Abstract
Background:
EXTEND (NCT01797965), an open-label extension study, evaluated the safety and efficacy of daclizumab beta in participants with relapsing multiple sclerosis (MS) who had completed the randomized DECIDE study.
Methods:
Eligible participants who received either daclizumab beta or interferon beta-1a in DECIDE received daclizumab beta 150 mg subcutaneously every 4 weeks for up to 5 years in EXTEND, followed by 24 weeks of post-dosing follow-up. Safety and tolerability were evaluated, as were clinical efficacy and magnetic resonance imaging (MRI). EXTEND was terminated ahead of schedule by the sponsors.
Results:
The total safety population (N = 1203) received at least one dose of daclizumab beta in EXTEND. In the DECIDE and EXTEND combined periods, the median number of doses of daclizumab beta was 53; median time on treatment was 196 weeks. By 24 September 2018, the end of the study, 110/1203 (9%) participants had completed the protocol-specified treatment period and 1101/1203 (92%) had experienced an adverse event (AE). The most commonly reported AEs were MS relapse, nasopharyngitis, and upper respiratory tract infection. Hepatic events (18%), cutaneous events (45%), and infections (62%) were common treatment-related AEs. The incidence of serious AEs was 29%, most commonly MS relapse and infections. The incidence of immune-mediated disorders was 2%; three of seven were encephalitis. Two of six deaths were considered treatment related. In participants who received continuous daclizumab beta throughout DECIDE and EXTEND, the treatment effects on clinical and MRI outcomes were maintained for up to 6 years.
Conclusion:
Results from the combined DECIDE-EXTEND study elucidate outcomes of longer-term treatment with daclizumab beta in the clinical trial setting and underscore the importance of pharmacovigilance with immunomodulatory therapies in the real-world setting.
Keywords: autoimmunity, daclizumab, encephalitis, multiple sclerosis
Introduction
Daclizumab beta is a humanized monoclonal antibody directed against CD25, the alpha subunit of the high-affinity interleukin-2 receptor. Treatment with daclizumab beta leads to a reduction of interleukin 2-mediated lymphocyte activation and expansion of immunoregulatory CD56bright natural killer cells.1–3 Daclizumab beta, a disease-modifying therapy (DMT) for multiple sclerosis (MS), was approved in 2016 by the US Food and Drug Administration and by the European Commission for relapsing forms of MS based on findings from phase IIb [SELECT4 (NCT00390221)] and pivotal phase III [DECIDE5 (NCT01064401)] clinical trials.6,7
DECIDE was a randomized trial comparing the efficacy and safety of daclizumab beta with intramuscular (IM) interferon (IFN) beta-1a over a period of 2–3 years.5 In DECIDE, daclizumab beta administered subcutaneously (SC) every 4 weeks for 2–3 years demonstrated superior efficacy versus IM IFN beta-1a in reducing clinical and radiological disease activity in patients with relapsing MS (RMS).5 Compared with IM IFN beta-1a, daclizumab beta reduced the annualized relapse rate (ARR) by 45% (p < 0.001) and the number of new/newly enlarging T2 hyperintense lesions at week 96 by 54% (p < 0.001).5 A higher incidence of infections, serious infections, cutaneous adverse events (AEs), serious cutaneous AEs, hepatic AEs (including transaminase elevations), and serious hepatic AEs was observed in patients treated with daclizumab beta versus IM IFN beta-1a.5
In February 2013, enrollment began for the clinical trial EXTEND8 (NCT01797965), an open-label extension study (for up to 5 years) to evaluate the long-term safety and efficacy of daclizumab beta monotherapy in participants with MS who had completed DECIDE. Participants with RMS who transitioned from SELECTED9 (NCT01051349; an open-label 3-year extension study of the parent study SELECT) or OBSERVE10 (NCT01462318; a phase III open-label study to assess the immunogenicity of daclizumab beta), were eligible to enroll in EXTEND. The majority (80%) of participants in EXTEND had transitioned from DECIDE.
During the course of EXTEND, the European Medicines Agency (EMA) Pharmacovigilance Risk Assessment Committee conducted a review of the hepatic effects of daclizumab beta following a case report of fatal fulminant liver failure in a patient with MS who had been treated as part of an ongoing phase IV observational study in the European Union.11 In October 2017, following a reassessment of its benefit–risk profile, the EMA recommended that daclizumab beta be restricted to patients who had an inadequate response to at least two DMTs and could not be treated with any other DMTs.12 Given the nature and complexity of AEs being reported, in the sponsors’ assessment, it was not possible to characterize the evolving benefit–risk profile of daclizumab beta in the limited number of patients being treated,9,13 and the sponsors voluntarily withdrew worldwide marketing authorizations for daclizumab beta on 2 March 2018.14,15 Although daclizumab beta is no longer commercially available for the treatment of RMS, it is important to disseminate the final long-term safety and efficacy findings from EXTEND to provide context to what we have learned from daclizumab beta regarding the interplay between innate and adaptive immunity in RMS.16 Here we report the safety and efficacy outcomes in participants who transitioned to EXTEND from DECIDE:5 one group received continuous daclizumab beta treatment for up to 8 years throughout DECIDE and EXTEND, and the other group received daclizumab beta up to 5 years in EXTEND after switching from IFN beta-1a in DECIDE.
Methods
Study design
EXTEND was an open-label, long-term extension study to evaluate the safety and efficacy of daclizumab beta in participants with RMS who had completed DECIDE or who transitioned from the SELECTED or OBSERVE daclizumab studies. The primary endpoint was the incidence of AEs and serious AEs (SAEs). Secondary endpoints included clinical outcomes [proportion of participants who were relapse free, confirmed disability progression (CDP) that was sustained for 24 weeks], and magnetic resonance imaging (MRI) outcomes [number of gadolinium-enhancing (Gd+) lesions, new/newly enlarging T2 hyperintense lesions, new T1 hypointense lesions]. A complete list of secondary endpoints and a summary table of MRI methods are provided in the online supplement.
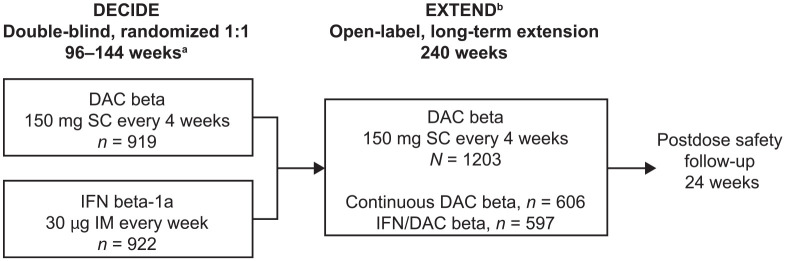
The analyses reported herein are limited to participants who were initially enrolled in DECIDE. Data from the SELECTED and OBSERVE trials were not included in the analysis for this paper due to differences in treatment regimens, doses, and duration, relative to DECIDE. Full details of the DECIDE study design have previously been published.5 Briefly, participants with active RMS who met key eligibility criteria were randomly assigned 1:1 to receive daclizumab beta 150 mg SC every 4 weeks or IM IFN beta-1a 30 µg once weekly for ⩾96 weeks, and up to 144 weeks (or until the last enrolled participant completed the week 96 study visit, whichever came first).5 Participants who received either daclizumab beta or IM IFN beta-1a in DECIDE were eligible to enroll in EXTEND and received open-label daclizumab beta 150 mg SC every 4 weeks for up to 5 years. EXTEND consisted of a 240-week treatment period followed by 24 weeks of post-dosing follow-up (Figure 1). The EXTEND protocol, which was amended five times (Supplemental Table 2), was approved by the relevant Ethics Committees (ECs) or Institutional Review Boards (IRBs; full list of EC/IRB names and approval numbers is available upon request). The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation guidelines on Good Clinical Practice. All participants provided written informed consent.
Figure 1.
EXTEND study design.
aIn DECIDE, participants with MS who met key eligibility criteria were randomly assigned 1:1 to receive DAC beta 150 mg SC every 4 weeks or IM IFN beta-1a 30 µg once weekly for ⩾96 weeks and up to 144 weeks (or until the last enrolled participant completed the week 96 study visit, whichever came first).
bApproximately 300 participants who transitioned from the long-term, open-label SELECTED17 extension study and the open-label OBSERVE10 immunogenicity and pharmacokinetics study entered EXTEND at week 144. This paper reports only on participants who enrolled from DECIDE; it does not include data from those who transitioned from SELECTED or OBSERVE.
