (HER2+), HER2-amplified, Luminal B (HER2–), and triple-negative subtype, respectively. Luminal B (HER2+) (OR = 0.20, 95% CI = 0.05–0.82) and HER2-amplified (OR = 0.19, 95% CI = 0.05–0.83) tumors were associated with lower ypN+ rates. Moreover, 100% of breast pCR patients with cT1-2N0 and HER2-positive disease achieved pathological pN0.
Conclusion:
In breast pCR patients after NAT, clinical stage and molecular subtype were significantly associated with ypN status. Patients with cT1-2N0 and HER2-positive disease who achieved breast pCR had a very low ypN+ rate, possibly indicating the possibility for de-escalation of axillary surgery in this patient subgroup.
Keywords: breast pathological complete remission, clinical stage, molecular subtype, neoadjuvant therapy, nodal residual burden
Introduction
Over the past decades, neoadjuvant therapy (NAT) has emerged as a preferred option for locally advanced breast cancer (LABC) and has been increasingly used in operable patients, especially those with a relatively large tumor or axillary lymph node (ALN)-positive, human epidermal growth factor receptor-2 (HER2)-positive, or triple-negative disease.1–3 NAT has a demonstrated ability to downstage the primary tumor, increase operability, facilitate breast conservation, and test in vivo treatment response.2
Patients who achieve pathological complete remission (pCR) after NAT have significantly superior clinical outcomes compared with those with residual disease.4–7 Therefore, achieving pCR is the foremost goal of NAT. As reported in previous studies, the total pCR rate is estimated to be 18–28% and to vary across different molecular subtypes, ranging from 8% to 11% in hormone receptor (HR)-positive/HER2-negative disease and 17–32% in HR-positive/HER2-positive disease to 33–51% in HR-negative/HER2-positive disease and 31–36% in triple-negative disease.1,2,8 The neoadjuvant treatment regimen can impact the pCR rate. For example, the addition of taxanes to doxorubicin and cyclophosphamide improved response to neoadjuvant therapy from 13% to 27%.9 The combination of dual anti-HER2-targeted therapy with standard chemotherapies significantly increased the pCR rate, subsequently ranging from 45% to 62%.10 Besides, clinical tumor stage is also an important predictor for pCR.11
In general, breast pCR can be achieved in 23–28% of patients and can reach a rate of around 70% or higher in HER2-positive/HR-negative patients treated with standard chemotherapy plus trastuzumab and pertuzumab.2,12,13 Of note, breast pCR was demonstrated to be associated with statistically significantly lower axillary residual burden.12,14 The rate of axillary ypN0 in breast pCR patients was twice higher than that in patients without breast pCR.12,14 In specific cN0 patients with HER2-positive or triple-negative disease, Barron et al.15 found that the ypN+ rate was less than 2% for those patients achieving breast pCR. Mougalian et al.16 found that axillary pCR was associated with statistically significantly better 10-year overall survival (84% versus 57%, p < 0.001) and recurrence-free survival (79% versus 50%, p < 0.001) compared with axillary residual disease.
While axillary node status is used to guide the subsequent selection of axillary surgery, optimal axillary management following NAT remains controversial. Currently, sentinel lymph node biopsy (SLNB) after NAT in cN0 patients is reliable and has a similar accuracy to that of upfront SLNB.17 Nevertheless, the use of SLNB in cN+ patients still lacks consensus, even though it shows reliable accuracy when dual tracers are used or ⩾3 sentinel nodes are removed.17,18 NAT has been shown to increase the rates of ypN0 compared with upfront surgery without NAT by 1.5–3.6-fold in cN0 patients and to convert 20–61% of cN+ patients with different molecular subtypes to ypN0 status.19 Given the decreased ypN+ rate after NAT, especially in breast pCR patients, the potential for reducing or eliminating axillary surgery warrants further research. Therefore, our study was designed to investigate the association of clinical stage and molecular subtype with ypN status after NAT and to identify potential predictors for ypN status in patients with different breast response to NAT, with the view to guide an optimal ALN surgical approach.
Patients and methods
Study population
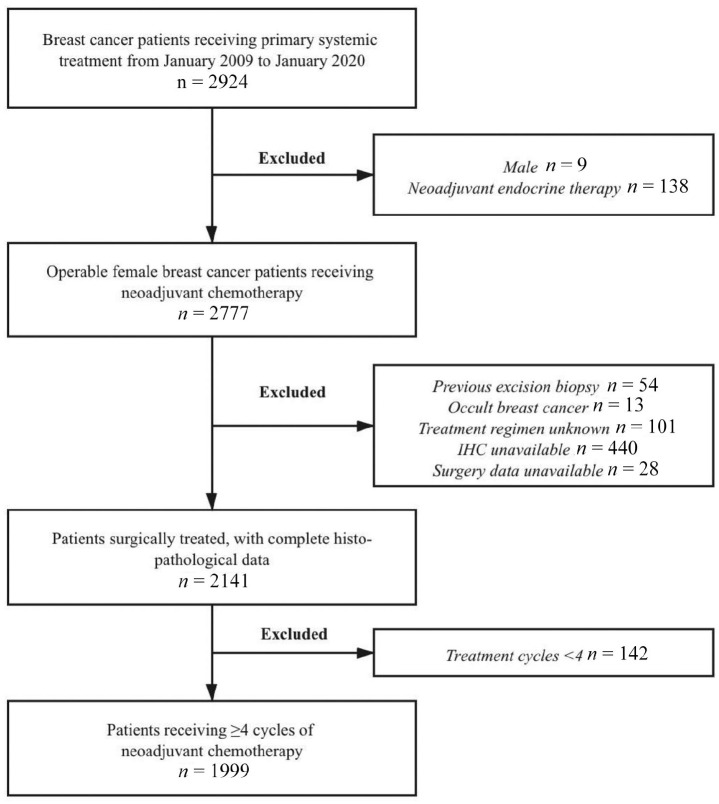
Consecutive patients diagnosed with invasive breast cancer between January 2009 and January 2020 were retrospectively reviewed. Data were derived from the Shanghai Jiao Tong University Breast Cancer Database (SJTU-BCDB), which included more than 30,000 breast cancer cases from 32 medical centers in China. Patients meeting the following criteria were enrolled: female patients; invasive breast cancer diagnosed by core needle biopsy (CNB) before NAT with complete clinicopathological information; receiving ⩾4 cycles of neoadjuvant chemotherapy with or without anti-HER2 targeted therapy (trastuzumab ± pertuzumab); undergoing standard breast and axillary surgery with complete histo-pathological data after NAT. Exclusion criteria were as follows: male patients; patients receiving neoadjuvant endocrine therapy alone; patients who underwent tumor excision biopsy before NAT; patients diagnosed with occult breast cancer; patients with unknown neoadjuvant regimens; patients without accurate pathological data or surgery information; and patients who received <4 cycles of NAT (Figure 1).
