Abstract
Poly (ADP-ribose) polymerase (PARP) inhibitors have demonstrated great promise for treating cancers with homologous recombination (HR) defects, such as germline BRCA1/2 mutation. Further studies suggest that PARP inhibitors (PARPi) can also exhibit efficacy in HR-competent cancers, by amplifying the DNA damage and inducing immunogenic cell death, and PARPi lead to increasing tumor neoantigen, upregulation of interferons and PD-L1, and modulation of the tumor microenvironment, which may facilitate a more profound antitumor immune response. Immune checkpoint inhibitors (ICIs) targeting PD-1/PD-L1 or CTLA-4 have achieved impressive success in the treatment of different malignancies. However, only a subset of populations derive clinical benefit, and the biomarkers and resistance mechanisms are not fully understood. Therefore, given that PARPi could potentiate the therapeutic effect of ICIs, PARPi combined with ICIs are becoming an alternative for patients who cannot benefit from ICI monotherapy. In this review, we focus on the mechanisms and immune role of PARPi and discuss the rationale and clinical studies of this combined regimen.
Keywords: PARP inhibitors, immune checkpoint inhibitors, BRCA, DNA damage response, cancer
Introduction
Poly (ADP-ribose) polymerase enzymes (PARPs) catalyze the poly ADP-ribosylation (PARylation), with nicotinamide adenine dinucleotide (NAD) serving as a substrate.1 Of all 17 members in the PARP family, only PARP1, PARP2, and PARP3 are thought to be involved in DNA repair, and PARP1 is the major one. PARP1 participates in both DNA base excision repair (BER)2 and DNA double-strand break (DSB) repair.3 PARP inhibitors (PARPi) have been demonstrated to be an effective therapeutic strategy against cancers with defects in DSB repair. In a decade to 2019, various PARPi have been involved in more than 70 clinical trials4 and approved by the US Food and Drug Administration (FDA), including olaparib,5 niraparib,6 rucaparib,7 and talazoparib.8 With further development, PARPi are not only used in breast cancer susceptibility gene (BRCA)-mutated patients with prior lines of chemotherapy; in 2018, olaparib was approved for first-line maintenance treatment in BRCA1/2-mutated, newly diagnosed advanced ovarian cancer after a complete response (CR)/partial response (PR) to platinum-based chemotherapy.9 Cancer types involved in PARPi-associated clinical studies included ovarian cancer, fallopian tube cancer, primary peritoneal cancer, high-grade endometrioid cancer, breast cancer, pancreatic cancer, prostate cancer, and lung cancer.4
The commonly recognized biomarker for PARPi is BRCA1/2, and most PARPi are approved by the FDA to be used for patients with BRCA mutation. In addition, some tumors without germline BRCA1/2 mutation exhibit “BRCAness”—a phenotype presenting similar molecular and biological characteristics to BRCA-deficient cancers.10 For example, patients with high-grade serous ovarian cancer and triple-negative breast cancer (TNBC) have a higher incidence of “BRCAness,” and those patients with wild-type BRCA1/2 could still benefit a lot from single-agent treatment,11 but the overall efficacy in patients with wild-type BRCA was weaker.12 And patients with germline BRCA1/2 mutation are still challenged by drug resistance and dose-limiting toxicities.13-15 Therefore, combination therapies based on synergistic effect are worth to be explored. Initial studies about PARPi-related combined therapeutics mostly focused on chemotherapy, radiotherapy, and a few target regimens. With further investigation, PARPi in combination with immunotherapeutics developed from preclinical models to clinical trials.
With the development of immune checkpoint inhibitors (ICIs) and next-generation sequencing, a growing number of medical guidelines for cancer treatment recommend carrying out molecular detection, and biomarkers such as PD-L1 expression, tumor mutation burden (TMB), microsatellite instability (MSI) and Epstein-Barr virus16-21 help us to select potential beneficiaries. However, patients without driven mutation and positive signatures for ICIs may turn to traditional chemotherapeutics. Although biomarkers for response to immunotherapy have not been fully studied and are not reliably predictive cancer types, the expression of PD-L1, TMB, and MSI status should be synthetically considered when making clinical strategies. If a patient’s disease is unlikely to benefit from immunotherapy as a monotherapy, combined therapy may become a choice. Emerging evidence has shown PARP inhibition can enhance the response of ICIs.22,23 Poly (ADP-ribose) polymerase inhibitors lead to the accumulation of DNA damage and trigger the interferon pathways.23,24 Thus, PARPi have the potential to improve response to ICIs by enhancing T-cell–mediated immune response.24,25
DNA Damage Response and PARPi
DNA damage repair
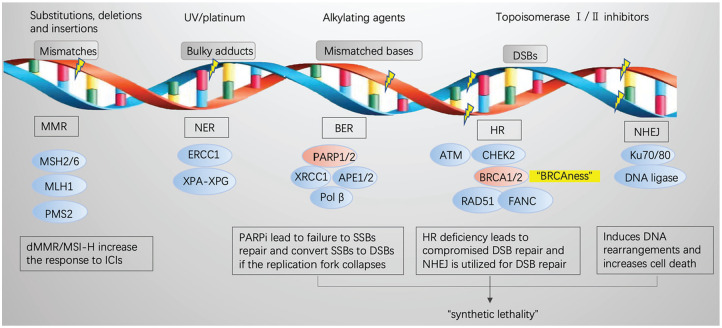
DNA is usually damaged when constantly exposed to endogenous or exogenous assaults, and the detection and repair of DNA damage is called “DNA damage response” (DDR).26 DNA damage includes DNA single-strand breaks(SSBs) and DSBs. While SSBs are managed mainly by 3 pathways27,28—(1) mismatch repair (MMR) mainly repairs mismatched DNA, escaping proofreading during replication; (2) nucleotide excision repair (NER) removes longer stretches of incorrect nucleotides, often resulting from UV/platinum; and (3) BER repairs DNA base lesions—DSBs are mainly repaired by homologous recombination (HR) and nonhomologous end joining (NHEJ). Homologous recombination is an effective repair approach to accurately and efficiently repair the DSB using the sister chromatid as a template,29 and NHEJ is an error-prone repair pathway that induces DNA rearrangements.30
The mechanisms of PARPi
The PARP family consists of 17 members,31 of which PARP1 is the most abundantly and extensively studied. PARP1 contains 4 domains with particular function: the DNA-binding domain (DBD) at N-terminus, the bipartite nuclear localization signal (NLS) domain, the auto-modification domain (AMD), and the catalytic domain (CD) at C-terminus.31,32 In response to DNA damage, PARP1 binds to DNA break sites through zinc finger I and II of the DBD, and the CD of PARP1 is activated by reliving the inhibition of α-helical subdomain (HD)32; then, PARP1 recruits DDR-related proteins, such as XRCC1, XPA, DNA polymerase β, and DNA ligase III.33-35 The activated PARP1 cooperates with other PARP enzymes to catalyze NAD+ to generate the polymer of ADP-ribose covalently on target proteins or itself in a linear or multibranched way, known as PARylation.35,36 PARP1 subsequently induces DDR, in which at least 450 proteins are involved,37 such as ATR/CHEK1/RAD51. While PARP1 contributes to 90% of total PARP activity,38 PARP2 contributes only 5% to 10%.39 Besides, PARP1, PARP2, and PARP3 also play an important role in DNA repair,40,41 whereas PARP4-PARP17 are not thought to be involved in DNA repair.
