Key Points
Question
What is the prevalence of inflammatory heart disease identified through implementation of recent return-to-play (RTP) cardiac screening recommendations in professional athletes with prior coronavirus disease 2019 (COVID-19) infection?
Findings
In this cross-sectional study of RTP cardiac testing performed on 789 professional athletes with COVID-19 infection, imaging evidence of inflammatory heart disease that resulted in restriction from play was identified in 5 athletes (0.6%). No adverse cardiac events occurred in the athletes who underwent cardiac screening and resumed professional sport participation.
Meaning
Using expert consensus RTP screening recommendations for athletes testing positive for COVID-19, few cases of inflammatory heart disease were detected and safe return to professional sport activity has thus far been achieved.
This cross-sectional study compiles data from multiple North American professional sports teams to assess the prevalence of detectable inflammatory heart disease in professional athletes with prior coronavirus disease 2019 infection.
Abstract
Importance
The major North American professional sports leagues were among the first to return to full-scale sport activity during the coronavirus disease 2019 (COVID-19) pandemic. Given the unknown incidence of adverse cardiac sequelae after COVID-19 infection in athletes, these leagues implemented a conservative return-to-play (RTP) cardiac testing program aligned with American College of Cardiology recommendations for all athletes testing positive for COVID-19.
Objective
To assess the prevalence of detectable inflammatory heart disease in professional athletes with prior COVID-19 infection, using current RTP screening recommendations.
Design, Setting, and Participants
This cross-sectional study reviewed RTP cardiac testing performed between May and October 2020 on professional athletes who had tested positive for COVID-19. The professional sports leagues (Major League Soccer, Major League Baseball, National Hockey League, National Football League, and the men’s and women’s National Basketball Association) implemented mandatory cardiac screening requirements for all players who had tested positive for COVID-19 prior to resumption of team-organized sports activities.
Exposures
Troponin testing, electrocardiography (ECG), and resting echocardiography were performed after a positive COVID-19 test result. Interleague, deidentified cardiac data were pooled for collective analysis. Those with abnormal screening test results were referred for additional testing, including cardiac magnetic resonance imaging and/or stress echocardiography.
Main Outcomes and Measures
The prevalence of abnormal RTP test results potentially representing COVID-19–associated cardiac injury, and results and outcomes of additional testing generated by the initial screening process.
Results
The study included 789 professional athletes (mean [SD] age, 25 [3] years; 777 men [98.5%]). A total of 460 athletes (58.3%) had prior symptomatic COVID-19 illness, and 329 (41.7%) were asymptomatic or paucisymptomatic (minimally symptomatic). Testing was performed a mean (SD) of 19 (17) days (range, 3-156 days) after a positive test result. Abnormal screening results were identified in 30 athletes (3.8%; troponin, 6 athletes [0.8%]; ECG, 10 athletes [1.3%]; echocardiography, 20 athletes [2.5%]), necessitating additional testing; 5 athletes (0.6%) ultimately had cardiac magnetic resonance imaging findings suggesting inflammatory heart disease (myocarditis, 3; pericarditis, 2) that resulted in restriction from play. No adverse cardiac events occurred in athletes who underwent cardiac screening and resumed professional sport participation.
Conclusions and Relevance
This study provides large-scale data assessing the prevalence of relevant COVID-19–associated cardiac pathology with implementation of current RTP screening recommendations. While long-term follow-up is ongoing, few cases of inflammatory heart disease have been detected, and a safe return to professional sports activity has thus far been achieved.