Figure 1.
Study population flowchart.
IHC, immunohistochemistry.
Neoadjuvant regimens were determined at the physicians’ discretion and classified into three categories: regimens containing anthracyclines, such as CEF [cyclophosphamide (C), epirubicin (E), and fluorouracil (F)] and EC; regimens containing taxanes, such as TC [docetaxel (T)], PCb [paclitaxel (P), carboplatin (Cb)] and TCb; and regimens combining anthracyclines and taxanes, such as EC-T, TEC, dose-dense EC-weekly P, and ET. HER2 positive breast cancer patients were also recommended to receive trastuzumab ± pertuzumab treatment as anti-HER2 targeted therapy.
This study was approved by the independent Ethical Committees of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (2020-309). Patients identity remained anonymous, and the requirement for informed consent was waived due to the observational nature of the study. All human-related procedures were in concordance with the 1964 Helsinki Declaration and its later amendments.
Clinical and pathological assessment
Clinical tumor and nodal status before NAT were determined through physical examination (PE) by experienced physicians and diagnostic ultrasound by at least two independent radiologists. No suspicious lymph node under PE and ultrasound or a negative cytological test result confirmed by ultrasound-guided fine-needle aspiration was defined as cN0. Clinical TNM staging of breast cancer was based on the American Joint Committee on Cancer cancer staging manual (eighth edition, 2017). Patients with clinical stage III or cT3N0 disease were classified as LABC.20 Comparisons were done among the cT1-2N0, cT1-2N1, and LABC groups.
Diagnosis of breast cancer was made by CNB before NAT. Histopathological and immunohistochemical (IHC) evaluation of tumors was accomplished by at least two independent experienced pathologists. The applied positivity criteria followed the 2018 American Society of Clinical Oncology/College of American Pathologists guidelines. Samples were defined as positive for estrogen receptor (ER) or progesterone receptor (PR) if no less than 1% of the invasive tumor cells stained positive by IHC. A 3+ IHC result for CerbB-2 or HER2 gene amplification confirmed by florescent in situ hybridization was regarded as HER2 positivity. According to the 2013 St Gallen international expert consensus,21 tumors were divided into five molecular subtypes: Luminal A (ER positive, PR ⩾20% positive, and Ki67 <14%); Luminal B (HER2–) (ER positive, PR <20% positive or Ki67 ⩾14%, and HER2 negative); Luminal B (HER2+) (ER and/or PR positive and HER2 positive); HER2-amplified (ER and PR negative and HER2 positive), and triple-negative breast cancer (ER, PR, and HER2 negative). Pathological response of the breast and lymph node was evaluated after standard surgery. Breast pCR was defined as the absence of invasive breast cancer in the primary breast tumor (ypT0/is).
Statistical analysis
Univariate analysis with two-sided Pearson Chi-square test was applied to compare categorical variables among tumors with different clinical stages, molecular subtypes, and pathological response to NAT. Multivariable logistic regression models were used to determine the impact factors for pathological nodal response and variables with p value < 0.05 in the univariate analysis were included. Data assessment was achieved by using IBM SPSS statistics software version 23 (SPSS, Inc., Chicago, IL, USA). GraphPad Prism version 7.0 (GraphPad Software, CA, USA) was used for image production. Two-sided p < 0.05 was considered statistically significant.
Results
Baseline characteristics
Altogether, 1999 breast cancer patients were included in the study (Figure 1). Patients’ clinicopathological characteristics are shown in Table 1. The median age was 50 (ranging from 21 to 83) years old. Invasive ductal carcinoma was diagnosed in 1880 (94.05%) of the enrolled population. Grade I–II and III tumors were found in 633 and 346 patients, respectively. A total of 540 patients were classified as cN0 cases. cT1-2N0, cT1-2N1, and LABC disease were found in 457 (22.9%), 884 (44.2%), and 658 (32.9%) patients, respectively. The Luminal A, Luminal B (HER2–), Luminal B (HER2+), HER2-amplified, and triple-negative molecular subtypes represented 5.7%, 43.0%, 21.9%, 14.1%, and 15.3% of the study population, respectively.
Table 1.