PARP1 is mainly involved in BER, but it is also critical for HR and NHEJ mechanisms,3 and BRCA1/2 are involved in the HR pathway.42 Therapeutic inhibitors of PARP1/2, such as olaparib, bind to the catalytic domain and inhibit the catalytic activity, which leads to the failure of SSB repair. If the replication fork collapses, a DSB might be created, and in tumor cells with HR deficiency such as BRCA1/2 mutation, NHEJ is used for DSB repair, which may determine eventual tumor cell death by increasing genetic instability without deleterious effects on normal cells.43,44 This is called the “synthetic lethality” effect,45 and subsequent immune response to dying tumor cells could potentiate antitumor efficacy of ICIs (Figure 1).
Figure 1.
DDR-associated pathways and “synthetic lethality” induced by PARPi.
ATM indicates ataxia telangiectasia-mutated gene; BER, base excision repair; BRCA, breast cancer susceptibility gene; CHEK2, checkpoint kinase 2; DDR, DNA damage response; DSB, double-strand break; HR, homologous recombination; ICI, immune checkpoint inhibitor; MMR, mismatch repair; MSI, microsatellite instability; NER, nucleotide excision repair; NHEJ, nonhomologous end joining; PARPi, poly (ADP-ribose) polymerase inhibitors; SSB, single-strand break.
In addition to catalytic inhibition, “PARP trapping” is another important mechanism for PARPi. It has been reported that PARPi are more cytotoxic than PARP depletion because of their ability to trap PARP enzymes on damaged DNA by way of a poisonous allosteric effect, and the authors detected PARP-DNA complexes, which interfered with the DNA replication.46 The capacity to trap PARP varies markedly among different PARPi, with talazoparib ≫ niraparib > olaparib or rucaparib ≫ veliparib, and this capacity may be associated with the extent to which PARPi interacts with the D-loop residues47-49
The immunological role of PARPs
Beyond maintaining genomic stability, PARPs play a significant role in both innate and adaptive immune responses. Multiple studies have demonstrated that PARPs are associated with cancer immunity. T cell is the principal part of antitumor immunity, and PARP inhibition significantly influences T cells in the tumor microenvironment (TME). In small cell lung cancer (SCLC),50 PARPi were reported to induce the activation and function of cytotoxic T lymphocytes via activating the STING/TBK1/IRF3 innate immune pathway and increasing levels of chemokines such as C-X-C motif chemokine ligand 10 (CXCL10) and C-C motif chemokine ligand 5 (CCL5). In ovarian cancer,51 it was revealed that PARPi could induce the upregulation of PD-L1 expression by promoting phosphorylation of CHK1, and antagonistic PD-L1 could reverse the inhibitory effect of PARPi on CD8+ T cells and had synergistic antitumor effect with PARPi. Moreover, it has been reported that natural killer (NK) cells and macrophages are indispensable for responsiveness to anti–PD-1 immunotherapy.52 Talazoparib (BMN673) is a PARP1/2 inhibitor, and Huang et al53 reported that BMN673 significantly increased the number of NK cells and their production of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) in a murine model of ovarian cancer. Other studies also showed that inhibition of PARP-1/2 maintained NK cell viability and increased tumor cell sensitivity to NK killing in various cancers, including breast, prostate, NSLC, and chronic myeloid leukemia.54,55 Besides, myeloid-derived suppressor cells (MDSCs) of patients who received PARPi/ICI combination treatment were also demonstrated to influence the efficacy.56
The effect of PARPi on immune cells was likely due to the immune system’s response to dying cancer cells, and this immune response was partly mediated by a series of transcriptional factors and chemokines. The release of IFN-γ by STING/TBK1/IRF3 signaling is a kind of typical immune response induced by PARPi.51 Nuclear Factor-κB (NF-κB) is a known essential coactivator for PARP-1, and previous literature has shown that PARP-1 could interact with the subunits of NF-κB, format the transcription complex, and ultimately influence NF-κB–dependent gene expression, which was independent of the enzymatic activity of PARP1.57,58 It was also reported that PARPs regulated a series of cytokines, such as Th1 cytokines (interleukin [IL]-2, IFN-γ), Th2 cytokines (IL-4, IL-5, IL-10), transforming growth factor-β (TGF-β) and the chemokines CXCL10, CCL5, CCL4, and CCL9.59-62
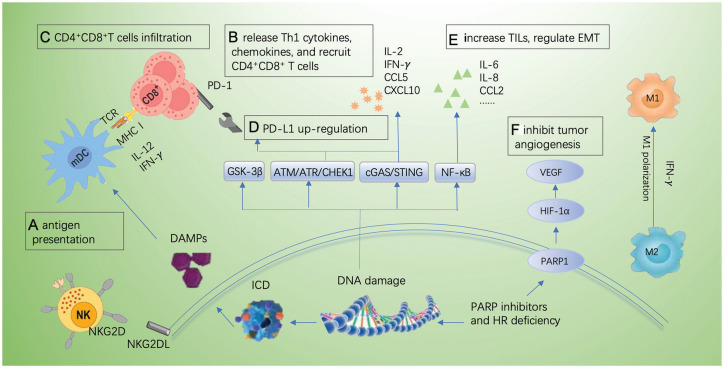
The PARP is also involved in a variety of biological processes, including chromatin remodeling, and the regulation of transcription factors and TME.63 It has been reported that PARPs participated in tumor cell proliferation, epithelial-mesenchymal transition (EMT) (Figure 2E), and apoptosis via coactivating NF-κB, mitogen-activated protein kinases (MAPKs), and TGF-β.64-66 A previous study has shown that overexpressed PARP-1 enhanced tumor angiogenesis by upregulating the vascular endothelial growth factor (VEGF)67 (Figure 2F); therefore, PARPi can also inhibit tumor angiogenesis.
Figure 2.
The mechanisms involved in DDR and checkpoint immunotherapies. (A) PARPi promote antigen presentation by ICD owing to the failure of DNA repair; (B) PARPi upregulate the release of Th1 cytokines and recruit CD4+CD8+ T cells via CCL5 and CXCL10; (C) PARPi promote T-cell infiltration; (D) PARPi increase PD-L1 expression by GSK-3β, ATM-ATR-CHK1, and cGAS-STING pathway; (E) PARPi regulate EMT; and (F) PARPi inhibit tumor angiogenesis by modulating PARP1/HIF1-α/VEGF signals.