DAC, daclizumab; IFN, interferon; IM, intramuscular; MS, multiple sclerosis; SC, subcutaneous.
Patients
To be eligible for EXTEND, participants must have either enrolled in DECIDE and completed 2–3 years of treatment with daclizumab beta or IM IFN beta-1a (week 96 or week 144 study visit), or transitioned from SELECTED or OBSERVE. Participants were excluded from the study if they: (a) permanently discontinued study treatment in DECIDE or terminated the study prematurely; (b) experienced a significant change in medical history that precluded administration of daclizumab beta; (c) had any other medical condition that, based on the opinion of the investigator, precluded participation in the study; or (d) were taking a protocol-defined prohibited concomitant therapy during DECIDE.
During the course of EXTEND, daclizumab beta received regulatory approval and was commercialized in several countries that were part of the study. As per the study protocol, all participants in those countries were considered to have completed study treatment on regulatory approval and commercial availability of daclizumab beta in their country, except in the United Kingdom, where this requirement was removed in an April 2013 protocol amendment. In March 2018, the last participant was dosed in EXTEND and the study was closed by the sponsors.15
Assessments
Safety
For the purpose of this report, the safety population consisted of all who participated in DECIDE and had received at least one dose of daclizumab beta during EXTEND. The treatment period referred to the time between the first dose date in EXTEND and the date of the last available measurement prior to the 180 days after the last dose in EXTEND. As per the study protocol, all remaining participants at the time of termination of dosing were to remain in the study for a 6-month follow-up period, which the last participants completed in September 2018.
In both DECIDE and EXTEND, liver function tests [LFTs; alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin, and gamma-glutamyltransferase (GGT)] were performed every 4 weeks. Results from an LFT performed within the protocol-defined window (7 days for DECIDE; 32 days for EXTEND) had to meet protocol-defined acceptable limits before the next dose of daclizumab beta could be administered.
In DECIDE, daclizumab beta treatment was temporarily suspended if a participant developed ALT or AST elevations >3 × the upper limit of normal (ULN) or total bilirubin >2 × ULN and could not resume until both resolved. Participants who suspended treatment for ⩾8 weeks due to abnormal LFTs were permanently discontinued from further daclizumab beta dosing. The protocol for EXTEND was amended in 2017 to revise the criteria for discontinuation; previously, the criteria for discontinuation were similar to those in DECIDE. Daclizumab beta treatment was temporarily suspended if a participant had ALT or AST elevations >3 to ⩽5 × ULN and total bilirubin ⩽2 × ULN; dosing could resume if a subsequent LFT showed ALT or AST <2 × ULN and the criteria for permanent discontinuation were not met. Daclizumab beta was permanently discontinued if a participant had ALT or AST elevations that were >5 × ULN and confirmed by a repeat test, or >3 × ULN with concomitant bilirubin >2 × ULN. Participants with treatment suspension or discontinuation were tested at least weekly until the LFT elevation resolved.
AEs and SAEs, coded using the Medical Dictionary for Regulatory Activities (MedDRA) system organ class (SOC), were monitored throughout the study. Hepatic AEs were defined using a standardized MedDRA query (SMQ) for drug-related hepatic disorders that included the SOC terms hepatobiliary disorders, investigations (also referred to as laboratory abnormalities), and benign and malignant neoplasms. Treatment-emergent AEs included any event that occurred or worsened in severity after the onset of treatment. AE severity and relatedness to study treatment were assessed by the investigator. The incidence of AEs, SAEs, and selected AEs of special interest (AESIs; hepatic AEs, infections, cutaneous AEs, lymphadenopathy events, and others) were summarized.
Efficacy
Relapses were defined as new or recurrent neurological symptoms not associated with fever or infection, lasting ⩾24 h, and accompanied by new objective neurological findings on examination by a neurologist. Participant performance on the Expanded Disability Status Scale (EDSS) was assessed at baseline and then every 12 weeks in DECIDE; and at baseline, week 12, week 24, and every 24 weeks thereafter in EXTEND until week 240. CDP was defined as a ⩾1.0-point increase in EDSS score from a baseline score of ⩾1.0 that was sustained for 24 weeks, or a ⩾1.5-point increase in EDSS score from a baseline score of 0 that was sustained for 24 weeks. MRI outcomes (number of Gd+ lesions, new/newly enlarging T2 hyperintense lesions, and new T1 hypointense lesions) were evaluated at baseline, week 24, week 96, and week 144 in DECIDE; and at baseline, week 48, and then every 48 weeks therafter to week 144 in EXTEND.
Statistical analyses
Safety analyses included all participants who received at least one dose of daclizumab beta as part of DECIDE or who switched from IM IFN beta-1a in DECIDE and received at least one dose of daclizumab beta in EXTEND. Safety data, including AEs and laboratory values, collected between the first dose date of daclizumab beta and up to 180 days after the last daclizumab beta dose date were considered in the safety analysis.
For efficacy analyses in EXTEND, the proportion of participants who were relapse free and the proportion who had 24-week CDP were analyzed in the intention-to-treat (ITT) population (including all those who received at least one dose of daclizumab beta in DECIDE) over the combined treatment period. The effects of continuous daclizumab beta treatment versus switching treatment (IM IFN beta-1a in DECIDE to daclizumab beta in EXTEND) were also assessed. ARR and MRI outcomes were analyzed in participants who completed DECIDE and received at least one dose of daclizumab beta in EXTEND (DECIDE-EXTEND ITT population), as new events were captured on a yearly basis. The adjusted ARR was estimated from a Poisson regression model adjusted for a history of prior IFN beta-1a use at DECIDE baseline, the number of relapses in the year prior to first dose in EXTEND, EDSS score (⩽2.5 versus >2.5) and age (⩽35 versus >35 years) at EXTEND baseline. The proportion of participants who were relapse free and the proportion with 24-week CDP were estimated using the Kaplan–Meier method. The number of new/newly enlarging T2 hyperintense lesions (or new T1 hypointense lesions, for T1 analyses) was estimated using a negative binomial regression model, including the prior treatment group in the DECIDE study, and was adjusted for the volume of T2 hyperintense lesions (or T1 hypointense lesions, for T1 analyses) at EXTEND baseline, a history of prior IFN beta-1a use at DECIDE baseline, and age (⩽35 versus >35 years) at EXTEND baseline.
In this report, subsequent descriptions pertaining to the DECIDE-EXTEND ITT population will be referred to as DECIDE-EXTEND.
Results
Patients
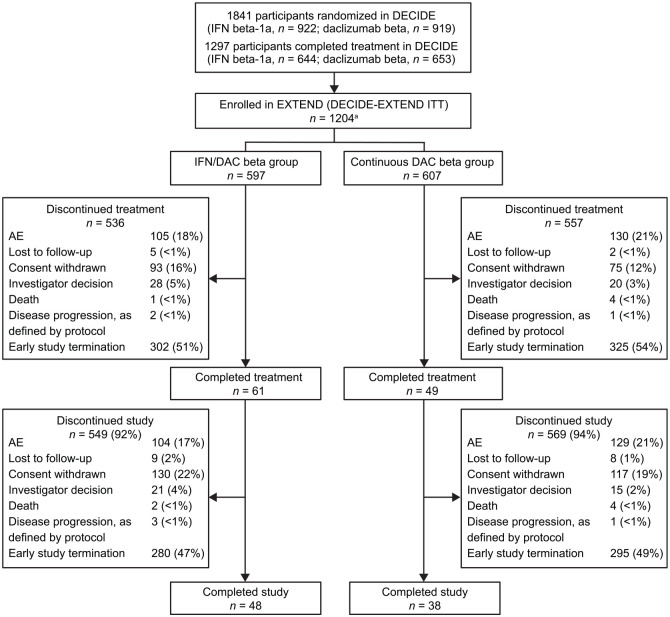
Of the 1841 participants who underwent random assignment in DECIDE (IM IFN beta-1a, n = 922; daclizumab beta, n = 919; DECIDE ITT population),5 1297 completed treatment, and 1203 enrolled and received at least one dose of daclizumab beta in EXTEND (Figure 2). The first participant was dosed with study treatment on 15 February 2013 and the last participant’s last study visit in EXTEND was 24 September 2018. Baseline participant demographics in DECIDE-EXTEND are shown in Table 1.12 As a result of the EMA recommendations in 2017 that restricted the indication for daclizumab beta to patients who had failed at least two other DMTs and could not be treated with any other DMT,12 the participant population in EXTEND no longer reflected the patient population for the modified indication for daclizumab beta. In January 2018, EXTEND was terminated by the sponsors ahead of schedule,8 to limit daclizumab beta-related clinical and data generation activities to the MS patient populations that were consistent with the approved indications across most regions where the drug was available. At that time, there were nine countries with patients remaining in EXTEND, representing 411 study participants. Study participants in EXTEND received the last dose of daclizumab beta on or before 30 March 2018. The maximum time on study in EXTEND was >260 weeks (represents up to 8 years of daclizumab beta treatment for participants who had received daclizumab beta for up to 144 weeks in DECIDE). In the final analysis, 1093 (91%) participants in EXTEND discontinued treatment (most of this is accounted for by early study closure); 20% of participants discontinued because of an AE.