Baseline characteristics of study population.
| Characteristics | Total |
cT1-2N0 |
cT1-2N1 |
LABC |
p-value |
|---|---|---|---|---|---|
| N = 1999 | n = 457 (%) | n = 884 (%) | n = 658 (%) | ||
| Age, years | 0.658 | ||||
| <50 | 976 | 230 (50.3) | 433 (49.0) | 313 (47.6) | |
| ⩾50 | 1023 | 227 (49.7) | 451 (51.0) | 345 (52.4) | |
| Menopausal status | 0.089 | ||||
| Premenopausal | 1093 | 255 (55.8) | 501 (56.7) | 337 (51.2) | |
| Postmenopausal | 906 | 202 (44.2) | 383 (43.3) | 321 (48.8) | |
| Histology | 0.027 | ||||
| IDC | 1880 | 418 (22.2) | 840 (44.7) | 622 (33.1) | |
| Other invasive | 119 | 39 (32.8) | 44 (37.0) | 36 (30.3) | |
| Tumor grade | <0.001 | ||||
| I–II | 633 | 211 (46.2) | 281 (31.8) | 141 (21.4) | |
| III | 346 | 67 (14.7) | 144 (16.3) | 135 (20.5) | |
| NA | 1020 | 179 (39.2) | 459 (51.9) | 382 (58.1) | |
| ER | 0.223 | ||||
| Negative | 626 | 150 (32.8) | 259 (29.3) | 217 (33.0) | |
| Positive | 1373 | 307 (67.2) | 625 (70.7) | 441 (67.0) | |
| PR | 0.163 | ||||
| Negative | 807 | 184 (40.3) | 339 (38.3) | 284 (43.2) | |
| Positive | 1192 | 273 (59.7) | 545 (61.7) | 374 (56.8) | |
| HER2 | 0.409 | ||||
| Negative | 1280 | 289 (63.2) | 580 (65.6) | 411 (62.5) | |
| Positive | 719 | 168 (36.8) | 304 (34.4) | 247 (37.5) | |
| Ki-67 (%) | 0.004 | ||||
| <14 | 282 | 84 (18.4) | 114 (12.9) | 84 (12.8) | |
| ⩾14 | 1670 | 356 (77.9) | 755 (85.4) | 559 (85.0) | |
| NA | 47 | 17 (3.7) | 15 (1.7) | 15 (2.2) | |
| Molecular subtype | 0.237 | ||||
| Luminal-A like | 115 | 33 (7.2) | 49 (5.5) | 33 (5.5) | |
| Luminal-B (HER2–) | 859 | 181 (39.6) | 398 (45.0) | 280 (42.6) | |
| Luminal-B (HER2+) | 437 | 103 (22.5) | 196 (22.2) | 138 (21.0) | |
| HER2-amplified | 282 | 65 (14.2) | 108 (12.2) | 109 (16.6) | |
| TNBC | 306 | 75 (16.4) | 133 (15.0) | 98 (14.9) | |
| NAC regimen | <0.001 | ||||
| Containing A | 174 | 72 (15.8) | 70 (7.9) | 32 (4.9) | |
| Containing T | 400 | 96 (21.0) | 175 (19.8) | 129 (19.6) | |
| A+T combination | 1425 | 289 (63.2) | 639 (72.3) | 497 (75.5) | |
| Targeted therapy | 0.041 | ||||
| Yes | 480 | 91 (19.9) | 215 (24.3) | 174 (26.4) | |
| No | 1519 | 366 (80.1) | 669 (75.7) | 484 (73.6) | |
| NAC cycles | <0.001 | ||||
| 4–6 | 1284 | 311 (68.1) | 592 (67.0) | 381 (57.9) | |
| >6 | 715 | 146 (31.9) | 292 (33.0) | 277 (42.1) | |
| Breast surgery | <0.001 | ||||
| BCS | 196 | 68 (14.9) | 86 (9.7) | 42 (6.4) | |
| Mastectomy | 1803 | 389 (85.1) | 798 (90.3) | 616 (93.6) | |
| ALN surgery | <0.001 | ||||
| SLNB | 119 | 82 (17.9) | 19 (2.1) | 18 (2.7) | |
| ALND | 1880 | 375 (82.1) | 865 (97.9) | 640 (97.3) |
A, anthracyclines; ALN, axillary lymph node; ALND, axillary lymph node dissection; BCS, breast conserving surgery; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IDC, invasive ductal carcinoma; LABC, locally advanced breast cancer; NA, not available; NAC, neoadjuvant chemotherapy; PR, progesterone receptor; SLNB, sentinel lymph node biopsy; T, taxanes; TNBC, triple negative breast cancer.
In terms of treatment regimens, 1425 (71.3%) patients were treated with NAT using a combination of anthracyclines and taxanes. Targeted therapy was given in 24.0% of the whole patient population, accounting for 66.8% of the HER2-positive patients. Moreover, 1284 (64.2%) patients received 4–6 cycles of NAT and 715 (35.8%) received more than six cycles. After NAT, mastectomy and axillary lymph node dissection were carried out in 90.2% and 94.1% of patients, respectively.
Univariate analysis showed that histologic type (p = 0.027), tumor grade (p < 0.001), Ki-67 (p = 0.004), neoadjuvant chemotherapy regimen (p < 0.001), targeted therapy application (p = 0.041), NAT cycles (p < 0.001), and breast surgery (p < 0.001) and axillary surgery (p < 0.001) types were differently distributed between cT1-2N0, cT1-2N1, and LABC disease (Table 1). Patients with higher clinical stage were more likely to receive a combination of anthracyclines and taxanes as NAT, more NAT cycles, targeted therapy, subsequent mastectomy, and axillary dissection.
Pathological response in the whole study population
Upon completion of NAT, 435 (21.8%) patients achieved breast pCR and 917 (45.9%) achieved ypN0. Breast pCR rates for cT1-2N0, cT1-2N1, and LABC disease were 20.6%, 24.2%, and 19.3%, respectively (p = 0.054). Meanwhile, the ypN+ rates were 24.5%, 60.3%, and 66.4% in these three categories (p < 0.001). Furthermore, the breast pCR rate was 11.3% for Luminal A, 18.5% for Luminal B (HER2–), 24.5% for Luminal B (HER2+), 28.4% for HER2-amplified, and 24.8% for triple negative breast cancer (TNBC) cases (p < 0.001) in the entire study population. Meanwhile, their ypN+ rates were 60.0%, 61.4%, 47.6%, 42.2%, and 52.0% (p < 0.001), respectively.