Introduction
Among the general population, a high prevalence of cardiac injury has been observed in patients hospitalized with severe acute respiratory syndrome coronavirus 2 viral infection.1 However, current data are sparse regarding the overall prevalence of adverse cardiovascular pathology associated with systemic coronavirus disease 2019 (COVID-19) illness or the extent to which occult myocardial injury may occur after asymptomatic or mildly symptomatic cases of prior COVID-19 infection.2,3,4 Among athletes, defining COVID-19–associated cardiac injury, particularly myocarditis, and distinguishing potential COVID-19–associated cardiac pathology from athletic cardiac adaptation has not yet been fully established. In the absence of robust data, the optimal approach to cardiac risk stratification for athletes returning to intensive sport activity after COVID-19 infection is not known. At present, a conservative return-to-play (RTP) approach for competitive athletes after COVID-19 infection has been recommended.5,6,7
The major North American professional sports leagues, including Major League Soccer (MLS), Major League Baseball (MLB), the National Hockey League (NHL), the National Football League (NFL), and the men’s and women’s National Basketball Associations (NBA and WNBA, respectively), were among the first sports organizations to return to full-scale sports activity in the setting of the COVID-19 pandemic, with provision of extensive health and safety measures as recommended by league medical staff and public health, infectious disease, and cardiac consultants. A program for preparticipation RTP cardiac testing, in alignment with the initial May 2020 recommendations of the American College of Cardiology (ACC) Sports and Exercise Cardiology Section,5 was implemented by each of these leagues for all athletes who tested positive for COVID-19. We are reporting the initial and collective results of systematic cardiac RTP COVID-19 testing used by these professional leagues to provide data assessing the prevalence of clinically detectable and relevant cardiac injury in athletes testing positive for COVID-19 and the efficacy of consensus screening recommendations in achieving a safe return to competitive sports.
Methods
In the spring of 2020, MLS, MLB, the NHL, the NFL, the WNBA, and the NBA implemented mandatory preparticipation cardiac screening requirements for all players testing positive for COVID-19 (either by antibody or polymerase chain reaction assay) prior to resumption of team-organized sport activities. The study protocol was approved by the institutional review board of Columbia University Irving Medical Center, and the collection and sharing of deidentified cardiac data for this study was approved by the NBA and the National Basketball Players Association, the WNBA and the Women’s National Basketball Players Association, MLS and the MLS Players Association, MLB and the Major League Baseball Players Association, and the NHL and the National Hockey League Players’ Association, as well as through the NFL and the National Football League Players Association Player Scientific and Medical Research Protocol. Pursuant to approval from the review board of Columbia University Irving Medical Center, informed consent was not required for this study.
The components of the cardiac screening protocol were in alignment with ACC RTP recommendations and included a troponin blood test, 12-lead electrocardiogram (ECG), and resting transthoracic echocardiogram, performed after resolution of symptoms and appropriate self-isolation.5 While the concurrent ACC algorithm recommended screening only for athletes with preceding symptoms associated with COVID-19 illness,5 the professional leagues’ screening process also included athletes who were asymptomatic or paucisymptomatic (minimally symptomatic)8 and had tested positive for COVID-19. Athlete testing was performed at team-affiliated or team-selected medical facilities across the US and Canada. Cardiac test results were initially interpreted by the respective professional team–affiliated cardiologists.
The high-sensitivity cardiac troponin assay was used in preference as the biomarker test, and earlier-generation assays for troponin (troponin I or T) were used if the high-sensitivity cardiac troponin test was not available in the local laboratory; an abnormal troponin level was defined as any level greater than the 99th percentile of the reference laboratory value. Resting ECGs were performed using standard lead placement; an abnormal ECG result was defined as meeting international recommendations9 and demonstrating findings raising concern for potential acute cardiac injury. Transthoracic echocardiograms were performed using commercially available systems; echocardiographic findings raising concern for potential acute cardiac injury were determined by examining physicians and in comparison with pre–COVID-19 echocardiogram study results when available. Athletes with abnormal screening test results were referred for additional testing, including cardiac magnetic resonance (CMR) imaging and/or stress echocardiography.
The team-affiliated health care professionals responsible for each individual athlete’s care evaluated the need for additional testing on a case-by-case basis. The individual athlete’s health care team managed the performance and interpretation of downstream testing, as well as decisions regarding athlete participation status after completion of these tests and consulted with experts in the interpretation of athlete cardiac study results chosen by respective teams or the applicable league.