ATM indicates ataxia telangiectasia-mutated gene; ATR, ataxia telangiectasia and Rad3 related; CCL2, C-C motif chemokine ligand 2; CCL5, C-C motif chemokine ligand 5; cGAS, cyclic GMP-AMP synthase; CHEK1, checkpoint kinase 1; CXCL10, C-X-C motif chemokine ligand 10; DAMP, damage-associated molecular pattern; DDR, DNA damage response; EMT, epithelial-mesenchymal transition; GSK-3β, glycogen synthase kinase-3β; HIF-1α, hypoxia-inducible factor-1 α; HR, homologous recombination; ICD, immunogenic cell death; IL, interleukin; INF-g, interferon-γ; MHC I, major histocompatibility complex class I; NF-κB, nuclear factor-kB; NKG2D(L), natural killer cells group 2D (ligand); PARPi, poly (ADP-ribose) polymerase inhibitors; SSB, single-strand breaks; PD-L1, programmed-death ligand 1; STING, stimulator of interferon genes; TILs, tumor-infiltrating lymphocytes; VEGF, vascular endothelial growth factor; TCR, T cell receptor; mDC, myeloid dendritic cells.
Rationale for Combination of PARPi and ICIs
To date, 4 PARPi have been approved by the FDA, mainly for the treatment of several solid cancers with BRCA1/2 mutation. However, the rate of BRCA1/2 mutation is relatively low, which means the population benefiting from PARPi is small. With further study, it was found that sporadic tumors exhibited “BRCAness,” a phenotype similar to BRCA1/2 mutation cancers, which resulted in DDR deficiency because of DDR-associated gene mutation or methylation.68 These mutated genes and downregulated proteins, including ATM, ATR, CHK1, CHK2, BARD1, BRIP1, DSS1, NBS1, PALB2, RAD51, CDK12, members of FANC family, EMSY, PALB2, XRCC2, XRCC3, TP53, or PTEN, may become predictive biomarkers for PARPi treatment,10,68-70 which lays the foundation for clinical application of PARPi in non-BRCA mutation cancers. Still, combined therapy is needed to improve efficacy. The rationale for PARPi in combination with ICIs mainly involves 4 aspects: tumor neoantigen production, enhanced antigen presentation, increasing tumor-infiltrating lymphocytes (TILs), upregulation of PD-L1, and reprogram of other molecules and immune cells involved in TME (Figure 2).
Neoantigen formation, TMB, and increased immunogenicity
The accumulated DNA damage by PARP inhibition fails to be repaired and results in tumor cell death, and dead tumor cells will release tumor neoantigen and increase immunogenicity, which lays the foundation for combination of PARPi with ICIs. DNA damage response–associated frameshift mutations contribute to neoantigen repertoire, and mismatch repair–deficient tumors are found to be sensitive to ICIs, regardless of the cancer types, which attributes to its large proportion of mutant neoantigens.21 Tumor mutation burden is regarded as a surrogate of neoantigen burden, which heralds the ICI therapeutic response, and in many malignancies, including non–small cell lung carcinoma (NSCLC) and melanoma, TMB was reported to be correlated with clinical response and survival.71,72 DNA damage response deficiency, including BRCA1/2, was found to be associated with higher tumor mutational load and predicted neoantigen in cancers such as ovarian cancer and NSCLC.72,73 Therefore, PARPi may facilitate a more profound antitumor immune response and synergize with ICIs by inducing DNA damage and neoantigen, which can increase immunogenicity.
Enhanced antigen presentation
Besides, neoantigen presentation by major histocompatibility complex class I (MHC I) is requisite for cytotoxic T-cell activation, which is accomplished by antigen-presenting cells (APCs), such as dendritic cells (DCs), monocytes/macrophages, and B lymphocytes. It was reported that DDR induced the expression of MHC I and antigen presenting,74 and PARP inhibition could upregulate MHC I.75 The PARPi lead to DDR and immunogenic cell death (ICD), thus inducing damage-associated molecular patterns (DAMPs) to promote recruiting APCs.76 Immune checkpoint inhibitors function to inhibit tumor growth by restoring and enhancing T-cell activation, and T-cell-DC crosstalk involving the cytokines IFN-γ and IL-12 is essential for improved ICI response.77,78 The PARPi have the ability to increase IFN-γ release via stimulator of interferon genes (STING) pathway,22,23 thus enhancing T-cell-DC crosstalk to promote antigen presentation.
Increasing TILs
T lymphocytes can be stimulated and recruited to tumors by tumor-specific neoantigens, and recent preclinical studies indicate that different PARPi can significantly increase the infiltration of CD4+ T cells and CD8+ T cells by activating the STING pathway in BRCA-deficient models.22,24,53 Increasing evidence suggests that the distribution, density, and phenotype of TILs influence the efficacy of ICI.79 Strickland et al73 demonstrated that BRCA1/2-mutated high-grade serous ovarian cancers exhibited significantly increased CD3+ and CD8+ TILs, compared with HR-proficient tumors, and the number of TILs was independently associated with patients’ survival outcome. Therefore, combined therapy of ICI and PARPi may extend durable responses for HR-deficient tumors.
Upregulation of PD-L1 and influence on other factors in TME
Although PD-L1 remains an imperfect biomarker owing to drug types, cancer types, cut-off value, and antibody for PD-L1 assay, many studies suggest PD-L1 is a biomarker for ICI response or overall survival (OS),17,80,81 and PD-L1 detection using immunohistochemistry has been widely used in clinical practice. Increasing evidence showed PARPi could increase the expression of PD-L1. The PARPi increase the DNA damage, and the stimulation of cytoplasmic DNA can result in the activation of cyclic GMP-AMP synthase (cGAS), and then cGAS catalyzes the generation of cyclic dinucleotide and promotes the activation of the STING pathway.82 Active STING upregulates the generation of type I IFNs mainly by initiating the downstream TBK1-IRF3-type I IFN pathway and NF-κB pathway.83 Type I IFN induces the activation of an antitumor immune response and increased PD-L1 expression. Therefore, PARPi could determine an increase in DNA damage and upregulate the PD-L1 expression by cGAS-STING pathway. In addition, PARPi-induced DSB could upregulate PD-L1 expression by ATM-ATR-CHK1 pathway.84 Preclinical studies also showed that PARP inhibition could upregulate PD-L1 by inactivating GSK-3β, and that PD-L1 upregulation may be a resistance mechanism of PARPi, and subsequent blockade of PD-L1 resensitized PARPi-treated cells to T-cell killing.85 Thus, PARPi-induced upregulation of PD-L1 expression may provide a theorical explanation for PARPi to combine with anti–PD-1/PD-L1 blockades.