Figure 2.
Disposition of participants in the EXTEND study.
AE, adverse event; DAC, daclizumab; IFN, interferon; ITT, intention-to-treat. aOne participant assigned to the Continuous DAC beta group discontinued before receiving treatment in EXTEND.
Table 1.
Baseline patient demographics and disease characteristics in the safety population.a
| Characteristic | IFN/DAC beta,b n = 597 | Continuous DAC beta,c n = 606 | Total, N = 1203 |
|---|---|---|---|
| Age, median (range), years | 39.0 (20–57) | 36.0 (18–55) | 37.0 (18–57) |
| Female, n (%) | 394 (66) | 400 (66) | 794 (66) |
| No. of DAC beta doses in combined study populations, mean (SD) | 39.9 (16.8) | 69.2 (18.8) | 54.7 (23.1) |
| No. of relapses in prior year, mean (SD) | 0.4 (0.63) | 0.2 (0.50) | 0.3 (0.57) |
| Range | 0–4 | 0–4 | 0–4 |
| EDSS score, mean (SD) | 2.50 (1.47) | 2.44 (1.41) | 2.47 (1.44) |
| ⩽2.5, n (%) | 360 (60) | 376 (62) | 736 (61) |
| >2.5, n (%) | 237 (40) | 230 (38) | 467 (39) |
| Total no. of Gd+ lesions, mean (SD) | 1.0 (3.09) | 0.5 (4.68) | 0.7 (3.97) |
| Total no. of T2 hyperintense lesions, mean (SD) | 62.7 (48.8) | 53.7 (38.9) | 58.1 (44.3) |
| Total no. of T1 hypointense lesions, mean (SD) | 38.6 (38.7) | 34.3 (35.1) | 36.5 (37.0) |
Baseline refers to the period from the last visit in DECIDE and within 28 days of the participants’ first dose of DAC beta in EXTEND.
Participants who received IM IFN beta-1a in DECIDE and switched to DAC beta 150 mg in EXTEND.
Participants who received DAC beta 150 mg in DECIDE and continued on DAC beta in EXTEND.
DAC, daclizumab; EDSS, Expanded Disability Status Scale; Gd+, gadolinium-enhancing; IFN, interferon; IM, intramuscular; SD, standard deviation.
Safety
Daclizumab beta exposure in the safety population (N = 1203) was 3566 patient-years in the EXTEND study period; the median (range) number of doses was 53 (1–96) in both DECIDE and EXTEND. The median (range) time on daclizumab beta treatment was 196 (0.1–237) weeks. In EXTEND, 14% of participants received daclizumab beta treatment for 0–48 weeks, 10% received daclizumab beta for 49–96 weeks, 14% for 97–144 weeks, 10% for 145–192 weeks, 51% for 193–236 weeks, and 1% for >236 weeks.
Overall safety summary and incidence of AEs in the safety population
In total, 1101/1203 (92%) participants in the safety population experienced an AE (Table 2); 48% of AEs were considered by the investigator to be related to the study treatment. Most participants (75%) had events that were mild or moderate in severity. The AEs by MedDRA SOC with the highest incidence (>20%) were infections and infestations (62%), nervous system disorders, including MS relapse and all other preferred terms within the SOC (46%), skin and subcutaneous tissue disorders (45%), musculoskeletal and connective tissue disorders (31%), gastrointestinal disorders (27%), investigations (i.e. laboratory abnormalities) (26%), and general disorders and administration site conditions (24%). SAEs were reported in 347/1203 (29%) participants. SAEs reported in at least three participants are listed in Table 3. The incidences of treatment-related SAEs were 9% in the IFN/daclizumab beta group and 10% in the continuous daclizumab beta group. The most commonly reported treatment-related SAEs were drug-induced liver injury [four participants (<1%)] in the IFN/daclizumab beta group and lymphadenopathy [eight participants (1%)] in the continuous daclizumab beta group.
Table 2.
| AEs, n (%) | IFN/DAC,a n = 597 | Continuous DAC beta, n = 606 | Total safety population,b N = 1203 |
|---|---|---|---|
| Any AE | 541 (91) | 560 (92) | 1101 (92) |
| AEs with the highest incidence (>20%) | |||
| Infections and infestations | 381 (64) | 370 (61) | 751 (62) |
| Nervous system disorders | 285 (48) | 271 (45) | 556 (46) |
| Skin and subcutaneous tissue disorders | 245 (41) | 292 (48) | 537 (45) |
| Musculoskeletal/connective tissue disorders | 186 (31) | 188 (31) | 374 (31) |
| Gastrointestinal disorders | 170 (28) | 160 (26) | 330 (27) |
| Investigations | 160 (27) | 152 (25) | 312 (26) |
| General disorders/administration site conditions | 153 (26) | 138 (23) | 291 (24) |
| AE severityc | |||
| Mild | 135 (23) | 146 (24) | 281 (23) |
| Moderate | 315 (53) | 307 (51) | 622 (52) |
| Severe | 90 (15) | 103 (17) | 193 (16) |
| AEs related to treatmentd | 279 (47) | 297 (49) | 576 (48) |
| AEs leading to treatment discontinuation | 105 (18) | 131 (22) | 236 (20) |
| Common AEs (⩾10% of participants) leading to treatment discontinuation and withdrawal from study | |||
| Abnormal LFT | 11 (2) | 12 (2) | 23 (2) |
| ALT increase | 10 (2) | 7 (1) | 17 (1) |
| Lymphadenopathy | 3 (<1) | 10 (2) | 13 (1) |
| AST increase | 4 (<1) | 7 (1) | 11 (<1) |
| MS relapse | 5 (<1) | 5 (<1) | 10 (<1) |
| Any SAE | 157 (26) | 190 (31) | 347 (29) |
| SAEs related to treatment | 55 (9) | 58 (10) | 113 (9) |
| Deathe | 2 (<1) | 4 (<1) | 6 (<1) |
Participants who received IM IFN beta-1a in DECIDE and switched to DAC beta 150 mg in EXTEND.
The total safety population (N = 1203) comprised participants who received at least one dose of daclizumab beta during DECIDE or who switched from IM IFN beta-1a in DECIDE and received at least one dose of DAC beta in EXTEND.
Maximum severity of all events for each participant; percentages are calculated using the total number of participants in the IFN/DAC group (n = 597) and total safety population (n = 1203), respectively.
Determined by the investigator.
One death each from the IFN/DAC and continuous DAC beta groups was deemed related to the study drug; the relationship to the study drug of one death from the continuous DAC beta group was unspecified.
AE, adverse event; ALT, alanine aminotransferase; AST, asparatate aminotransferase; DAC, daclizumab; IFN, interferon; IM, intramuscular; LFT, liver function test; MS, multiple sclerosis; SAE, serious adverse event.
Table 3.