Each professional league collected from their respective member teams a report of the cardiac data for the athletes who tested positive for COVID-19 and underwent RTP cardiac screening and prepared a deidentified league summary prior to data analysis. The 789 athletes included in this study represent the total number of athletes with both COVID-19 and complete cardiac testing available for review. The deidentified data from each league were sent to Columbia University Irving Medical Center via secure electronic transfer, and the interleague data were pooled for collective analysis.
This study is a cross-sectional study of cardiac testing performed between May and October 2020 on the professional athletes in the major North American professional sports leagues who tested positive for COVID-19. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies. This is a descriptive study, and comparisons between groups and statistical analyses were not performed. Data analysis was completed with Excel version 2016 (Microsoft).
Results
This study included 789 professional athletes who tested positive for COVID-19 and underwent RTP cardiac screening; the mean (SD) athlete age was 25 (3) years (range, 19-41 years), and 777 (98.5%) were men. A total of 460 athletes (58.3%) had prior symptoms reflecting COVID-19 illness, and 329 (41.7%) were asymptomatic or paucisymptomatic but had tested positive for the virus; COVID-19 positivity was diagnosed by polymerase chain reaction assay in 587 athletes (74.4%) and antibody testing in 202 athletes (25.6%). For athletes testing positive for COVID-19 via polymerase chain reaction assay, cardiac screening was performed a mean (SD) of 19 (17) days (range, 3-156 days) after the positive COVID-19 test. No athlete was deemed to have severe COVID-19 viral illness as determined by team physicians. One athlete was hospitalized overnight for observation, but no athlete was admitted to a hospital for cardiopulmonary symptoms. Using the specified RTP cardiac screening algorithm, 6 athletes (0.8%) had an abnormal troponin level (defined as a level greater than the 99th percentile of the reference laboratory value), 10 athletes (1.3%) had ECG abnormalities warranting further cardiac evaluation, and 20 athletes (2.5%) had an echocardiographic finding necessitating additional testing to exclude acute cardiac injury. These results are summarized in Table 1.
Table 1. Return-to-Play Cardiac Screening in Professional Athletes Testing Positive for Coronavirus Disease 2019 (COVID-19).
| Characteristic | Individuals, No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| National Basketball Association | Major League Soccer | Major League Baseball | National Hockey League | National Football League | Total professional athlete cohort | ||
| Men | Women | ||||||
| Total athletes positive for COVID-19, No. | 109 | 12 | 70 | 181 | 68 | 349 | 789 |
| Age, mean (range), y | 25 (19-35) | 27 (21-33) | 25 (18-31) | 25 (19-38) | 25 (19-41) | 25 (21-37) | 25 (19-41) |
| COVID-19 symptom burden | |||||||
| Preceding viral symptoms | 71 (65.1) | 8 (67) | 33 (47) | 109 (60.2) | 51 (75) | 188 (53.9) | 460 (58.3) |
| Asymptomatic or paucisymptomatic | 38 (34.9) | 4 (33) | 37 (53) | 72 (39.8) | 17 (25) | 161 (46.1) | 329 (41.7) |
| COVID-19 test | |||||||
| Polymerase chain reaction | 75 (68.8) | 9 (75) | 51 (73) | 172 (95.0) | 54 (79) | 226 (64.8) | 587 (74.4) |
| Antibody | 34 (31.2) | 3 (25) | 19 (27) | 9 (5.0) | 14 (21) | 123 (35.2) | 202 (25.6) |
| Days between COVID-19 polymerase chain reaction test and cardiac screen, mean (range) | 32 (9-124) | 23 (14-60) | 14 (13-16) | 21 (3-90) | 18 (9-97) | 17 (3-156) | 19 (3-156) |
| Abnormal cardiac testing results | |||||||
| Troponina | 3 (2.8) | 1 (8) | 1 (1) | 0 | 0 | 1 (0.3) | 6 (0.8) |
| Electrocardiogramb | 0 | 0 | 0 | 5 (2.8) | 0 | 5 (1.4) | 10 (1.3) |
| Echocardiogramc | 2 (1.8) | 3 (25) | 3 (4) | 6 (3.3) | 1 (1) | 5 (1.4) | 20 (2.5) |
An abnormal troponin level was defined as a level greater than the 99th percentile of the reference laboratory value.
