Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS–CoV-2) has given rise to a pandemic of unprecedented proportions in the modern era because of its highly contagious nature and impact on human health and society: coronavirus disease 2019 (COVID-19). Patients with cardiovascular (CV) risk factors and established CV disease (CVD) are among those initially identified at the highest risk for serious complications, including death. Subsequent studies have pointed out that patients with cancer are also at high risk for a critical disease course. Therefore, the most vulnerable patients are seemingly those with both cancer and CVD, and a careful, unified approach in the evaluation and management of this patient population is especially needed in times of the COVID-19 pandemic. This review provides an overview of the unique implications of the viral outbreak for the field of cardiooncology and outlines key modifications in the approach to this ever-increasing patient population. These modifications include a shift toward greater utilization of cardiac biomarkers and a more focused CV imaging approach in the broader context of modifications to typical practice pathways. The goal of this strategic adjustment is to minimize the risk of SARS–CoV-2 infection (or other future viral outbreaks) while not becoming negligent of CVD and its important impact on the overall outcomes of patients who are being treated for cancer.
Keywords: best practice, cancer, cardiac safety, cardio-oncology, coronavirus disease 2019 (COVID-19), pandemic, recommendations, virus
Introduction
On December 31, 2019, Chinese officials informed the World Health Organization of a cluster of cases of pneumonia in Wuhan, central China, related to a novel coronavirus (2019-nCoV, later designated severe acute respiratory syndrome coronavirus 2 [SARS–CoV-2]).1 The viral outbreak has been marked by the rapidity of spread to pandemic proportions (coronavirus disease 2019 [COVID-19]) and the spectrum of presentations from asymptomatic supercarrier status to life-threatening respiratory failure.1,2 Individuals with SARS–CoV-2 at highest risk for morbidity and mortality include the elderly, those with cardiovascular (CV) risk factors and established CV disease (CVD), and those with cancer.3–8 Accordingly, patients with both cancer and CVD are likely to be at highest risk to experience morbidity and mortality from SARS–CoV-2. The purpose of this review from the International Cardio-Oncology Society (www.IC-OS.org) is to address and provide general guidance for the assurance of cardiac safety among patients with cancer that can be practically useful for oncologists, hematologists, and cardiologists during and after this COVID-19 pandemic. Importantly, the need for such guidance is underscored by the projection that COVID-19 will remain a legitimate concern for health care systems and the practice of medicine for the foreseeable future. The expected timeline for the development of an effective vaccine is unknown, and reports are surfacing that there may be a relatively short-lasting immunity among patients who have had the SARS–CoV-2 infection (compared with other viral illnesses).9
Therefore, this review is appropriate not only for immediate utilization but also for the new reality that this pandemic has created: cardio-oncology (C-O) care in the era of COVID-19 (Fig. 1). As a disclaimer, as the evidence base in this area is rapidly evolving, a complete understanding is still in its early stages, and many specific aspects still need to be conclusively defined.
FIGURE 1.
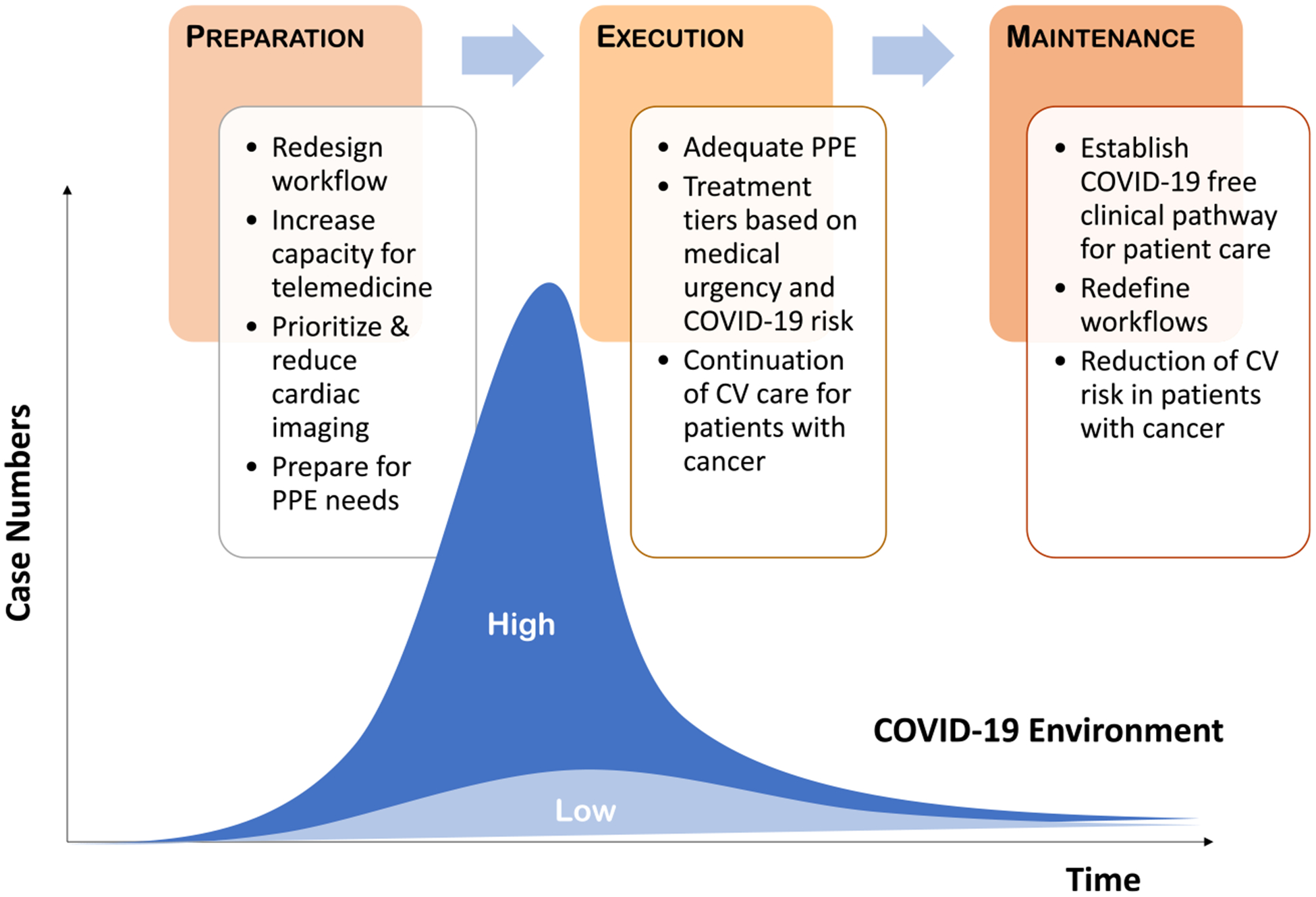
Cardio-Oncology Care in the Era of Coronavirus Disease 2019 (COVID-19). This is an overview of the changes in medical practice for patients with cancer in the setting of a viral pandemic such as COVID-19. These include proper preparation, execution of modified workflows, and maintenance of excellent care. Some modified practices evolving before and during peak times of a pandemic will likely remain in place to address persistent residual risk and resurges and/or those recognized to contribute to practice optimization in general. CV indicates cardiovascular; PPE, personal protection equipment.
Impact of COVID-19 on the CV System
CV injury and dysfunction are leading causes of death and disability among patients with SARS–CoV-2. For example, Guo et al demonstrated that 27.8% of patients with SARS–CoV-2 had myocardial injury (defined by an elevation in troponin), 20% had arrhythmias, and 16% needed hemodynamic support.10 Importantly, cardiac troponin elevations indicate worse outcomes; for instance, a >60% mortality rate among patients with CVD.2 Interestingly, elevated cardiac troponin levels are correlate with high C-reactive protein levels and an exaggerated inflammatory response, perhaps similar to that seen after chimeric antigen receptor-T cell (CAR-T) therapy.11 This exaggerated inflammatory response/cytokine storm is postulated to be one of the primary mechanisms leading to cardiac injury and dysfunction. However, there are multiple potential mechanisms for cardiac injury with COVID-19 beyond an exaggerated inflammatory response, including acute coronary syndrome, microvascular dysfunction, thrombosis, and even myocarditis. It is interesting to note that the cellular receptor for viral entry into the human body is the angiotensin-converting enzyme-2 receptor (ACE2), which is expressed on lung epithelial cells (type 2 pneumocytes) as well as vascular endothelial cells, pericytes, cardiomyocytes, and macrophages. Accordingly, SARS–CoV-2 may be viewed not only as a respiratory virus but also as a CV virus, which can result in both severe acute lung injury and severe CV injury.12–17 Indeed, several autopsy-based studies have now conclusively reported arterial and venous thrombosis, including pulmonary thrombosis and infarction, in addition to acute lung injury.18–20
These prominent thrombotic aspects are a distinguishing feature of SARS–CoV-2. The mechanisms are not fully detailed but may relate to the inflammatory response and endothelial injury. From the initial reports, there appears to be a link between markers of inflammation (interleukin-6 [IL-6]), myocardial injury (troponin), and thrombosis/fibrinolysis (D-dimer) for identifying patients who are suffering important CV consequences.4 In fact, a D-dimer level >1μg/mL was the only biomarker independently and most potently predictive of mortality in one of the first studies on patients hospitalized with COVID-19.4 The value of D-Dimer levels in this regard has subsequently been confirmed in other studies.21
Impact of COVID-19 on Patients With Cancer
While the course of COVID-19 in patients with cancer continues to be defined, current data suggest that patients with cancer are at higher risk of acquiring the infection and experiencing a more severe course and higher mortality.6,22,23 Indeed, various factors might influence susceptibility to COVID-19 among patients with cancer, including the type and stage of cancer, its location, the immunosuppressive effects of the therapeutic choices, the degree of bone marrow suppression, the frequent visits to the health care system, and the prevalence of COVID-19 in the region.