Univariate and multivariate analyses identified clinical stage (p < 0.001), grade (p = 0.045), neoadjuvant targeted therapy (p < 0.001), and the achievement of breast pCR (p < 0.001) as independent impact factors for nodal response in the whole study population (Supplemental material Table S1 online and Table 2). Compared with cT1-2N0 patients, the ypN+ rate was significantly higher in cT1-2N1 [odds ratio (OR) = 5.48, 95% confidence interval (CI) = 3.77–7.97, p < 0.001] and LABC (OR = 10.90, 95% CI = 7.12–16.70, p < 0.001) cases. Grade III tumors had higher odds of nodal residual disease compared with grade I–II tumors (OR = 1.39, 95% CI = 1.01–1.92, p = 0.045). Neoadjuvant targeted therapy greatly reduced the risk of ypN+ (OR = 0.40, 95% CI = 0.27–0.59, p < 0.001). Moreover, patients achieving breast pCR had a significantly lower ypN+ rate compared with patients without breast pCR (23.9% versus 62.5%, univariate p < 0.001; OR = 0.14, 95% CI = 0.09–0.21, p < 0.001).
Table 2.
Multivariate analysis of impact factors for ypN+ in the whole population.
| Factors | OR | 95% CI | p-value |
|---|---|---|---|
| Clinical stage | <0.001 | ||
| cT1-2N0 | 1 | ||
| cT1-2N1 | 5.48 | 3.77–7.97 | <0.001 |
| LABC | 10.90 | 7.12–16.70 | <0.001 |
| ER | 0.092 | ||
| Negative | 1 | ||
| Positive | 1.32 | 0.96–1.82 | |
| PR | 0.617 | ||
| Negative | 1 | ||
| Positive | 0.89 | 0.58–1.39 | |
| HER2 | 0.270 | ||
| Negative | 1 | ||
| Positive | 0.81 | 0.56–1.17 | |
| Molecular subtypes | 0.326 | ||
| Luminal-A like | 1 | ||
| Luminal-B (HER2–) | 1.19 | 0.64–2.25 | 0.583 |
| Luminal-B (HER2+) | 0.82 | 0.41–1.64 | 0.579 |
| HER2-amplified | 0.87 | 0.42–1.81 | 0.703 |
| TNBC | 0.81 | 0.40–1.63 | 0.552 |
| Grade | 0.045 | ||
| I–II | 1 | ||
| III | 1.39 | 1.01–1.92 | |
| NAC regimens | 0.232 | ||
| A contained | 1 | ||
| T contained | 0.81 | 0.44–1.48 | 0.491 |
| A+T combination | 1.17 | 0.72–1.89 | 0.531 |
| Neoadjuvant targeted therapy | <0.001 | ||
| No | 1 | ||
| Yes | 0.40 | 0.27–0.59 | |
| Breast pCR | <0.001 | ||
| No | 1 | ||
| Yes | 0.14 | 0.09–0.21 |
A, anthracyclines; CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; LABC, locally advanced breast cancer; NAC, neoadjuvant chemotherapy; OR, odds ratio; pCR, pathological complete remission; PR, progesterone receptor; T, taxanes; TNBC, triple negative breast cancer.
Axillary pathological response in breast pCR patients
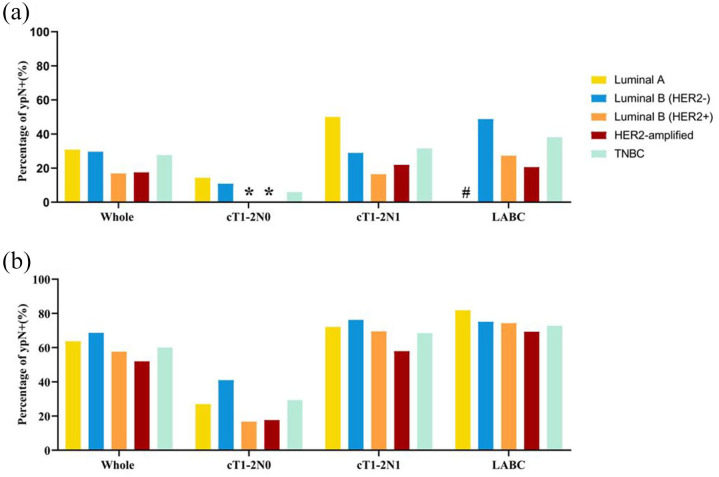
In patients with breast pCR, the ypN+ rate was 6.4%, 25.7%, and 33.9% in cT1-2N0, cT1-2N1, and LABC patients, respectively (p < 0.001; Table 3 and Figure 2).
Table 3.
Pathologic axillary lymph node status stratified by clinical stage and molecular subtype between patients with breast pathological complete remission (pCR) and non-pCR.