An abnormal electrocardiogram was defined as meeting international recommendations9 and demonstrating findings raising concern for potential acute cardiac injury.
An abnormal echocardiogram was defined by ventricular dysfunction or another finding raising concern for potential acute cardiac injury.
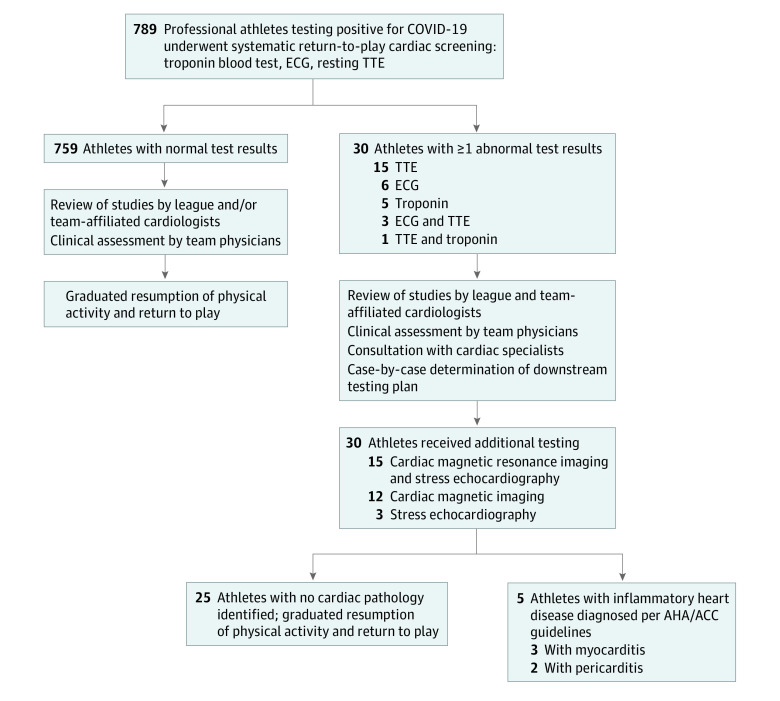
Thirty athletes (3.8%) were sent for additional cardiac testing as a result of abnormalities on the initial cardiac screening tests that raised concern for potential COVID-19–associated cardiac injury. Cardiac magnetic resonance imaging was performed in 27 of these 30 athletes (90.0%). Downstream testing confirmed diagnoses of inflammatory heart disease in 5 of 27 athletes (18.5%; 0.6% of the total athlete cohort): 3 athletes with CMR-confirmed myocarditis (0.4% of the total cohort) and 2 athletes with CMR-confirmed pericarditis (0.3% of the total cohort). These athletes were held out from sport participation in accordance with expert task force guidelines.10 The remaining 25 of 30 athletes (83.3%) who underwent additional testing did not ultimately have findings to suggest acute cardiac injury and returned to play. These results are summarized in Table 2. A flow diagram of the testing process and results is provided in the Figure. As of late December 2020, no clinical cardiac events have occurred in any of the athletes who have undergone cardiac screening and resumed full professional sporting activity.
