Abstract
Background
Teleneurology for multiple sclerosis (MS) care was considered feasible, but utilization was limited.
Objective
To describe how the existing teleneurology populations at two academic MS Centers changed during the COVID-19 pandemic.
Methods
In this cross-sectional study, we captured all in-person and teleneurology visits at two academic MS Centers between January 2019 and April 2020. We compared group differences between the Centers, and COVID-related changes using T-, chi-squared Kruskal-Wallis and Fisher exact tests.
Results
2268 patients completed 2579 teleneurology visits (mean age 48.3 ± 13.3 years, 72.9% female). Pre-COVID, the Centers’ teleneurology populations were similar for age, sex, MS type, and disability level (all p > 0.1), but differed for race (96.5% vs 80.7% white, p ≤ 0.001), MS treatment (49.1% vs 32.1% infusible, p ≤ 0.001), and median distance from Center (72 vs 186 miles, p ≤ 0.001). Post-COVID, both Centers’ teleneurology populations had more black (12.7% vs 4.37%, p ≤ 0.001) and local (median 34.5 vs 102 miles, p ≤ 0.001) patients.
Conclusion
Teleneurology visits in 2019 reflected the organizational and local teleneurology reimbursement patterns of our Centers. Our post-COVID-19 changes illustrate the potential for payors and policy to change disparities in access to, or utilization of, remote care. Patients’ perception of care quality and value following this shift warrants study.
Keywords: Multiple sclerosis, health disparities, COVID-19, models of care
Introduction
The landscape of multiple sclerosis (MS) diagnosis and treatment has evolved dramatically over the last century, leading to marked improvements in patient outcomes, but access to these innovations is uneven.1 Advances in MS care have resulted in earlier diagnosis, reduced disability and slowed progression.1 Despite these advances, patients with MS face significant barriers to accessing specialized care, including financial, insurance-based, physical and geographical limitations, compounded by a shortage of neurologists.2
Telehealth has the potential to improve access, quality of life, and outcomes for patients with neurological conditions including MS, while reducing costs.3 In other chronic health conditions, telehealth has been shown to increase access to specialty care among patients who are minorities and those with geographical limitations, but it could also exacerbate health disparities.4–6
The COVID-19 pandemic has highlighted the need for novel ways to care for patients, and has catapulted telehealth to the forefront.7,8 It has also shed light on the striking societal and racial inequalities that exist across healthcare, including worse access to care and outcomes for minority populations.9,10 Both Cleveland Clinic (CC) and University of California San Francisco (UCSF) MS Centers had integrated teleneurology visits into routine MS care before the COVID-19 pandemic.11,12 The aim of this study was to compare teleneurology use at these two large MS Centers before and following the COVID-19 pandemic, to understand changes in both access to and utilization of teleneurology.
Methods
In this two-site retrospective observational study, we identified all in-person clinic visits and teleneurology visits performed for the neuroimmunology clinics at CC and UCSF from January 2019 through April 2020.
Patient population
The CC Mellen Center for MS Treatment and Research and the UCSF MS and Neuroinflammation Center are tertiary care centers providing care to Ohio and California respectively, as well as nationally. We retrospectively reviewed all visits completed by clinicians [neurologists, advanced practice practitioners (APPs)] within both Centers as part of routine care. Data for both in-clinic and teleneurology visits were collected and defined as either “pre-COVID19” or “post-COVID19” if completed after 3/15/20, given major statewide closures within both Ohio and California and restricted access to in-clinic care.13,14
In-clinic visits: We included all nonprocedural in-person neurology visits to describe practice patterns for all patients >18 years seen at UCSF during the study period, and at CC those who completed the standardized intake process of neuroperformance testing and patient reported outcomes (approximately 77% of the entire follow-up MS clinical population15). Neurological diagnosis was based on clinical documentation. If a patient completed both in-person and teleneurology visits, they were included only in the teleneurology population.
Teleneurology visits: We included all teleneurology visits completed by MS clinicians. Patients were offered teleneurology visits pre-COVID19; thereafter, most visits were required to be conducted via teleneurology. The primary platforms utilized at both institutions were encrypted, compliant with the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
At CC, teleneurology visits were completed initially using the synchronous televideo Cleveland Clinic Express Care Online® (ECO) platform supported by American Well, and post-COVID19 for patients unable to use ECO, other widely available applications (e.g. Facetime®, Google Duo®) or telephone only. Visits were conducted with both in-state and out-of-state patients, and were billed to insurance payors or the patient directly depending on the individuals’ coverage. State licensing was properly addressed by all clinicians wherever required. Following the intensification of the COVID-19 pandemic, the co-pay for teleneurology visits was waived for this timeframe and all visits were billed to insurance payors.16
At UCSF, the teleneurology visits were completed using the synchronous televideo Zoom platform. All teleneurology visits were billed to insurance payors, and prior to the COVID-19 were conducted only for patients living in California. Post-COVID-19, the Coronavirus Preparedness and Response Supplemental Appropriations Act included provisions facilitating telehealth visits to be offered to in and out of state patients both new to, and with an established relationship at, the Centers All visits continued to be billed to insurance payors.