| Response in breast |
||||
|---|---|---|---|---|
| pCR |
Non-pCR |
|||
| ypN0 n (%) | ypN+ n (%) | ypN0 n (%) | ypN+ n (%) | |
| Whole population | 331 (76.1) | 104 (23.9) | 586 (37.5) | 978 (62.5) |
| cT1-2N0 | 88 (93.6) | 6 (6.4) | 257 (70.8) | 106 (29.2) |
| cT1-2N1 | 159 (74.3) | 55 (25.7) | 192 (28.7) | 478 (71.3) |
| LABC | 84 (66.1) | 43 (33.9) | 137 (25.8) | 394 (74.2) |
| Luminal-A like | 9 (69.2) | 4 (30.8) | 37 (36.3) | 65 (63.7) |
| cT1-2N0 | 6 (85.7) | 1 (14.3) | 19 (73.1) | 7 (26.9) |
| cT1-2N1 | 3 (50.0) | 3 (50.0) | 12 (27.9) | 31 (72.1) |
| LABC | 0 (0.0) | 0 (0.0) | 6 (18.2) | 27 (81.8) |
| Luminal-B like (HER2–) | 112 (70.4) | 47 (29.6) | 220 (31.4) | 480 (68.6) |
| cT1-2N0 | 33 (89.2) | 4 (10.8) | 85 (59.0) | 59 (41.0) |
| cT1-2N1 | 59 (71.1) | 24 (28.9) | 75 (23.8) | 240 (76.2) |
| LABC | 20 (51.3) | 19 (48.7) | 60 (24.9) | 181 (75.1) |
| Luminal-B like (HER2+) | 89 (83.2) | 18 (16.8) | 140 (42.4) | 190 (57.6) |
| cT1-2N0 | 19 (100.0) | 0 (0.0) | 70 (83.3) | 14 (16.7) |
| cT1-2N1 | 46 (83.6) | 9 (16.4) | 43 (30.5) | 98 (69.5) |
| LABC | 24 (72.7) | 9 (27.3) | 27 (25.7) | 78 (74.3) |
| HER2-amplified | 66 (82.5) | 14 (17.5) | 97 (48.0) | 105 (52.0) |
| cT1-2N0 | 14 (100.0) | 0 (0.0) | 42 (82.4) | 9 (17.6) |
| cT1-2N1 | 25 (78.1) | 7 (21.9) | 32 (42.1) | 44 (57.9) |
| LABC | 27 (79.4) | 7 (20.6) | 23 (30.7) | 52 (69.3) |
| TNBC | 55 (72.4) | 21 (27.6) | 92 (40.0) | 138 (60.0) |
| cT1-2N0 | 16 (94.1) | 1 (5.9) | 41 (70.7) | 17 (29.3) |
| cT1-2N1 | 26 (68.4) | 12 (31.6) | 30 (31.6) | 65 (68.4) |
| LABC | 13 (61.9) | 8 (38.1) | 21 (27.3) | 56 (72.7) |
HER2, human epidermal growth factor receptor 2; LABC, locally advanced breast cancer; pCR, pathological complete remission; TNBC, triple negative breast cancer.
Figure 2.
Pathologic nodal residual burden in patients with breast pathological complete remission (pCR) and non-pCR.
(a) Percentage of ypN+ in patients with breast pCR. (b) Percentage of ypN+ in patients with breast non-pCR.
*No residual nodal burden in breast pCR patients with the Luminal B (HER2+) or the HER2-amplified subtype.
#No patients with the Luminal A subtype in the LABC/breast pCR subgroup.
HER2, human epidermal growth factor receptor-2; LABC, locally advanced breast cancer; pCR, pathological complete remission; TNBC, triple-negative breast cancer.
When stratified by molecular subtype, the ypN+ rate was 30.8%, 29.6%, 16.8%, 17.5%, and 27.6% in the Luminal A, Luminal B (HER2–), Luminal B (HER2+), HER2-amplified, and triple-negative subtypes, respectively (p = 0.081). The ypN+ rate was similar in HER2+ breast pCR patients treated with or without anti-HER2 targeted therapy (16.9% versus 17.9%, p = 0.876; Supplemental Figure S1). Of note, in patients with cT1-2N0 and breast pCR after NAT, the ypN0 rate was 100% in Luminal B (HER2+) or HER2-amplified disease and 94.1% in triple-negative disease (Table 3 and Figure 2). A similar result was found when breast pCR was defined as ypT0, with patients with cT1-2N0 and HER2-positive disease who achieved breast pCR also having a 100% ypN0 rate (Supplemental Table S4 and Supplemental Figure S2).
Multivariate analyses found that initial clinical stage (p < 0.001), PR status (p = 0.036), HER2 status (p = 0.001), and molecular subtype (p = 0.008) were substantially correlated to the ypN+ rate in the breast pCR population (Table 4). Compared with cT1-2N0 patients, the ypN+ rate was significantly higher in the cT1-2N1 (OR = 5.64, 95% CI = 2.31–13.76, p < 0.001) and the LABC (OR = 9.80, 95% CI = 3.88–24.77, p < 0.001) subgroup. PR positivity increased the odds of having nodal residual disease (OR = 1.66, 95% CI = 1.03–2.67, p = 0.036). In contrast, HER2 positivity was associated with lower proportion of ypN+ (OR = 0.45, 95% CI = 0.27–0.73, p = 0.001). Meanwhile, the ypN+ rate was significantly lower in the Luminal B (HER2+) (OR = 0.20, 95% CI = 0.05–0.82, p = 0.025) and HER2-amplified (OR = 0.19, 95% CI = 0.05–0.83, p = 0.026) subtypes, compared with the Luminal A subtype.
Table 4.
Multivariate analysis of impact factors for ypN+ in breast pathological complete remission patients.
| Factors | OR | 95% CI | p-value |
|---|---|---|---|
| Clinical stage | <0.001 | ||
| cT1-2N0 | 1 | ||
| cT1-2N1 | 5.64 | 2.31–13.76 | <0.001 |
| LABC | 9.80 | 3.88–24.77 | <0.001 |
| PR | 0.036 | ||
| Negative | 1 | ||
| Positive | 1.66 | 1.03–2.67 | |
| HER2 | 0.001 | ||
| Negative | 1 | ||
| Positive | 0.45 | 0.27–0.73 | |
| Molecular subtypes | 0.008 | ||
| Luminal-A like | 1 | ||
| Luminal-B (HER2–) | 0.48 | 0.12–1.85 | 0.286 |
| Luminal-B (HER2+) | 0.20 | 0.05–0.82 | 0.025 |
| HER2-amplified | 0.19 | 0.05–0.83 | 0.026 |
| TNBC | 0.42 | 0.10–1.71 | 0.226 |
| Neoadjuvant targeted therapy | 0.483 | ||
| Yes | 1 | ||
| No | 1.30 | 0.63–2.69 |
CI, confidence interval; HER2, human epidermal growth factor receptor 2; LABC, locally advanced breast cancer; OR, odds ratio; PR, progesterone receptor; TNBC, triple negative breast cancer.