An overview of studies on patients with cancer who had COVID-19 published in the first one-half of 2020 is provided in Table 1.6,24–31 The majority of these studies confirm the notion that patients with cancer have a more severe course of COVID-19 and are also at a higher mortality risk. Increasing age has been the most consistent predictor of poor outcomes in these patients, followed by male sex. The presence of comorbidities, in particular CVD, have been identified as risk factors for a more severe and even fatal course of COVID-19. No particular cancer type has been identified to pose the highest risk, although lung cancer and hematologic malignancies have been discussed. Patients with active, progressive, and metastatic cancer seem to be more vulnerable to poor outcomes. No specific type of cancer therapy has been identified to pose a risk for a complicated or fatal course of COVID-19.
TABLE 1.
Studies on Coronavirus Disease 2019 (COVID-19) in Patients With Cancer (Status as of June 2020)
| STUDY | COUNTRY | CANCER COHORT | NO. | COVID-19+ | ICU | MV | MORTALITY | SEVERE | INDEPENDENT PREDICTORS |
|---|---|---|---|---|---|---|---|---|---|
| Liang 20206 | 5 Districts, China | 17 Solid cancers, 1 lymphoma; 4 on active therapy | 18 Patients with cancer and 1572 patients without cancer | 1 % of patients with cancer vs 0.29% in general population | 39% in patients with cancer vs 8% in general population (P < .001) | For severe events:
|
|||
| Zhang 202024 | Hubei, China | Solid cancer only; 6 received anticancer therapy within 14 d (1 radiation, 5 drug) | 28 | 2.2% of all admitted patients | 21.4% | 35.7% | 28.6% | 53.6% | For severe events:
|
| Dai 202025 | Hubei, China | 96 Solid cancers, 9 hematologic malignancies; 48 with cancer therapy within 40 d (8 surgery, 13 radiation therapy) | 105 Patients with cancer; 536 matched patients without cancer | NA | 19.05% | 9.52% | 11.43% | 34.3% | Death:
|
| Yang 202026 | Hubei, China | 183 Solid tumors, 22 hematologic malignancies; 54 with cancer therapies in the 4 wk prior (31 chemotherapy, 12 targeted therapy, 4 immunotherapy, 9 radiation, 2 surgery) | 205 | NA | 15% | 10% | 20% | Mortality:
|
|
| Rogado 202027 | Madrid, Spain | 45 Solid cancers; 71.1% on active treatment | 45 | 4.2% (vs 0.6% in the overall population) | 0% | 42.2% (vs 13.2% in the overall population) | 64% | Mortality:
|
|
| Mehta 202028 | New York City, USA | 164 Solid cancers, 54 hem. malignancies; 92 with active cancer <1 y (49 on radiation therapy, 47 on drug therapy) | 218 With cancer; 1090 age-matched and sex-matched controls | NA | 10.5% | 20.6% | 28% (14% in control group) | Mortality:
HTN, CAD, CHF, chronic lung disease |
|
| Garassino 202029 | USA, UK, Spain, Italy, France, Switzerland, China, Netherlands | Thoracic malignancies, 151 NSCLC, 29 SCLC, 8 thymomas, 2 carcinoids, 1 mesothelioma; 147 on therapy |
200 | NA | 10% | 6% | 33% | Mortality:
|
|
| Kuderer202030 | USA, Canada, Spain | 758 Solid cancers, 204 hematologic malignancies; 366 with cancer therapy within 4 wk of COVID-19 diagnosis (160 cytotoxic therapy, 72 targeted therapy, 38 immunotherapy, 12 radiation, 2 surgery) | 928 | NA | 14% | 12% | 13% | 26% | 30-D mortality:
|
| Lee 202031 | UK | 693 Solid tumors, 169 hematologic malignancies, 47 unspecified; 518 on cancer therapy within 4 wk of COVID-19 diagnosis (281 chemotherapy, 72 targeted therapy, 44 immunotherapy, 76 radiation, 29 surgery) | 800 | NA | 7% | NA | 28% | 23% (critical, 22%) | Mortality:
|
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disorder; CVC, cardiovascular disease; DM, diabetes mellitus; ECOG, Eastern Cooperative Oncology Group; Hem. malignancy, hematologic malignancy; HR, hazard ratio; HTN, hypertension; ICU, intensive care unit; Met. cancer, metastatic cancer; MV, mechanical ventilation; NA, not available; NSCLC, nonsmall cell lung cancer; OR, odds ratio; SCLC, small cell lung cancer.
Interestingly, calls to prepare for the care of patients with cancer in times of a pandemic were issued over a decade ago and were updated more recently.32 These include preparation for a change in the delivery of cancer care, including type, timing, and setting. Such an approach may shift away from surgery, radiation therapy, and conventional infusion chemotherapy toward therapies that can be given as a tablet or injection. More recently, specific recommendations for the care of patients with cancer in times of COVID-19 have emerged. A key concept is the designation of patients with cancer as high-risk or low-risk for disease progression without cancer therapy and thus at high need or low need for urgent treatment.33,34 This concept of prioritization is depicted in Figure 2.
FIGURE 2.
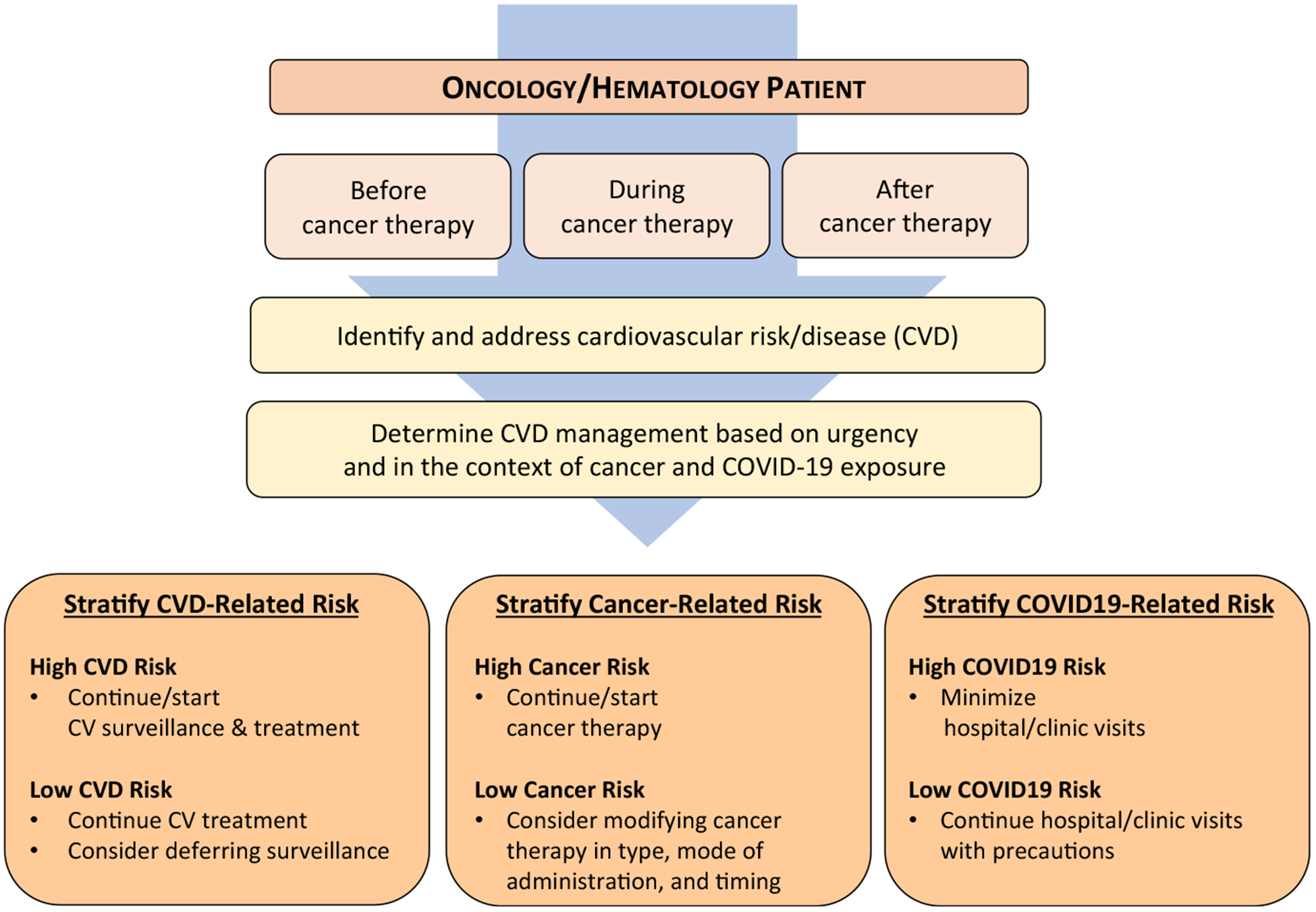
Workflow Outline for Cardio-Oncology in Times of a Viral Pandemic. Initial triage should take cardiovascular (CV) disease (CVD) acuity into consideration as well as the broader scope of the patient’s cancer care and the specific viral pandemic practice environment. COVID-19 indicates coronavirus disease 2019.
Impact of COVID-19 on the Patient With Cancer and CV Disease
Contemporary cancer therapy has expanded beyond traditional chemotherapeutics, radiation therapy, and surgery to include targeted therapies as well as immunotherapy, resulting in a range of potential CV toxicities that require more complex CV monitoring.11,35–37 Specific cancer therapies are associated with worsening CV risk factors, such as hypertension and dyslipidemia, as well as the development of CVD, including heart failure, reduced left ventricular ejection fraction (LVEF), accelerated coronary artery disease, myocardial infarction, increased thrombosis, and (uncommonly) myocarditis. Patients with cancer or those who have been previously treated may be immunocompromised and have increased susceptibility to SARS–CoV-2; however, more than half of the patients reported previously from Wuhan who had cancer were survivors and were not necessarily undergoing active therapy. This suggests that the adverse outcomes in patients with cancer may relate, in part, to a reduced cardiopulmonary reserve or the presence of CVD. Importantly, in terms of acute CVD related to cancer therapy, it remains to be seen whether patients exposed to potentially cardiotoxic cancer therapies are at an even higher risk with COVID-19. Indeed, the CV effects of certain cancer therapies may be unmasked or heightened by the current pandemic, especially when newer cancer therapy has the potential to cause endothelial damage and reduce CV reserve.38 For this very reason, a multidisciplinary C-O approach is needed for patients with cancer during COVID-19.