All teleneurology visits were conducted with the patient in their home setting. For all of the televideo visits, a neurological exam was able to be performed, as per prior experience17 and the examination components conducted and patient reported outcomes collected were determined by the treating clinician per usual clinical practice.
Data collected (EMR)
We extracted demographics (age, sex, race, distance from Center), visit characteristics (clinician type visit duration, reason for visit), and MS disease characteristics (disease duration, disease course, current DMT, disability rating). At CC a PDDS (Patient Determined Disease Steps)18,19 and at UCSF an EDSS (Expanded Disability Status Scale)20 are usually recorded clinically. To allow for disability level comparisons across these measures, the following categories were made: no disability (PDDS 0 and EDSS 0–1.5), mild disability with no gait impairment (PDDS 1–2 and EDSS: 2-3.5), gait impairment with no aide (PDDS 3 and EDSS: 4–5.5), gait impairment requires aide (PDSS 4–6 and EDSS: 6–6.5), non-ambulatory (PDSS 7–8 and EDSS: 7–10). Driving distance was calculated using a SAS macro from Google Maps. Visits from locations not reachable via driving had air distance calculated.21
Statistical analysis
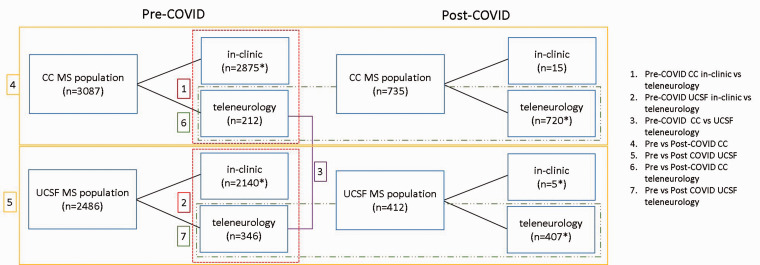
To evaluate group differences between sites, differences between teleneurology and in-person visit populations, and differences across pre- and post- COVID19 cohorts (Figure 1), we used t-test or Wilcoxon rank sum test, chi-squared test or Fisher exact test as appropriate.
Figure 1.
Main comparisons performed in the MS populations across two academic Centers.
*Patients who completed both in-clinic and teleneurology visits during the study period were only included in the teleneurology population. Only first visit was included for patients had multiple visits.
We used two different categorizations of patients’ primary self-identified race. We first categorized patients as either white or BIPOC (Black, Indigenous, People of Color) - a recent, inclusive and descriptive term22 that included patients who self-identified as American Indian or Alaska Native, Asian, Black or African American, and Native Hawaiian or Other Pacific Islander. Whenever statistical models allowed more precision, we used a second categorization of race to specifically allow us to understand changes among black patients, our second most common racial group who face unique challenges with respect to MS care and course:23 white, black and other (non-black BIPOC).
To further evaluate which factors were associated with utilization of teleneurology versus in-person visits pre-COVID, we performed a logistic regression model with a stepwise model selection. UCSF in-person and teleneurology populations were previously compared.11 To explore, post hoc, the relationship of race and distance from Center pre- and post- COVID, linear regression models of the teleneurology combined CC and UCSF populations was performed, adjusting for age and sex. Log transformation was applied to distance. Significance of statistical analyses was set at p < 0.05. Statistical analysis was performed using R Statistical Software (version 3.6.0), compareGroups (v4.0, Subirana et al., 2014) and stats (v 3.6.0, R core team, 2019) packages.
Standard protocol approvals, registrations, and patient consents
This study was approved by the CC Institutional Review Board (#19-1505) and the UCSF Committee of Human Research (#13-11686).
Results
During the entire study period (January 2019 through April 2020), a total of 15,419 visits were conducted at CC and 7,511 were conducted at UCSF. Of these, 2,268 patients completed 2,579 teleneurology visits (1,384 CC, 1,195 UCSF). Comparing the pre- and post-COVID19 observation periods, the proportion of teleneurology visits among all visits increased from 2.6% to 94.0% at CC and 8.1% to 95.8% at UCSF (Table 1). For the study period, most visits were conducted via the primary televideo visit (CC 83%, UCSF 100%). We evaluated the entire pre-COVID clinic populations between the Center (Table 2) and compared the entire population pre-COVID to the post-COVID at each Center. The remaining analyses focused specifically on the MS-only population, as summarized in Figure 1.
Table 1.
Distribution of teleneurology and in-person visits by center.
|
CC |
UCSF |
|||
|---|---|---|---|---|
| Before COVID19* | After COVID19* | Before COVID19* | After COVID19* | |
| Teleneurology | 377 (6.0) | 1007 (152.6) | 552 (8.8) | 643 (97.4) |
| In-person clinic | 13,966 (222.7) | 69 (10.5) | 6288 (100.3) | 28 (4.2) |
| Proportion teleneurology | 2.6% | 94.0% | 8.1% | 95.8% |
*Statistics present as total visits (visits per week).