SAEs reported in at least three participants in the total safety population.a
| SAE, n (%) | IFN/DAC,a n = 597 | Continuous DAC beta, n = 606 | Total safety population, N = 1203 |
|---|---|---|---|
| Multiple sclerosis relapse | 61 (10) | 65 (11) | 126 (10) |
| Lymphadenopathy | 4 (<1) | 10 (2) | 14 (1) |
| Pneumonia | 5 (<1) | 8 (1) | 13 (1) |
| Urinary tract infection | 6 (1) | 3 (<1) | 9 (<1) |
| Fall | 1 (<1) | 7 (1) | 8 (<1) |
| Cholelithiasis | 5 (<1) | 2 (<1) | 7 (<1) |
| Multiple sclerosis | 3 (<1) | 3 (<1) | 6 (<1) |
| Pyrexia | 3 (<1) | 3 (<1) | 6 (<1) |
| Drug-induced liver injury | 4 (<1) | 1 (<1) | 5 (<1) |
| Epilepsy | 2 (<1) | 3 (<1) | 5 (<1) |
| Lymphadenitis | 2 (<1) | 3 (<1) | 5 (<1) |
| Lymphoid tissue hyperplasia | 4 (<1) | 1 (<1) | 5 (<1) |
| Psoriasis | 3 (<1) | 2 (<1) | 5 (<1) |
| Suicide attempt | 2 (<1) | 3 (<1) | 5 (<1) |
| Breast cancer | 3 (<1) | 1 (<1) | 4 (<1) |
| Cholecystitis | 2 (<1) | 2 (<1) | 4 (<1) |
| Hemolytic anemia | 2 (<1) | 2 (<1) | 4 (<1) |
| Inguinal hernia | 2 (<1) | 2 (<1) | 4 (<1) |
| Uterine leiomyoma | 0 | 4 (<1) | 4 (<1) |
| Agranulocytosis | 1 (<1) | 2 (<1) | 3 (<1) |
| ALT increased | 1 (<1) | 2 (<1) | 3 (<1) |
| Appendicitis | 1 (<1) | 2 (<1) | 3 (<1) |
| AST increased | 1 (<1) | 2 (<1) | 3 (<1) |
| Dermatitis contact | 3 (<1) | 0 | 3 (<1) |
| Eczema | 2 (<1) | 1 (<1) | 3 (<1) |
| Encephalitis autoimmune | 3 (<1) | 0 | 3 (<1) |
| Hepatitis toxic | 1 (<1) | 2 (<1) | 3 (<1) |
| Pancreatitis | 2 (<1) | 1 (<1) | 3 (<1) |
| Pancreatitis acute | 3 (<1) | 0 | 3 (<1) |
| Pulmonary sarcoidosis | 0 | 3 (<1) | 3 (<1) |
| Rash generalized | 0 | 3 (<1) | 3 (<1) |
| Rash maculopapular | 2 (<1) | 1 (<1) | 3 (<1) |
| Respiratory tract infection | 1 (<1) | 2 (<1) | 3 (<1) |
| Septic shock | 2 (<1) | 1 (<1) | 3 (<1) |
| Syncope | 1 (<1) | 2 (<1) | 3 (<1) |
| Thrombocytopenia | 3 (<1) | 0 | 3 (<1) |
Based on the MedDRA preferred term (version 16.1).
ALT, alanine aminotransferase; AST, asparatate aminotransferase; DAC, daclizumab; IFN, interferon; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event.
AEs leading to discontinuation of the study drug occurred in 20% of participants, and 19% withdrew from the study. Discontinuations and withdrawals were most frequently (⩾10 participants) attributed to abnormal LFT (2%), increased ALT (1%), lymphadenopathy (1%), increased AST (<1%), and MS relapse (<1%). Except for lymphadenopathy, which was experienced by a slightly greater number of participants in the continuous daclizumab beta group compared with the IFN/daclizumab beta group (2% versus <1%), there were no remarkable group differences in the incidence of AEs or SAEs leading to treatment and study discontinuation. Six participants died during the EXTEND treatment period (all from the DECIDE-EXTEND safety population). The causes of death for four participants, not considered related to study treatment, were septic shock due to perforated and ischemic colon, brain edema and dislocation (post-trauma), respiratory failure, and exacerbation of heart failure. Two deaths, both occurring after treatment discontinuation and study withdrawal, were considered to be related to study treatment: a 30-year-old man from the IFN/daclizumab beta group died from septic shock; and a 27-year-old woman from the continuous daclizumab beta group experienced leukopenia, pancytopenia, fever, and multi-organ failure, which led to death.
AESIs
Important identified risks included hepatic AEs (including transaminase elevations, serious hepatic injury, and hepatic failure), cutaneous events, serious cutaneous events, depression, infections, serious infections, immune-mediated disorders, autoimmune hemolytic anemia, and colitis (Table 4). These AESIs are summarized in this section. Important potential risks included anaphylaxis, opportunistic infections, malignancies, and lymphadenopathy. Other AESIs include hypersensitivity, angioedema, lymphopenia, sarcoidosis, and injection site AEs.
Table 4.
AEs of special interest.
| AEs, n (%) | Total safety population, N = 1203 |
|---|---|
| Hepatic AEsa | |
| Any AE | 222 (18) |
| Mild | 111/222 (50) |
| Moderate | 84/222 (38) |
| Severe | 27/222 (12) |
| SAEs | 16 (1) |
| AEs leading to treatment discontinuation | 12 (<1) |
| Infectionb | |
| Any AE | 751 (62) |
| Mild | 347/751 (46) |
| Moderate | 364/751 (48) |
| Severe | 39/751 (5) |
| SAEs | 62 (5) |
| AEs leading to treatment discontinuation | 15 (1) |
| Cutaneousc,d | |
| Any AE | 537 (45) |
| Mild | 273/537 (51) |
| Moderate | 241/537 (45) |
| Severe | 23/537 (4) |
| SAEs | 38 (3) |
| AEs leading to treatment discontinuation | 60 (5) |
| Lymphadenopathye | |
| Any AE | 136 (11) |
| Mild | 78/136 (57) |
| Moderate | 51/136 (38) |
| Severe | 7/136 (5) |
| SAEs | 23 (2) |
| AEs leading to treatment discontinuation | 18 (1) |
| Depression | |
| Any AE | 117 (10) |
| SAEs | 7 (<1) |
| Lymphopenia | |
| Any AE | 57 (5) |
| SAEs | 2 (<1) |
| Angioedema | |
| Any AE | 53 (4) |
| SAEs | 0 |
| Immune-mediated disorder | |
| Any AE | 30 (2) |
| SAEs | 15 (1) |
| Autoimmune encephalitis | 3 (<1) |
| Colitis | |
| Any AE | 18 (1) |
| SAEs | 5 (<1) |
| Malignancy | |
| Any AE | 16 (1) |
| Sarcoidosis | |
| Any AE | 6 (<1) |
| SAEs | 4 (<1) |
| Autoimmune hemolytic anemia | |
| Any AE | 4 (<1) |
| SAEs | 4 (<1) |
Defined using an SMQ for drug-related hepatic disorders, which is a sublevel of SMQ hepatic disorders and includes the preferred terms hepatobiliary disorders and investigations, among others.
MedDRA SOC of infections and infestations.
MedDRA SOC of skin and subcutaneous tissue disorders.
Hypersensitivity AEs were mainly cutaneous [n = 366/399 (92%)].
Customized MedDRA search under selected high-level terms.
AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event; SMQ, standardized Medical Dictionary for Regulatory Activities query; SOC, system organ class.
Hepatic AEs
Patients with a history of any significant hepatic disease or clinically significant laboratory abnormalities from the most recently available test in DECIDE were excluded from participating in EXTEND. Of the 1203 patients in the safety population, hepatic AEs were reported in 222 (18%) participants (Table 4). Of 222 participants with hepatic AEs, 195 (88%) events were mild or moderate in severity (Table 4). Hepatic AEs led to discontinuation of study drug in <1% of participants (Table 4). Most of the study participants had no pre-existing liver dysfunction (n = 1158; Table 5). The incidence of hepatic AEs was slightly higher in participants with a pre-existing liver impairment (n/N = 11/45; 24%) than in those without a pre-existing condition (n/N = 211/1158; 18%), although not statistically significant. The incidence of hepatic AEs leading to treatment discontinuation and withdrawal from the study was higher in participants without pre-existing conditions than in those with pre-existing conditions (6% versus 2%). The number of participants who were taking potential hepatotoxic medications was comparable to the number who were not taking potential hepatotoxic medications (574 versus 629, respectively); no remarkable group differences in hepatic AEs were observed.
Table 5.
Summary of hepatic AEs.
| Hepatic AEs, n (%)a,b | Total safety population, N = 1203 |
|---|---|
| Any hepatic AE | 222 (18) |
| Without pre-existing liver conditions | 211/1158 (18) |
| With pre-existing liver conditions | 11/45 (24) |
| Without potential hepatotoxic medication | 101/629 (16) |
| With potential hepatotoxic medication | 121/574 (21) |
| Serious hepatic AEs | 16 (1) |
| Drug-induced liver injury | 5 (<1) |
| Toxic hepatitis | 3 (<1) |
| ALT increase | 3 (<1) |
| AST increase | 3 (<1) |
| Liver injury | 2 (<1) |
| Chronic hepatitis | 1 (<1) |
| Hepatic necrosis | 1 (<1) |
| Hepatitis cholestatic | 1 (<1) |
| Jaundice | 1 (<1) |
| Blood bilirubin increase | 1 (<1) |
| Hepatic enzyme increase | 1 (<1) |
| Hepatic AEs by SOC | |
| Laboratory abnormalitiesc | 203 (17) |
| Hepatobiliary disorders | 36 (3) |
| Neoplasm – hemangiomas of the liver | 4 (<1) |
Defined using an SMQ for drug-related hepatic disorders, which is a sublevel of SMQ hepatic disorders and includes the preferred terms hepatobiliary disorders and investigations, among others.