Table 2. Results of Additional Cardiac Testing and Participation Status of Athletes With Abnormalities on Baseline Cardiac Screening Examinationsa.
| Athlete No. | Symptomatic infection | COVID-19 test | Abnormal results | Echo abnormality | Additional testing | Participation statuse | |||
|---|---|---|---|---|---|---|---|---|---|
| Troponin levelb | ECGc | Echod | Cardiac MRI | Stress echo | |||||
| 1 | Yes | PCR | Yes | No | No | NA | Normal LVEF; no LGE; no CMR evidence of myocarditis | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 2 | Yes | PCR | Yes | No | No | NA | Normal LVEF; no LGE; no CMR evidence of myocarditis | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 3 | Yes | PCR | No | No | Yes | Small pericardial effusion | Normal LVEF; no LGE; no CMR evidence of myocarditis/pericarditis | NA | Return to play |
| 4 | Yes | PCR | No | No | Yes | Small pericardial effusion | CMR pericardial enhancement diagnostic of pericarditis; no LGE; no CMR evidence of myocarditis | NA | Held out |
| 5 | Yes | PCR | No | No | Yes | LVEF, 53% | NA | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 6 | No | IgG | No | No | Yes | LVEF, 53% | NA | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 7 | No | IgG | Yes | No | No | NA | Normal LVEF; no LGE; no CMR evidence of myocarditis | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 8 | No | IgG | No | No | Yes | Mildly reduced LVEF, 45%-50% | CMR-calculated LVEF, 53%; no LGE; no CMR evidence of myocarditis | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 9 | Yes | IgG | No | No | Yes | Mildly reduced LVEF, 45% | CMR-calculated LVEF, 56%; no LGE; no CMR evidence of myocarditis | NA | Return to play |
| 10 | No | IgG | Yes | No | No | NA | NA | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 11 | Yes | PCR | No | No | Yes | Mildly reduced LVEF, 48% | CMR-calculated LVEF, 53%; no LGE; no CMR evidence of myocarditis | NA | Return to play |
| 12 | Yes | PCR | Yes | No | Yes | Mildly reduced LVEF, 50% | CMR-calculated LVEF, 44%; patchy LGE present; CMR findings consistent with myocarditis | NA | Held out |
| 13 | Yes | PCR | No | Yes | No | NA | CMR-calculated LVEF, 78%; no LGE; no CMR evidence of myocarditis | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 14 | Yes | PCR | No | No | Yes | Mildly reduced LVEF, 45% | CMR-calculated LVEF, 52%; no LGE; no CMR evidence of myocarditis | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 15 | Yes | PCR | No | No | Yes | Small pericardial effusion | Normal LVEF; no LGE; no CMR evidence of myocarditis or pericarditis | NA | Return to play |
| 16 | No | IgG | No | Yes | No | NA | Normal LVEF; no LGE; no CMR evidence of myocarditis | NA | Return to play |
| 17 | Yes | PCR | No | Yes | Yes | Impaired LV relaxation | Normal LVEF; no LGE; no CMR evidence of myocarditis | NA | Return to play |
| 18 | No | IgG | No | No | Yes | Mild global LV hypokinesis | CMR-calculated LVEF, 46%; no LGE; no CMR evidence of myocarditis or pericarditis | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 19 | Yes | PCR | Yes | No | No | NA | CMR pericardial enhancement diagnostic of pericarditis; no LGE; no CMR evidence of myocarditis | NA | Held out |
| 20 | Yes | PCR | No | No | Yes | Mild global LV hypokinesis | No CMR evidence of myocarditis | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 21 | Yes | PCR | No | Yes | No | NA | No CMR evidence of myocarditis | NA | Return to play |
| 22 | No | IgG | No | No | Yes | Dilated right ventricle | No CMR evidence of myocarditis | NA | Return to play |
| 23 | Yes | PCR | No | Yes | No | NA | No CMR evidence of myocarditis | NA | Return to play |
| 24 | Yes | PCR | No | No | Yes | Frequent PVCs | No CMR evidence of myocarditis | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 25 | Yes | PCR | No | No | Yes | Decreased GLS | No CMR evidence of myocarditis | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 26 | Yes | PCR | No | Yes | No | NA | No CMR evidence of myocarditis | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 27 | Yes | PCR | No | Yes | Yes | Decreased GLS | No CMR evidence of myocarditis | Normal LV augmentation with exercise; no exercise-induced arrhythmias | Return to play |
| 28 | Yes | PCR | No | Yes | Yes | New regional wall motion abnormality in comparison with prior echocardiographic studies; preserved LVEF | Normal LVEF; LGE present in corresponding territory as echocardiogram; CMR findings consistent with myocarditis | NA | Held out |
| 29 | Yes | PCR | No | No | Yes | Dilated right ventricle | Normal LVEF and RV systolic function; focal T2 hyperintensity in the mid-ventricular inferior wall; CMR findings consistent with myocarditis | NA | Held out |
| 30 | Yes | PCR | No | Yes | No | NA | No CMR evidence of myocarditis | NA | Return to play |
Abbreviations: CMR, cardiac magnetic resonance imaging; COVID-19, coronavirus disease 2019; ECG, electrocardiogram; echo, echocardiogram; GLS, global longitudinal strain; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; NA, not applicable; PCR, polymerase chain reaction; PVC, premature ventricular contractions.