The central conundrum for C-O care in the era of COVID-19 is how to deliver optimal care with fewer resources and at the lowest (possible) risk. Important questions include the following: 1) Which patients need attention, and by what means? 2) Is there a need to modify published algorithms and protocols? 3) Should there be a switch from CV imaging to serum biomarkers? 4) Which innovative approaches for remote monitoring can be quickly and effectively used? The answers to many of these questions may vary, depending on the local impact of COVID-19 or any other pandemic at the time, and these questions should be approached with vibrant and ongoing communication between oncology, hematology, and C-O providers alike. In fact, it is advisable that the directors of C-O clinics and service lines be proactive in approaching and working out potential new workflows completely integrated with the cancer team (Figs. 2 and 3). Some of these newly developed strategies in response to a pandemic can be found in other areas of cardiology, such as the care of patients with acute coronary syndrome and stroke, the need for cardioversion, and imaging.16,39–41 It is important to emphasize that postponing or neglecting any appropriate care because of the COVID-19 pandemic is as detrimental as not considering potential exposures to COVID-19 and its impact on the patient and the health care system.
FIGURE 3.
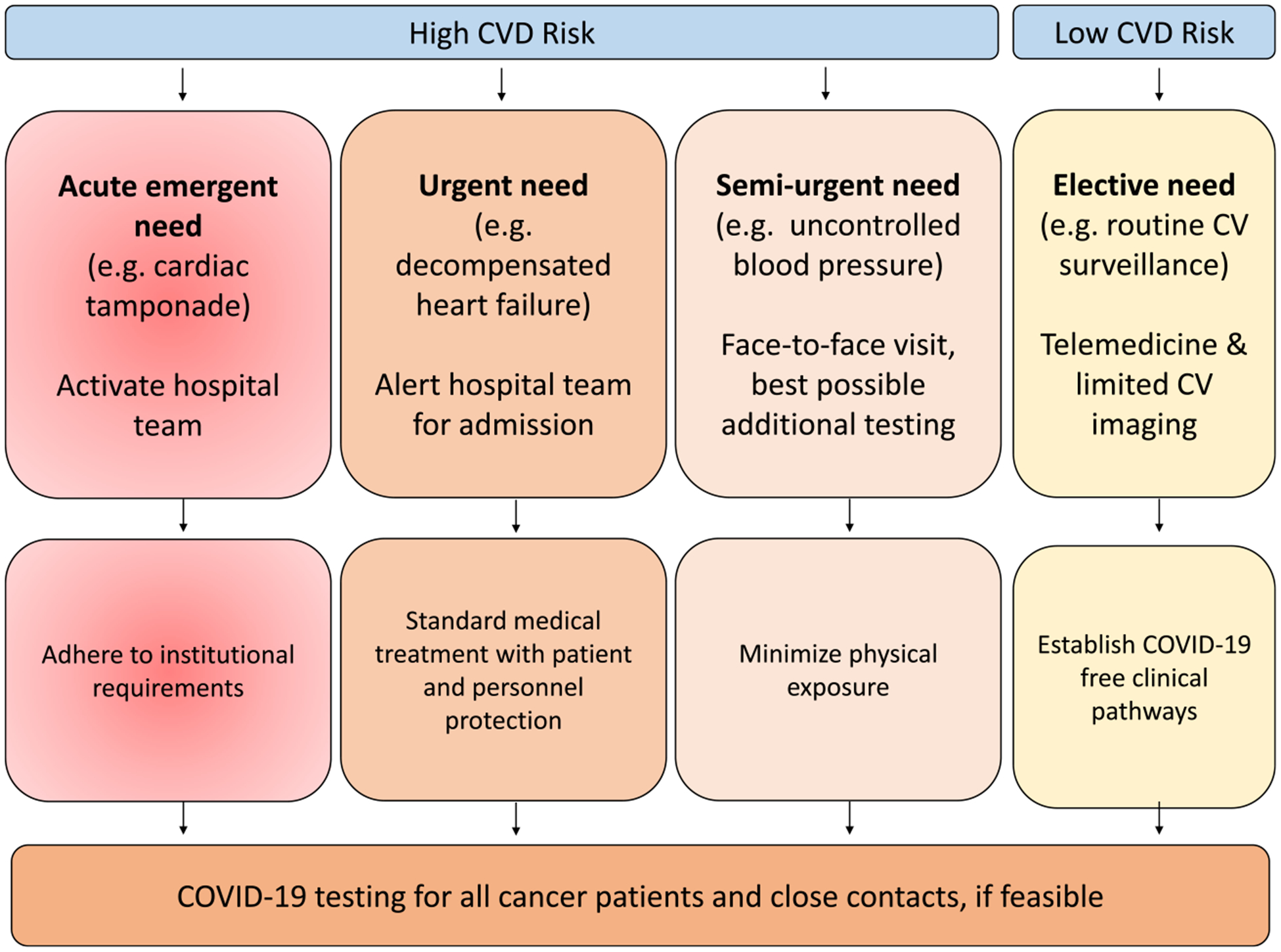
Outline of High-Risk and Low-Risk Cardiovascular (CV) Conditions. All cases should be addressed on an individual basis. The type of clinical encounter is driven by the clinical presentation. Coronavirus disease 2019 (COVID-19) screening should be obtained for any patient to be seen in person and, ideally, for all patients proceeding with cancer therapy. Repeat and serial testing might be required. CVD indicates cardiovascular disease.
General Recommendations
One of the key traits of SARS–CoV-2 is the high level of contagiousness.42–44 The primary route of transmission from person to person is via droplets generated by a cough or sneeze of an infected person. These droplets are propelled through the air, often several meters, and can deposit on the mucous membranes of the mouth, nose, or eyes of individuals nearby. The virus also can spread when a person touches a surface or object contaminated with infectious droplets and then touches his or her mouth, nose, or eye(s). Of note, the virus can survive outside the body for several days, including on common surfaces (cardboard for 24 hours, stainless steel for 48 hours, and plastic for 72 hours).45 Of particular concern is the fact that infected individuals may maintain high viral loads in the upper respiratory tract without any symptoms, leading to asymptomatic viral shedding and transmission. In the medical setting, the risk of viral transmission is particularly high when aerosol-generating procedures are pursued, including nebulizer treatment, highflow oxygen, bronchoscopy, open tracheal suctioning, intubation, extubation, noninvasive positive-pressure ventilation, endoscopy, and transesophageal echocardiography.
A systematic review and meta-analysis concluded that face masks are the most effective measure of protection.46 Health care systems thus should adopt a universal face mask policy with an increased level of respiratory protection for health care personnel in high-risk exposure scenarios. Other measures include eye protection. Physical distancing may be difficult in the office setting, especially in the examination room, but any degree of one meter or more is helpful.46 Along these lines, the indications for close contact, as required for physical examination and certain clinical studies, need to be carefully considered. Hand hygiene, especially hand washing before and after seeing and examining a patient, is key. Soap is highly effective, although evaporating disinfectant dispenser bottles are very convenient.47,48 Universal gloving, as needed (eg, for neutropenic patients), should include only new gloves used once per patient.
Recommendation
Patients with cancer should be shielded as much as possible from infection risks, including those from SARS–CoV-19, by proper hygiene standards in the health care system, such as proper hand washing, cleaning of examination rooms, and cleaning of instruments for clinical examination.
Universal face masking is recommended, especially during periods of high virus spread and/or risk, as with any community exposure to a viral pandemic such as COVID-19.
Depending on clinical exposure risk, personal protection equipment (PPE) for health care personnel needs to be maximized, including N95 masks, eye protection, gowns, and gloves.
Physical distancing is recommended at all times, including limiting close contacts, such as those needed for physical examination and clinical testing, including echocardiography (Echo).
Specific Recommendations
Strategies to Optimize CV Care of Patients With Cancer During a Pandemic
When cardiology input is needed in the care of a patient with cancer, the first consideration should be appropriate triage based on clinical acuity and practice environment (Fig. 2). Patients with CV emergencies still need to be treated as such, with the right protective precautions and resultant workflow modifications (Fig. 3). On the other end of the spectrum, those with routine care needs can be rescheduled or followed using non-face-to-face (virtual) formats. Telehealth (including video consultations) might be the ideal tool to connect physicians and other health care professionals with patients in this setting. The advantages and disadvantages of the various consultations types are summarized in Table 2.
TABLE 2.
Types of Cardio-Oncology Consultations
| TYPE OF CONSULTATION | DESCRIPTION | ADVANTAGES AND DISADVANTAGES |
|---|---|---|
| E-consultation | Virtual chart review: Examine the past medical history, active problem list, ongoing cardiovascular concerns E-messaging: Maintain bidirectional communication between patients and providers, including report of symptoms, vital signs, laboratory test results |
Advantages:
|
| Telephone consultation | Telephone appointment: Requires a dedicated time for a phone call with direct, real-time communication | Advantages:
|
| Video consultation | Live streaming: Provides limited but direct visual communication between the patient and the provider | Advantages:
|
| Face-to-face consultation | Traditional appointment: Allows a direct interaction between the patient and provider, including ability to perform a physical examination | Advantages:
|
Recommendation
During periods of high virus spread and/or risk, C-O consultation before, during, or after cancer therapy should be performed virtually if clinically feasible; in-person consultation should be limited to emergencies for which physical examination is essential in determining clinical management.
