Abstract
Background:
Hyaluronic acid (HA) and leukocyte-poor platelet-rich plasma (LP-PRP) are 2 nonoperative treatment options that have been studied in patients with hip osteoarthritis (OA).
Purpose:
To compare the efficacy of intra-articular injections of low–molecular weight (LMW) HA and LP-PRP in patients with hip OA.
Study Design:
Randomized controlled trial; Level of evidence, 1.
Methods:
A total of 34 patients (36 hips) presenting with signs of hip OA were randomized to receive 3 blinded, weekly intra-articular injections of either LP-PRP or LMW-HA. Patients were prospectively evaluated before injections and at 6 weeks and then at 3, 6, 12, and 24 months. The primary outcome, conversion to total hip arthroplasty (THA) or a hip resurfacing procedure, was analyzed along with secondary outcomes including the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score and hip range of motion.
Results:
The final analysis included 33 hips (mean Kellgren-Lawrence grade, 2.73) (LMW-HA: n = 14; LP-PRP: n = 19) in 31 patients (18 male; mean age, 53.8 years). Significantly more patients converted to THA or a hip resurfacing procedure in the LMW-HA group (7/14; 50.0%) (mean, 1.3 years after first injection) than the LP-PRP group (3/19; 15.8%) (mean, 0.73 years after first injection) (P = .035). There was no significant improvement or decline in any outcome scores within the LMW-HA group from before injections to 6 weeks or 3, 6, and 12 months. For the LP-PRP group, WOMAC overall (P = .032), joint (P = .030), and function scores (P = .025) significantly improved from before injections to 6 weeks, and WOMAC joint scores significantly improved from before injections to 6 months (P = .036). When comparing the difference between groups in internal rotation at 90° of hip flexion from before injections to 6 months, the LP-PRP group demonstrated a mean 5.0° improvement, while the LMW-HA group showed a mean 1.5° decrease (P = .028).
Conclusion:
Intra-articular hip injections of LP-PRP in patients with hip OA resulted in an improvement in WOMAC scores and hip internal rotation at 6 months and delayed the need for THA or a hip resurfacing procedure compared with treatment with LMW-HA. A longer follow-up is necessary to further compare the effects of LP-PRP and LMW-HA injections in patients with hip OA.
Registration:
NCT01920152 (ClinicalTrials.gov identifier).
Keywords: hip, osteoarthritis, platelet-rich plasma, hyaluronic acid, intra-articular injection
Osteoarthritis (OA) is one of the most common painful conditions affecting adults, frequently impairing mobility and reducing quality of life.8,10,12,32,34,37,48 Obesity,12,17,18,25,26,32 older age, female sex,12,17,25,33,37 White ethnicity,25,35 and genetics27,45,49,53 have been reported to increase the risk for developing OA. No treatment methods have been shown to reduce joint articular cartilage degeneration in OA,39,48 but biological approaches including platelet-rich plasma (PRP) have shown promise as an effective treatment option for OA in multiple joints outside of the hip.4,9
PRP is rich in growth factors8,13,39 that stimulate the body’s natural healing process and interact with the various tissues affected by OA, including cartilage and synovium.29,39 PRP can be formulated to have a high (leukocyte-rich PRP [LR-PRP]) or low (leukocyte-poor PRP [LP-PRP]) concentration of white blood cells. At this time, there is evidence to support the use of LP-PRP but insufficient evidence to support LR-PRP for knee OA.4,23,42
Another advantage of PRP is that it improves the quality of synovial fluid by inducing the endogenous secretion of hyaluronic acid (HA).2,41 Synovial HA is a glycosaminoglycan normally present in synovial fluid that possesses anti-inflammatory and analgesic properties33 and is thought to restore viscoelasticity to the joint.8,33,40,50 HA is approved by the US Food and Drug Administration for the treatment of early knee OA and comes in both high–molecular weight (HMW; 6-7 million Da) and low–molecular weight (LMW; 0.5-1.5 million Da) formulations. LMW-HA is known to provide an anti-inflammatory effect,41 whereas HMW-HA may protect cartilage from degradation through the inhibition of aggrecanase.36 Multiple studies in patients with knee OA have found no significant differences in the efficacy of HMW-HA versus LMW-HA.14,24,46
It remains to be determined whether LP-PRP is a superior treatment method to LMW-HA for hip OA.11,23,54 The purpose of this study was to compare the efficacy of intra-articular injections of LMW-HA and LP-PRP in patients with hip OA.
Methods
Study Design
This study was approved by an institutional review board and was registered at ClinicalTrials.gov (NCT01920152). Patients with hip OA, defined as grade 2 or 3 on the Kellgren-Lawrence scale, were enrolled in the study from 2013 to 2018.20 All patients had presented to a dedicated hip preservation service with issues of hip pain and/or functional limitations. Once enrolled, patients completed the baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).5 Patients with polyarticular disease or major health conditions such as poorly controlled diabetes, congestive heart failure, chronic obstructive pulmonary disease, untreated depression, or known blood disorders; pregnant or nursing patients; patients with inflammatory arthritic conditions; non–English speaking patients; patients with additional disabilities in any of the lower limbs that would interfere with any of the clinical assessments; those with chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or chemotherapy drugs; those who had treated symptoms with aspirin or NSAIDs within 7 days before randomization; and those with a body mass index over 30 kg/m2 were excluded from the study. Patients who had undergone previous hip surgery, received intra-articular treatment with steroids within 6 months of the beginning of the study, or received more than 3 previous intra-articular steroid injections to the affected hip were also excluded. Patients who received all 3 injections of PRP or HA and completed a minimum 6-week follow-up were included in the study. A preliminary physical examination and radiographic assessment with anteroposterior pelvic radiography were performed for each patient to document baseline parameters including range of motion (ROM) at both 90° of hip flexion and neutral hip and the Kellgren-Lawrence grade,20 respectively.