Axillary pathological response in breast non-pCR patients
The total ypN+ rate was 62.5% in breast non-pCR patients and 29.2%, 71.3%, and 74.2% in the cT1-2N0, cT1-2N1, and LABC subgroups (p < 0.001), respectively (Table 3 and Figure 2). Regarding molecular subtypes, the ypN+ rate was 63.7% in the Luminal A, 68.6% in the Luminal B (HER2–), 57.6% in the Luminal B (HER2+), 52.0% in the HER2-amplified, and 60% in the TNBC subtype (p < 0.001) (Table 2 and Figure 2).
Multivariate analyses showed that only initial clinical stage (p < 0.001) and neoadjuvant targeted therapy (p = 0.001) were independent impact factors for nodal response in breast non-pCR patients (Supplemental Table S2). As previously seen in the breast pCR population, the ypN+ rate in breast non-pCR patients was significantly higher in the cT1-2N1 (OR = 5.51, 95% CI = 3.71–8.16, p < 0.001) and LABC (OR = 10.63, 95% CI = 6.76–16.72, p < 0.001) subtypes compared with the cT1-2N0 subtype. The omission of targeted therapy in the neoadjuvant setting was associated with increased risk of ypN+ (OR = 2.15, 95% CI = 1.39–3.32, p = 0.001) (Supplemental Table S2). Molecular subtype was no longer independently associated with axillary response in the breast non-pCR population (p = 0.634).
Discussion
In our large cohort of breast cancer patients who received NAT, the overall breast pCR rate was 21.8% and the ypN0 rate was 45.9%. Our results suggest that clinical stage and molecular subtype are significantly associated with ypN status in breast pCR patients. Furthermore, in breast pCR patients with cT1-2N0 and HER2-positive disease, 100% of patients were found to achieve ypN0. Our findings thus support that elimination of axillary surgery should be considered in these sub-populations.
Both breast pCR and ypN0 status were significantly associated with survival in breast cancer patients.2 Many studies have investigated possible factors related to pCR with the view to further improve treatment efficacy. Tumor biology has been an important factor for predicting pathological response in both the breast and axilla. The American College of Surgeons Oncology Group Z1071 study found that the pCR rate was the highest in the HER2-positive subtype (45.4%) and the lowest in the HR-positive/HER2-negative subtype (11.4%), with an intermediate value for the triple-negative subtype (38.2%) (p < 0.001). A similar pattern across tumor subtypes was found in one of our previous studies assessing breast pCR and ypN0 rates separately.8 In addition, in a cohort of 148 cN0 patients treated with neoadjuvant chemotherapy, Shi et al.22 observed that the ypN0 rates were significantly higher in HER2-positive (95.5%) and triple-negative (94.6%) breast cancer patients, compared with HR-positive/HER2-negative patients (p < 0.05). Consistent with our findings, they also found molecular subtype (OR = 2.37, p = 0.033) to be an independent predictor for ypN0 after full-course NAT.22 In our study, both the highest breast pCR and ypN0 rates were found in HER2-positive patients, and the lowest pCR rates were found in the HR-positive/HER2-negative subgroup. Multivariate analysis showed that molecular subtype was significantly associated with ypN status only in breast pCR patients, and not in the breast non-pCR population. Across our entire study cohort, HER2-positive patients treated with targeted therapy had a lower ypN+ rate than patients without targeted therapy (Supplemental Figure S1). Interestingly, in breast pCR patients with HER2-positive disease, anti-HER2-targeted therapy did not significantly impact the ypN0 rate, given that breast pCR was the strongest influencing factor for the ypN+ rate, regardless of the use of targeted therapy.
In clinical practice, the status of nodal residual disease is used to guide the choice of subsequent axillary surgery upon the completion of NAT. Currently, SLNB is recommended in cN0 patients after NAT, with a high detection accuracy and relatively low false-negative rate (FNR).23,24 The ypN+ rate was 24.5% in cT1-2N0 patients in our study, which was relatively higher than other studies.14,15 The reason might be attributed to insufficient treatment for pathological response assessment in these patients, as 68.1% of cT1-2N0 patients in our study received only 4–6 cycles of NAT. A retrospective study from Tadros et al.25 found a ypN0 rate of 100% in patients with cN0 and HER2-positive or triple-negative disease who reached breast pCR after NAT. In a larger cohort study, Barron et al.15 found a ypN+ rate <2% in breast pCR patients with cN0 HER2-positive or triple-negative disease. In our study, the ypN+ rate was extremely low (6.4%) in cT1-2N0 patients with breast pCR after NAT and a 100% ypN0 rate was found in the HER2-positive subgroup. In the triple-negative subgroup, the ypN0 rate was 94.1%, with only one patient out of 17 showing nodal residual disease. The same results were found when breast pCR was defined as the absence of invasive carcinoma or ductal carcinoma in situ in the breast (ypT0) (Supplemental Table S4 and Supplemental Figure S2). It remains to be determined whether ALN surgery is necessary for cN0 patients who achieved breast pCR, especially for patients with HER2-positive or triple-negative disease. To date, there is still insufficient evidence on the safety of omitting axillary surgery in these subpopulations, and further clinical trials should be conducted. Furthermore, the accuracy of breast pCR prediction is important for patients’ selection and current methods still lack sufficient sensitivity and specificity.26,27 Due to the low comorbidity of SLNB and limited accuracy methods to predict breast pCR, SLNB is still recommended and axillary lymph node dissection (ALND) can be avoided in SLNB negative patients. Moreover, Kuerer et al.27 reported that combined fine-needle aspiration and vacuum-assisted core biopsy had an accuracy of 98% for breast pCR prediction preoperatively. Therefore, prospective studies with accurate breast biopsy after NAT before surgery should be conducted to evaluate the feasibility and safety of completely avoiding axillary surgery in cT1-2N0 patients with HER2+ breast cancer achieving pCR in the breast.