Cardiovascular Surveillance
Cardiac imaging plays an important role in treatment decisions for C-O patients and, as a result, at least 2 groups, the European Association of Cardiovascular Imaging and the American Society of Echocardiography, have produced recommendations on precautions, indications, prioritization, and protection for patients and health care personnel during the time of the COVID-19 pandemic (key points are highlighted in Table 3).41,49 A key aspect to consider is not only the frequency of imaging but also the type and duration. Echo involves much closer and longer patient-staff contact than cardiac magnetic resonance imaging, nuclear scans, computed tomographic angiography, or coronary computed tomographic angiography. To decrease exposure time and risk, many imaging centers have incorporated abbreviated protocols to decrease the duration of Echo scanning: the focused cardiac ultrasound study. For instance, if the only interest is in cardiac function or LVEF, this can commonly be obtained in 5 minutes, even when both right and left ventricular function are assessed, which is recommended in the context of COVID-19.50 A summary of the advantages and disadvantages of the various cardiac imaging techniques is provided in Table 4.51
TABLE 3.
Key Points With Regard to Cardiac Imaging in Response to Coronavirus Disease 2019 (COVID-19)
General recommendations
|
Specific considerations
|
Modified from: Skulstad H, Cosyns B, Popescu BA, et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020; 21:592–59841; and Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak. J Am Coll Cardiol. 2020; 75:3078–3084.49
TABLE 4.
Strengths and Limitations of Different Imaging Modalities for Diagnosis and Monitoring of Cardiotoxicitya
| IMAGING MODALITY | VOLUME/FUNCTION | TISSUE/MASS | MYOCARDITIS/INFLAMMATION | VALVE DISEASE | PERICARDIAL DISEASE | CORONARY DISEASE/ISCHEMIA | RADIATION EXPOSURE | REPRODUCIBILITY ACCURACY | COST | AVAILABILITY | BEDSIDE TEST OPTION | EXAMINATION TIME | VIRAL INFECTION RISK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2D Echo | + | + | 0 | +++ | ++ | 0 | None | + | + | +++ | +++ | + to +++ | ++ |
| TEE | + | + | 0 | +++ | + | 0 | None | ++ | ++ | ++ | +++ | + to ++ | +++ |
| CMR | +++b | +++b | +++ | ++ | +++ | +++ | None | +++ | +++ | ++ | 0 | ++ | + |
| Nuclearc | + + | + | + | 0 | ++ | ++ | +++ | ++ | + | ++ | 0 | + | + |
| CCTA | + | + | 0 | + | ++ | +++d | +/++ | +++ | ++ | ++ | 0 | +++ | + |
Abbreviations: 2D Echo, 2-dimensional echocardiography; CCTA, coronary computed tomography angiogram; CMR, cardiac magnetic resonance; TEE, transesophageal echocardiography.
Modalities are scored as follows: +++, excellent diagnostic accuracy or features/high cost; ++, intermediate diagnostic accuracy or features/intermediate cost; +, reasonable diagnostic accuracy or features/low cost; 0, unable to diagnose.
This is the established gold standard.
Nuclear imaging modalities include single-photon emission computed tomography and multigated acquisition.
CCTA is the only noninvasive test that provides anatomic information with regard to the presence of coronary disease, and it also allows for imaging of the lung parenchyma, which shows characteristic changes in coronavirus disease 2019 and in the pulmonary vasculature and aorta (triple rule out); furthermore, it can identify pericardial effusion.
Modified from: Seraphim A, Westwood M, Bhuva AN, et al. Advanced imaging modalities to monitor for cardiotoxicity. Curr Treat Options Oncol. 2019; 20:73.51
Previous algorithms on the cardiac safety and monitoring of patients with cancer who are exposed to potentially cardiotoxic medications have varied greatly, but a contemporary and comprehensive consensus has recently been published by the European Society of Medical Oncology (ESMO).35 The American Society of Clinical Oncology (ASCO) had previously published a guideline that was focused specifically on cardiac dysfunction in survivors of cancer therapy, particularly those treated with anthracyclines and trastuzumab.52 During the current COVID-19 pandemic, the requirement for social distancing has broad impact within medical care to the degree that virtual and/or telemedicine visits are emphasized. In previous ESMO recommendations, a cardiac serum biomarker approach using troponin I was considered a valid alternative to echocardiographic imaging during anthracycline-based therapy.53 This strategy is certainly appealing in principle, but efforts to validate this approach in a broad range of patients receiving treatment for cancer has varied significantly based on malignancy and type of cancer therapy.54,55 In addition, the length and timing of the screening is important, as outlined for natriuretic peptides (NPs), ie, B-type NP (BNP) and N-terminal pro-BNP (NT-proBNP), which have been successfully used in patients with multiple myeloma (MM) undergoing treatment with carfilzomib.56 For cardiotoxicity surveillance measures, serum biomarker measurements would appear to provide a safe and cost-effective alternative to cardiac imaging, considering the potential exposure risks of patients and health care providers to SARS–CoV-19 (Fig. 4). Serial cardiac biomarker assessments allow for the recognition of trends over time and reassurance when no change is noted. Even more, taking the high negative predictive value into consideration, patients with values below the institutional laboratory cutoff for BNP, NT-proBNP, and cardiac troponin assays (below the 99th percentile) would be expected to present a rather low-risk constellation. Thus a cardiac biomarker-based surveillance approach holds merit, particularly to identify those patients who are not expected to benefit from cardiac imaging to such a degree that justifies the potential risk of SARS–CoV-19 exposure. The section below is based on the current ESMO consensus document with updated modifications that are in line with alterations in medical care necessitated by the current and any possible future pandemics (Table 5).27
FIGURE 4.
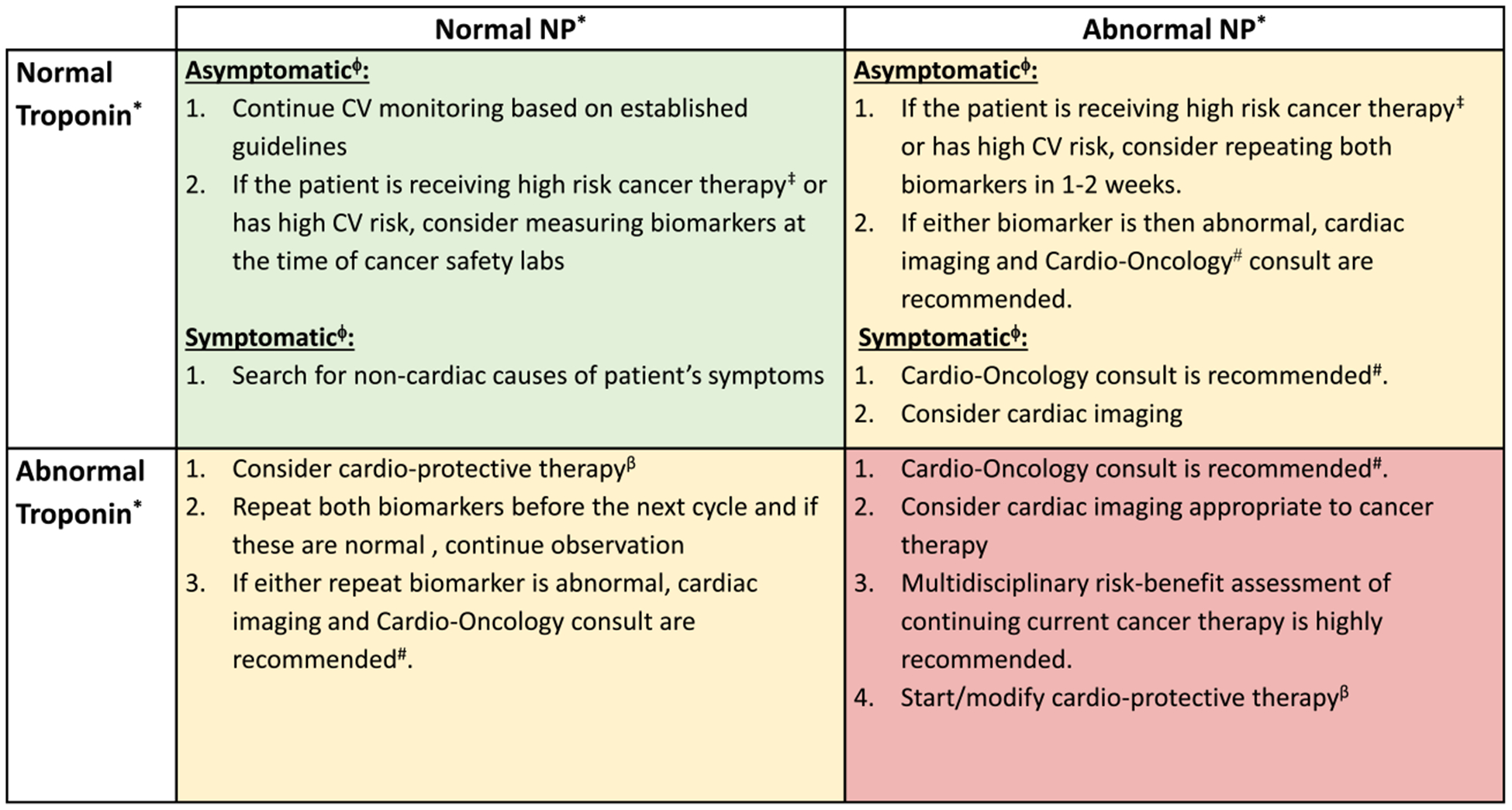
Action Grid for Cardiac Biomarker Profile. This is an outline of recommendations based on the constellation of troponin and natriuretic peptide values and within the context of the clinical presentation. CV indicates cardiovascular. *NP indicates natriuretic peptides (B-type natriuretic peptide [BNP] and N-terminal pro-BNP [NT-proBNP]); troponin, troponin I or troponin T (normal vs abnormal levels are based on the institutional reference range). ϕAsymptomatic indicates the absence of symptoms of heart failure (HF) or acute coronary syndrome (ACS); symptomatic, symptoms of HF or ACS. #A consultation can happen in person or by telemedicine. Discussion should include initiation of cardioprotective therapy. ‡High-risk cancer therapy is that known to be associated with HF or left ventricular dysfunction (LVD). βCardioprotective therapy indicates that an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker and/or carvedilol with or without a statin (if the patient is receiving anthracycline-containing chemotherapy) is recommended.