Table 2.
Pre-COVID neuroimmunology teleneurology populations.
| CC N = 344 | UCSF N = 470 | p-Value | |
|---|---|---|---|
| Patient Age‡ | 46.3 (12.7) | 48.0 (13.3) | 0.073a |
| Patient Sex‡ | 1.000b | ||
| Female | 254 (73.8%) | 306 (73.9%) | |
| Male | 90 (26.2%) | 108 (26.1%) | |
| Race‡ | <0.001c | ||
| White | 305 (93.8%) | 277 (79.8%) | |
| Black | 14 (4.31%) | 20 (5.76%) | |
| Other | 6 (1.85%) | 50 (14.4%) | |
| Ethnicity | <0.001c | ||
| Not Hispanic | 322 (99.1%) | 326 (91.8%) | |
| Hispanic | 3 (0.92%) | 29 (8.17%) | |
| Clinician Type‡ | <0.001c | ||
| Physician | 151 (44.3%) | 459 (97.7%) | |
| APP | 190 (55.7%) | 11 (2.34%) | |
| Diagnosis‡ | <0.001c | ||
| MS | 212 (69.7%) | 346 (73.6%) | |
| CIS | 6 (1.97%) | 12 (2.55%) | |
| MOG | 4 (1.32%) | 0 (0.00%) | |
| NMO | 2 (0.66%) | 8 (1.70%) | |
| ON | 0 (0.00%) | 7 (1.49%) | |
| Other autoimmune | 27 (8.88%) | 97 (20.6%) | |
| Unknown | 53 (17.4%) | 0 (0.00%) | |
| Disease Course*‡ | 0.249b | ||
| PPMS | 27 (12.8%) | 33 (9.88%) | |
| RRMS | 153 (72.5%) | 263 (78.7%) | |
| SPMS | 31 (14.7%) | 38 (11.4%) | |
| Current DMT‡: | 0.001c | ||
| Infusion | 70 (31.0%) | 171 (47.8%) | |
| Oral | 54 (23.9%) | 71 (19.8%) | |
| Injectable | 29 (12.8%) | 39 (10.9%) | |
| other | 3 (1.33%) | 2 (0.56%) | |
| none | 70 (31.0%) | 75 (20.9%) | |
| Disability Level‡ | 0.199c | ||
| Non-ambulatory | 2 (6.06%) | 23 (9.13%) | |
| Gait impairment requires aide | 4 (12.1%) | 42 (16.7%) | |
| Gait Impairment with no aide | 2 (6.06%) | 21 (8.33%) | |
| Mild disability with no gait impairment | 9 (27.3%) | 99 (39.3%) | |
| No disability | 16 (48.5%) | 67 (26.6%) | |
| Reason for Visit‡ | <0.001c | ||
| Routine | 225 (66.0%) | 464 (98.9%) | |
| New | 105 (30.8%) | 2 (0.43%) | |
| Urgent | 8 (2.35%) | 3 (0.64%) | |
| Other | 3 (0.88%) | 0 (0.00%) | |
| Drive distance‡ | 216 [96.2;367] | 74.0 [23.2;185] | <0.001d |
Statistics presented as Mean (SD), Median [1st, 3rd] or N (column %).
P-value: a = t-test; b = chi-square test; c = Fisher exact test; d = Wilcoxon rank sum test.
*Subgroup analysis only includes MS patients.
‡Data not available for all subjects. Missing values: patient age: 56; patient gender: 56; race: 142; clinician type: 3; diagnosis: 40; current DMT: 230; disability level: 529; reason for visit: 4; drive distance: 101.
CC: Cleveland Clinic; CIS: clinically isolated syndrome; MOG: Myelin oligodendrocyte glycoprotein antibody disorder, MS: multiple sclerosis; NMO: neuromyelitis optica spectrum disorder, ON: optic neuritis; PPMS: primary progressive MS, RRMS: relapsing remitting, SPMS: secondary progressive MS.
Entire center neuromimmunology populations: Pre-COVID teleneurology populations
The entire pre-COVID teleneurology populations (MS and other neuroimmunology conditions) were compared between Centers (Table 2). CC teleneurology patients, when compared with UCSF, included a higher proportion of white patients (93.8% vs 79.8%), a lower proportion of patients with other autoimmune conditions (8.9% vs 20.6%), and patients lived further from the Center (216 vs 74 miles). CC had a greater proportion of visits conducted by APPs (55.7% vs 2.34%) and for patients new to the Center (30.8% vs 0.43%). For the MS population, UCSF had a higher proportion of patients on infusible DMTs (47.8% vs 31.0%); disease course and disability were similar (p ≥ 0.1).