There were 4 women and 26 men ranging in age from 19 to 35 years. Age and sex are not specified for each athlete in this table to maintain confidentiality.
Abnormal troponin levels were defined as those greater than the 99th percentile upper limit of normal.
An abnormal ECG was defined as a new ECG abnormality meeting international recommendations9 and demonstrating findings raising concern for potential acute cardiac injury.
An abnormal echocardiogram was defined as new ventricular dysfunction or another echocardiographic finding raising concern for potential acute cardiac injury.
Following American Heart Association/American College of Cardiology myocarditis or pericarditis guidelines.10
Figure. Flow Diagram of the Systematic Return-to-Play Cardiac Screening Process Used for Professional Athletes Testing Positive for Coronavirus Disease 2019 (COVID-19).
Thirty of 789 athletes (3.8%) had abnormal cardiac screening test results necessitating additional evaluation and downstream testing; 5 athletes (0.6%) were detected to have findings raising concern for COVID-19–associated inflammatory heart disease that resulted in restriction from sport participation per American Heart Association (AHA)/American College of Cardiology (ACC) guidelines.10 ECG indicates electrocardiogram; TTE, transthoracic echocardiogram.
Discussion
Through RTP cardiac screening for professional athletes testing positive for COVID-19, 0.6% (5 of 789 athletes) had imaging findings suggestive of inflammatory heart disease that resulted in restriction from play in alignment with American Heart Association/ACC guidelines.10 With implementation of current expert consensus–based cardiovascular risk stratification practices, safe return to professional sporting activity has thus far been achieved, with no cardiovascular events occurring within these professional leagues during and on completion of competitive play in 2020.
The results of this study provide an early opportunity to assess the potential for clinical effectiveness of current athletic RTP screening recommendations and the prevalence of relevant COVID-19–associated cardiac pathology in a large-scale setting. The low prevalence of clinically detectable inflammatory heart disease in this athlete population with nonsevere COVID-19 illness offers a counterpoint to findings of higher rates of COVID-19 myocarditis and pericarditis reported in recent small cohort and CMR-based observational studies in athletes.3,11 The difference in detected and reported abnormalities between the current and prior studies highlight inherent difficulties in using CMR as a standalone screening tool in COVID-19 RTP cardiovascular risk stratification. In concert with the challenges in CMR interpretation to differentiate attributes of athletic cardiac remodeling from potential cardiac pathology and lack of athlete-specific CMR standards, our findings add support for the use of CMR as a clinically indicated and selective downstream test, rather than a tool to be applied in frontline and widespread screening, especially if clinical pretest probability is low.4,12
In this athlete cohort, 5 athletes (0.6%) had an isolated abnormal troponin test result without other clinical or imaging evidence of cardiac injury. The low but not inconsequential rate of an isolated false-positive troponin level observed in this study underscores the fact that established 99th-percentile reference ranges for troponin levels do not include elite athletes and supports recommendations that biomarker interpretation must be incorporated with additional cardiac test results and assessed in context with supporting clinical factors.13 We also observed that ECG results in this elite athlete population provided low specificity, especially without other concomitant screening abnormalities, for the detection of subclinical inflammatory heart disease. Our experience suggests that the addition of an ECG in isolation to a preparticipation physical evaluation as a method of COVID-19 RTP cardiac screening, which could be conceived as an expedient and cost-effective alternative to current RTP screening recommendations, would not be effective in elite athletes with nonsevere forms of COVID-19 illness. Echocardiograms, on the other hand, did yield more clinical information that aided in the detection of inflammatory heart disease. Echocardiographic findings interpreted as abnormal, however, also led to