TABLE 5.
Best Practices for the Prevention and Management of Cardiac Complications in Patients With Cancer in the Coronavirus Disease 2019 (COVID-19) Era
| 2020 ESMO CONSENSUS RECOMMENDATION | ICOS CONSENSUS RECOMMENDATION IN THE COVID-19 ERA |
|---|---|
| 1. General principles | |
| 1.1. Screening for known CV risk factors in patients with cancer is recommended; treatment of identified CV risk factors according to current guidelines is recommended (LOE, I; GOR, A) | 1.1. Continuing approval without change |
| 1.2. Many types of cancer therapy, especially mediastinal and left-sided chest radiation and certain chemotherapy and targeted agents, can substantially affect the heart and vascular system, and it is recommended that CV safety be monitored (LOE, I; GOR, A) | 1.2. Continuing approval without change |
| 1.3. Close and early collaboration between cardiologists, oncologists, hematologists, and radiation oncologists is recommended to ensure lifelong CV health and to avoid unnecessary discontinuation of cancer therapy (LOE, III; GOR, C) | 1.3. Continuing approval without change |
| 2. Baseline CV risk assessments (precancer therapy) | |
| 2.1. Routine use of cardiac biomarkers (cardiac troponins I or T [TnI or TnT] and B-type natriuretic peptide [BNP], or N-terminal pro-BNP [NT pro-BNP]) for patients undergoing potentially cardiotoxic chemotherapy is not well established; however, for high-risk patients (with preexisting, significant CVD) and those receiving high doses of cardiotoxic chemotherapy such as an anthracycline, baseline measurement of such cardiac biomarkers should be considered (LOE, III; GOR, A) | 2.1. Continuing approval without change |
| 2.2. For patients with a cancer diagnosis that requires treatment with a potentially cardiotoxic treatment, a baseline electrocardiogram (ECG), including measurement of a heart rate-corrected QT interval (QTc), is recommended (LOE, I; GOR, A): QTc should be calculated by either of the 2 most standardized methods—Bazett or Fridericia—and the comparative measurements during treatment should all be done using the same method | 2.2. Continuing approval without change |
| 2.3. In patients scheduled to undergo cancer therapy associated with heart failure or left ventricular (LV) dysfunction, baseline evaluation of left ventricular ejection fraction (LVEF), with strain imaging when possible, and diastolic function according to accepted comprehensive imaging practice is recommended (LOE, I; GOR, A) | 2.3. Transthoracic echocardiogram (with or without strain imaging) and cardiac MRI (with or without strain) are the recommended imaging modalities |
| 3. Primary prevention therapy | |
| 3.1. In patients with a normal LVEF scheduled to undergo cancer therapy with known cardiotoxic agents and risk factors for cardiac toxicity, prophylactic use of angiotensin-converting enzyme inhibitors (ACE-Is) or, alternatively, angiotensin receptor blockers (ARBs), and/or selected β-blockers (BBs) may be considered to reduce the risk of cardiotoxicity; dexrazoxane has been validated as a cardioprotectant in selected populations who are receiving anthracycline-based chemotherapy (LOE, II; GOR, B) | 3.1. Continuing approval without change |
| 3.2. Patients with evidence of hyperlipidemia may benefit from treatment during active cancer therapy, especially cardiotoxic chemotherapy (LOE, II; GOR, C) | 3.2. Continuing approval without change |
| 4. During cancer treatment: Cardiac safety surveillance | |
| 4.1. The following general principles are recommended for medical imaging in patients with cancer at risk for cardiac complications particularly for the periodic assessment of LV systolic function: | 4.1. Continuing approval without change |
| 4.1.1. Highly reproducible, quantitative volumetric, nonirradiating imaging with quality control is recommended (quantitative 2-dimensional [2D] and 3-dimensional [3D] echocardiography, and cardiac MRI) (LOE, I; GOR, A) | |
| 4.1.2. For each patient, the same imaging modality at the same facility is highly recommended for serial testing (LOE, I; GOR, A) | |
| 4.1.3. LV global longitudinal strain (GLS) imaging may be considered, when available, for baseline and serial LV systolic function monitoring (LOE, III; GOR, C) | |
| 4.2. Asymptomatic patients receiving anthracycline-based chemotherapy with no cardiac biomarker or imaging abnormalities should undergo surveillance for risk stratification and the early detection of cardiac toxicity | 4.2. Measurement of cardiac biomarkers (TnI or TnT and BNP, or NT-proBNP) should be considered in conjunction with cancer treatment safety laboratories if obtained with each cycle of chemotherapy; if either cardiac biomarker (TnI or TnT and BNP or NT-proBNP) is abnormal at the assay-specific cutoff and/or the patient becomes symptomatic, reassessment of LVEF with or without GLS should be performed using either 2D or 3D echocardiography, or cardiac MRI |
| 4.2.1. Periodic (every 3–6 wk or before each cycle) measurement of TnI or TnT and BNP or NT-proBNP using the same institutional laboratory using an acceptable 99% upper limit of normal reference range as the threshold for abnormal (LOE, III; GOR, C) | |
| 4.2.2. Reassessment of LVEF and GLS (when possible) following the general imaging principles is recommended after a cumulative dose of 250 mg/m2 of doxorubicin or its equivalent anthracycline, after approximately each additional 100 mg/m2 (or approximately 200 mg/m2 of epirubicin) beyond 250 mg/m2, and at the end of therapy, even if the cumulative dose is <400 mg/m2 (LOE, I; GOR, A) | |
| 4.3. Aligned to the current recommendations by the FDA for asymptomatic, nonmetastatic patients undergoing adjuvant trastuzumab treatment, routine surveillance, consisting of cardiac imaging every 3 mo, should be considered for the early detection of cardiac toxicity; however, the effective-ness of this strategy in patients at low CV risk with no evidence of early LV dysfunction has not been demonstrated (LOE, II; GOR, B) | 4.3. In patients who remain asymptomatic and who have normal or less than a 50% increase in cardiac biomarkers during monitoring, cardiac imaging is not recommended |
| 4.4. Cardiac biomarker assessment may be considered as a valuable tool for cardiac safety surveillance in patients receiving adjuvant anti-HER2–based treatment (LOE, III; GOR, C) | 4.4. Cardiac imaging should be performed only when patients become symptomatic or have abnormal cardiac biomarkers |
| 4.5. Asymptomatic patients receiving anti-HER2–based treatment (trastuzumab, pertuzumab, T-DM1) for metastatic disease should have general surveillance for cardiac toxicity that may consist of periodic physical examination, cardiac biomarkers, and/or cardiac imaging (LOE, I; GOR, B) | 4.5. Continuing approval without change; the threshold to obtain cardiac imaging should be higher in the COVID-19 era, but continued surveillance for the detection of new symptoms or elevation of cardiac biomarkers is unchanged |
| 4.6. In patients receiving cancer therapeutics associated with a risk of systemic hypertension, especially anti-VEGF-based therapy, establishment of a baseline blood pressure measurement and serial blood pressure monitoring is recommended along with surveillance for the early detection of CV toxicity that may consist of periodic cardiac examination, cardiac biomarkers, and/or cardiac imaging (LOE, I; GOR, A) | |
| 5. During cancer treatment: Asymptomatic, new laboratory abnormalities (or preclinical toxicity) | |
| 5.1. In asymptomatic patients receiving treatment with anthracyclines who have an LVEF decrease of ≥10% from baseline to <50% or decrease in LVEF to ≥40% but <50%, the following evaluations are recommended (LOE, III; GOR, A): | 5.1. This recommendation remains the same, except for repeating LVEF with or without GLS after every other dose of anthracycline-based chemotherapy; it is recommended to monitor patients by checking cardiac biomarkers (TnI or TnT and BNP or NT-proBNP) every other cycle, and reassessing LV function with imaging only if either biomarker is abnormal or the patient develops heart failure symptoms. The determination for the presence of heart failure symptoms can be obtained by telemedicine |
| 5.1.1. Cardiology consultation (preferably a cardio-oncology specialist) | |
| 5.1.2. Consider initiation of cardioprotective treatment (ACE-I or ARB with or without BB, and perhaps a statin), if not already prescribed | |
| 5.1.3. Consider measuring cardiac biomarkers (TnI or TnT and BNP or NT-proBNP) and perform a cardiac-focused physical examination after each dose of anthracycline | |
| 5.1.4. Repeat LVEF with or without GLS measurement after every other dose of anthracycline-based chemotherapy | |
| 5.1.5. If further anthracycline therapy is planned, the benefit-risk assessment of continued anthracycline-based therapy as well as options of nonanthracycline regimens should be discussed, and there should be consideration for the use of dexrazoxane and/or liposomal doxorubicin | |
| 5.2. In asymptomatic patients receiving treatment with trastuzumab who have an LVEF decrease of ≥10% from baseline to <50% or have a decrease in LVEF to ≥40% but <50%, the following evaluations are recommended (LOE, III; GOR, A): | 5.2. If the baseline LVEF with or without GLS is within normal range and the patient is asymptomatic while on treatment with trastuzumab, cardiac biomarkers (TnI or TnT and BNP or NT-proBNP) can be measured every other cycle; cardiac imaging with either echocardiography or cardiac MRI is recommended only if any cardiac biomarker is abnormal or the patient develops heart failure symptoms |
| 5.2.1. Cardiology consultation (preferably a cardio-oncology specialist) | |
| 5.2.2. Consider initiation of cardioprotective treatment (ACE-I or ARB with or without BB), if not already prescribed | |
| 5.2.3. Consider measuring cardiac biomarkers (TnI or TnT and BNP or NT-proBNP) at a suggested interval of every 4–6 wk and periodic cardiac-focused physical examination for ongoing monitoring of cardiac toxicity | |
| 5.2.4. Repeat LVEF with or without GLS measurement within 3–6 wk after holding trastuzumab, and, if LVEF with or without GLS has normalized, trastuzumab therapy can be resumed | |
| 5.3. In asymptomatic patients receiving treatment with any cardiotoxic cancer therapy who have a normal LVEF but a decrease in average GLS from baseline assessment (≥12% relative decrease or ≥5% absolute decrease), the following evaluation and treatment should be considered (LOE, III; GOR, B): | 5.3. Continuing approval without change |
| 5.3.1. Consider initiation of cardioprotective treatment (ACE-I or ARB with or without BB), if not already prescribed | |