A computer-generated randomization table (QuickCalcs random number generator; GraphPad Software)15 was utilized to randomize patients to either the PRP or the HA treatment groups (Figure 1). Patients enrolled for bilateral hip OA were randomized to the same treatment group for both hips. Time intervals between injections were held constant for both groups, with patients receiving 3 injections at 1-week intervals. Before all injections, patients in both treatment groups had their blood drawn to maintain the double-blind methodology. If the patient was randomized to the LMW-HA group, the blood draw was discarded, and the patient received weekly injections of 2.5 mL of 1% (10 mg) sodium hyaluronate (Supartz; Smith & Nephew). If the patient was randomized to the LP-PRP group, the blood was immediately processed to produce LP-PRP for the injection using a validated method, resulting in a 2- to 3-fold increase in platelet concentration without leukocytes.48 The LP-PRP group received weekly injections of PRP obtained using the Endoret kit (PRGF; BTI Biotechnology Institute) that was prepared in a sterile fashion as described by Sanchez et al.43 A total of 36 mL of peripheral blood was collected into four 9-mL extraction tubes containing 3.8% (wt/vol) sodium citrate. Tubes were centrifuged at 640 rpm for 8 minutes at room temperature. The 1- to 2-mL plasma fraction located just above the buffy coat (this volume can be different between patients) was then manually aspirated from each tube and dispensed into the fractioning tube under laminar airflow conditions. Immediately before the injection, calcium chloride was added to the LP-PRP fractioning tube with a final concentration of 50 µL for every 1 mL of LP-PRP. The activated LP-PRP was injected in its entirety into the hip joint.
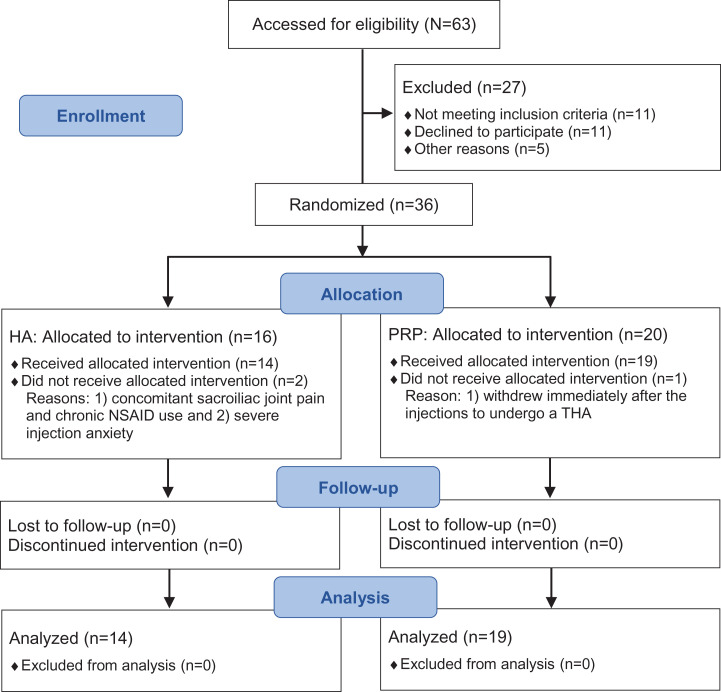
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials)31 flow diagram. Sample sizes (n) refer to the number of hips included at each stage. HA, hyaluronic acid; NSAID, nonsteroidal anti-inflammatory drug; PRP, platelet-rich plasma; THA, total hip arthroplasty.
Non–image guided intra-articular hip injections were given based on anatomic landmarks as previously described.22,30 In a previous study using this same technique, we found that 96% of patients with intra-articular hip abnormalities experienced at least 70% improvement in pain after a corticosteroid injection, thereby proving the efficacy of the non–image guided technique.22 Patients were advised to significantly reduce physical activity for 2 to 3 days after each injection and were instructed to avoid blood-thinning medications 2 days before and 5 days after the injections, including aspirin and NSAIDs. The use of pain medications or NSAIDs was not restricted after that time.
Survivorship
To measure the duration of clinical benefit, survivorship was analyzed by investigating the frequency of patient withdrawal to undergo surgery (total hip arthroplasty [THA] or hip resurfacing procedure). This served as the primary outcome measure for the study.
Efficacy Measurements
The WOMAC5 was designated as the secondary outcome measure. It is a normalized patient-reported outcome measure in which 0 represents the worst possible score and 100 represents the best possible score. The WOMAC is primarily used in OA clinical trials to evaluate the effects of arthroplasty and drug interventions in patients with hip OA.1,5,28,52 Function, ROM, and adverse events were assessed at the time of enrollment and at 6 weeks and then at 3, 6, 12, and 24 months after the initial injection by an examiner who was blinded to the applied treatment. During follow-up visits, a physical examination was performed to assess function and ROM of the hip by visual estimation, including external rotation, internal rotation, and flexion. Examiners were blinded to the treatment group during all follow-up assessments. The degree of agreement between visual estimation and goniometric methods of measuring hip ROM was previously evaluated in a pilot study of 100 consecutive hips using 2-way, mixed, absolute-agreement, single-measures intraclass correlation coefficients.7,21 The intraclass correlation coefficient was 0.976 (95% CI, 0.727-0.992), indicating excellent reliability.