additional cardiac testing in 15 athletes (1.9% of the cohort) in whom no acute pathology was ultimately detected. Given published reports14,15,16 demonstrating low normal to mildly reduced resting left and right ventricular systolic function in a proportion of athletes who are highly trained, significant challenges remain with using echocardiography in elite athletes to distinguish athletic remodeling from potential COVID-19–associated cardiac pathology. The ability to compare echocardiographic findings with prior, pre–COVID-19 examinations, if available, is valuable when interpreting RTP screening echocardiograms. The limitations observed with echocardiography presented in this study, along with the need to balance resource management across health care systems, will be essential elements to incorporate in the refinement of RTP screening practices.
It is important to note that none of the athletes in this cohort were clinically assessed as having severe COVID-19 viral illness. All of the 5 athletes that were identified as having inflammatory heart disease, however, had preceding symptoms that exceeded empirical definitions of mild COVID-19 illness (such as loss of taste and smell, nonspecific fatigue, and cough without dyspnea).17 Our data support the concept that ascertained symptom burden should be a primary reference point to guide the next steps in the evaluation of the athlete testing positive for COVID-19. The findings from this study support updated expert consensus ACC recommendations, which do not advocate for cardiovascular risk stratification in athletes who were asymptomatic or athletes with mild COVID-19 viral illness who remain asymptomatic after completion of appropriate self-isolation.7
Limitations
This study has important limitations that are inherent in its design as a multicenter, retrospective cross-sectional analysis. First, RTP screening examinations were performed in a clinical setting and in most leagues were analyzed and adjudicated by team physicians and cardiologists across the US and Canada, allowing for varying determinations of potential cardiac pathology and need for downstream testing. Cardiac magnetic resonance imaging was not performed uniformly as part of the initial screening process, potentially allowing for some cases of subclinical inflammatory heart disease to be missed. However, the clinical significance of such potential cases of subclinical cardiac inflammation, in the absence of corroborating clinical data or abnormalities in standard RTP testing, is not certain. In all instances in which downstream testing was performed, consultation with experts in athlete cardiac imaging was obtained to assist in diagnosis and decision-making regarding athlete participation status. Because of the decentralized structure of this process across and within most leagues, variations in the interpretation of test results inevitably limit our ability to provide uniform criteria to diagnose COVID-19–associated inflammatory heart disease. While athlete testing across the professional leagues could not be subjected to a core laboratory analysis, the format of this study is reflective of the realities embodied in multicenter and large-scale clinical cardiac testing, and the results of this study should reasonably reflect outcomes of widespread clinical screening. These features and limitations of this study highlight the need to include cardiologists with expertise in the cardiac imaging of athletes into the screening process to optimize the proper identification of disease and minimize unnecessary disqualification or delays in RTP. A further limitation of this study is the variability in time between COVID-19 testing and cardiac screening. Some athletes tested positive for COVID-19 early in the pandemic and did not have to report to league-organized training camps until many weeks after the initial viral illness, leaving open the possibility that early manifestations of COVID-19 cardiac injury could have resolved by the time cardiac testing was performed in the cases in which the time between COVID-19 and cardiac testing was longer. However, similar patterns of COVID-19 and RTP cardiac testing of athletes are likely to be continually encountered in most athletic scenarios, in which an infection may occur in an athlete’s off-season period. An additional limitation of this study is that nearly all of the athletes (98.5%) were men.