Percentages out of total AEs reported.
Includes abnormalities of transaminases, bilirubin, liver function tests, hepatic enzyme, and gamma-glutamyl transferase.
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SMQ, standardized Medical Dictionary for Regulatory Activities query; SOC, system organ class.
Laboratory abnormalities were the most frequent AE among participants with hepatic AEs [203/222 (91%)]; those occurring in ⩾1% of participants were increased ALT, increased AST, abnormal LFT, and increased GGT. Among the 1202 participants with at least one measurement of concurrent total bilirubin and ALT/AST, 11% of participants had ALT or AST elevations ⩾3 × ULN, 7% had elevations >5 × ULN, and 3% had elevations >10 × ULN (the incidence of elevated LFTs was based on clinical laboratory tests and may not have been reported as AEs).
Serious hepatic AEs (based on the SMQ for drug-related hepatic disorders) occurred in 16 (1%) participants (Table 3). Among these, serious hepatobiliary disorders (as reported by MedDRA preferred term in at least two participants) each occurred in <1% of the study population. The most common serious hepatic events were drug-induced liver injury, toxic hepatitis, increased ALT and AST levels, and liver injury. Except for the drug-induced liver injury SAE (four participants in the IFN/daclizumab beta group versus one participant in the continuous daclizumab beta group), there were no remarkable differences in the incidence of hepatic events between the two treatment groups.
Cutaneous AEs
Cutaneous AEs were reported in 537/1203 (45%) participants; the vast majority of participants with cutaneous AEs (93%) had events that were mild to moderate (Table 4). Most hypersensitivity events [366/399 (92%)] were manifested as skin and subcutaneous tissue disorders. Cutaneous AEs with an incidence of ⩾4% were rash (9%), eczema (7%), erythema (5%), seborrheic dermatitis (4%), maculopapular rash (4%), and psoriasis (4%). Sixty participants (5%) had AEs that led to treatment discontinuation; 59 (5%) reported AEs that led to withdrawal from the study, the three most common of which were eczema, maculopapular rash, and rash (each <1% incidence). There was one reported case of Stevens–Johnson syndrome and one case of toxic epidermal necrolysis, both from the IFN/daclizumab beta group. One participant from each treatment group experienced drug reactions with eosinophilia and systemic symptoms (DRESS); however, both of these events were reviewed and were adjudicated as not DRESS. There were no remarkable differences in the incidence of serious skin reactions between the IFN/daclizumab beta and continuous daclizumab beta groups.
Infections
Infections were reported in 751/1203 (62%) participants; 97% (2731/2808) of all infectious AEs resolved by the end of the study. The median (range) onset of the first infection relative to the first daclizumab beta dose was 217 (1–1626) days; the median duration of events that resolved was 19 (1–840) days. The majority (94%) of participants with infections had events that were of mild or moderate severity (Table 5).
The most common infections (occurring in ⩾10% of participants) were nasopharyngitis (20%), upper respiratory tract infection (19%), and urinary tract infection (12%). There were no reported cases of progressive multifocal leukoencephalopathy, and the overall incidence of potential opportunistic infections was 3%, with the most common being vulvovaginal or oral candidiasis. Five per cent of participants (n = 62) experienced a serious infection, most commonly pneumonia (n = 13; 1%) or urinary tract infection (n = 9; <1%). Ninety per cent of serious infections had resolved by the end of the study. The median (range) duration of events that resolved was 18 (1–245) days.
There were four cases of serious opportunistic infection during the EXTEND treatment period, all from the IFN/DAC group: two cases of pulmonary tuberculosis (<1%), and one case each of cytomegalovirus infection and cytomegaloviral pneumonia. Two of these events had resolved by the end of the study. The mean [standard deviation (SD)] and median durations of events that resolved were 17.5 (12.02) and 17.5 days, respectively. Eighty-one (7%) participants had an infection that led to dose interruption, and 15 (1%) had an infection that led to treatment discontinuation. Infections leading to discontinuation were spread across a number of conditions and included hepatitis E (n = 3), pulmonary tuberculosis (n = 2), and septic shock (n = 2). The remaining infections leading to discontinuation of treatment were reported in one participant each. There were no remarkable differences for infections between the IFN/daclizumab beta and continuous daclizumab beta groups.
Immune-mediated disorders (including immune-mediated encephalitis)
Immune-mediated disorders were reported in 30/1203 (2%) participants. The most frequent events by MedDRA SOC were nervous system disorders, gastrointestinal disorders, skin and subcutaneous tissue disorders, and musculoskeletal and connective tissue disorders [six participants each (<1%)]. Immune-mediated AEs that were reported in more than one participant were celiac disease (n = 6; <1%), encephalitis autoimmune (n = 3; <1%), uveitis (n = 3; <1%), rheumatoid arthritis (n = 3; <1%), Guillain–Barré syndrome (n = 2; <1%), pemphigoid (n = 2; <1%), and vitiligo (n = 2; <1%). Serious immune-mediated disorders of the aforementioned organ systems, plus the gastrointestinal system, were reported in 15 (1%) participants; the only SAEs with at least one event were autoimmune encephalitis (n = 3; <1%), Guillain–Barré syndrome (n = 2; <1%), and celiac disease (n = 2; <1%). All reported cases of encephalitis were from the IFN/daclizumab beta group: two men (aged 35 years and 39 years) and one woman (aged 31 years). Two of these three cases were deemed related to treatment, and led to withdrawal from the study; the other case was not considered treatment related (patient eventually died of septic shock due to perforated and ischemic colon). There were no cases of immune-mediated liver failure.
Lymphadenopathy events
Lymphadenopathy was reported in 136 (11%) of participants. Lymphadenopathy could be localized or present in multiple bilateral regions. In five cases, lymphadenopathy was diagnosed as lymphoma, but on subsequent lymph node biopsy and evaluation, there were no confirmed cases of lymphoma. The most frequently reported lymphadenopathy events (at least five participants) included lymphadenopathy (n = 120; 10%), lymphadenitis (n = 16; 1%), and lymphoid tissue hyperplasia (n = 5; <1%). Fifty-eight (5%) participants had events that were moderate or severe. Eighteen (1%) participants had lymphadenopathy events that led to discontinuation of study treatment and withdrawal from the study. Serious lymphadenopathy events occurred in 23 (2%) participants; nine (<1%) discontinued study treatment and withdrew from the study. Lymphadenopathy events were more frequently reported among participants in the continuous daclizumab beta group. However, serious lymphadenopathy events were experienced by a similar percentage of participants in both treatment groups (2%).
Malignancies
Malignancies were reported in 18 (1%) participants in EXTEND. Basal cell carcinoma and breast cancer were reported in four participants each; adenocarcinoma of the colon, adrenal neoplasm, B-cell lymphoma, breast neoplasm, invasive ductal breast carcinoma, metaplastic breast carcinoma, mycosis fungoides, ovarian cancer, post-transplant lymphoproliferative disorder, and uterine leiomyosarcoma were reported in one participant each. A case initially reported as B-cell lymphoma was later diagnosed as reactive lymphadenopathy by two independent experts. An equal number of participants in the IFN/daclizumab beta and continuous daclizumab beta groups experienced malignancies.
Sarcoidosis
Sarcoidosis was reported in six (<1%) participants: one from the IFN/daclizumab beta group and five from the continuous daclizumab beta group, consisting of pulmonary sarcoidosis (n = 3; <1%), sarcoidosis (n = 3; <1%), and cutaneous sarcoidosis (n = 1; <1%). Four (<1%) participants, all from the continuous daclizumab beta group, had serious sarcoidosis events. These SAEs included pulmonary sarcoidosis (n = 3; 1%), and sarcoidosis (n = 1; <1%).
Autoimmune hemolytic anemia
There were four (<1%) reported cases of autoimmune hemolytic anemia, with two (<1%) participants each in the IFN/daclizumab beta and continuous daclizumab beta groups. All were SAEs and were considered by the investigator to be related to study treatment.
Other AEs
Depression was reported in 10% of participants; serious events of depression were experienced by <1%. Colitis was reported in 1%.