Statistical Analysis
Statistical analyses were blinded and performed according to the intention-to-treat principle. The Student t test was used to compare outcomes between baseline and each follow-up interval within each treatment group. Additionally, the Student t test was used to compare differences between baseline and all time points. For categorical variables, the chi-square test was used. Survival analysis of the duration of clinical benefit provided by the injection treatment was performed using the Kaplan-Meier method19; the Mantel-Haenszel test was used to compare the 2 injection treatments. A P value <.05 was considered statistically significant. Survival curve differences were calculated using the survdiff function16 of the survival package51 in RStudio statistical software (Version 1.1.456).
To determine the power of this study, a post hoc power analysis was performed based on survivorship, which was defined as the primary outcome measure. On the basis of the chi-square goodness-of-fit test, an effect size (w) of 0.650 was calculated. With this effect size, we ascertained that the size of our cohort was sufficient to obtain a 96.2% chance of detecting a 5% difference in survivorship. The post hoc analysis supported the results obtained and the sample size empirically used in the present study.
Results
A total of 34 patients (36 hips) met inclusion criteria and were enrolled in the study from 2013 to 2018. There were 2 patients in the LMW-HA group who did not complete the injection intervention and were thus excluded. Also, 1 patient in the LP-PRP group discontinued the intervention and was thus excluded; this patient withdrew before completing the intervention protocol to undergo THA, as she was offered surgery earlier in place of a canceled patient. Thus, 31 patients (18 male, 13 female) with 33 symptomatic hips completed the treatment protocol and subsequent follow-up assessments and were included in the analysis (see Figure 1).
The LMW-HA group comprised 13 patients (14 hips) and the LP-PRP group comprised 18 patients (19 hips), all of whom completed a minimum 6-month follow-up. There were 2 patients who underwent bilateral treatments, with 1 in each group. No significant differences were found in demographics between the 2 groups, although the LMW-HA group demonstrated a trend toward a larger proportion of male patients (P = .070) (Table 1). No adverse events were documented for patients in either treatment group.
Table 1.
Demographic Dataa
| LMW-HA | LP-PRP | P Value | |
|---|---|---|---|
| Age, y | 53.6 ± 7.6 | 53.3 ± 8.4 | .72 |
| Body mass index, kg/m2 | 23.5 ± 2.0 | 23.7 ± 2.1 | .81 |
| Sex, n (%) | .070 | ||
| Male | 10 (77) | 8 (44) | |
| Female | 3 (23) | 10 (56) | |
| Side, n (%) | .65 | ||
| Right | 7 (50) | 11 (58) | |
| Left | 7 (50) | 8 (42) | |
| Kellgren-Lawrence grade, n (%) | .15 | ||
| 2 | 2 (14) | 7 (37) | |
| 3 | 12 (86) | 12 (63) |
aData are reported as mean ± SD unless otherwise indicated. LMW-HA, low–molecular weight hyaluronic acid; LP-PRP, leukocyte-poor platelet-rich plasma.
Significantly more patients who received HA injections (7/14; 50.0%) (mean, 1.3 years after first injection) withdrew to undergo THA or a hip resurfacing procedure compared with patients who received PRP injections (3/19; 15.8%) (mean, 0.73 years after first injection) (P = .035) (Table 2). Furthermore, 64.3% of patients in the LMW-HA group withdrew from the study for any reason compared with only 21.1% of patients in the LP-PRP group (P = .012) (Table 2).
Table 2.
Reasons for Study Withdrawala
| Time Point | LMW-HA (n = 14 Hips) | LP-PRP (n = 19 Hips) | ||
|---|---|---|---|---|
| Surgery | Other | Surgery | Other | |
| 3 mo | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) |
| 6 mo | 1 (7.1) | 0 (0.0) | 1 (5.3) | 0 (0.0) |
| 12 mo | 5 (35.7) | 2 (14.3)b | 1 (5.3) | 1 (5.3)b |
| 18 mo | 1 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 24 mo | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 7 (50.0) | 2 (14.3)b | 3 (15.8) | 1 (5.3)b |
| Overall | 9 (64.3) | 4 (21.1) | ||
aData are reported as n (%). Surgery comprised total hip arthroplasty or hip resurfacing procedure. LMW-HA, low–molecular weight hyaluronic acid; LP-PRP, leukocyte-poor platelet-rich plasma.
bOther treatment included corticosteroid or PRP injection.
In terms of survivorship, the LP-PRP group demonstrated a significantly lower conversion rate to surgical management compared with the LMW-HA group (P = .038) (Figure 2). The 2-year survival probability estimate for the LP-PRP group was 0.84 (95% CI, 0.69-1.00) versus 0.41 (95% CI, 0.20-0.83) for the LMW-HA group. The 1-year survival probability estimate for the LP-PRP group was 0.89 (95% CI, 0.77-1.00) versus 0.86 (95% CI, 0.69-1.00) for the LMW-HA group.
