Abstract
This cohort study examines treatment with immune checkpoint inhibitors for patients with metastatic renal cell carcinoma.
An elevated body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) has been associated with an increased risk of renal cell carcinoma (RCC).1 Previously, higher BMI was shown to be a positive prognostic factor for patients with metastatic clear cell RCC (mRCC) who were treated during the vascular endothelial growth factor (VEGF)–targeted therapy era.2 However, the treatment landscape has shifted to include immune checkpoint inhibitors (ICIs) for most patients. We investigated this obesity paradox in patients with mRCC who were treated with programmed cell death 1 protein/programmed cell death 1 ligand 1 (PD-1/PD-L1)–based ICIs and explored potential genomic alterations according to BMI status.
Methods
Using the International Metastatic RCC Database Consortium (IMDC) database, we included patients treated with PD-1/PD-L1–based ICI alone or in combinations. Institutional review board approval was obtained from the Dana-Farber Cancer Institute and each participating site within the IMDC consortium, and participants provided written informed consent. Comparisons between patients who were defined as having a high BMI (≥25) vs low (<25) were conducted using χ2 and Fisher tests. We investigated the association of BMI with overall survival (OS; time from ICI initiation to death or censoring at last follow-up), time to treatment failure (TTF; time from ICI initiation to treatment cessation, progression, death, or censoring), and objective response (complete/partial response, by Response Evaluation Criteria in Solid Tumor, version 1.1]). Associations of BMI were assessed in multivariable logistic (objective response rate [ORR]) and Cox (TTF; OS) regression, which were adjusted for IMDC risk classifications (favorable, intermediate, or poor), age, sex, race/ethnicity, histology, sarcomatoid features, line, and type of ICI. In patients with available next-generation sequencing data (OncoPanel, 275-447 genes3), genomic alteration frequencies (nonsense, insertions/deletions, and missense by a Polyphen-2/Mutation Assessor4), and tumor mutational burden were compared by BMI status using Fisher exact and Mann-Whitney U tests. Statistical tests were 2-sided and performed using SAS, version 9.4 (SAS Institute), and R, version 3.6.1 (R Foundation). Results were considered statistically significant if P < .05 or q < 0.05.
Results
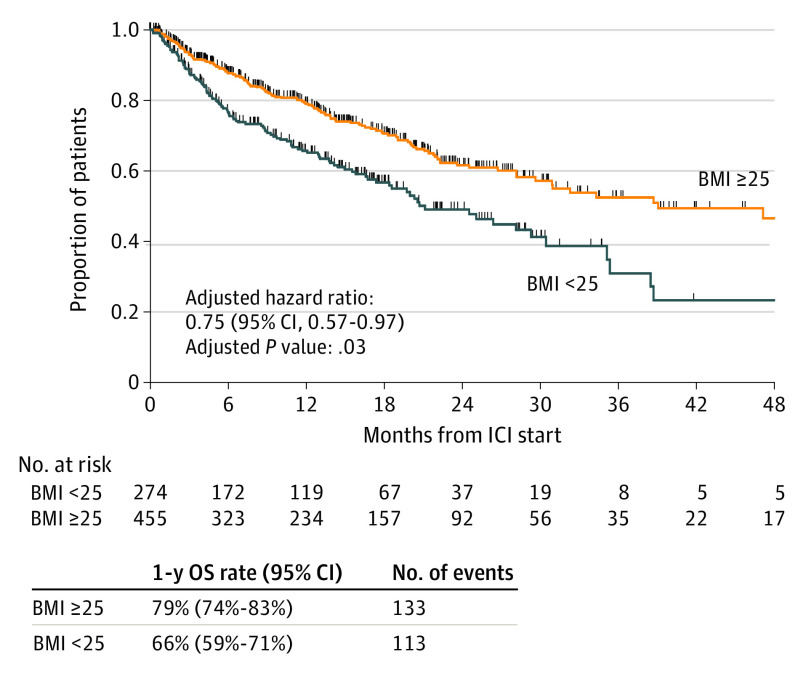
Within the IMDC database, 735 patients with mRCC with a recorded BMI were treated with PD-1/PD-L1–based ICI. Overall, 229 (31%) received first-line ICI, and 230 (31%) received combination ICI (142 [19%] with VEGF; 88 [12%] with CTLA-4 (cytotoxic T-lymphocyte–associated protein 4)/other therapies). At ICI initiation, 274 patients (37%) had what was considered low BMI and 461 (63%) a high BMI (Table). Median follow-up was 13.5 months (range, <1 to 78.6 months). Patients with a high BMI displayed significantly improved OS compared with those with a low BMI (1-year OS: 79% vs 66%; adjusted hazard ratio, 0.75; 95% CI, 0.57-0.97; P = .03) (Figure). This association was consistent in subgroup analysis by sex, IMDC group, histology, and type/line of therapy. Patients with higher BMIs also had a numerically higher ORR (30% vs 21%) and TTF (median [95% CI], 7.4 [6.7-9.0] vs 4.9 [3.8-6.9] months), although these were not statistically significant in multivariable models (ORR: adjusted odds ratio, 1.51; 95% CI, 0.98-2.32; P = .06; TTF: adjusted hazard ratio, 0.98; 95% CI, 0.80-1.20; P = .83). In 319 patients with available next-generation sequencing data, genomic alteration frequencies (all q > 0.50), and tumor mutational burden (6.8 vs 6.8 mutations per megabase; P = .90) were found to be similar between BMI groups.