Optimal axilla management following NAT in cN+ patients is still controversial. Although the use of SLNB has increased since 2013,18,28,29 several prospective studies have indicated that a low SLNB FNR (<10%) would be difficult to achieve, unless more than two sentinel lymph nodes are examined or dual tracers are applied.30–32 In the current study, all cN+ patients received upfront axillary dissection, with a ypN0 rate of 74.3% in the cT1-2N1 subgroup and of 66.1% in the LABC patients with breast pCR. Nearly two-thirds of axillary dissections might be spared if SLNB was properly done, while elimination of axillary surgery was not supported, even for those HER2+ or TNBC subtype. A study from Piltin et al.33 showed that SLNB surgery alone in selected patients with an excellent response to NAT was not inferior to ALND during short-term follow-up. Hence, SLNB might be considered in those patients who have complete breast response. On the other hand, in cT1-2N1 or LABC patients without breast pCR, the ypN+ rate was more than 70%. Among these ypN+ patients, 52.7% in the cT1-2N1 and 64.5% in the LABC subgroup had four or more lymph nodes involved (Supplemental Table S3), showing higher ypN+ rates than those in Choi’s study (45.3% for cN1 and 48.6% for cN2/3).12 Currently, axillary dissection might thus not be spared for cT1-2N1 or LABC patients without breast pCR.
Molecular subtypes and clinical stages also influenced the subsequent adjuvant therapies significantly (Supplemental Table S5). Among the 1532 patients with adjuvant treatment data, anti-HER2 targeted therapy was administrated in 68.8% of HER2 positive patients. Use of adjuvant chemotherapy was significantly associated with NAT cycles, 57.8% in these treated with 4–6 cycles and only 23.5% in patients receiving >6 cycles of NAT (OR = 0.30, p < 0.001), which was due to the not full courses of NAT for those with 4–6 cycles (Supplemental Table S6). Molecular subtypes were also significantly associated with adjuvant chemotherapy choice, and more patients with TNBC received adjuvant chemotherapy than patients with Luminal HER2– disease (OR = 2.00, p < 0.001). In terms of radiotherapy, clinical stage was an important factor. The proportions of radiation were higher in patients with LABC (OR = 3.98, p < 0.001) and cT1-2N1 (OR = 1.92, p < 0.001) disease compared with patients with cT1-2N0 breast cancer.
This study has some limitations. First, as a retrospective study, not all of the patients who were included had finished their scheduled NAT regimens. Second, the data in our study were derived from different medical centers, with the lack of a commonly defined protocol for the evaluation of clinical stage and pathological response. Third, some patients who received NAT were excluded due to missing data on treatment, IHC, or surgery, which may have affected the results.
Conclusions
Our study demonstrated that in breast pCR patients after NAT, clinical stage and molecular subtype were significantly associated with ypN status. More importantly, 100% of breast pCR patients with cT1-2N0 and HER2-positive disease achieved ypN0, indicating the possibility of omitting axillary surgery in those selected patients. For other patients with cT1-2N0 disease or breast pCR, SLNB was a recommended procedure under certain conditions. Meanwhile, in breast non-pCR patients with cT1-2N1 or LABC disease, axillary dissection should not be spared.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_1758835921996673 for Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery by Jin Hong, Yiwei Tong, Jianrong He, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-2-tam-10.1177_1758835921996673 for Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery by Jin Hong, Yiwei Tong, Jianrong He, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-3-tam-10.1177_1758835921996673 for Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery by Jin Hong, Yiwei Tong, Jianrong He, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-4-tam-10.1177_1758835921996673 for Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery by Jin Hong, Yiwei Tong, Jianrong He, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-5-tam-10.1177_1758835921996673 for Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery by Jin Hong, Yiwei Tong, Jianrong He, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-6-tam-10.1177_1758835921996673 for Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery by Jin Hong, Yiwei Tong, Jianrong He, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors are grateful to all the patients who participated in this study. The authors also want to thank all the doctors across the country who contributed patients’ data to the SJTU-BCDB for their work and support for this study.
Footnotes
Author contributions: KWS and XSC developed the main concept and contributed to the study design. JH, YWT, and JRH performed the data analysis and interpretation. JH and YWT drafted the manuscript. XSC and KWS contributed to the editing and critical revision of the manuscript. All authors reviewed and approved the final draft.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant number 81772797); the Shanghai Municipal Education Commission (20172007); and Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (GCQN-2017-A18, GCQN-2018-B11). All these financial sponsors had no role in the study design, data collection, analysis, or interpretation.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jin Hong, Department of General Surgery, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Yiwei Tong, Department of General Surgery, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Jianrong He, Department of General Surgery, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Xiaosong Chen, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, 197 Ruijin Er Road, Shanghai 200025, China.
Kunwei Shen, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, 197 Ruijin Er Road, Shanghai 200025, China.