Figure 2.
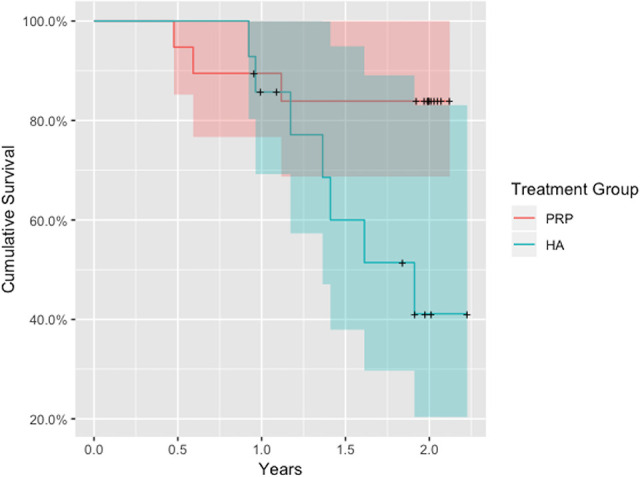
Survivorship of treatment groups. HA, hyaluronic acid; PRP, platelet-rich plasma.
Mean WOMAC joint (P = .030), function (P = .025), and overall (P = .032) scores increased (improved) from before injections to 6 weeks for the LP-PRP group. There was also a significant improvement in WOMAC joint scores from before injections to 6 months (P = .036) in the LP-PRP group. Otherwise, there were no significant differences in improvement for any other WOMAC outcome measure from before injections to any follow-up interval within the LP-PRP group. Within the LMW-HA group, no significant improvements in any WOMAC scores were demonstrated from before injections to any follow-up interval.
When comparing the difference in WOMAC function scores from before injections to 6 weeks between groups, the LP-PRP group demonstrated a significantly greater improvement compared with the LMW-HA group (P = .020) (Table 3). When comparing the difference in WOMAC scores from before injections to 12 months between groups, the LP-PRP group (17 hips) demonstrated a significant improvement in WOMAC joint (54.4 ± 27.9 to 65.8 ± 25.6) and function (68.7 ± 21.5 to 77.2 ± 22.3) scores, while the LMW-HA group (13 hips) showed a decline in both WOMAC joint (70.2 ± 25.3 to 56.7 ± 31.3; P = .003) and WOMAC function (80.8 ± 13.4 to 74.4 ± 20.7; P = .006) scores. When comparing the difference in WOMAC pain scores from 6 to 12 months, the LMW-HA group demonstrated a significant decline in scores (84.6 ± 15.1 to 71.5 ± 20.2), while the LP-PRP group maintained similar scores (75.6 ± 15.5 to 74.4 ± 24.1) (P = .019).
Table 3.
WOMAC Scores: Time × Group Interactiona
| LMW-HA (n = 14 Hips) | LP-PRP (n = 19 Hips) | P Valueb | |||
|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | ||
| WOMAC pain | |||||
| 0 wk | 14 | 78.9 ± 14.3 | 19 | 72.1 ± 18.0 | .23 |
| 6 wk | 12 | 80.0 ± 17.1 | 18 | 80.8 ± 12.4 | .89 |
| 12 wk | 13 | 79.2 ± 18.0 | 18 | 80.8 ± 12.0 | .78 |
| 24 wk | 14 | 84.3 ± 14.5 | 18 | 76.1 ± 15.2 | .13 |
| WOMAC joint | |||||
| 0 wk | 14 | 68.8 ± 24.9 | 19 | 55.3 ± 26.5 | .14 |
| 6 wk | 13 | 75.0 ± 23.4 | 18 | 71.5 ± 15.9c | .65 |
| 12 wk | 13 | 71.2 ± 29.5 | 18 | 68.8 ± 19.8 | .80 |
| 24 wk | 14 | 74.1 ± 22.2 | 18 | 72.9 ± 22.8c | .88 |
| WOMAC function | |||||
| 0 wk | 13 | 81.2 ± 12.9 | 18 | 69.6 ± 20.4 | .06 |
| 6 wk | 13 | 83.7 ± 15.2 | 18 | 82.9 ± 12.5c | .88 |
| 12 wk | 13 | 79.1 ± 17.8 | 18 | 80.1 ± 14.8 | .86 |
| 24 wk | 14 | 84.1 ± 15.7 | 18 | 80.2 ± 16.4 | .50 |
| WOMAC overall | |||||
| 0 wk | 13 | 77.9 ± 14.4 | 18 | 67.6 ± 19.7 | .10 |
| 6 wk | 12 | 79.5 ± 16.2 | 18 | 79.7 ± 10.9c | .97 |
| 12 wk | 13 | 77.5 ± 19.0 | 18 | 78.0 ± 13.0 | .93 |
| 24 wk | 14 | 82.1 ± 15.1 | 18 | 77.0 ± 15.8 | .36 |
aLMW-HA, low–molecular weight hyaluronic acid; LP-PRP, leukocyte-poor platelet-rich plasma; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
bP values compare scores between groups at each time point.
cValue indicates a significant difference (P < .05) compared with the preinjection value.