The PARP inhibition extends durable ICI response by influencing the integral TME, including the regulation of NK cells, a series of cytokines and chemokines, angiogenesis, and oxidative stress. Olaparib was proved to improve the killing activity of NK cells,55 and NK-DC axis defined ICI response.52 The knockout of PARPi and PARP-1 was also found to impair angiogenesis and abrogate migration of tumor cells by modulating PARP1/HIF1-α/VEGF signals.86 Given the effect of PARPi on the proliferation, apoptosis, and migration of TILs, it is promising to combine it with ICIs.
Studies about PARPi combined with checkpoint blockades
Preclinical studies suggest that PARPi, including olaparib, niraparib, rucaparib, and talazoparib, synergize with PD-1/PD-L1 blockade regardless of the BRCA status,22,23,85,87 and it has been reported that veliparib, a PARP inhibitor, can enhance the therapeutic efficacy of CTLA-4 blockade and contribute to tumor clearance and long-term survival in BRCA−/− mouse models.88 Based on the above promising data from preclinical studies, a series of clinical trials are currently underway (Table 1).
Table 1.
Clinical studies about PARPi/ICIs.
| ClinicalTrials.gov identifier | Combination | Phase | Cancer type | References |
|---|---|---|---|---|
| NCT02657889 | Niraparib + pembrolizumab | I/II | Basket study in TNBC and ovarian cancer | Konstantinopoulos et al,89 Vinayak et al90 |
| NCT02849496 | Veliparib + atezolizumab | II | HR-deficient and HER-2–negative TNBC | N/A |
| NCT03101280 | Rucaparib + atezolizumab | I | BRCA+ ovarian cancer and TNBC | N/A |
| NCT03598270 | Niraparib + atezolizumab | III | Maintenance treatment of recurrent ovarian cancer | N/A |
| NCT03522246 | Rucaparib + nivolumab | III | Front-line ovarian cancer | N/A |
| NCT03642132 | Talazoparib + avelumab | III | Front-line ovarian cancer | N/A |
| NCT03602859 | Niraparib + TSR-042 | III | Front-line ovarian cancer | N/A |
| NCT03307785 | Niraparib + TSR-042 | I/II | Solid tumors | N/A |
| NCT03565991 | Talazoparib + avelumab | II | BRCA/ATM-mutant solid tumors | N/A |
| NCT03330405 | Talazoparib + avelumab | II | Basket study in ovarian cancer, HER2- breast cancer, mCRPC, bladder cancer, and NSCLC | N/A |
| NCT02660034 | Pamiparib + tislelizumab | I | Basket study in TNBC, ovarian cancer, mCRPC, SCLC, bladder cancer, HER2-gastric cancer, pancreatic cancer, and other solid tumors | Friedlander et al91 |
| NCT02734004 | Olaparib + durvalumab | II | Basket study in germline BRCA-mutant ovarian, HER2-breast cancer, gastric cancer, and relapsed SCLC | Krebs et al92 |
| NCT02484404 | Olaparib + durvalumab | II | Basket study in ovarian cancer, TNBC, NSCLC, SCLC, mCRPC, and microsatellite stable colorectal cancer | Thomas et al,56 Karzai et al93 |
| NCT03572478 | Rucaparib + nivolumab | I/IIa | Prostate/endometrial cancers | N/A |
| NCT03338790 | Rucaparib + nivolumab | II | Umbrella study in mCRPC | N/A |
| NCT02861573 | Olaparib + pembrolizumab |
I | Umbrella study in mCRPC | N/A |
| NCT02546661 | Olaparib + durvalumab | Ib | Umbrella study in HR-deficient muscle invasive bladder cancer | N/A |
| NCT03459846 | Olaparib + durvalumab | II | Cisplatin-ineligible bladder cancer | N/A |
| NCT03534492 | Olaparib + durvalumab | II | Study before surgery of resectable urothelial bladder cancer | N/A |
| NCT03334617 | Olaparib + durvalumab | II | Umbrella study in patients with NSCLC who have progressed on anti–PD-1/PD-L1 | N/A |
| NCT03308942 | Niraparib + PD-1 inhibitor | II | NSCLC | N/A |
Abbreviations: ATM, ataxia telangiectasia mutated gene; BRCA, breast cancer susceptibility gene; HER-2, human epidermal growth factor receptor-2; HR, homologous recombination; ICI, immune checkpoint inhibitor; mCRPC, metastatic castration-resistant prostate cancer; NSCLC, non–small cell lung cancer; PARPi, poly (ADP-ribose) polymerase inhibitors; PD-1, programmed cell death 1; SCLC, small cell lung cancer; TNBC, triple-negative breast cancer.
The first reported clinical study about the combined therapy of PARPi and checkpoint blockade is TOPACIO trial (NCT02657889). In this study, researchers analyzed the efficacy and safety of niraparib in combination with pembrolizumab in the treatment of recurrent ovarian carcinoma and TNBC. Totally, 62 patients were enrolled in ovarian cancer cohort and 60 patients were evaluable, and the integrated objective response rate (ORR) was 18% and the disease control rate (DCR) was 65%. Further analysis showed the ORRs of tumor PD-L1 expression, BRCA or HR status, and other biomarker-based subgroups were similar.89 In the TNBC cohort, 47 of 55 patients were evaluable, and the ORR and DCR were 21% and 49%, respectively. Different from the ovarian cancer cohort, patients with BRCA mutation significantly benefited more compared with those without, with a better ORR of 47% versus 11%, DCR of 80% versus 33%, and median progression-free survival (mPFS) of 8.3 months versus 2.1 months.90 Different data from several clinical studies about ovarian cancers suggested PARPi/PD-1 or PD-L1 combinations89,94 contributed to similar ORR with PARPi treatment in the same settings.95
Besides, olaparib/durvalumab combination also showed excellent efficacy in mCRPC,96 9 of 17 patients (53%) demonstrated a prostate-specific antigen (PSA) response (⩾50% declination), and patients with DDR defects acquired better mPFS of 16.1 months, whereas mPFS of those without was 4.8 months. These data compare favorably with those of monotherapy, despite results from different trials; pembrolizumab monotherapy showed a 6% PSA response rate,97 whereas olaparib led to a 22% PSA response rate, an ORR of 33%, and a mPFS of 9.8 months in DDR-deficient patients and 2.1 months in DDR-proficient patients.98 In platinum-resistant/refractory SCLC, the olaparib/durvalumab combination displayed a clinical benefit in 4 (21.1%) of 19 patients, including 2 patients with PR or CR and 2 patients with prolonged stable disease for more than 8 months, and the 2 with CRs showed an inflamed phenotype in pretreated tumors.56 The details are shown in Table 2.