References
- 1. Houssami N, Macaskill P, von Minckwitz G, et al. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 2012; 48: 3342–3354. [DOI] [PubMed] [Google Scholar]
- 2. von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30: 1796–1804. [DOI] [PubMed] [Google Scholar]
- 3. Mamounas EP. Impact of neoadjuvant chemotherapy on locoregional surgical treatment of breast cancer. Ann Surg Oncol 2015; 22: 1425–1433. [DOI] [PubMed] [Google Scholar]
- 4. Spring L, Greenup R, Niemierko A, et al. Pathologic complete response after neoadjuvant chemotherapy and long-term outcomes among young women with breast cancer. J Natl Compr Canc Netw 2017; 15: 1216–1223. [DOI] [PubMed] [Google Scholar]
- 5. Wang-Lopez Q, Chalabi N, Abrial C, et al. Can pathologic complete response (pCR) be used as a surrogate marker of survival after neoadjuvant therapy for breast cancer? Crit Rev Oncol Hematol 2015; 95: 88–104. [DOI] [PubMed] [Google Scholar]
- 6. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164–172. [DOI] [PubMed] [Google Scholar]
- 7. Broglio KR, Quintana M, Foster M, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol 2016; 2: 751–760. [DOI] [PubMed] [Google Scholar]
- 8. Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) prospective multicenter clinical trial. Ann Surg 2014; 260: 608–614 and 614–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 2008; 26: 778–785. [DOI] [PubMed] [Google Scholar]
- 10. Harbeck N, Gluz O. Neoadjuvant therapy for triple negative and HER2-positive early breast cancer. Breast 2017; 34: S99–S103. [DOI] [PubMed] [Google Scholar]
- 11. Goorts B, van Nijnatten TJ, de Munck L, et al. Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat 2017; 163: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi HJ, Ryu JM, Kim I, et al. Prediction of axillary pathologic response with breast pathologic complete response after neoadjuvant chemotherapy. Breast Cancer Res Treat 2019; 176: 591–596. [DOI] [PubMed] [Google Scholar]
- 13. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013; 24: 2278–2284. [DOI] [PubMed] [Google Scholar]
- 14. Samiei S, van Nijnatten TJA, de Munck L, et al. Correlation between pathologic complete response in the breast and absence of axillary lymph node metastases after neoadjuvant systemic therapy. Ann Surg 2020; 271: 574–580. [DOI] [PubMed] [Google Scholar]
- 15. Barron AU, Hoskin TL, Day CN, et al. Association of low nodal positivity rate among patients with ERBB2-positive or triple-negative breast cancer and breast pathologic complete response to neoadjuvant chemotherapy. JAMA Surg 2018; 153: 1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mougalian SS, Hernandez M, Lei X, et al. Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol 2016; 2: 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pilewskie M, Morrow M. Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol 2017; 3: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tee SR, Devane LA, Evoy D, et al. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br J Surg 2018; 105: 1541–1552. [DOI] [PubMed] [Google Scholar]
- 19. Al-Hilli Z, Hoskin TL, Day CN, et al. Impact of neoadjuvant chemotherapy on nodal disease and nodal surgery by tumor subtype. Ann Surg Oncol 2018; 25: 482–493. [DOI] [PubMed] [Google Scholar]
- 20. Tryfonidis K, Senkus E, Cardoso MJ, et al. Management of locally advanced breast cancer-perspectives and future directions. Nat Rev Clin Oncol 2015; 12: 147–162. [DOI] [PubMed] [Google Scholar]
- 21. Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 2013; 24: 2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi Zq, Qiu Pf, Liu Yb, et al. Neo-adjuvant chemotherapy and axillary de-escalation management for patients with clinically node-negative breast cancer. Breast Journal 2019; 25: 1154–1159. [DOI] [PubMed] [Google Scholar]
- 23. Classe JM, Bordes V, Campion L, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol 2009; 27: 726–732. [DOI] [PubMed] [Google Scholar]
- 24. Tan VK, Goh BK, Fook-Chong S, et al. The feasibility and accuracy of sentinel lymph node biopsy in clinically node-negative patients after neoadjuvant chemotherapy for breast cancer–a systematic review and meta-analysis. J Surg Oncol 2011; 104: 97–103. [DOI] [PubMed] [Google Scholar]
- 25. Tadros AB, Yang WT, Krishnamurthy S, et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg 2017; 152: 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van la Parra RF, Kuerer HM. Selective elimination of breast cancer surgery in exceptional responders: historical perspective and current trials. Breast Cancer Res 2016; 18: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuerer HM, Rauch GM, Krishnamurthy S, et al. A clinical feasibility trial for identification of exceptional responders in whom breast cancer surgery can be eliminated following neoadjuvant systemic therapy. Ann Surg 2018; 267: 946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong SM, Weiss A, Mittendorf EA, et al. Surgical management of the axilla in clinically node-positive patients receiving neoadjuvant chemotherapy: a national cancer database analysis. Ann Surg Oncol 2019; 26: 3517–3525. [DOI] [PubMed] [Google Scholar]
- 29. Srour MK, Tseng J, Luu M, et al. Patterns in the use of axillary operations for patients with node-positive breast cancer after neoadjuvant chemotherapy: a National Cancer Database (NCDB) analysis. Ann Surg Oncol 2019; 26: 3305–3311. [DOI] [PubMed] [Google Scholar]
- 30. Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 2015; 33: 258–264. [DOI] [PubMed] [Google Scholar]
- 31. Boughey JC. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer. JAMA 2013; 310: 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013; 14: 609–618. [DOI] [PubMed] [Google Scholar]
- 33. Piltin MA, Hoskin TL, Day CN, et al. Oncologic outcomes of sentinel lymph node surgery after neoadjuvant chemotherapy for node-positive breast cancer. Ann Surg Oncol 2020; 27: 4795–4801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_1758835921996673 for Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery by Jin Hong, Yiwei Tong, Jianrong He, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-2-tam-10.1177_1758835921996673 for Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery by Jin Hong, Yiwei Tong, Jianrong He, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-3-tam-10.1177_1758835921996673 for Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery by Jin Hong, Yiwei Tong, Jianrong He, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-4-tam-10.1177_1758835921996673 for Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery by Jin Hong, Yiwei Tong, Jianrong He, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-5-tam-10.1177_1758835921996673 for Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery by Jin Hong, Yiwei Tong, Jianrong He, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-6-tam-10.1177_1758835921996673 for Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery by Jin Hong, Yiwei Tong, Jianrong He, Xiaosong Chen and Kunwei Shen in Therapeutic Advances in Medical Oncology