Ultimately, 14 patients (15 hips; 78.9%) in the LP-PRP group reached a final follow-up of 2 years, although only 5 patients (5 hips; 35.7%) in the LMW-HA group reached 2-year follow-up. Thus, no analysis between groups was possible at this time point. However, the LP-PRP group maintained higher scores from baseline to 2 years on all WOMAC measures (Table 4).
Table 4.
WOMAC Scores: Time × Group Interaction for Patients With Minimum 2-Year Follow-upa
| LMW-HA (n = 5 Hips) | LP-PRP (n = 15 Hips) | |||
|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | |
| WOMAC pain | ||||
| 0 y | 5 | 84.0 ± 11.9 | 15 | 72.7 ± 19.5 |
| 2 y | 5 | 84.0 ± 23.3 | 15 | 79.3 ± 13.9 |
| WOMAC joint | ||||
| 0 y | 5 | 77.5 ± 22.4 | 15 | 54.2 ± 29.8 |
| 2 y | 5 | 75.0 ± 19.8 | 15 | 66.7 ± 23.0 |
| WOMAC function | ||||
| 0 y | 5 | 87.1 ± 8.7 | 15 | 68.9 ± 22.2 |
| 2 y | 5 | 84.4 ± 22.6 | 15 | 78.7 ± 15.3 |
| WOMAC overall | ||||
| 0 y | 5 | 83.8 ± 12.1 | 15 | 67.4 ± 21.4 |
| 2 y | 5 | 82.3 ± 20.3 | 15 | 76.4 ± 15.8 |
aLMW-HA, low–molecular weight hyaluronic acid; LP-PRP, leukocyte-poor platelet-rich plasma; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
There was no statistically significant improvement in any ROM measurement within the LMW-HA or LP-PRP group from before injections to any follow-up interval (Table 5). However, when comparing the difference between groups in internal rotation at 90° of hip flexion from before injections to 24 weeks, the LP-PRP group demonstrated a significantly greater improvement, with a mean increase in internal rotation of 5.0° compared with a mean 1.5° decrease seen in the LMW-HA group (P = .028). There were no significant differences in improvement in any other ROM measurements between the groups.
Table 5.
ROM: Time × Group Interactiona
| LMW-HA (n = 14 Hips) | LP-PRP (n = 19 Hips) | P Valueb | |||
|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | ||
| Internal rotationc | |||||
| 0 wk | 14 | 2.3 ± 13.5 | 18 | 2.8 ± 7.0 | .90 |
| 6 wk | 13 | 0.9 ± 14.1 | 18 | 3.3 ± 6.6 | .58 |
| 12 wk | 14 | 0.7 ± 14.5 | 18 | 4.7 ± 7.9 | .37 |
| 24 wk | 13 | 0.8 ± 11.3 | 18 | 7.8 ± 10.3 | .09 |
| External rotation | |||||
| 0 wk | 14 | 36.4 ± 10.8 | 18 | 36.1 ± 12.9 | .94 |
| 6 wk | 13 | 36.2 ± 9.2 | 18 | 37.6 ± 9.2 | .66 |
| 12 wk | 14 | 35.7 ± 8.7 | 18 | 34.6 ± 12.2 | .76 |
| 24 wk | 13 | 38.5 ± 7.5 | 18 | 36.8 ± 10.1 | .60 |
| Flexion | |||||
| 0 wk | 14 | 101.1 ± 11.3 | 18 | 102.4 ± 8.2 | .72 |
| 6 wk | 13 | 99.2 ± 9.8 | 18 | 99.6 ± 7.8 | .92 |
| 12 wk | 14 | 98.2 ± 9.3 | 18 | 101.9 ± 12.2 | .35 |
| 24 wk | 13 | 97.3 ± 10.1 | 18 | 103.5 ± 13.3 | .15 |
aLMW-HA, low–molecular weight hyaluronic acid; LP-PRP, leukocyte-poor platelet-rich plasma; ROM, range of motion.
bP values compare measurements between groups at each time point.
cSome patients were unable to reach 0° of hip internal rotation, and therefore, the value was recorded as negative.
Discussion
The principal findings of this double-blind, randomized trial comparing the treatment of hip OA symptoms with intra-articular injections of LP-PRP versus LMW-HA were the following: (1) significantly more patients treated with LMW-HA injections failed to improve and underwent hip replacement procedures compared with patients who received LP-PRP injections, (2) patients treated with LP-PRP demonstrated significant improvements on more outcome measures compared with those treated with LMW-HA, and (3) patients treated with LP-PRP demonstrated a significantly greater improvement in internal rotation at 6 months compared with those treated with LMW-HA.