MS patients: Entire center populations: Pre-COVID to post-COVID
At CC, the entire pre and post-COVID MS populations did not differ for age (50.1 vs 49.6, p = 0.40), sex (72.1% vs 73.5% female, p = 0.49), ethnicity (1.78% vs 1.40% Hispanic, p = 0.58), disability level (33.8% vs 34,% no disability, p = 0.89), or income (55,385 vs 53,831 p = 0.28). The post-COVID population had a higher proportion of black patients (14.2% vs 10.6%, p < 0.001), high proportion of RRMS patients (75.0% vs 66.1%, p < 0.001) and lived closer to the Center (38.8 vs 32.5, p < 0.001). At UCSF the two populations only differed for age (50.0 vs 48.5, p = 0.035), but did not differ for sex (71.3% vs 70.4% female, p = 0.75), race (6.44% vs 9.44% black, p = 0.083) or ethnicity (11.1% vs 10.8% Hispanic, p = 0.905). Disease course and distance from the Center were not available for the in-clinic population at UCSF.
MS patients: Comparison of Pre-COVID in-person and teleneurology populations
At CC, among patients with MS, 2875 unique patients completed only in-person visits, and 212 patients completed at least one teleneurology visit. The teleneurology patients at CC pre-COVID were younger (46.0 vs 50.4 years, p ≤ 0.001), were mostly white (96.5% vs 83.9%, p ≤ 0.001), had RRMS (72.5% vs 65.6%, p ≤ 0.001), and lived further from the Center (186 vs 35. 6 miles, p ≤ 0.001) than patients completing in-person visits. The populations did not not differ for sex or disability level, but a disability score was only available 36% of teleneurology patients. The UCSF MS teleneurology population also was younger (48.1 vs 50.3 p ≤ 0.004), and had more white patients (80.7% vs 73.4%, p = 0.019) than the in-clinic population.
We further evaluated potential predictors of completing a teleneurology visit in the pre-COVID population using a logistic regression model (variables only available for the CC population). Using stepwise selection, younger age (OR 0.97; 95% CI 0.96 – 0.98; P < 0.001), white race (OR 5.35; 95% CI 2.53 – 13.90; P < 0.001) and longer distance (OR 1.004; 95% CI 1.003 – 1.005; P < 0.001) were associated with completing a teleneurology visit. Sex, median household income for zip code, and disease course were not significant; disability level was removed due to level of missingness (86.3%) in the teleneurology population.
MS patients: Comparison of pre- and post-COVID populations
Overall, changes in the MS population were noted pre- and post-COVID19 at each Center (Table 3). Comparing the teleneurology only MS patients (Table 3), at both Centers there was a significantly higher proportion of BIPOC patients (pooled: 12.7% vs 4.37%, p ≤ 0.001), and reduced driving distance to clinic (pooled: 34.5 vs 102 miles, p ≤ 0.001). At CC, the post-COVID population was also older; a higher proportion of APPs completed the visits, and there was a higher proportion of both routine follow-up visits and new patient visits (Table 3).
Table 3.
MS Teleneurology populations pre and post-COVID by Center.
| CC before, N = 212 | CC after, N = 720 | p-Value | UCSF before, N = 346 | UCSF after, N = 407 | p-Value | |
|---|---|---|---|---|---|---|
| Patient Age | 46.0 (11.8) | 49.7 (13.0) | <0.001a | 48.1 (12.9) | 48.5 (13.3) | 0.721a |
| Patient Sex | 0.581b | 0.240b | ||||
| Female | 161 (75.9%) | 531 (73.8%) | 259 (74.9%) | 288 (70.8%) | ||
| Male | 51 (24.1%) | 189 (26.2%) | 87 (25.1%) | 119 (29.2%) | ||
| Race | <0.001c | 0.007b | ||||
| White | 193 (96.5%) | 588 (84.4%) | 263 (80.7%) | 274 (70.8%) | ||
| Black | 6 (3.00%) | 101 (14.5%) | 17 (5.2%) | 37 (9.6%) | ||
| Other | 1 (0.50%) | 8 (1.15%) | 46 (14.1%) | 76 (19.6%) | ||
| Ethnicity | 0.472c | 0.325b | ||||
| Not Hispanic | 198 (99.5%) | 690 (98.6%) | 304 (87.9%) | 351(86.2%) | ||