Table 2.
Efficacy comparison of PARPi/ICI combination therapy with single agents.
| Cancer | Clinical trials | Drugs | ORR, % | DCR, % | mPFS, mo | mOS, mo | References |
|---|---|---|---|---|---|---|---|
| TNBC | NCT02657889 | Niraparib + pembrolizumab | Total: 21 BRCA+: 47 BRCA−: 11 PD-L1+: 32 PD-L1−: 8 |
Total: 49 BRCA+: 80 BRCA−: 33 |
Total: 2.3 BRCA+: 8.3 BRCA−: 2.1 |
NA | Vinayak et al90 |
| NCT01848834 | Pembrolizumab | 18.5 | 25.9 | 1.9 | 11.2 | Nanda et al99 | |
| NCT02447003 | Pembrolizumab | PD-L1+: 5.3 PD-L1−: 5.7 |
PD-L1+: 7.6 PD-L1−: 9.5 |
2.0 | 9.0 | Adams et al100 | |
| NCT00749502 | Niraparib | BRCA+: 5 | NA | NA | NA | Sandhu et al15 | |
| Ovarian cancer | NCT02657889 | Niraparib + pembrolizumab | Total: 18 BRCA+: 18 BRCA−: 19 PD-L1+: 21 PD-L1−: 10 |
65 | 3.4 | NA | Konstantinopoulos et al89 |
| NCT02674061 | Pembrolizumab | 9.9 | 37.4 | 2.1 | 17.6 | Cohort B101 | |
| NCT02054806 | Pembrolizumab | 11.5 | NA | 1.9 | 13.1 | Varga et al102 | |
| SCLC | NCT02484404 | Olaparib + durvalumab | 10.5 | NA | 1.8 | 4.1 | Thomas et al56 |
| NCT02734004 | Olaparib + durvalumab | 11 | 29 | NA | NA | Krebs et al92 | |
|
NCT02054806 NCT02628067 |
Pembrolizumab | 19.3 | 37.4 | 2.0 | 7.7 | Chung et al103 | |
| NCT01928394 | Nivolumab | 10 | 32 | NA | 4.4 | Antonia et al104 | |
| mCRPC | NCT02484404 | Olaparib + durvalumab | 23.5 | 70.6 | 16.1 | NA | Karzai et al96 |
| NCT02787005 | Pembrolizumab | 5 | 10 | 2.1 | 9.6 | Antonarakis et al97 |
Abbreviations: BRCA, breast cancer susceptibility gene; DCR, disease control rate; ICI, immune checkpoint inhibitor; mCRPC, metastatic castration-resistant prostate cancer; mOS, median overall survival; mPFS, median progression-free survival; ORR, objective response rate; PARPi, poly (ADP-ribose) polymerase inhibitors; PD-L1, programmed cell death ligand 1; SCLC, small cell lung cancer; TNBC, triple-negative breast cancer.
About safety, the most common adverse effects (AEs) included hematologic-related toxicities, such as anemia, lymphopenia, leukopenia, thrombocytopenia, fatigue, nausea, and constipation, and immune-related adverse effects (irAEs).56,89,90,96 According to available safety data from clinical trials about PARPi (niraparib) combined with anti–PD-1/PD-L1 (pembrolizumab), the incidences of irAEs of any grade and severe grade were 15% to 19% and 4% to 6%, respectively,89,90 and these data were similar to those observed with PD-1/PD-L1 monotherapy, which showed that in 18.5% and 5.1% of patients any grade and severe-grade irAEs occurred.105 And for olaparib/durvalumab combination, the incidences of all grade and grade 3+ irAEs were 23.5% to 25.0% and 11.8%, respectively,56,96 and the incidences were lower than those reported in durvalumab monotherapy, which were 53.8% and 21.5% of any grade and grade 3+ irAEs.106 Although many clinical trials reported the maximum tolerated dose and demonstrated that the toxicity of these combinations was acceptable, the combination of BGB-A317/BGB-290 was reported to show an increased rate of autoimmune hepatitis and elevated aspartate transaminase/alanine transaminase91; this hepatic toxicity of PARPi/ICI combinations may vary according to the agents used in different combination settings.
Conclusions and Perspectives
The PARPi have exhibited remarkable antitumor efficacy in BRCA1/2 mutant solid tumors, mainly through catalytic inhibition-induced synthetic lethality and PARP trapping.45,46 In tumors, emerging evidence has suggested that PARPi modify the immune context. Given the immune role of PARPi, especially the recruitment and priming of CD4+/CD8+ T cells through neoantigen production and releasing cytokines and chemokines, such as CCL5 and CXCL10,23,61 the PARPi/ICI combination may have potential to extend benefit populations and broaden durable responses of both PARPi and immune checkpoint blockades. Preclinical studies show that PARPi/ICI combinations synergize via STING-associated signal pathways,22,23 which are responsible for releasing INF-γ, recruiting CD8+ T cells, and upregulating the expression of PD-L1.24,107 And both preclinical studies and clinical trials demonstrate that PARPi in combination with ICIs improve antitumor efficacy compared with single regimen. Still, there are several questions to be explored and answered. How to choose the potential benefit patients? How to choose the different drug combinations? What is the timing to apply this combination?
With the development of precision medicine, biomarker-guided treatment is urgently needed. It is significant to choose the patients who are likely to benefit from the combinations. Because DDR defects, especially BRCA mutation, are related to high response to PARP inhibition, detection of DDR defects is important for guiding therapeutic decisions. However, different DDR gene mutations may have distinct effects on immunogenicity. More mutated genes of different DDR pathways may be more likely to result in DDR dysfunction, and heterozygous or homozygous mutation, and germline or somatic mutation have different influences on tumor development and susceptibility to PARP inhibition. It has been demonstrated that loss of heterozygosity (LOH) led to biallelic BRCA inactivation,108 so we should pay attention to distinguish the gene mutation numbers and forms. Besides, many other mutations were reported to be associated with PARPi and/or PD-1/PD-L1 blockades, such as ATM, ATR, POLE, POLD1, CHECK1/2, WEEK1, JAK2, ATK11, and MMR-related genes.79 It is also reported that the expression of PD-L1 and TILs are somehow related to the efficacy of PARPi/PD-1 or PD-L1 therapeutics, in which PARPi may play a vital role to modulate the tumor immune microenvironment, especially to regulate the expression of PD-L1 through STING-associated signal pathways. Therefore, dynamic detection and analysis of tumor-related immune cells, PD-L1 expression, gene mutation, and TMB with pretreatment and posttreatment samples is a significant step to seek the appropriate biomarker. Nevertheless, it is a long way to go to find the precise biomarker.