| 5.3.2. Repeat LVEF with or without GLS measurement every 3 mo, or sooner, if cardiac symptoms develop | 5.3.2. Cardiac imaging should be performed in patients with abnormal cardiac biomarkers or in those who develop cardiac symptoms |
| 5.3.3. Consider measuring cardiac biomarkers (TnI or TnT and BNP or NT-proBNP) as needed based on the cancer treatment’s risk of cardiac toxicity | |
| 5.3.4. Life-saving cancer treatment should not (?) be altered solely based on changes in LV strain | |
| 5.4. In asymptomatic patients undergoing treatment with cardiotoxic anticancer therapy and an have elevation in cardiac troponin, the following measures should be considered (LOE, III; GOR, C): | 5.4. Continuing approval without change |
| 5.4.1. Cardiology consultation, preferably a cardio-oncology specialist | |
| 5.4.2. Consider LVEF and GLS assessment with echocardiography | |
| 5.4.3. Appropriate evaluation to exclude ischemic heart disease as a comorbidity | |
| 5.4.4. Consider initiation of cardioprotective treatments (ACE-Is, ARBs and/or BBs), if not already prescribed | |
| 5.4.5. Consider initiation of dexrazoxane in patients undergoing anthracycline-based chemotherapy | |
| 5.4.6. It is possible that anticancer therapy may be continued without interruption if only mild elevations in cardiac biomarkers occur without significant LV dysfunction | |
| 6. During cancer treatment: Clinical cardiac dysfunction | |
| 6.1. In patients with an abnormal LVEF <50% but ≥40%, medical therapy with an ACE-I (or ARB) and/or BB is recommended before starting potential cardiotoxic treatment (LOE, I; GOR, A) | 6.1. Continuing approval without change |
| 6.2. For those with an LVEF <40%, anthracycline therapy, in particular, is not recommended unless there is no other effective alternative cancer treatment option (LOE, IV; GOR, A) | 6.2. Continuing approval without change |
| 6.3. For a patient receiving treatment with any cardiotoxic agent who presents with potentially cardiac-related but unexplained signs and symptoms, such as (but not limited to) sinus tachycardia, rapid weight gain, dyspnea, peripheral edema, or ascites, it is recommended to obtain a cardio-oncology consultation, reassess LVEF with or without GLS, and potentially measure cardiac biomarkers (LOE, III; GOR, A) | 6.3. It is recommended to obtain cardiac biomarkers and discuss with a cardio-oncologist if cardiac imaging is warranted |
| 6.4. For a patient receiving treatment with trastuzumab (or any HER2-targeted molecular therapy) who has signs and symptoms of heart failure, or for an asymptomatic patient with an LVEF <40%, the same assessments as those for an LVEF ≥40% are recommended; in addition, trastuzumab (or any HER2-targeted molecular therapy) should be held until the cardiac status has stabilized, and a discussion regarding the risk/benefits of continuation should be held with the multidisciplinary team and the patient (LOE, I; GOR, A) | 6.4. It is recommended to obtain cardiac biomarkers and discuss with a cardio-oncologist if cardiac imaging is warranted |
| 6.5. For a patient in whom trastuzumab (or any HER2-targeted molecular therapy) has been interrupted, whose LVEF has increased to ≥40%, and/or whose signs and symptoms of heart failure have resolved, resumption of trastuzumab (or any HER2-targeted molecular therapy) should be considered, along with the following recommendations (LOE, III; GOR, B): | 6.5. Continuing approval without change |
| 6.5.1. Continued medical therapy for heart failure and ongoing cardiology care | |
| 6.5.2. Periodic cardiac biomarker (BNP or NT-proBNP) assessment | 6.5.2. Periodic cardiac biomarker (BNP or NT-proBNP) assessment should be performed at a frequency determined by the treating physician considering the patient’s clinical presentation and risk of cardiac toxicity |
| 6.5.3. Periodic LVEF assessments during ongoing treatment | 6.5.3. LVEF assessment is recommended only in the setting of an abnormal cardiac biomarker and/or worsening symptoms of heart failure |
| 6.6. For a patient in whom trastuzumab (or any HER2-targeted molecular therapy) has been interrupted, whose signs and symptoms of heart failure do not resolve, cardiac biomarker does not normalize, and/or LVEF remains <40%, resumption of trastuzumab may be considered if no alternative therapeutic options exist; the risk-benefit assessment of prognosis from cancer versus heart failure should be discussed with the multidisciplinary team and the patient (LOE, IV; GOR, C) | 6.6. Continuing approval without change |
| 6.7. For a patient receiving treatment with sunitinib (or any other anti-VEGF based therapy) with signs and symptoms of heart failure, assessment of blood pressure control is recommended and measurement of LVEF and/or cardiac biomarkers should be considered; in addition, sunitinib (or any other anti-VEGF–based therapies) should be interrupted until the appropriateness of reinstituting this therapy has been fully assessed (LOE, III; GOR, A) | 6.7. It is first recommended to assess blood pressure control and cardiac biomarkers; if cardiac biomarkers are abnormal and/or symptoms of heart failure worsen despite achieving adequate blood pressure control, measurement of LVEF is then recommended; early involvement of a cardio-oncologist is strongly recommended to guide choice of antihypertensive therapy |
| 7. Survivors of cancer therapy (posttreatment) | |
| 7.1. For asymptomatic patients who have been treated with cardiotoxic agents and have normal cardiac function, periodic screening for the development of new asymptomatic LV dysfunction with cardiac biomarkers and, potentially, cardiac imaging should be considered at 1-y and 2-y posttreatment, and periodic reassessment thereafter should be considered (LOE, III; GOR, B) | 7.1. A thorough history, including assessment of CV risk factor control, should be performed along with cardiac biomarkers; these should then guide the decision about assessing LVEF with or without GLS; early consultation with a cardiologist or cardio-oncologist is strongly recommended; if prior imaging was significantly abnormal (ie, valvular or pericardial disease), the interval for reassessment should not be excessively delayed |
| 7.2. For patients who developed asymptomatic LV dysfunction or heart failure because of trastuzumab (or any other HER2-targeted molecular therapy), anthracycline, or other cancer therapy, CV care, including medical treatment with ACE-I (or ARB) and/or BB and regular CV evaluation (eg, annually, if asymptomatic), should be continued indefinitely, regardless of improvement in LVEF or symptoms; any decision to withdraw guideline-based medical therapy should only be done after a period of stability, no active cardiac risk factors, and no further active cancer therapy (LOE, II; GOR, B) | 7.2. Decision about withdrawing any cardiac medications should first be discussed with the patient’s cardio-oncologist |
| 7.3. For patients with a history of mediastinal or chest radiation, evaluation for coronary artery disease and myocardial ischemia as well as valvular heart disease is recommended, even if asymptomatic, starting at 5 y posttreatment and then at least every 3–5 y thereafter (LOE, I; GOR, A) | 7.3. Any routine CV imaging should be postponed until COVID-19 restrictions are suspended as long as the patient is asymptomatic; symptom awareness, optimal CV risk factor management, and occasional cardiac biomarker assessment should continue at the recommended intervals |
| 8. Immune checkpoint inhibitor-associated CV toxicity | |
| 8.1. For patients who develop new CV symptoms or are incidentally noted to have any arrhythmia, conduction abnormality on ECG, or LVSD on echocardiogram while undergoing (or after recent completion) of immune checkpoint inhibitor (ICI) therapy, further appropriate work-up (ECG, troponin, BNP or NT-pro-BNP, C-reactive protein, viral titer, echocardiogram with GLS, cardiac MRI) for ICI-associated CV toxicity, particularly myocarditis and other common differential diagnoses, should be carried out promptly (LOE, IV; GOR, C) | 8.1. Continuing approval without change |
| 8.2. Endomyocardial biopsy for diagnosis should be considered if the diagnosis is highly suspected with otherwise negative work-up (LOE, IV; GOR, C) | 8.2. Continuing approval without change |
| 8.3. With either suspicion or confirmation of ICI-associated myocarditis, further therapy with ICIs should be withheld, and high-dose corticosteroids (methylprednisolone 1000 mg/d followed by oral prednisone 1 mg/kg/d) should be initiated promptly; corticosteroids should be continued until resolution of symptoms and normalization of troponin, LV systolic function, and conduction abnormalities (LOE, IV; GOR, C) | 8.3. Continuing approval without change |
| 8.4. For steroid-refractory or high-grade myocarditis with hemodynamic instability, other immunosuppressive therapies, such as antithymocyte globulin, infliximab (except in patients with HF), mycophenolate mofetil, or abatacept, should be considered (LOE, IV; GOR, C) | 8.4. Continuing approval without change |
| 8.5. For patients with cardiomyopathy and/or HF, appropriate guideline-directed medical therapy and hemodynamic support should be provided as indicated (LOE, IV; GOR, C) | 8.5. Continuing approval without change |
| 8.6. For patients with atrial or ventricular tachyarrhythmia or heart block, appropriate medical and supportive care should be provided as indicated (LOE, IV; GOR, C) | 8.6. Continuing approval without change |
| 8.7. ICI therapy should be permanently discontinued with any clinical myocarditis; the decision regarding restarting ICI therapy in the absence of alternative available antineoplastic therapy needs to be individualized with multidisciplinary discussion considering the cancer status, response to prior therapy, severity of cardiotoxicity, regression of toxicity with immunosuppressive therapy, and patient preference after weighing the risks and benefits; if ICI therapy needs to be restarted, monotherapy with an antiprogrammed cell death protein 1 (anti-PD-1) agent might be considered with very close surveillance for cardiotoxicity development (LOE, V; GOR, C) | 8.7. Continuing approval without change |
| Level of evidence (LOE) |
|
| Grading of recommendation (GOR) |
|
Abbreviations: CV, cardiovascular; ESMO, European Society of Medical Oncology; FDA, US Food and Drug Administration; HF, heart failure; ICOS, International Cardio-Oncology Society; LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; MRI, magnetic resonance imaging; T-DM1, trastuzumab emtansine. Modified from Rogado J, Obispo B, Pangua C, et al. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin Transl Oncol. Published online May 25, 2020.27
Recommendations
All cardiac imaging should be reduced in frequency to that which is critical and necessary for clinical decision making.