| Hispanic | 1 (0.50%) | 10 (1.43%) | 27 (7.8%) | 43 (10.6%) | ||
| Disease Course | 0.267c | 0.871b | ||||
| RRMS | 153 (72.5%) | 540 (75.1%) | 33 (9.9%) | 39 (11.0%) | ||
| SPMS | 31 (14.7%) | 117 (16.3%) | 263 (78.7%) | 278 (78.3%) | ||
| PPMS | 27 (12.8%) | 61 (8.48%) | 38 (11.4%) | 38 (10.7%) | ||
| RIS | 0 (0.00%) | 1 (0.14%) | 0 | 0 | ||
| Disability Level | 0.596c | 0.173b | ||||
| Non-ambulatory | 2 (6.90%) | 28 (6.59%) | 23 (9.5%) | 22 (7.7%) | ||
| Gait impairment requires aide | 3 (10.3%) | 92 (21.6%) | 42 (17.4%) | 31 (10.8%) | ||
| Gait Impairment with no aide | 2 (6.90%) | 41 (9.65%) | 20 (8.3%) | 31 (10.8%) | ||
| Mild disability with no gait impairment | 9 (31.0%) | 117 (27.5%) | 96 (39.8%) | 129 (45.1%) | ||
| No disability | 13 (44.8%) | 147 (34.6%) | 60 (24.9%) | 73 (25.5%) | ||
| Clinician Type: | <0.001c | 0.791c | ||||
| Physician | 85 (40.5%) | 178 (25.2%) | 337 (97.4%) | 394 (96.8%) | ||
| APP | 125 (59.5%) | 522 (73.8%) | 9 (2.6%) | 13 (3.2%) | ||
| Oher | 0 (0.00%) | 7 (0.99%) | ||||
| Reason for Visit: | <0.001c | <0.001c | ||||
| Routine | 154 (72.6%) | 650 (90.3%) | 0 (0.0%) | 14 (3.4%) | ||
| New | 48 (22.6%) | 30 (4.17%) | 343 (99.1%) | 392 (96.3%) | ||
| Urgent | 8 (3.77%) | 13 (1.81%) | 3 (0.87%) | 1 (0.25%) | ||
| Other | 2 (0.94%) | 27 (3.75%) | 0 | 0 | ||
| Drive distance | 186 [82.3;334] | 31.9 [15.6;77.5] | <0.001d | 72.2 [22.6;182] | 39.1 [15.8;109] | <0.001d |
| Income* | 56,652 [48875;70514] | 53,831 [44426;69628] | 0.049d | na | na |
na: not available; CC: Cleveland Clinic; CIS: clinically isolated syndrome; MS: multiple sclerosis; ON: optic neuritis; PPMS: primary progressive MS, RRMS: relapsing remitting, SPMS: secondary progressive MS; RIS: radiologically isolated syndrome.P-value: a = t-test; b = chi-square test; c = Fisher exact test; d = Wilcoxon rank sum test.*input with median income by zip code.
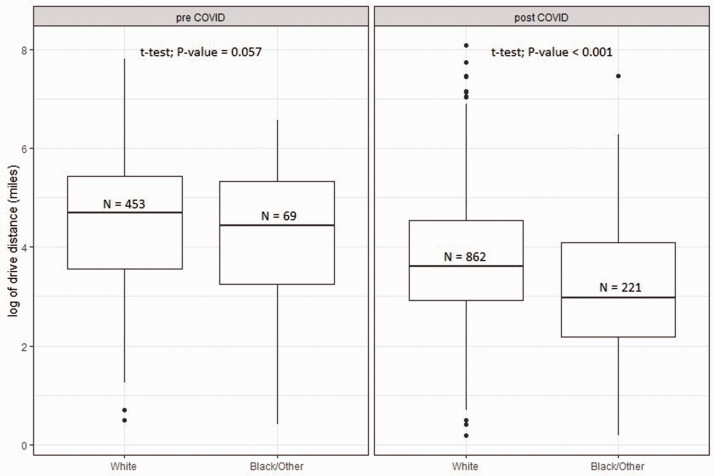
Post-hoc sensitivity analyses of race and geographical/socioeconomic characteristics. Since we noted a significant increase in the proportion of BIPOC patients in the Centers’ teleneurology populations post-COVID19, we further evaluated several hypotheses. We first asked whether racial differences in distance to the MS clinics could exist and be reflected in their teleneurology utilization pre-COVID. In the combined Center populations, driving distance among MS teleneurology patients was shorter for BIPOC patients than white patients (Figure 2); post-COVID, BIPOC patients had a 48.5% shorter drive distance than white patients (95% CI 38.2% – 57.0%; P < 0.001).
Figure 2.
Comparison of distances from Centers by race in the combined CC and UCSF teleneurology populations.
CC cohort only. More black than white patients lived under 50 miles from the Center both pre-COVID (71.7% v. 51.5%, p < 0.001) and post-COVID (79.8% vs. 59.7%, p < 0.001); and in both racial groups, the proportion of patients living near the Center increased post-COVID. We repeated our stepwise regression analysis of factors contributing to teleneurology vs. in-clinic visits, including not only race and driving distance (both significant) but also an interaction term, which was not significant (p > 0.05).
Since socioeconomic differences could also contribute to teleneurology utilization, we utilized median income for zipcode as an indicator of socieoeconomic status (SES). Pre-COVID, white race remained a significant predictor of completing a teleneneurology visit even after adjusting for SES. While for the entire MS population median SES did not differ pre- and post-COVID, for the teleneurology population the median SES did decrease post-COVID ($53,831 vs $56,652 p = 0.049).