PRP can be formulated to have a high (LR-PRP) or low (LP-PRP) concentration of white blood cells. LP-PRP induces an anti-inflammatory effect by reducing the effect of interleukin 1 beta (IL-1β),47 whereas LR-PRP is proinflammatory and has higher concentrations of multiple growth factors, including platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF).55 For this reason, LR-PRP is more appealing for clinical cases in which increased inflammation and pain are present.55 At this time, there is evidence to support the use of LP-PRP for early knee OA but insufficient evidence to support PRP for hip OA.4,23,42
Few studies3,8,10,11 have aimed to compare the efficacy of intra-articular injections of HA and PRP in patients with hip OA, with no analysis of hip survivorship. In a study by Battaglia et al,3 intra-articular injections of LR-PRP with a moderate leukocyte concentration (8300/µL) were found to be efficacious in terms of functional improvement and pain relief, although they were not superior to HMW-HA in patients with symptomatic hip OA at 12-month follow-up. In a randomized controlled study by Dallari et al,8 the therapeutic effects of either PRP (the authors of that study did not comment on the PRP formulation) or HMW-HA or a combination of PRP and HA were investigated. The PRP group had the lowest (best) pain visual analog scale scores at all follow-up time points (2, 6, and 12 months) and significantly better WOMAC scores at 2- and 6-month follow-ups but not at 12-month follow-up.8
To our knowledge, no previous studies have addressed survivorship within the context of intra-articular injections of LP-PRP versus LMW-HA in the treatment of hip OA. This aspect of analysis is an interesting addition to traditionally analyzed outcome measures, as it could be used to study the cost-effectiveness of the treatment algorithm. While it is understood that no current treatment methods are able to reverse the progression of OA, survivorship could be used as a way of measuring the effectiveness of biological treatment methods, and functional magnetic resonance imaging analysis could be used to evaluate potential slowing or halting of the progression of OA. In addition to the anti-inflammatory properties of LP-PRP, there is evidence that PRP improves the quality of synovial fluid by inducing the endogenous secretion of HA.2,41 Specifically, PRP stimulates synovial cell proliferation and migration as well as the autocrine release of hepatocyte growth factors and HA.44 Thus, PRP may hold some of the same biological advantages of HA alone in addition to the effects of its growth factors. Interestingly, we found similar results of hip survivorship at 1 year after injection therapy (1-year survival probability estimates: PRP, 0.89; HA, 0.86), with a marked change in results at 2-year follow-up (2-year survival probability estimates: PRP, 0.84; HA, 0.41). Thus, the benefits of PRP in treating hip OA may be primarily related to its long-lasting effects compared with HA. It is possible that a second round of HA injections after 1 year may further improve hip survivorship in these patients, although further studies would be necessary to test this hypothesis.
In the present study, WOMAC subscores showed a statistically significant improvement at various time points in the LP-PRP group compared with no significant improvements in the LMW-HA group. Even when WOMAC scores did not demonstrate a statistically significant improvement (because of the relatively low sample size), the LP-PRP group frequently showed a clinically superior improvement in these scores in comparison with the LMW-HA group. Furthermore, there was a significantly greater improvement in hip internal rotation in the LP-PRP group. In this patient population, limited hip internal rotation is primarily caused by cartilage loss, bony osteophytes, and capsular tightness, thereby providing further clinical evidence of the biological effect of PRP on the hip joint.
Although we found improved clinical outcomes in the LP-PRP group, it should be noted that 3 of 19 hips (15.8%) later underwent hip arthroplasty at a mean 0.73 years after the first injection. Longer term follow-up is needed to further analyze the true outcomes of intra-articular hip injections of PRP or HA in patients with hip OA, although our results seem to demonstrate an overall delay (rather than elimination) in the need for hip arthroplasty with LP-PRP injections. Furthermore, PRP injections are expensive and may not be affordable for most patients (US$1500 for 3 injections at our institution). In addition, unlike HA treatments, PRP injections require an in-office blood draw, which may be painful and anxiety producing for some patients. Thus, patients with hip OA considering PRP injections must weigh these drawbacks with their potential clinical benefit.
The strengths of this study include the double-blind, randomized study design and minimization of assignment bias through the randomization and blinding of patients, treating physician, data outcome assessors, and collectors. The limitations of the current study should also be noted. This includes the absence of a true control group, such as sham treatment with saline. Previous randomized controlled trials have demonstrated improvements in pain and stiffness with the use of placebo in patients with hip OA, and this must be taken into account when considering our results.6,38 In addition, a larger sample size and longer follow-up time may help clarify the comparative efficacy of these 2 treatment methods. Although we used a PRP kit that has previously been shown to deliver LP-PRP,43 we did not analyze the PRP specimens in this study to ensure that they were LP-PRP or to characterize its growth factors. The inclusion/exclusion criteria of our study were strict (eg, exclusion of patients with a body mass index >30 kg/m2) and may not be applicable to the general population presenting with hip OA. Although not statistically significant, baseline WOMAC scores were lower in the LP-PRP group, despite the randomization process. This could have affected the level of improvement in WOMAC scores between the 2 groups at the various follow-up intervals. Also, the use of analgesic medication was not recorded during the 2-year follow-up period. Finally, the underlying cause of the patients’ hip OA (femoroacetabular impingement, dysplasia, trauma, etc) was not known and may have differed between the treatment groups.
Conclusion
Intra-articular hip injections of LP-PRP in patients with hip OA resulted in an improvement in WOMAC scores and hip internal rotation at 6 months and delayed the need for THA or a hip resurfacing procedure compared with treatment with LMW-HA. Longer follow-up is necessary to further compare the effects of LP-PRP and LMW-HA injections in patients with hip OA.