When performed, the cardiac imaging technique that addresses the clinical question in the most effective and efficient manner should be used, decreasing exposure time and risk as much as possible; modified, focused cardiac imaging protocols (eg, focused cardiac ultrasound study) are encouraged, especially for Echo.
A cardiac biomarker-based approach, using cardiac troponins and/or NPs, in combination with accurate symptom assessment should allow adequate monitoring of cardiac safety to avoid unnecessary cardiac imaging.
Anthracycline-Based Therapy
Baseline (pretherapy) assessment of cardiac function in an asymptomatic patient rarely leads to withholding of cancer therapy. However, the baseline LVEF evaluation does serve the important purpose of a point of reference in the event of evolving cardiac dysfunction/heart failure and to establish a causal association with cancer therapy. This is particularly true in patients considered at high risk for cardiotoxicity. According to ASCO practice guidelines, this includes 3 groups of patients: 1) those receiving either ≥250 mg/m2 of doxorubicin (or its equivalent), or radiation therapy ≥30 grays in which the heart is in the treatment field, or any dose combination of anthracycline and chest radiation therapy; 2) those receiving sequential anthracycline and trastuzumab therapy; and 3) those receiving anthracycline or trastuzumab therapy who are either aged ≥60 years or have ≥2 CV risk factors, or an LVEF ≤55%, or a history of myocardial infarction, or moderate or greater valvular heart disease.52 To document evidence of substantial clinical cardiotoxicity, early recognition of a decline in LVEF to <50%, even if asymptomatic (American College of Cardiology/American Heart Association stage B heart failure), is important in terms of timely initiation of cardioprotective therapy as well as overall prognosis.57,58 As such, current consensus guidelines support cardiac follow-up evaluations 6 to 12 months in high risk patients, especially after full completion of anthracycline-based cancer therapy.52 In the COVID era, this practice should remain although the timing of surveillance imaging may vary based on availability and safety.
Some data indicate that a persistent increase in cardiac biomarkers, including cardiac troponin and NPs, measured repeatedly during (cardiac troponins in particular) and after (NPs) anthracycline administration, is associated with a subsequent decline in cardiac function over time and thus may serve as a gatekeeper.55,59 Subject to further studies, cardiac biomarkers drawn during routine laboratory assessments in the context of oncology/hematology care may help risk-stratify patients with a more urgent need for cardiac imaging.
Recommendation
Routine precancer therapy cardiac function assessment should continue to be performed in individuals at high risk (eg, ASCO guidelines for high risk,52 prior CVD, symptoms of cardiac dysfunction), whereas routine assessment of cardiac function (although recommendable) could be deferred otherwise.
Routine postanthracycline therapy imaging should still be considered in those with heart failure symptoms, prior CVD, multiple CV risk factors, and persistent elevation in serum cardiac biomarkers or if there is a need to continue anthracycline therapy with doses exceeding ≥250 mg/m2 of doxorubicin (or its equivalent). For cancer survivors beyond the acute period, all routine cardiac surveillance should be postponed unless patients have a history of or express new signs and symptoms of heart failure.
Human Epidermal Receptor-2–Directed Therapy
Similar to anthracyclines, trastuzumab (and other human epidermal growth factor receptor 2 [HER2]-based therapies, such as pertuzumab) carry a black-box warning for cardiotoxicity, and LVEF assessment is recommended before and during every 3 months therapy in all settings, ie, neoadjuvant, adjuvant, and metastatic, per the package insert. The role of cardiac biomarkers as substitutes for cardiac function imaging has not been well defined in patients with breast cancer undergoing HER2-directed therapy. Cardiac troponin elevations related to HER2-directed therapy are most commonly noted early after anthracycline exposure.60 The absence of troponin elevation makes irreversible LVEF declines very unlikely but cannot exclude the possibility that reductions in LVEF may occur. The published literature on NPs in patients with breast cancer actively undergoing trastuzumab therapy is not robust enough to endorse its complete substitution for cardiac imaging surveillance during standard care.35,62–74 However, there is substantial experience that serial use of either BNP or NT-proBNP can allow for adequate cardiac safety assessment during this time.66 As stated previously, there is evidence in the general population that NPs have high negative predictive value for excluding heart failure, and assessing NPs in an asymptomatic patient may allow deferring any cardiac imaging in a high-risk COVID-19 environment.61,66
Recommendation
Routine pretherapy cardiac function assessment should continue to be performed in individuals at high risk (eg, ASCO guidelines for high risk,52 prior CVD, symptoms of cardiac dysfunction), whereas routine assessment of cardiac function (although recommendable) could be deferred otherwise.
During therapy with HER-2–based treatment, cardiac biomarkers may be useful to identify those patients who do not require cardiac imaging to establish cardiac safety. In patients at low CV risk who remain asymptomatic and have stable or normal cardiac biomarkers during monitoring, cardiac function imaging may be deferred.66,75
Tyrosine Kinase Inhibitors Inhibiting the Vascular Endothelial Growth Factor Pathway
Consensus recommendations, such as the 2014 American Society of Echocardiography statement, outlined that, for various tyrosine kinase inhibitors (TKIs) associated with potential cardiotoxicity, a strategy for LVEF assessment should be undertaken.76 For vascular endothelial growth factor signaling pathway inhibitors (VSPs), the primary issue is hypertension management.77,78 The use of NPs has been proposed as a more relevant tool for identifying those at risk of CV events with VSP inhibitor-based therapy.79,80 In times of a viral pandemic, home-based (remote care, including telemedicine) approaches are preferred to obtain vital signs, especially serial (up to daily) blood pressure measurements in any patient on VSP-based TKIs and to focus attention on the medical management of hypertension.
Recommendation
For patients receiving VSP inhibitors, serial (daily) home blood pressure monitoring is paramount, and the periodic use of NPs can effectively screen for those patients at risk for the development of heart failure. Routine cardiac function imaging is not recommended.
Proteasome Inhibitors
Carfilzomib, an irreversible inhibitor of the proteasome, is a drug with a notable risk of cardiotoxicity, especially in patients with relapsed MM.56,81 Interestingly, echocardiography (LVEF) is not of high value, and risk-benefit analysis does not support its use in the COVID-19 era for screening.56,81 In contrast, there is demonstrated value in the measurement of NPs, even as early as mid-first cycle.56 CVD is highly prevalent in the MM population and is associated with adverse events that affect long-term outcomes, including those after a stem cell transplantation.82 Patients with amyloidosis (light-chain disease [AL]) with or without MM are commonly treated with bortezomib, ixazomib, and (less commonly) carfilzomib, and these patients frequently have complex CVD as well as multiple CV complications during therapy.83 The use of cardiac biomarkers is essential in the AL amyloidosis population, and these cardiac biomarkers are used to stage and prognosticate in this disease.84 As a result of the high prevalence of CVD in patients with MM and AL amyloidosis, these patients are considered high-risk for the development of cardiac adverse events. Furthermore, cardiac biomarkers are an essential and proven tool for assessing cardiac safety during treatment in these patients, whereas cardiac imaging is not apparently useful.
Recommendation
Cardiac biomarker assessments before and during cancer treatment are essential components of ensuring cardiac safety in patients with MM and AL amyloidosis. The utility of cardiac imaging for early detection of CV adverse events with ongoing cancer treatment in these patients is not established or recommended.
Immune Checkpoint Inhibitors
The use of immune checkpoint inhibitors (ICIs) has expanded significantly but may be associated with immune-related adverse events (irAEs). Although one of the least common irAEs, myocarditis with an ICI is one of the most fatal.37,85 Different degrees (high-level and lowlevel) of lymphohistiocytic myocardial inflammation can be seen, and the exact mechanisms of this presentation are not yet defined.86 Confirmation of the diagnosis can be challenging because neither magnetic resonance imaging nor endomyocardial biopsy is sufficiently sensitive, and a diagnosis is often made based on the overall clinical picture (in keeping with proposed consensus definitions).37,87,88 As SARS–CoV-2 can directly infect the myocardium, with a resultant inflammatory response, SARS–CoV-2 should be considered in the differential diagnosis of a patient with concern for ICI-associated myocarditis.13,14 There are no data to suggest caution for the continued use of ICIs during this COVID-19 pandemic, especially considering the dramatic cancer responses and overall safety profiles that have been reported with these therapies in previously recalcitrant cancer conditions.89,90 Persistent viral infections or excessive viral antigen load may cause T-cell exhaustion, with increased expression of PD-1 and PD-L1. In this setting, the effect of ICI directed against PD-1/PD-L1 is unknown. However, in contradistinction, supporting the immune response with a CTLA-4 agonist has been shown to be helpful in other models of acute infection. Although various CV adverse events have been reported with ICI therapy, including serious rhythm issues, the optimal strategy for monitoring cardiac safety before, during, and after ICI therapy for cancer has not been standardized or recommended.37,38,91 Serial electrocardiography (ECG) and cardiac troponin and/or NP levels have been considered, but the value is unclear.37,92 A complete baseline assessment, including biomarkers and an ECG, would be prudent given the range of potential CV adverse events, but it is unclear whether an assessment of LVEF is necessary or helpful.