Conclusions
In this multicenter cross-sectional study of professional athletes with prior COVID-19 infection, we observed only rare cases of athletes having potential cardiac involvement. This reporting of systematic RTP cardiac screening, while not generalizable to all athletic populations, can provide clinical guidance for other athletic organizations who are preparing and optimizing RTP protocols. Similar studies of other athletes, including pediatric, collegiate, and masters-level athletes, are required. Longitudinal assessment of athletes with prior COVID-19 infection remains necessary to enhance our understanding of the short-term and potential long-term pathologic cardiac sequelae of COVID-19 infection.
References
- 1.Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648-1655. doi: 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
- 2.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265-1273. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajpal S, Tong MS, Borchers J, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starekova J, Bluemke DA, Bradham WS, et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. Published online January 14, 2021. doi: 10.1001/jamacardio.2020.7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phelan D, Kim JH, Chung EH. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) infection. JAMA Cardiol. 2020;5(10):1085-1086. doi: 10.1001/jamacardio.2020.2136 [DOI] [PubMed] [Google Scholar]
- 6.Baggish A, Drezner JA, Kim J, Martinez M, Prutkin JM. Resurgence of sport in the wake of COVID-19: cardiac considerations in competitive athletes. Br J Sports Med. 2020;54(19):1130-1131. doi: 10.1136/bjsports-2020-102516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JH, Levine BD, Phelan D, et al. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021;6(2):219-227. doi: 10.1001/jamacardio.2020.5890 [DOI] [PubMed] [Google Scholar]
- 8.Meyerowitz EA, Richterman A, Bogoch II, Low N, Cevik M. Towards an accurate and systematic characterisation of persistently asymptomatic infection with SARS-CoV-2. Lancet Infect Dis. 2020;S1473-3099(20)30837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma S, Drezner JA, Baggish A, et al. International recommendations for electrocardiographic interpretation in athletes. J Am Coll Cardiol. 2017;69(8):1057-1075. doi: 10.1016/j.jacc.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 10.Maron BJ, Udelson JE, Bonow RO, et al. ; American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology, Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and American College of Cardiology . Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3, hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132(22):e273-e280. [DOI] [PubMed] [Google Scholar]
- 11.Brito D, Meester S, Yanamala N, et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging. 2020;S1936-878X(20)30946-3. doi: 10.1016/j.jcmg.2020.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH. Screening athletes for myocarditis with cardiac magnetic resonance imaging after COVID-19 infection—lessons from an English philosopher. JAMA Cardiol. 2021. doi: 10.1001/jamacardio.2020.7463 [DOI] [PubMed] [Google Scholar]
- 13.Phelan D, Kim JH, Elliott MD, et al. Screening of potential cardiac involvement in competitive athletes recovering from COVID-19: an expert consensus statement. JACC Cardiovasc Imaging. 2020;13(12):2635-2652. doi: 10.1016/j.jcmg.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engel DJ, Schwartz A, Homma S. Athletic cardiac remodeling in US professional basketball players. JAMA Cardiol. 2016;1(1):80-87. doi: 10.1001/jamacardio.2015.0252 [DOI] [PubMed] [Google Scholar]
- 15.Abergel E, Chatellier G, Hagege AA, et al. Serial left ventricular adaptations in world-class professional cyclists: implications for disease screening and follow-up. J Am Coll Cardiol. 2004;44(1):144-149. doi: 10.1016/j.jacc.2004.02.057 [DOI] [PubMed] [Google Scholar]
- 16.Teske AJ, Prakken NH, De Boeck BW, et al. Echocardiographic tissue deformation imaging of right ventricular systolic function in endurance athletes. Eur Heart J. 2009;30(8):969-977. doi: 10.1093/eurheartj/ehp040 [DOI] [PubMed] [Google Scholar]
- 17.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate COVID-19. N Engl J Med. 2020;383(18):1757-1766. doi: 10.1056/NEJMcp2009249 [DOI] [PubMed] [Google Scholar]