Discussion
Prior to the COVID-19 pandemic there were distinct differences in teleneurology visits between the two Centers, reflecting geographic, organizational, and reimbursement patterns. The UCSF teleneurology population had a higher proportion of BIPOC patients, perhaps reflecting geographic racial distributions between Ohio and California (whites constitute 81.7% in Ohio and 71.9% in California).24 Further, since all California payors reimbursed teleneurology visits for California residents only, while Ohio had a variable rate of payor reimbursement, UCSF patients who used teleneurology lived overall closer to the Center. The COVID-19 pandemic prompted changes in care delivery, and this study supports a higher utilization of teleneurology at two large MS referral Centers.
During the COVID-19 pandemic there were multiple nationwide and organizational changes leading to a dramatic change in teleneurology utilization.25 At the nationwide level, Congress waved certain Medicare restrictions and requirements regarding telehealth, resulting in improved reimbursement and access. The American Academy of Neurology also strongly advocated for teleneurology to maintain access to neurological care. These changes were reflected in significant increases in the teleneurology populations at both Centers post-COVID, including a higher proportion of patients living closer to each Center, and also from outside California (UCSF only).
After COVID19, the racial composition of our Centers’ MS teleneurology patients also changed, with a significant increase in BIPOC patients. We were positioned to evaluate only one among many possible factors for these increases in teleneurology utilization by BIPOC patients: whether they were more likely to be local and hence to have only utilized teleneurology once physical access to the Centers was limited. However, we suspect that pre-COVID19, there were racial disparities in utilization of teleneurology that were only partially explained by geographical distance from the Center. At CC pre-COVID, white race predicted teleneurology visits even after adjusting for both white patients’ greater driving distance from the Center and higher SES. Therefore other factors could have played a role in these race differences. Challenges to utilization, such as perception of quality and trust, privacy concerns for patients with lower SES and crowded living conditions,26 low bandwidth in rural areas precluding satisfactory videoconferencing, and lower technological literacy among some older adults11 will need to be evaluated in future studies.
Our findings are important given the known racial disparities in access to MS care.27 BIPOC patients also face worse MS outcomes, and potentially even increasing incidence.28,29 As sharply illustrated when examining disparities in clinical outcomes related to the COVID-19 pandemic in the general population, these disparities are linked to differences in access, SES and discrimination. Outside of the United States as well, studies have linked low SES and minority racial group status with increased risk of MS-related disability.30
To our knowledge this is the largest reported experience with teleneurology for MS care. Both Centers had established teleneurology programs for MS since at least 2016, which allowed us to develop an understanding of how COVID-era changes affected utilization. It is clear from the literature that regulations and precautions due to the COVID-19 pandemic resulted in a significant increase in telehealth services overall, but the pre- and post- analysis performed in this study begins to help us evaluate how these regulatory and institutional changes impacted patient access and utilization of these services. The study has several limitations. First it is an observational retrospective study with differences in data collection and variables assessed at the two sites, which limited some comparisons. Thus, we only had data to perform pre-COVID predictive model at CC, resulting in some loss of generalizability. In that same predictive model zipcode was used as a representation of SES, but this has many known limitations and a more robust measure of SES would be valuable in future studies. Additionally, the post-COVID timeframe was only 6 weeks, and our finding that there were a higher proportion of RRMS patients and local patients post-COVID suggests that certain groups including more disabled and remote patients, could have been underrepresented in this sample. As regulatory changes continue to evolve, a long-term study to determine the differential utilization of telehealth across populations is needed. For some statistical analyses, we had to collapse certain race categories despite distinct sociocultural forces influencing healthcare access in each group. Additionally, a disability score was only available for 36% of teleneurology patients, which limits the interpretation of disability level of patients utilizing telehealth. Finally, some adjustments to teleneurology care due to the pandemic, such as use of alternative televideo platforms (e.g. FaceTime) and waiving of co-pays at CC, further limit generalizability.
This study suggests that rapid shifts in telehealth utilization can drive increased utilization of technology by BIPOC patients. Prior to COVID-19, telehealth in several chronic conditions was shown to improve access, reduce hospitalization rates, and have lower costs to the patient than traditional in-person visits.26,31–33 While telehealth is embraced by many neurology sub-disciplines, the American Academy of Neurology Telemedicine Work Group highlights large gaps in evidence basis for its impact on clinical outcomes.34 Teleneurology for MS has been shown to provide a reasonable assessment of MS-related disability and can reduce caregiver burden, while maintaining high clinician and patient satisfaction.11,17 It is not known whether overall, increased BIPOC utilization of teleneurology visits is perceived as an improvement in access to MS care, or conversely, necessary for maintaining access to care but inferior to in-person visits.