The question of the optimal combination and drug dose is not easy to answer. To date, available clinical data only focus on PD-1/PD-L1 blockade in combination with PARPi therapies. A previous animal study indicated that the combination of veliparib with anti–CTLA-4 blockade enhanced tumor clearance and improved long-term survival in BRCA1-deficient mouse models; however, the combination with anti–PD-1/PD-L1 failed to improve survival in this study.88 These different results may be due to the higher drug activity of CTLA-4 antibody compared with PD-1/PD-L1 blockades under the immune context of BR5 mouse ovarian cancer model and may because of the activation of new CD8+ T cells, but not reversal of exhausted T cells, promote the selective efficacy of CTLA-4 antibody. Another preclinical study conducted in BRCA1-mutant, but not BRCA wild-type, syngeneic models showed the combination led to better tumor shrinkage and improved survival compared with single-agent treatment,87 and this might be explained by the different immune context of different mouse models, such as TILs, and another reason that might lead to these contrast results was that the activity of different PARPi varies because of different capacity of PARP trapping and immune modulation. Current clinical trials about PARPi/ICI combination mainly focus on anti–PD-1/PD-L1 blockades, such as pembrolizumab, nivolumab, and durvalumab; anti–CTLA-4 blockade has not been studied. Therefore, randomized controlled trials (RCT) are necessary to further compare and explain the efficacy of combinations and single agent in different cancer types. Besides, the drug dose of different combinations has not reached a consensus. Taking clinical experience and limited data from literature, it may become a choice to deliver PARPi in a pulsatile way or by decreasing PARPi administration, and enzyme-linked immunosorbent assay can be used as a biomarker assay to measure PARP activity.109 Overall, it is essential to explore different agents and their dose combinations to find an optimal balance between the efficacy and safety.
It is a question worthy to explore when to apply this combined therapy. The ICI monotherapy only has a small number of benefit population, and it usually takes effect slowly; even some patients may experience “hyper-progressive disease” which means the faster growth of tumor. Given the ability of PARPi to promote inflammation and immune priming, it is a potential choice to combine PARPi for PD-1/PD-L1 blockade-resistant settings, and there has been a related clinical trial focusing on patients with NSCLC who progressed on anti–PD-1/PD-L1 containing therapy. Besides, PD-L1 upregulation is one of the resistance mechanisms for PARPi,84,85 so it can be addressed through the combination of PARPi and ICI. Under the different immune contexture and mutation milieu, we should consider personalized treatment plans.
Finally, the multiple links between PARPi and tumor immune response suggest PARPi/ICI combinations have potential to improve cancer patient responses, and clinical trials investigating this combination showed preliminary promising results; still, it is a long way to go to further explore the precision biomarker and choose potential benefit patients. Greater clarity of the above key questions will bring new insights to better develop PARPi and immunotherapeutic agents to guide clinical treatment.
Acknowledgments
We thank Professor Wang for linguistic polish.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Basic Research Program of China (973 program) (2017YFC0907904 to YH).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors contributed to study design. ZW, HT and SZ collected the literature. ZW and PC collected the data of clinical trials and drew the figures. ZW and YH conceived the discussion part. ZW drafted the manuscript, and the other authors revised the manuscript. All authors have read and approved the final manuscript.
ORCID iDs: Zhaozhen Wu  https://orcid.org/0000-0001-8962-5741
https://orcid.org/0000-0001-8962-5741
References
- 1. Sonnenblick A, de Azambuja E, Azim HA, Jr, Piccart M. An update on PARP inhibitors—moving to the adjuvant setting. Nat Rev Clin Oncol. 2015;12:27-41. [DOI] [PubMed] [Google Scholar]
- 2. Nogueira A, Assis J, Faustino I, Pereira D, Catarino R, Medeiros R. Base excision repair pathway: PARP1 genotypes as modulators of therapy response in cervical cancer patients. Biomarkers. 2017;22:70-76. [DOI] [PubMed] [Google Scholar]
- 3. Beck C, Robert I, Reina-San-Martin B, Schreiber V, Dantzer F. Poly(ADP-ribose) polymerases in double-strand break repair: focus on PARP1, PARP2 and PARP3. Exp Cell Res. 2014;329:18-25. [DOI] [PubMed] [Google Scholar]
- 4. Lin KY, Kraus WL. PARP inhibitors for cancer therapy. Cell. 2017;169:183. [DOI] [PubMed] [Google Scholar]
- 5. Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30:372-379. [DOI] [PubMed] [Google Scholar]
- 6. Oza AM, Tinker AV, Oaknin A, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: integrated analysis of data from study 10 and ARIEL2. Gynecol Oncol. 2017;147:267-275. [DOI] [PubMed] [Google Scholar]
- 7. Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75-87. [DOI] [PubMed] [Google Scholar]
- 8. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505. [DOI] [PubMed] [Google Scholar]
- 10. Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16:110-120. [DOI] [PubMed] [Google Scholar]
- 11. Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852-861. [DOI] [PubMed] [Google Scholar]
- 12. Ledermann JA, Harter P, Gourley C, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17:1579-1589. [DOI] [PubMed] [Google Scholar]
- 13. Drean A, Williamson CT, Brough R, et al. Modeling therapy resistance in BRCA1/2-mutant cancers. Mol Cancer Ther. 2017;16:2022-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhawan MS, Bartelink IH, Aggarwal RR, et al. Differential toxicity in patients with and without DNA repair mutations: phase I study of carboplatin and talazoparib in advanced solid tumors. Clin Cancer Res. 2017;23:6400-6410. [DOI] [PubMed] [Google Scholar]
- 15. Sandhu SK, Schelman WR, Wilding G, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882-892. [DOI] [PubMed] [Google Scholar]
- 16. Zhou J, Mahoney KM, Giobbie-Hurder A, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res. 2017;5:480-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018-2028. [DOI] [PubMed] [Google Scholar]
- 18. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Domingo E, Camps C, Kaisaki PJ, et al. Mutation burden and other molecular markers of prognosis in colorectal cancer treated with curative intent: results from the QUASAR 2 clinical trial and an Australian community-based series. Lancet Gastroenterol Hepatol. 2018;3:635-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449-1458. [DOI] [PubMed] [Google Scholar]