Table. Patient Characteristics.
| Characteristics | BMI <25 | BMI ≥25 | P valuea |
|---|---|---|---|
| No. (%) | 274 (37%) | 461 (63%) | NA |
| Age at therapy initiation, median (range), y | 63 (14-88) | 63 (22-88) | .49 |
| Sex | |||
| Male | 201 (73.4) | 361 (78.3) | .13 |
| Female | 73 (26.6) | 100 (21.7) | |
| Race/ethnicity | |||
| Asian | 54 (19.7) | 21 (4.6) | <.001 |
| Black | 5 (1.8) | 7 (1.5) | |
| Hispanic | 6 (2.2) | 8 (1.7) | |
| White | 166 (60.6) | 354 (76.8) | |
| ECOG score | |||
| 0-1 | 197 (84.2) | 397 (93.2) | <.001 |
| 2/3/4 | 37 (15.8) | 29 (6.8) | |
| Nephrectomy, yes | 168 (81.2) | 276 (88.5) | .02 |
| Pathology | |||
| Clear cell | 214 (79.9) | 404 (88.4) | .002 |
| Nonclear cell | 54 (20.1) | 53 (11.6) | |
| Sarcomatoid/rhabdoid component, yes | 33 (14.0) | 69 (16.2) | .47 |
| IMDC risk group | |||
| Favorable | 22 (9.6) | 91 (22.5) | <.001 |
| Intermediate | 124 (54.4) | 253 (62.6) | |
| Poor | 82 (36.0) | 60 (14.9) | |
| Line of therapy | |||
| 1 | 70 (25.6) | 159 (34.5) | .01 |
| 2 | 123 (44.9) | 202 (43.8) | |
| 3-4 | 81 (29.6) | 100 (21.7) | |
| Type of anti–PD-1/PD-L1 therapy | |||
| Anti–PD-1/PD-L1 monotherapy | 199 (72.6) | 306 (66.4) | .08 |
| Anti–PD-1/PD-L1 + anti-VEGF | 46 (16.8) | 96 (20.8) | |
| Anti–PD-1/PD-L1 + anti–CTLA-4 | 25 (9.2) | 47 (10.2) | |
| Anti–PD-1 + other | 4 (1.5) | 12 (2.6) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared);CTLA-4, cytotoxic T-lymphocyte– associated protein 4; ECOG, Eastern Cooperative Oncology Group; IMDC, International Metastatic RCC Database Consortium; NA, not applicable; PD-1, programmed cell death 1 protein; PD-L1, PD-1 ligand 1; VEGF, vascular endothelial growth factor.
Excluded unknown group in the comparisons (by BMI status): race (n = 43 vs 71); ECOG (n = 40 vs 35); pathology (n = 6 vs 4); sarcomatoid/rhabdoid component (n = 39 vs 34); IMDC groups (n = 46 vs 57); and nephrectomy status (n = 67 vs 149).
Figure. Kaplan-Meier Curves for Overall Survival (OS) According to Body Mass Index (BMI)a Status.
ICI indicates immune checkpoint inhibitor.
aCalculated as weight in kilograms divided by height in meters squared.
Discussion
In this multinational analysis from the IMDC, an elevated BMI was independently associated with improved OS in patients with mRCC who were treated with PD-1/PD-L1–based ICIs. These findings are consistent with the obesity paradox that was previously seen during the VEGF-targeted therapy era.2 Several hypotheses have attempted to explain this clinical observation in RCC. Low fatty acid synthase gene expression, which is inversely correlated with BMI, was associated with longer OS in VEGF-treated patients.2 Transcriptomic analysis suggests that patients with obesity have tumors with increased angiogenesis gene signatures and peritumoral adipose tissues with increased hypoxia, inflammation, and immune cell infiltration signatures.5 The limitations of this study include biases that were associated with the retrospective analysis and lack of robust gene-expression profiling. While baseline characteristics differed between groups, we adjusted for key prognostic variables in multivariable models. Further, BMI may have limitations as a surrogate marker of adiposity; more sophisticated, although cumbersome, radiological measurements could better identify sarcopenic obesity.6 Ultimately, further correlative work is required to explore the biological underpinnings for similar findings across other solid tumors that are treated with ICIs.
References
- 1.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579-591. doi: 10.1038/nrc1408 [DOI] [PubMed] [Google Scholar]
- 2.Albiges L, Hakimi AA, Xie W, et al. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J Clin Oncol. 2016;34(30):3655-3663. doi: 10.1200/JCO.2016.66.7311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia EP, Minkovsky A, Jia Y, et al. Validation of OncoPanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141(6):751-758. doi: 10.5858/arpa.2016-0527-OA [DOI] [PubMed] [Google Scholar]
- 4.Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res. 2011;39(17):e118. doi: 10.1093/nar/gkr407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez A, Furberg H, Kuo F, et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol. 2020;21(2):283-293. doi: 10.1016/S1470-2045(19)30797-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan CL, Heng DYC. New insights into the obesity paradox in renal cell carcinoma. Nat Rev Nephrol. 2020;16(5):253-254. doi: 10.1038/s41581-020-0264-y [DOI] [PubMed] [Google Scholar]