Efficacy
Efficacy outcomes were assessed in DECIDE-EXTEND participants who received continuous daclizumab beta treatment in both studies [continuous daclizumab beta group (n = 606)] and in those who received IM IFN beta-1a in DECIDE and switched to daclizumab beta in EXTEND [IFN/daclizumab beta group (n = 597)]. The median (range) times between the last dose in DECIDE and the first dose in EXTEND were 1.1 (0.1–22.6) weeks for the IFN/daclizumab beta group and 4.29 (2.4–27.4) weeks for the continuous daclizumab beta group.
Clinical outcomes
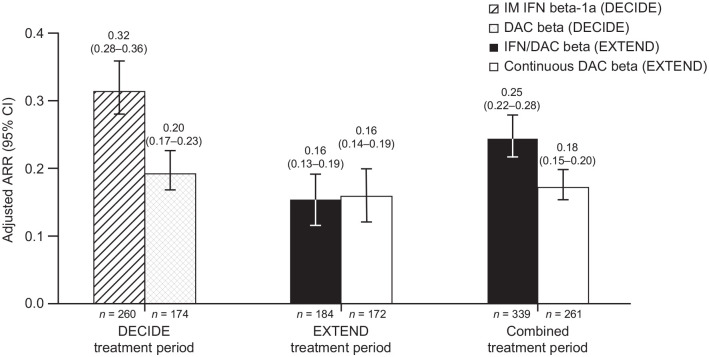
The ARR was analyzed for the ITT population in the combined DECIDE-EXTEND study period, as well as the ITT population in the DECIDE treatment period and the EXTEND treatment period. In the continuous daclizumab beta group, ARR [95% confidence interval (CI)] remained low in the EXTEND treatment period [0.16 (0.14–0.19)] and combined treatment period [0.18 (0.15–0.20)], similar to what was observed in daclizumab beta-treated participants in DECIDE [0.20 (0.17–0.23); Figure 3]. One year into EXTEND, the ARR of participants who switched from IM IFN beta-1a in DECIDE to daclizumab beta in EXTEND was about half the ARR in DECIDE prior to the switch [0.16 (0.13–0.19) versus 0.32 (0.28–0.36)]; the ARR of IFN/daclizumab beta in the EXTEND period was comparable to the ARR of the continuous daclizumab beta group [0.16 (0.13–0.19) versus 0.16 (0.14–0.19), respectively]. However, in the combined DECIDE-EXTEND treatment period, the continuous daclizumab beta group had a lower ARR than the IFN/daclizumab beta group [0.18 (0.15–0.20) and 0.25 (0.22–0.28), respectively].
Figure 3.
ARR for treatment groups in DECIDE and for IFN/DAC beta and continuous DAC beta-treated participants in EXTEND.
ARR, annualized relapse rate; CI, confidence interval; DAC, daclizumab; IFN, interferon; IM, intramuscular.
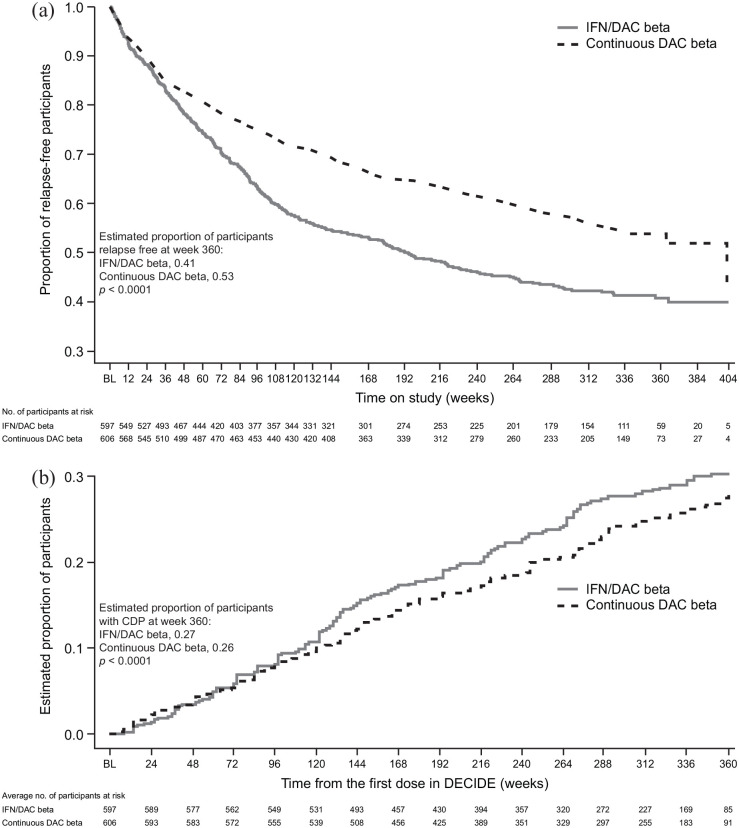
Time to first relapse and time to 24-week CDP were analyzed from DECIDE baseline through the transition into EXTEND and study completion. At week 360, more participants in the continuous daclizumab beta group of the combined study population were relapse free [53% versus 41%; Figure 4(a)] and fewer had experienced 24-week CDP [26% versus 27%; Figure 4(b)] than those in the IFN/daclizumab beta group.
Figure 4.
Time to (a) first relapse and (b) 24-week CDPa among IFN/DAC beta- and continuous DAC beta-treated participants in the DECIDE-EXTEND population.b,c
aCDP was defined as a ⩾1.0-point increase in EDSS score from a DECIDE baseline EDSS score of ⩾1.0 sustained for 24 weeks or a ⩾1.5-point increase in EDSS score from a DECIDE baseline EDSS score of 0 sustained for 24 weeks.
bIFN/DAC beta refers to participants who received IM IFN beta-1a in DECIDE and switched to DAC beta in EXTEND and participants who received IM IFN beta-1a in DECIDE and did not enroll in EXTEND. Continuous DAC beta refers to participants who received DAC beta in DECIDE and EXTEND and participants who received DAC beta in DECIDE and did not enroll in EXTEND.
cParticipants in DECIDE were treated with IM IFN beta-1a or DAC beta for 96–144 weeks before they enrolled in EXTEND open-label treatment with DAC beta.
BL, baseline; CDP, confirmed disability progression; DAC, daclizumab; EDSS, Expanded Disability Status Score; IFN, interferon; IM, intramuscular.
MRI outcomes
In EXTEND, the mean (95% CI) number of Gd+ lesions was lower in both treatment groups at week 48 versus baseline [EXTEND IFN/daclizumab beta, 0.4 (0.3, 0.5) and continuous daclizumab beta, 0.3 (0.2, 0.4) versus baseline IFN/daclizumab beta, 1.0 (3.09) and continuous daclizumab beta, 0.5 (4.68); Supplemental Figure 1(A)]. Overall, the number of MRI lesions (including number of Gd+, new or newly enlarging T2 hyperintense lesions, and new T1 hypointense lesions) remained low through week 96 in EXTEND compared with week 96 in DECIDE [Supplemental Figure 1(B–C)]. There were limited data on brain volume change due to the timing of the protocol amendment.
Discussion
The overall rates and nature of AEs during the combined treatment period with daclizumab beta (up to 8 years in participants who received continuous daclizumab beta in DECIDE-EXTEND) were generally consistent with those observed in previous clinical studies of daclizumab.4,5,9,13 The most commonly reported AEs were MS relapse, nasopharyngitis, and upper respiratory tract infection. No remarkable trends were observed in the distribution of AEs between participants who had continued exposure and new exposure to daclizumab beta on entering EXTEND. The majority of participants had events that were mild to moderate in severity; the incidence of severe events was 16%. The most frequently reported treatment-related AEs included lymphadenopathy and increases in liver transaminase levels. The incidence of SAEs was 29%, most commonly MS relapse and infections. About 20% of participants either discontinued treatment or withdrew from the study due to AEs, frequently due to hepatic AEs (mostly increased liver transaminase levels), lymphadenopathy, and MS relapse. These frequencies observed in EXTEND are consistent with the safety profile of daclizumab beta observed in the SELECT and DECIDE studies that also showed a higher incidence of hepatic laboratory abnormalities, serious hepatic events (DECIDE only), cutaneous events, serious cutaneous events, infections, and serious infections in participants treated with daclizumab beta 150 mg SC than those treated with placebo in SELECT or IM IFN beta-1a in DECIDE.4,5
Serum transaminase elevations and serious drug-induced hepatic injury, including autoimmune hepatitis and liver failure, were observed across the clinical development program and post-marketing setting.5 Although the majority of hepatic events in the clinical development program were asymptomatic transaminase elevations, some were serious and/or fatal. The incidence of serious hepatic AEs (1%) was consistent with the incidence reported in DECIDE.5 Serious drug-related liver injury was reported in 1% of participants across the clinical development program. In the SELECTION18 study (NCT00870740), a washout/re-initiation extension of SELECT,4 a case of fatal autoimmune hepatitis occurred in a patient re-initiating treatment with daclizumab beta 300 mg after a planned 6-month treatment interruption period.18 A case of fulminant liver failure with a fatal outcome was reported from non-interventional study EUR-ZIN-16-11024; based on this case, an Article 20 referral (a benefit–risk reassessment procedure for marketed products in Europe) of daclizumab beta was initiated in August 2017 in the European Union. An additional four cases of serious liver injury in EXTEND were disclosed in the first periodic safety update report (covering between 27 May 2016 through 27 November 2016).11 In all four cases, the hepatic events resolved and were not considered by the investigator to be life threatening. In EXTEND, there were no cases of immune-mediated liver failure and none of the six deaths that occurred in EXTEND were determined to be drug-related liver deaths.