- 21. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Z, Sun K, Xiao Y, et al. Niraparib activates interferon signaling and potentiates anti-PD-1 antibody efficacy in tumor models. Sci Rep. 2019;9:1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shen J, Zhao W, Ju Z, et al. PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Res. 2019;79:311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pantelidou C, Sonzogni O, De Oliveria Taveira M, et al. PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 2019;9:722-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vikas P, Borcherding N, Chennamadhavuni A, Garje R. Therapeutic potential of combining PARP inhibitor and immunotherapy in solid tumors. Front Oncol. 2020;10:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caldecott KW. DNA single-strand break repair. Exp Cell Res. 2014;329:2-8. [DOI] [PubMed] [Google Scholar]
- 28. Chatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen. 2017;58:235-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moynahan ME, Jasin M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat Rev Mol Cell Biol. 2010;11:196-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882-893. [DOI] [PubMed] [Google Scholar]
- 32. Dawicki-McKenna JM, Langelier MF, DeNizio JE, et al. PARP-1 activation requires local unfolding of an autoinhibitory domain. Mol Cell. 2015;60:755-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eustermann S, Videler H, Yang JC, et al. The DNA-binding domain of human PARP-1 interacts with DNA single-strand breaks as a monomer through its second zinc finger. J Mol Biol. 2011;407:149-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sousa FG, Matuo R, Soares DG, et al. PARPs and the DNA damage response. Carcinogenesis. 2012;33:1433-1440. [DOI] [PubMed] [Google Scholar]
- 35. Schreiber V, Ame JC, Dolle P, et al. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J Biol Chem. 2002;277:23028-23036. [DOI] [PubMed] [Google Scholar]
- 36. Daniels CM, Ong SE, Leung AK. The promise of proteomics for the study of ADP-ribosylation. Mol Cell. 2015;58:911-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pearl LH, Schierz AC, Ward SE, Al-Lazikani B, Pearl FM. Therapeutic opportunities within the DNA damage response. Nat Rev Cancer. 2015;15:166-180. [DOI] [PubMed] [Google Scholar]
- 38. Langelier MF, Riccio AA, Pascal JM. PARP-2 and PARP-3 are selectively activated by 5’ phosphorylated DNA breaks through an allosteric regulatory mechanism shared with PARP-1. Nucleic Acids Res. 2014;42:7762-7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Menissier de, Murcia J, Ricoul M, Tartier L, et al. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO J. 2003;22:2255-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yelamos J, Farres J, Llacuna L, Ampurdanes C, Martin-Caballero J. PARP-1 and PARP-2: new players in tumour development. Am J Cancer Res. 2011;1:328-346. [PMC free article] [PubMed] [Google Scholar]
- 41. Loseva O, Jemth AS, Bryant HE, et al. PARP-3 is a mono-ADP-ribosylase that activates PARP-1 in the absence of DNA. J Biol Chem. 2010;285:8054-8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913-917. [DOI] [PubMed] [Google Scholar]
- 44. O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60:547-560. [DOI] [PubMed] [Google Scholar]
- 45. Lord CJ, Tutt AN, Ashworth A. Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu Rev Med. 2015;66:455-470. [DOI] [PubMed] [Google Scholar]
- 46. Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hopkins TA, Shi Y, Rodriguez LE, et al. Mechanistic dissection of PARP1 trapping and the impact on in vivo tolerability and efficacy of PARP inhibitors. Mol Cancer Res. 2015;13:1465-1477. [DOI] [PubMed] [Google Scholar]
- 48. Shen Y, Aoyagi-Scharber M, Wang B. Trapping poly(ADP-ribose) polymerase. J Pharmacol Exp Ther. 2015;353:446-457. [DOI] [PubMed] [Google Scholar]
- 49. Murai J, Huang SY, Renaud A, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther. 2014;13:433-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sen T, Rodriguez BL, Chen L, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019;9:646-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xue C, Xu Y, Ye W, et al. Expression of PD-L1 in ovarian cancer and its synergistic antitumor effect with PARP inhibitor. Gynecol Oncol. 2020;157:222-233. [DOI] [PubMed] [Google Scholar]
- 52. Barry KC, Hsu J, Broz ML, et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. 2018;24:1178-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang J, Wang L, Cong Z, et al. The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1(-/-) murine model of ovarian cancer. Biochem Biophys Res Commun. 2015;463:551-556. [DOI] [PubMed] [Google Scholar]
- 54. Aurelius J, Martner A, Riise RE, et al. Chronic myeloid leukemic cells trigger poly(ADP-ribose) polymerase-dependent inactivation and cell death in lymphocytes. J Leukoc Biol. 2013;93:155-160. [DOI] [PubMed] [Google Scholar]
- 55. Fenerty KE, Padget M, Wolfson B, et al. Immunotherapy utilizing the combination of natural killer- and antibody dependent cellular cytotoxicity (ADCC)-mediating agents with poly (ADP-ribose) polymerase (PARP) inhibition. J Immunother Cancer. 2018;6:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thomas A, Vilimas R, Trindade C, et al. Durvalumab in combination with olaparib in patients with relapsed SCLC: results from a phase II study. J Thorac Oncol. 2019;14:1447-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zerfaoui M, Errami Y, Naura AS, et al. Poly(ADP-ribose) polymerase-1 is a determining factor in Crm1-mediated nuclear export and retention of p65 NF-kappa B upon TLR4 stimulation. J Immunol. 2010;185:1894-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hassa PO, Covic M, Hasan S, Imhof R, Hottiger MO. The enzymatic and DNA binding activity of PARP-1 are not required for NF-kappa B coactivator function. J Biol Chem. 2001;276:45588-45597. [DOI] [PubMed] [Google Scholar]
- 59. Olabisi OA, Soto-Nieves N, Nieves E, et al. Regulation of transcription factor NFAT by ADP-ribosylation. Mol Cell Biol. 2008;28:2860-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ghonim MA, Pyakurel K, Ibba SV, et al. PARP inhibition by olaparib or gene knockout blocks asthma-like manifestation in mice by modulating CD4(+) T cell function. J Transl Med. 2015;13:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rosado MM, Bennici E, Novelli F, Pioli C. Beyond DNA repair, the immunological role of PARP-1 and its siblings. Immunology. 2013;139:428-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mulligan AM, Raitman I, Feeley L, et al. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin Cancer Res. 2013;19:336-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mangerich A, Burkle A. Pleiotropic cellular functions of PARP1 in longevity and aging: genome maintenance meets inflammation. Oxid Med Cell Longev. 2012;2012:321653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hunter JE, Willmore E, Irving JA, Hostomsky Z, Veuger SJ, Durkacz BW. NF-kappaB mediates radio-sensitization by the PARP-1 inhibitor, AG-014699. Oncogene. 2012;31:251-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hocsak E, Szabo V, Kalman N, et al. PARP inhibition protects mitochondria and reduces ROS production via PARP-1-ATF4-MKP-1-MAPK retrograde pathway. Free Radic Biol Med. 2017;108:770-784. [DOI] [PubMed] [Google Scholar]