Footnotes
Final revision submitted June 19, 2020; accepted July 2, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by BTI Biotechnology Institute and Bioventus (distributor of Supartz); BTI Biotechnology Institute donated the platelet-rich plasma kits, and Bioventus donated hyaluronic acid for the study. T.G. has received consulting fees from Stryker and hospitality payments from Smith & Nephew. J.L.D. has received research support from Conmed Linvatec, Ossur, RTI Surgical, Terumo BCT, and Zimmer; consulting fees from Arthrex, Becton Dickinson, Biomet, Bioventus, Breg, Conmed Linvatec, DePuy, DJO, Exactech, Flexion Therapeutics, Genzyme, Harvest Technologies, Joint Restoration Foundation, KCRN Research, Moximed, Ossur, RTI Surgical, RNL Bio, Sideline Sports Doc, and Zimmer; speaking fees from Ossur; and other financial support from EmCyte and Harvest Technologies. O.M.-D. has received research support from Stryker, has received educational support from Arthrex, has received consulting fees from Smith & Nephew and Stryker, has received nonconsulting fees from Smith & Nephew, has received royalties from Stryker, and has stock/stock options in MITA. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the University of Colorado (CRV006-1).
References
- 1. Angst F, Ewert T, Lehmann S, Aeschlimann A, Stucki G. The factor subdimensions of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) help to specify hip and knee osteoarthritis: a prospective evaluation and validation study. J Rheumatol. 2005;32(7):1324–1330. [PubMed] [Google Scholar]
- 2. Anitua E, Sanchez M, Nurden AT, et al. Platelet-released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology (Oxford). 2007;46(12):1769–1772. [DOI] [PubMed] [Google Scholar]
- 3. Battaglia M, Guaraldi F, Vannini F, et al. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics. 2013;36(12):e1501–e1508. [DOI] [PubMed] [Google Scholar]
- 4. Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, McCarty EC. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. Published online April 17, 2020. doi: 10.1177/0363546520909397 [DOI] [PubMed] [Google Scholar]
- 5. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 6. Bennell KL, Egerton T, Martin J, et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311(19):1987–1997. [DOI] [PubMed] [Google Scholar]
- 7. Chadayammuri V, Garabekyan T, Bedi A, et al. Passive hip range of motion predicts femoral torsion and acetabular version. J Bone Joint Surg Am. 2016;98(2):127–134. [DOI] [PubMed] [Google Scholar]
- 8. Dallari D, Stagni C, Rani N, et al. Ultrasound-guided injection of platelet-rich plasma and hyaluronic acid, separately and in combination, for hip osteoarthritis: a randomized controlled study. Am J Sports Med. 2016;44(3):664–671. [DOI] [PubMed] [Google Scholar]
- 9. de Girolamo L, Kon E, Filardo G, et al. Regenerative approaches for the treatment of early OA. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1826–1835. [DOI] [PubMed] [Google Scholar]
- 10. Di Sante L, Villani C, Santilli V, et al. Intra-articular hyaluronic acid vs platelet-rich plasma in the treatment of hip osteoarthritis. Med Ultrason. 2016;18(4):463–468. [DOI] [PubMed] [Google Scholar]
- 11. Doria C, Mosele GR, Caggiari G, Puddu L, Ciurlia E. Treatment of early hip osteoarthritis: ultrasound-guided platelet rich plasma versus hyaluronic acid injections in a randomized clinical trial. Joints. 2017;5(3):152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. [DOI] [PubMed] [Google Scholar]
- 13. Filardo G, Kon E, Buda R, et al. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):528–535. [DOI] [PubMed] [Google Scholar]
- 14. Gigis I, Fotiadis E, Nenopoulos A, Tsitas K, Hatzokos I. Comparison of two different molecular weight intra-articular injections of hyaluronic acid for the treatment of knee osteoarthritis. Hippokratia. 2016;20(1):26–31. [PMC free article] [PubMed] [Google Scholar]
- 15. GraphPad Software. QuickCalcs random number generator. Accessed November 26, 2013. https://www.graphpad.com/quickcalcs/randomize1.cfm
- 16. Harrington DP, Fleming TR. A class of rank test procedures for censored survival data. Biometrika. 1982;69:553–566. [Google Scholar]
- 17. Jackson KA, Glyn-Jones S, Batt ME, Arden NK, Newton JL, Delphi P. Assessing risk factors for early hip osteoarthritis in activity-related hip pain: a Delphi study. BMJ Open. 2015;5(9):e007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang L, Rong J, Wang Y, et al. The relationship between body mass index and hip osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2011;78(2):150–155. [DOI] [PubMed] [Google Scholar]
- 19. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kraeutler MJ, Chadayammuri V, Garabekyan T, Mei-Dan O. Femoral version abnormalities significantly outweigh effect of cam impingement on hip internal rotation. J Bone Joint Surg Am. 2018;100(3):205–210. [DOI] [PubMed] [Google Scholar]
- 22. Kraeutler MJ, Garabekyan T, Fioravanti MJ, Young DA, Mei-Dan O. Efficacy of a non-image-guided diagnostic hip injection in patients with clinical and radiographic evidence of intra-articular hip pathology. J Hip Preserv Surg. 2018;5(3):220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le ADK, Enweze L, DeBaun MR, Dragoo JL. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11(4):624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee PB, Kim YC, Lim YJ, et al. Comparison between high and low molecular weight hyaluronates in knee osteoarthritis patients: open-label, randomized, multicentre clinical trial. J Int Med Res. 2006;34(1):77–87. [DOI] [PubMed] [Google Scholar]