Overall in EXTEND, the type and pattern of infections observed were consistent with those typically seen in an MS population.19,20 By the end of the study, ⩾95% of events had resolved. This is consistent with published data that indicate daclizumab beta may cause reversible, mild to modest lymphocyte reductions that are not associated with an increased risk of infection or opportunistic infection.21
The reported hypersensitivity events and cutaneous events were consistent with previous clinical experience and included rash, eczema, macupapular rash, allergic dermatitis, urticaria, contact dermatitis, and dermatitis. Cutaneous AEs generally resolved and treatment discontinuation due to cutaneous AEs was low, similar to findings from DECIDE.22 Although the potential link between underlying mechanisms of action for daclizumab beta and cutaneous AEs is unclear, cutaneous adverse reactions have been posited to be directly or indirectly associated with the biological activity of daclizumab in modulating the immune system, including innate lymphoid cells such as natural killer cells.23 CD25 blockade by daclizumab beta may have resulted in the reduction of skin-resident regulatory T cells, which typically counteract inflammatory T cell responses.22,23
Following a full assessment of cases of autoimmune encephalitis, including the three cases in this study, immune-mediated encephalitis was identified as a risk of daclizumab beta therapy.15 Following this, the EMA recommended the immediate suspension of daclizumab beta based on reports of serious inflammatory brain disorders worldwide, including encephalitis, some of which were fatal.24
With regard to treatment efficacy, EXTEND showed that, in participants who received continuous daclizumab beta throughout DECIDE and EXTEND, treatment effects on clinical and MRI outcomes were maintained for up to 6 years. At the conclusion of DECIDE (and prior to entry in EXTEND), participants who were treated with daclizumab beta showed better clinical outcomes than those treated with IFN beta-1a.25,26 The participants treated with IFN beta-1a in DECIDE who switched to daclizumab beta in EXTEND demonstrated improvement in clinical outcomes in EXTEND in comparison with levels demonstrated by that group in DECIDE. Overall, during daclizumab beta treatment in EXTEND, the clinical and MRI outcomes were comparable between the IFN/daclizumab beta and continuous daclizumab beta treatment groups. In the SELECT trilogy (SELECT,4 SELECTION,18 SELECTED9) of clinical studies, in which some participants received up to 6 years of continuous treatment with daclizumab beta, the yearly incidence of AEs had not increased over time and efficacy was maintained for up to 6 years. Data from EXTEND confirm these findings, showing sustained efficacy and no increased incidence of overall AEs with up to 8 years of daclizumab beta treatment throughout DECIDE and EXTEND.15
In a pharmacodynamic analysis of four clinical trials of participants with MS who were treated with daclizumab beta, significant increases in CD56bright natural killer cells and reductions in skin-resident regulatory T cells relative to baseline levels were noted in the first 2 weeks of treatment; these alterations and CD25 occupancy saturation were sustained during the treatment period.27 Such changes have been associated with the clinical and radiological efficacy of the drug.28,29 The link between the underlying therapeutic mechanisms of daclizumab beta and the associated AEs is unclear and may involve interaction between daclizumab beta and the innate immune system.16
Worldwide market approval of daclizumab beta was based on pivotal clinical trial data showing that the treatment benefits outweighed the risks.6,7 Following the long-term follow-up in EXTEND, new safety signals were identified that were not apparent in the pivotal trials. This is because identification of rare AEs may require that more patients be treated for longer periods of time before such events can be recognized. Post-marketing reports of complex SAEs along with the small number of patients being treated with daclizumab beta made it difficult to assess the ongoing benefit–risk profile.15 In March 2018, the Marketing Authorisation Holder made the decision voluntarily to withdraw the marketing authorization for daclizumab beta from the market.15 The marketing experience of daclizumab beta underscores the importance of phase IV monitoring of newly approved treatments. Patient populations in clinical trials are not as heterogeneous as those in the real world, and rare AEs may remain undetected. More studies are needed to elucidate the effects of immunomodulators on the dynamic between innate and adaptive immune mechanisms in autoimmune diseases such as RMS to provide therapeutic options with better clinical efficacy and manageable AEs.
Conclusion
In EXTEND, treatment with daclizumab beta over up to 6 years resulted in a robust and sustained treatment effect on well-validated clinical and radiographic measures. Nevertheless, although the overall rate and nature of AEs of daclizumab beta treatment was generally consistent with that previously observed in the controlled clinical trial setting, additional rare but serious autoimmune AEs were observed in both EXTEND and post-marketing and eventually led to drug withdrawal. These observations underscore the importance of pharmacovigilance in the post-marketing setting and the need for better understanding of the effects of immunomodulating treatments and the potential therapeutic value of modulating the innate immune system in MS.
Supplemental Material
Supplemental material, sj-pdf-1-tan-10.1177_1756286420987941 for Safety and efficacy of daclizumab beta in patients with relapsing multiple sclerosis in a 5-year open-label study (EXTEND): final results following early termination by Ludwig Kappos, Stanley Cohan, Douglas L. Arnold, Randy R. Robinson, Joan Holman, Sami Fam, Becky Parks, Shan Xiao and Wanda Castro-Borrero in Therapeutic Advances in Neurological Disorders
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Biogen (Cambridge, MA, USA) and AbbVie Inc. (Redwood City, CA, USA). Biogen and AbbVie provided funding for medical writing support in the development of this manuscript. Susan Chow and Bess Reinoso from Excel Scientific Solutions wrote the first draft of the manuscript based on input from the authors, and Nathaniel Hoover from Excel Scientific Solutions copyedited and styled the manuscript as per journal requirements. Biogen reviewed and provided feedback on the paper to the authors. The authors had full editorial control of the paper, and provided their final approval of all content.
Conflict of interest: LK reports funding to his institution (University Hospital Basel) in the last 3 years used exclusively for research support; steering committee, advisory board, and consultancy fees from Actelion, Addex, Bayer, Biogen, Celgene, Genzyme, Merck, Mitsubishi, Novartis, Pfizer, Sanofi, and Santhera; speaker fees from Biogen, Merck, Novartis, Sanofi, and Teva; support of educational activities from Bayer, Biogen, CSL Behring, Genzyme, Merck, Novartis, Sanofi, and Teva; license fees for Neurostatus products; and grants from Bayer, Biogen, European Union, Innosuisse, Merck, Novartis, Roche Research Foundation, Swiss MS Society, and Swiss National Research Foundation.
SC reports speaking honoraria from and/or advisory boards for Biogen, Bristol Myers Squibb, EMD Serono, Novartis, Pear Therapeutics, Roche Genentech, and Sanofi Genzyme; and research support from AbbVie, Acorda, Actelion, Biogen, MedDay, Novartis, Roche Genentech, and Sanofi Genzyme.
DLA reports consultant fees and/or grants from Acorda, Adelphi, Alkermes, Biogen, Canadian Institutes of Health Research, Celgene, Frequency Therapeutics, Genentech, Genzyme, Immune Tolerance Network, Immunotec, International Progressive MS Alliance, MedDay, Merck Serono, MS Society of Canada, Novartis, Pfizer, Receptos, Roche, and Sanofi and an equity interest in NeuroRx Research.
RRR is an employee of and holds stock/stock options in AbbVie.
JH was a former employee of and holds stock/stock options in AbbVie.
BP was a former employee of and holds stock/stock options in Biogen.
SF, SX and WC-B are employees of and hold stock/stock options in Biogen.