- 66. Schacke M, Kumar J, Colwell N, et al. PARP-1/2 inhibitor olaparib prevents or partially reverts EMT induced by TGF-beta in NMuMG cells. Int J Mol Sci. 2019;20:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wei W, Li Y, Lv S, Zhang C, Tian Y. PARP-1 may be involved in angiogenesis in epithelial ovarian cancer. Oncol Lett. 2016;12:4561-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Turner N, Tutt A, Ashworth A. Hallmarks of “BRCAness” in sporadic cancers. Nat Rev Cancer. 2004;4:814-819. [DOI] [PubMed] [Google Scholar]
- 69. Keung MYT, Wu Y, Vadgama JV. PARP inhibitors as a therapeutic agent for homologous recombination deficiency in breast cancers. J Clin Med. 2019;8:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cerrato A, Morra F, Celetti A. Use of poly ADP-ribose polymerase [PARP] inhibitors in cancer cells bearing DDR defects: the rationale for their inclusion in the clinic. J Exp Clin Cancer Res. 2016;35:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Anagnostou V, Smith KN, Forde PM, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 2017;7:264-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:13587-13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tang ML, Khan MK, Croxford JL, Tan KW, Angeli V, Gasser S. The DNA damage response induces antigen presenting cell-like functions in fibroblasts. Eur J Immunol. 2014;44:1108-1118. [DOI] [PubMed] [Google Scholar]
- 75. Seyedin SN, Hasibuzzaman MM, Pham V, et al. Combination therapy with radiation and PARP inhibition enhances responsiveness to anti-PD-1 therapy in colorectal tumor models. Int J Radiat Oncol Biol Phys. 2020;108:81-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brown JS, Sundar R, Lopez J. Combining DNA damaging therapeutics with immunotherapy: more haste, less speed. Br J Cancer. 2018;118:312-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lichtenegger FS, Rothe M, Schnorfeil FM, et al. Targeting LAG-3 and PD-1 to enhance T cell activation by antigen-presenting cells. Front Immunol. 2018;9:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Garris CS, Arlauckas SP, Kohler RH, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-gamma and IL-12. Immunity. 2018;49:1148-1161.e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rui X, Gu TT, Pan HF, Zhang HZ. Evaluation of PD-L1 biomarker for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatments for urothelial carcinoma patients: a meta-analysis. Int Immunopharmacol. 2019;67:378-385. [DOI] [PubMed] [Google Scholar]
- 81. Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hartlova A, Erttmann SF, Raffi FA, et al. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity. 2015;42:332-343. [DOI] [PubMed] [Google Scholar]
- 83. Li A, Yi M, Qin S, Chu Q, Luo S, Wu K. Prospects for combining immune checkpoint blockade with PARP inhibition. J Hematol Oncol. 2019;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sato H, Niimi A, Yasuhara T, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017;8:1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jiao S, Xia W, Yamaguchi H, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23:3711-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Martin-Oliva D, Aguilar-Quesada R, O’Valle F, et al. Inhibition of poly(ADP-ribose) polymerase modulates tumor-related gene expression, including hypoxia-inducible factor-1 activation, during skin carcinogenesis. Cancer Res. 2006;66:5744-5756. [DOI] [PubMed] [Google Scholar]
- 87. Robillard L, Nguyen M, Loehr A, et al. Preclinical evaluation of the PARP inhibitor rucaparib in combination with PD-1 and PD-L1 inhibition in a syngeneic BRCA1 mutant ovarian cancer model. Paper presented at: AACR Annual Meeting; April 1-5, 2017; Washington, DC. https://discovery.ucl.ac.uk/id/eprint/1553486/ [Google Scholar]
- 88. Higuchi T, Flies DB, Marjon NA, et al. CTLA-4 blockade synergizes therapeutically with PARP inhibition in BRCA1-deficient ovarian cancer. Cancer Immunol Res. 2015;3:1257-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Konstantinopoulos PA, Waggoner S, Vidal GA, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;13:1141-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vinayak S, Tolaney SM, Schwartzberg L, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 2019;5:1132-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Friedlander M, Meniawy T, Markman B, et al. A phase 1b study of the anti-PD-1 monoclonal antibody BGB-A317 (A317) in combination with the PARP inhibitor BGB-290 (290) in advanced solid tumors. J Clin Oncol. 2018;36:48-48. [Google Scholar]
- 92. Krebs M, Ross K, Kim S, et al. An open-label, multitumor phase II basket study of olaparib and durvalumab (MEDIOLA): results in patients with relapsed SCLC. J Thorac Oncol. 2017;12:S2044-S2045. [Google Scholar]
- 93. Karzai F, Madan RA, Owens H, et al. A phase 2 study of olaparib and durvalumab in metastatic castrate-resistant prostate cancer (mCRPC) in an unselected population. J Clin Oncol. 2018;36:163-163. [Google Scholar]
- 94. Drew Y, de Jonge M, Hong SH, et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): results in germline BRCA-mutated (gBRCAm) platinum-sensitive relapsed (PSR) ovarian cancer (OC). Gynecol Oncol. 2018;149:246-247. [Google Scholar]
- 95. Kamel D, Gray C, Walia JS, Kumar V. PARP inhibitor drugs in the treatment of breast, ovarian, prostate and pancreatic cancers: an update of clinical trials. Curr Drug Targets. 2018;19:21-37. [DOI] [PubMed] [Google Scholar]
- 96. Karzai F, VanderWeele D, Madan RA, et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J Immunother Cancer. 2018;6:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38:395-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:397-404. [DOI] [PubMed] [Google Scholar]
- 101. Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30:1080-1087. [DOI] [PubMed] [Google Scholar]
- 102. Varga A, Piha-Paul SA, Ott PA, Mehnert JM, Matei D. Pembrolizumab in patients (pts) with PD-L1–positive (PD-L1 +) advanced ovarian cancer: updated analysis of KEYNOTE-028. J Clin Oncol. 2017;35:5513-5513. [Google Scholar]
- 103. Chung HC, Piha-Paul SA, Lopez-Martin J, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol. 2020;15:618-627. [DOI] [PubMed] [Google Scholar]
- 104. Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883-895. [DOI] [PubMed] [Google Scholar]
- 105. Wang PF, Chen Y, Song SY, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. El Osta B, Hu F, Sadek R, Chintalapally R, Tang SC. Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol. 2017;119:1-12. [DOI] [PubMed] [Google Scholar]
- 107. Lee SJ, Jang BC, Lee SW, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett. 2006;580:755-762. [DOI] [PubMed] [Google Scholar]
- 108. Maxwell KN, Sloover DD, Wubbenhorst B, et al. Abstract 2990: evidence for diverse mechanisms of tumorigenesis in breast and ovarian tumors of BRCA1/2 carriers. Cancer Res. 2015;75:2990-2990. [Google Scholar]
- 109. Liu X, Palma J, Kinders R, et al. An enzyme-linked immunosorbent poly(ADP-ribose) polymerase biomarker assay for clinical trials of PARP inhibitors. Anal Biochem. 2008;381:240-247. [DOI] [PubMed] [Google Scholar]