We demonstrate that teleneurology, for both established and new MS patient evaluations, can be rapidly scaled to meet patient needs in the face of evolving public health emergencies. This includes expanded access also for local, and BIPOC patient populations. Such approaches will be informative for other CNS autoimmune conditions. The data presented here lay the groundwork for continued utilization of teleneurology in MS but also highlight specific research gaps, including systematic comparisons between in-person and teleneurology visits relating to patient access, costs of care, patient perceptions and experience, and quality of outcomes for diverse, representative patient populations.
Acknowledgements
We would like to thank the clinicians at the Mellen Center and UCSF for the in person and teleneurology care and documentation during clinical visits.
Footnotes
Authors’ contributions: Marisa P McGinley: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data; Study concept or design; Analysis or interpretation of data. Shauna Gales: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data; Analysis or interpretation of data. William Rowles: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data. Zhini Wang: Analysis or interpretation of data. Wan-Yu Hsu: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data. Lilyana Amezcua: Drafting/revision of the manuscript for content, including medical writing for content. Riley Bove: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data; Study concept or design; Analysis or interpretation of data.
Conflict of Interests: M. McGinley has served on scientific advisory boards for Genzyme and Genentech, received research funding from Novartis, and receives funding via a KL2 (KL2TR002547) grant from Clinical and Translational Science Collaborative of Cleveland, from the National Center for Advancing Translational Sciences (NCATS) component of the NIH. S. Gales reports no disclosures relevant to the manuscript. W Rowles reports no disclosure relevant to the manuscript. Z. Wang reports no disclosures relevant to the manuscript. W. Hsu reports no disclosures relevant to the manuscript. L. Amezcua reports research funding from Biogen. She has served on advisory boards and is a consultant for Alexion, Biogen and Genzyme. R. Bove is a recipient of the National Multiple Sclerosis Society Harry Weaver Award. She has received research support from the National Multiple Sclerosis Society, the Hilton Foundation, the California Initiative to Advance Precision Medicine, the Sherak Foundation, Akili Interactive and Roche Genentech. She has received personal compensation for consulting from Alexion, Biogen, EMD Serono, Novartis, Pear Therapeutics, Roche Genentech and Sanofi.
Funding: KL2 (KL2TR002547) grant from Clinical and Translational Science Collaborative of Cleveland, from the National Center for Advancing Translational Sciences (NCATS) component of the NIH.
Contributor Information
Shauna Gales, Mellen Center, Cleveland Clinic, Cleveland, USA.
William Rowles, Department of Neurology and Weill Institute for Neurosciences, University of California, San Francisco, USA.
Zhini Wang, Mellen Center, Cleveland Clinic, Cleveland, USA.
Wan-Yu Hsu, Department of Neurology and Weill Institute for Neurosciences, University of California, San Francisco, USA.
Lilyana Amezcua, Department of Neurology, University of Southern California, Los Angeles, USA.
ORCID iDs
Marisa P McGinley https://orcid.org/0000-0002-7463-6787
Wan-Yu Hsu https://orcid.org/0000-0003-1410-8429
Lilyana Amezcua https://orcid.org/0000-0003-1542-7819
Riley Bove https://orcid.org/0000-0002-2034-8800
References
- 1.Cree BAC, Gourraud PA, Oksenberg JR, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol 2016; 80: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu C, Bishop M, Pionke JJ, et al. Barriers to the accessibility and continuity of Health-Care services in people with multiple sclerosis: a literature review. Int J MS Care 2017; 19: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatcher-Martin JM, Adams JL, Anderson ER, et al. Telemedicine in neurology: Telemedicine Work Group of the American Academy of Neurology update. Neurology 2020; 94(1): 30–38. [DOI] [PubMed]
- 4.Tong T, Myers AK, Bissoonauth AA, et al. Identifying the barriers and perceptions of non-Hispanic black and Hispanic/Latino persons with uncontrolled type 2 diabetes for participation in a home telemonitoring feasibility study: a quantitative analysis of those who declined participation, withdrew or were non-adherent. Ethn Health 2020; 25: 485–494. [DOI] [PubMed] [Google Scholar]
- 5.George S, Hamilton A, Baker RS. How do low-income urban African Americans and Latinos feel about telemedicine? A diffusion of innovation analysis. Int J Telemed Appl 2012; 2012: 715194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obermeyer Z, Powers B, Vogeli C, et al. Dissecting racial bias in an algorithm used to manage the health of populations. Science 2019; 366: 447–453. [DOI] [PubMed] [Google Scholar]