The EXTEND study and these analyses were funded by Biogen (Cambridge, MA, USA) and AbbVie (Redwood City, CA, USA).
ORCID iD: Ludwig Kappos  https://orcid.org/0000-0003-4175-5509
https://orcid.org/0000-0003-4175-5509
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ludwig Kappos, Research Center Clinical Neuroimmunology and Neuroscience Basel (RC2NB), Departments of Medicine, Clinical Research, and Biomedicine and Biomedical Engineering, University Hospital and University of Basel, Petersgaben 4, Basel, CH-4031, Switzerland.
Stanley Cohan, Providence Multiple Sclerosis Center, Providence Brain and Spine Institute, Providence St. Joseph Health, Portland, OR, USA.
Douglas L. Arnold, Montreal Neurological Institute, McGill University, Montreal, QC, Canada NeuroRx Research, Montreal, QC, Canada
Randy R. Robinson, AbbVie Inc., Redwood City, CA, USA
Joan Holman, AbbVie Inc., North Chicago, IL, USA.
Sami Fam, Biogen, Cambridge, MA, USA.
Becky Parks, Biogen, Cambridge, MA, USA.
Shan Xiao, Biogen, Cambridge, MA, USA.
Wanda Castro-Borrero, Biogen, Cambridge, MA, USA.
References
- 1. Cohan S. Therapeutic efficacy of monthly subcutaneous injection of daclizumab in relapsing multiple sclerosis. Biologics 2016; 10: 119–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Preiningerova JL, Vachova M. Daclizumab high-yield process in the treatment of relapsing–remitting multiple sclerosis. Ther Adv Neurol Disord 2017; 10: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baldassari LE, Rose JW. Daclizumab: development, clinical trials, and practical aspects of use in multiple sclerosis. Neurotherapeutics 2017; 14: 842–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gold R, Giovannoni G, Selmaj K, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet 2013; 381: 2167–2175. [DOI] [PubMed] [Google Scholar]
- 5. Kappos L, Wiendl H, Selmaj K, et al. Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2015; 373: 1418–1428. [DOI] [PubMed] [Google Scholar]
- 6. US Food and Drug Administration. FDA approves Zinbryta to treat multiple sclerosis. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm504000.htm (2016; accessed 12 March 2020).
- 7. Inacio P. Zinbryta (daclizumab) approved in Europe to treat relapsing MS. https://multiplesclerosisnewstoday.com/2016/07/07/zinbryta-daclizumab-approved-as-ms-treatment-in-european-union/ (2016; accessed 12 March 2020).
- 8. ClinicalTrials.gov. Long-term extension study in participants with multiple sclerosis who have completed study 205MS301 (NCT01064401) to evaluate the safety and efficacy of BIIB019 (EXTEND). https://clinicaltrials.gov/ct2/show/NCT01797965 (2017; accessed 12 March 2020).
- 9. Gold R, Radue EW, Giovannoni G, et al. Safety and efficacy of daclizumab in relapsing-remitting multiple sclerosis: 3-year results from the SELECTED open-label extension study. BMC Neurol 2016; 16: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ClinicalTrials.gov. An open-label immunogenicity and pharmacokinetics study of daclizumab high yield process prefilled syringe in relapsing remitting multiple sclerosis (OBSERVE). https://clinicaltrials.gov/ct2/show/NCT01462318 (2017; accessed 12 March 2020).
- 11. European Medicines Agency. Procedure under Article 20 of Regulation (EC) No 726/2004 resulting from pharmacovigilance data. https://www.ema.europa.eu/en/documents/referral/zinbryta-article-20-referral-assessment-report-provisional-measures_en.pdf (2017; accessed 24 April 2020).
- 12. European Medicines Agency. EMA concludes review of Zinbryta and confirms further restrictions to reduce risk of liver damage. https://www.ema.europa.eu/en/news/ema-concludes-review-zinbryta-confirms-further-restrictions-reduce-risk-liver-damage (2017; accessed 12 March 2020).
- 13. Giovannoni G, Kappos L, Gold R, et al. Safety and tolerability profile of daclizumab in patients with relapsing-remitting multiple sclerosis: an integrated analysis of clinical studies. Mult Scler Relat Disord 2016; 9: 36–46. [DOI] [PubMed] [Google Scholar]
- 14. Biogen. Zinbryta (daclizumab): restrictions of use due to the risk of fulminant liver failure. https://www.hpra.ie/docs/default-source/default-document-library/important-safety-information—zinbryta-(daclizumab).pdf (2017; accessed 12 March 2020).
- 15. Biogen. Biogen and AbbVie announce the voluntary worldwide withdrawal of marketing authorizations for Zinbryta® (daclizumab) for relapsing multiple sclerosis. http://investors.biogen.com/news-releases/news-release-details/biogen-and-abbvie-announce-voluntary-worldwide-withdrawal (2018; accessed 12 March 2020).
- 16. Cohan SL, Lucassen EB, Romba MC, et al. Daclizumab: mechanisms of action, therapeutic efficacy, adverse events and its uncovering the potential role of innate immune system recruitment as a treatment strategy for relapsing multiple sclerosis. Biomedicines 2019; 7: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ClinicalTrials.gov. Safety and efficacy extension study of daclizumab high yield process (DAC HYP) (BIIB019) in participants who have completed study 205MS202 (NCT00870740) to treat relapsing remitting multiple sclerosis (SELECTED). https://clinicaltrials.gov/ct2/show/NCT01051349 (accessed 12 March 2020).
- 18. Giovannoni G, Gold R, Selmaj K, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECTION): a multicentre, randomised, double-blind extension trial. Lancet Neurol 2014; 13: 472–481. [DOI] [PubMed] [Google Scholar]
- 19. Venkatesan A, Johnson RT. Infections and multiple sclerosis. Handb Clin Neurol 2014; 122: 151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Winkelmann A, Loebermann M, Reisinger EC, et al. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol 2016; 12: 217–233. [DOI] [PubMed] [Google Scholar]
- 21. Giovannoni G, Wiendl H, Turner B, et al. Circulating lymphocyte levels and relationship with infection status in patients with relapsing–remitting multiple sclerosis treated with daclizumab beta. Mult Scler 2018; 24: 1725–1736. [DOI] [PubMed] [Google Scholar]
- 22. Krueger JG, Kircik L, Hougeir F, et al. Cutaneous adverse events in the randomized, double-blind, active-comparator DECIDE study of daclizumab high-yield process versus intramuscular interferon beta-1a in relapsing-remitting multiple sclerosis. Adv Ther 2016; 33: 1231–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cortese I, Ohayon J, Fenton K, et al. Cutaneous adverse events in multiple sclerosis patients treated with daclizumab. Neurology 2016; 86: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. European Medicines Agency. Multiple sclerosis medicine Zinbryta suspended in the EU: evidence indicates risk of serious inflammatory brain disorders. https://www.ema.europa.eu/en/documents/referral/zinbryta-article-20-procedure-multiple-sclerosis-medicine-zinbryta-suspended-eu_en.pdf (2018; accessed 12 March 2020).
- 25. Cohan S, Kappos L, Giovannoni G, et al. Efficacy of daclizumab beta versus intramuscular interferon beta-1a on disability progression across patient demographic and disease activity subgroups in DECIDE. Mult Scler 2018; 24: 1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kappos L, Havrdova E, Giovannoni G, et al. No evidence of disease activity in patients receiving daclizumab versus intramuscular interferon beta-1a for relapsing-remitting multiple sclerosis in the DECIDE study. Mult Scler 2017; 23: 1736–1747. [DOI] [PubMed] [Google Scholar]
- 27. Diao L, Hang Y, Othman AA, et al. Population PK-PD analyses of CD25 occupancy, CD56bright NK cell expansion, and regulatory T cell reduction by daclizumab HYP in subjects with multiple sclerosis. Br J Clin Pharmacol 2016; 82: 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elkins J, Sheridan J, Amaravadi L, et al. CD56bright natural killer cells and response to daclizumab HYP in relapsing–remitting MS. Neurol Neuroimmunol Neuroinflamm 2015; 2: e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bielekova B, Howard T, Packer AN, et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch Neurol 2009; 66: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tan-10.1177_1756286420987941 for Safety and efficacy of daclizumab beta in patients with relapsing multiple sclerosis in a 5-year open-label study (EXTEND): final results following early termination by Ludwig Kappos, Stanley Cohan, Douglas L. Arnold, Randy R. Robinson, Joan Holman, Sami Fam, Becky Parks, Shan Xiao and Wanda Castro-Borrero in Therapeutic Advances in Neurological Disorders