- 25. Lespasio MJ, Sultan AA, Piuzzi NS, et al. Hip osteoarthritis: a primer. Perm J. 2018;22:17–084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lohmander LS, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Engstrom G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann Rheum Dis. 2009;68(4):490–496. [DOI] [PubMed] [Google Scholar]
- 27. MacGregor AJ, Antoniades L, Matson M, Andrew T, Spector TD. The genetic contribution to radiographic hip osteoarthritis in women: results of a classic twin study. Arthritis Rheum. 2000;43(11):2410–2416. [DOI] [PubMed] [Google Scholar]
- 28. McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum. 2001;45(5):453–461. [DOI] [PubMed] [Google Scholar]
- 29. Mei-Dan O, Carmont MR, Laver L, Mann G, Maffulli N, Nyska M. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40(3):534–541. [DOI] [PubMed] [Google Scholar]
- 30. Mei-Dan O, McConkey MO, Petersen B, McCarty E, Moreira B, Young DA. The anterior approach for a non-image-guided intra-articular hip injection. Arthroscopy. 2013;29(6):1025–1033. [DOI] [PubMed] [Google Scholar]
- 31. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357(9263):1191–1194. [PubMed] [Google Scholar]
- 32. Moss AS, Murphy LB, Helmick CG, et al. Annual incidence rates of hip symptoms and three hip OA outcomes from a U.S. population-based cohort study: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2016;24(9):1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murphy NJ, Eyles JP, Hunter DJ. Hip osteoarthritis: etiopathogenesis and implications for management. Adv Ther. 2016;33(11):1921–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nevitt MC, Xu L, Zhang Y, et al. Very low prevalence of hip osteoarthritis among Chinese elderly in Beijing, China, compared with whites in the United States: the Beijing osteoarthritis study. Arthritis Rheum. 2002;46(7):1773–1779. [DOI] [PubMed] [Google Scholar]
- 36. Ohtsuki T, Asano K, Inagaki J, et al. High molecular weight hyaluronan protects cartilage from degradation by inhibiting aggrecanase expression. J Orthop Res. 2018;36(12):3247–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38(8):1134–1141. [DOI] [PubMed] [Google Scholar]
- 38. Olsen NJ, Branch VK, Jonnala G, Seskar M, Cooper M. Phase 1, placebo-controlled, dose escalation trial of chicory root extract in patients with osteoarthritis of the hip or knee. BMC Musculoskelet Disord. 2010;11:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ornetti P, Nourissat G, Berenbaum F, et al. Does platelet-rich plasma have a role in the treatment of osteoarthritis? Joint Bone Spine. 2016;83(1):31–36. [DOI] [PubMed] [Google Scholar]
- 40. Palmieri B, Rottigni V, Iannitti T. Preliminary study of highly cross-linked hyaluronic acid-based combination therapy for management of knee osteoarthritis-related pain. Drug Des Devel Ther. 2013;7:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Papalia R, Russo F, Torre G, et al. Hybrid hyaluronic acid versus high molecular weight hyaluronic acid for the treatment of osteoarthritis in obese patients. J Biol Regul Homeost Agents. 2017;31(4_suppl_2):103–109. [PubMed] [Google Scholar]
- 42. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792–800. [DOI] [PubMed] [Google Scholar]
- 43. Sanchez M, Anitua E, Azofra J, Aguirre JJ, Andia I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol. 2008;26(5):910–913. [PubMed] [Google Scholar]
- 44. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070–1078. [DOI] [PubMed] [Google Scholar]
- 45. Sandell LJ. Etiology of osteoarthritis: genetics and synovial joint development. Nat Rev Rheumatol. 2012;8(2):77–89. [DOI] [PubMed] [Google Scholar]
- 46. Shewale AR, Barnes CL, Fischbach LA, Ounpraseuth ST, Painter JT, Martin BC. Comparison of low-, moderate-, and high-molecular-weight hyaluronic acid injections in delaying time to knee surgery. J Arthroplasty. 2017;32(10):2952–2957.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simental-Mendia M, Vilchez-Cavazos F, Garcia-Garza R, et al. The matrix synthesis and anti-inflammatory effect of autologous leukocyte-poor platelet rich plasma in human cartilage explants. Histol Histopathol. 2018;33(6):609–618. [DOI] [PubMed] [Google Scholar]
- 48. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411–417. [DOI] [PubMed] [Google Scholar]
- 49. Spector TD, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study. BMJ. 1996;312(7036):940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sudha PN, Rose MH. Beneficial effects of hyaluronic acid. Adv Food Nutr Res. 2014;72:137–176. [DOI] [PubMed] [Google Scholar]
- 51. Therneau T. A Package for Survival Analysis in S [computer program]. Version 2.38. Vienna: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 52. Thomsen MG, Latifi R, Kallemose T, Barfod KW, Husted H, Troelsen A. Good validity and reliability of the forgotten joint score in evaluating the outcome of total knee arthroplasty. Acta Orthop. 2016;87(3):280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsezou A. Osteoarthritis year in review 2014: genetics and genomics. Osteoarthritis Cartilage. 2014;22(12):2017–2024. [DOI] [PubMed] [Google Scholar]
- 54. Ye Y, Zhou X, Mao S, Zhang J, Lin B. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279–287. [DOI] [PubMed] [Google Scholar]
- 55. Ziegler CG, Van Sloun R, Gonzalez S, et al. Characterization of growth factors, cytokines, and chemokines in bone marrow concentrate and platelet-rich plasma: a prospective analysis. Am J Sports Med. 2019;47(9):2174–2187. [DOI] [PubMed] [Google Scholar]