- 7.Hollander JE, Carr BG. Virtually perfect? Telemedicine for covid-19. N Engl J Med 2020; 382: 1679–1681. [DOI] [PubMed] [Google Scholar]
- 8.McGinley MP, Ontaneda D, Wang Z, et al. Teleneurology as a solution for outpatient care during the COVID-19 pandemic. Telemed e-Health. Epub ahead of print 16 June 2020. [DOI] [PMC free article] [PubMed]
- 9.Moore JT, Ricaldi JN, Rose CE, et al. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5–18, 2020 – 22 states, February–June 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manchanda EC, Couillard C, Sivashanker K. Inequity in crisis standards of care. N Engl J Med 2020; 383 1–2. [DOI] [PubMed]
- 11.Bove R, Garcha P, Bevan CJ, et al. Clinic to in-home telemedicine reduces barriers to care for patients with MS or other neuroimmunologic conditions. Neurol Neuroimmunol Neuroinflamm 2018; 5: e505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross L, Bena J, Bermel R, et al. Implementation and patient experience of outpatient teleneurology. Telemed e-Health. Epub ahead of print 23 June 2020. [DOI] [PMC free article] [PubMed]
- 13.State of Ohio Executive Order 2020-01D. Available at: https://governor.ohio.gov/wps/portal/gov/governor/media/executive-orders/executive-order-2020-01-d
- 14.State of California Executive Order N-33-20. Available at: https://www.gov.ca.gov/wp-content/uploads/2020/03/EO-N-33-20-COVID-19-HEALTH-ORDER-03.19.2020-002.pdf
- 15.Macaron G, Moss BP, Li H, et al. Technology-enabled assessments to enhance multiple sclerosis clinical care and research. Neurol Clin Pract 2020; 10: 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.H.R.6074 – Coronavirus Preparedness and Response Supplemental Appropriations Act. 2020. Available at: https://www.congress.gov/bill/116th-congress/house-bill/6074
- 17.Bove R, Bevan C, Crabtree E, et al. Toward a low-cost, in-home, telemedicine-enabled assessment of disability in multiple sclerosis. Mult Scler 2019; 25: 1526–1534. [DOI] [PubMed] [Google Scholar]
- 18.Marrie RA, Goldman M. Validity of performance scales for disability assessment in multiple sclerosis. Mult Scler 2007; 13: 1176–1182. [DOI] [PubMed] [Google Scholar]
- 19.Hohol MJ, Orav EJ, Weiner HL, et al. Disease steps and EDSS to evaluate disease progression. Mult Scler 1999; 5: 349–354. [DOI] [PubMed] [Google Scholar]
- 20.Kappos L, D’Souza M, Lechner-Scott J, et al. On the origin of neurostatus. Mult Scler Relat Disord 2015; 4: 182–185. [DOI] [PubMed] [Google Scholar]
- 21.Zdeb M. Driving distances and drive times using SAS and Google Maps. India: Coders’ Corner. 2010. (Paper 50).
- 22.Clark US, Hurd YL. Addressing racism and disparities in the biomedical sciences. Nat Hum Behav 2020; 4: 774–777. [DOI] [PubMed] [Google Scholar]
- 23.Amezcua L, McCauley JL. Race and ethnicity on MS presentation and disease course. Mult Scler 2020; 26: 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Census Population Estimates, www.census.gov/quickfacts/fact/table/US/PST045219 (accessed 9 October 2020).
- 25.Grossman SN, Han SC, Balcer LJ, et al. Rapid implementation of virtual neurology in response to the COVID-19 pandemic. Neurology 2020; 94: 1077–1087. [DOI] [PubMed] [Google Scholar]
- 26.Brown EM. The Ontario telemedicine network: a case report. Telemed J E Health 2013; 19: 373–376. [DOI] [PubMed] [Google Scholar]
- 27.Roddam H, Rog D, Janssen J, et al. Inequalities in access to health and social care among adults with multiple sclerosis: a scoping review of the literature. Mult Scler Relat Disord 2019; 28: 290–304. [DOI] [PubMed] [Google Scholar]
- 28.Langer-Gould A, Brara SM, Beaber BE, et al. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology 2013; 80: 1734–1739. [DOI] [PubMed] [Google Scholar]
- 29.Romanelli RJ, Huang Q, Lacy J, et al. Multiple sclerosis in a multi-ethnic population from Northern California: a retrospective analysis, 2010–2016. BMC Neurol 2020; 20: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calocer F, Dejardin O, Kwiatkowski A, et al. Socioeconomic deprivation increases the risk of disability in multiple sclerosis patients. Mult Scler Relat Disord 2020; 40: 101930. [DOI] [PubMed] [Google Scholar]
- 31.Uscher-Pines L, Mehrotra A. Analysis of teladoc use seems to indicate expanded access to care for patients without prior connection to a provider. Health Aff (Millwood) 2014; 33: 258–264. [DOI] [PubMed] [Google Scholar]
- 32.Reed ME, Parikh R, Huang J, et al. Real-time patient-provider video telemedicine integrated with clinical care. N Engl J Med 2018; 379: 1478–1479. [DOI] [PubMed] [Google Scholar]
- 33.Gordon AS, Adamson WC, DeVries AR. Virtual visits for acute, nonurgent care: a claims analysis of episode-level utilization. J Med Internet Res 2017; 19: e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine SR, Gorman M. “ Telestroke”: the application of telemedicine for stroke. Stroke 1999; 30: 464–469. [DOI] [PubMed] [Google Scholar]