Recommendation
In patients about to receive ICI therapy for cancer, baseline assessment for CVD, including cardiac biomarkers and ECG, should be completed as well as SARS–CoV-2 status. The need or value of cardiac imaging before initiating ICI therapy is not established. The optimal protocol for monitoring cardiac safety during treatment with ICIs is unknown at present. Serious cardiac adverse events related to ICI therapy have been reported, and any suspicious symptoms or clinical findings of CV toxicity should be aggressively investigated. SARS–CoV-2 has been noted to cause myocarditis, which needs to be differentiated from ICI myocarditis in patients on ICI therapy.
CAR-T and Bispecific T-Cell–Engager Therapy
Adoptive immunotherapy for cancer may result in a systemic inflammatory response of varying severity, referred to as the cytokine release syndrome (CRS).93 This has been associated with neurotoxicity and CV toxicity.11,94 The value of cardiac imaging at baseline before therapy or during the monitoring phase after treatment is still quite uncertain. The measurement of cardiac biomarkers has been identified as a useful strategy for ensuring cardiac safety, although the experience remains quite limited in general.11 In the current COVID-19 pandemic, the virus itself can lead to a severe and overwhelming CRS, thus treatment with these cancer therapies may likely lead to added CV risk for CRS. Interestingly, IL-6 antagonists, typically used to treat CAR-T therapy-associated CRS, are also currently being tested in severely ill patients with COVID-19 who display an overwhelming inflammatory response that is a harbinger of poor outcomes.95 Recently, the Society for Immunotherapy in Cancer proposed that efforts should be made to maximize the availability of anti–IL-6 agents, including tocilizumab and sarilumab, for use on a compassionate basis to critically ill, hospitalized, COVID-19–infected patients.96
Recommendation
In patients receiving CAR-T or bispecific T-cell–engager therapy, it is recommended to screen for active COVID-19 infection before initiation of treatment. A low threshold for the use of an anti-IL-6 agent, such as tocilizumab, should be considered in patients who develop CRS in the current pandemic, given the potential for unrecognized coinfection with SARS–CoV-2.
Patients on active cancer therapy that is compromising the immune system should receive maximal precautions and protection against SARS–CoV-2 exposure.
Cancer Therapies With Vascular Toxicity
SARS–CoV-2 uses the ACE2 receptor for cellular entry, which may explain why certain organs are preferentially affected in COVID-19.97 ACE2 is expressed on lung alveolar epithelial cells and enterocytes of the small intestine as well as on the arterial and venous endothelium.97 As outlined above, patients with preexisting CVD and/or CV risk factors are at the highest risk of experiencing a more severe course with COVID-19, and it is hypothesized that this may be related to reduced endothelial reserve. Along these lines, several cancer therapies, including chemotherapies (eg, cisplatin, cyclophosphamide), targeted therapies (eg, VSP inhibitors), immune therapies (eg, ICIs), and radiation therapy, can activate and injure the endothelium.98 Although this vascular injury may often go unnoticed in clinical practice, it may become more evident in the setting of a potent endothelial stressor such as COVID-19. Indeed, viral infection of endothelial cells has been observed, and the vascular injury is considered to contribute to several complicating manifestations of COVID-19.99 Recent data suggest that statin therapy may be an important adjunct for minimizing the major adverse vascular effects and inflammation that appears to be critically important during COVID-19.100 It is unknown whether aspirin is a beneficial or harmful adjunctive therapy unless otherwise indicated for patients presenting with classic acute myocardial infarction, stroke, or acute limb ischemia, as per standard practice guidelines. Trials are currently ongoing to address the thrombotic aspects of COVID-19, which include microthrombi in various organs, and the merit of anticoagulation strategies.
Recommendation
In patients with cancer who present with acute vascular events, such as acute myocardial infarction or stroke, the possibility of an infection with SARS–CoV-2 should be considered. This is especially important for patients who are being considered for invasive procedures. Myopericarditis is an important condition that should be considered in patients with SARS–CoV-2 and can mimic even ST-segment elevation myocardial infarction.
Statins should be continued in patients already on therapy and should be considered in patients undergoing therapies with potential CV toxicity.
Radiation Therapy
Patients who received prior chest radiation, especially those treated >2 decades ago, are at particular risk for radiation-induced heart and lung disease and represent a patient population expected to have a significantly reduced reserve should they develop COVID-19. For patients who received radiation therapy in the more recent era, the risk is likely lower. For those who are currently undergoing or are planned to undergo radiation therapy, acute injury and inflammation may pose a risk. Furthermore, it is recommended that, once radiation therapy is started, the full course of treatment should be completed.33
Recommendation
In patients receiving active radiation therapy, clinicians should be aware that symptoms related to radiation-induced pneumonitis may mimic symptoms of COVID-19.
Patients who have received prior radiation therapy may have a reduced cardiopulmonary reserve and may be especially susceptible to a complicated course of COVID-19; strict safety measures should be in place for these patients.
Use of ACE Inhibitors/Angiotensin Receptor Blockers During COVID-19
ACE2 is a coreceptor for SARS–CoV-2, thus there has been a debate regarding the safety of ACE inhibitors (ACE-Is) and angiotensin 2 receptor blockers (ARBs) during the COVID pandemic.101,102 A higher proportion of patients with adverse outcomes had hypertension and diabetes, and it has been speculated that ACE2 levels are upregulated in these patients, putting them at greater risk. One hypothesis is that ACE2 upregulation may be related to the pathogenesis of these CV risk factors or to other related comorbidities, such as obesity.103–105 Alternatively, treatment of hypertension and diabetes with ACE-Is and ARBs may increase ACE2 expression, thus increasing the risk of viral entry and propagation.106 However, in animal models, SARS-CoV infection led to reduced ACE2 expression and acute lung injury that was rescued by blocking the renin-angiotensin-aldosterone pathway. Clinical trials are ongoing that testing whether losartan, an ARB, is protective in COVID-19.107 Furthermore, ACE-Is and ARBs have established efficacy for the treatment of cardiomyopathy and heart failure, even in patients with cancer, and should be a preferred agent when used for cardioprotection in patients receiving cardiotoxic therapies. Current statements by CV societies (acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statementaddresses-concerns-re-using-raas-antagonists-in-covid-19 and escardio.org/Councils/Council-on-Hypertension(CHT)/News/position-statement-of-the-esc-council-onhypertension-on-ace-inhibitors-and-ang) do not indicate that there are adequate data to recommend stopping or withholding these therapies in patients with cancer who have established indications for their use.108 This view is supported by the observation that clinical outcomes did not differ between patients with a history of hypertension who were hospitalized for COVID-19 in Wuhan, China, and were treated either with an ACE-I/ARB or with an alternative antihypertensive regimen.10,109
Recommendation
ACE-Is and ARBs should be continued in patients with cancer who are receiving them for established indications during the COVID-19 pandemic.
ACE-Is and ARBs, the β-blocker carvedilol, and statins should continue to be appropriate strategies for cardioprotection while patients are receiving cancer therapy during the COVID-19 pandemic.
Cancer Therapies With Arrhythmic and QT-Prolonging Potential
Malignant ventricular arrhythmias have been recognized in patients with COVID-19 infection and lead to many management challenges.40 Moreover, various investigational treatments for COVID-19, including hydroxychloroquine and azithromycin, are associated with significant QT prolongation, which can increase the likelihood of ventricular tachyarrhythmia, particularly torsades de pointes. On the basis of these considerations and emerging data, the US Food and Drug Administration determined that the benefits of chloroquine and hydroxychloroquine no longer outweigh their potential risks within the anticipated framework of use, and they revoked the emergency use authorization for these medications on June 15, 2020. It should be added that the arrhythmic risk of these medications is likely increased in patients receiving active cancer therapy because many targeted agents may have an effect on the QT interval during treatment. For example, cancer therapeutics that are known to have important effects on the QT interval include arsenic trioxide, certain TKIs (vandetanib, nilotinib, vemurafenib), as well as ribociclib (Table 6). In addition, other risk factors during cancer therapy, such as nausea and diarrhea, may further exacerbate any changes in the QT interval as a result of hypomagnesemia or hypokalemia. In patients with QT prolongation who are receiving cancer therapeutics (ie, QTc ≥500 msec or ΔQT >60 msec from baseline), caution should be exercised when initiating COVID-19 therapies that may be have an important effect on the QT interval. Moreover, electrolytes should be continually repleted, and any other nonessential QT-prolonging medications should be discontinued. In patients with significant QT prolongation (>500 msec) in whom both cancer-related and COVID-19-related therapies are considered essential and cannot be altered, additional protective measures may be considered, including topical patch monitors and/or external wearable defibrillators, especially if the patient is treated in the outpatient setting.110
TABLE 6.
Cardiovascular Adverse Effects From Cancer Therapies
| ANTICANCER AGENT | CANCER USE | IMPORTANT COMMON CV ADVERSE EFFECT |
|---|---|---|
| Anthracyclines | ||
| Doxorubicin | Breast, sarcoma, lung, bladder, gastric, prostate, leukemia, lymphoma, others | Heart failure, cardiomyopathy, arrhythmia |
| Daunorubicin | ||
| Epirubicin | ||
| Idarubicin | ||
| Mitoxantrone | ||
| Alkylating agents | ||
| Cisplatin | Lung, bladder, testicular, breast, esophageal, head and neck | Venous and arterial thromboembolism, hypertension |
| Melphalan | Multiple myeloma, ovarian, neuroblastoma | Arrhythmia |
| Antimetabolites | ||
| Capecitabine | Breast, colon, gastric, pancreatic | Arterial (coronary) vasospasm, (myocardial) ischemia, Takotsubo |
| Fluorouracil | ||
| Monoclonal antibodies | ||
| Bevacizumab | Colorectal, cervical, glioblastoma, ovarian, renal, endometrial, sarcoma, breast | Hypertension, arterial and venous thromboembolism, bleeding |
| Rituximab | Lymphoma, leukemia | Hypotension (infusion reaction) |
| Ofatumumab | Hypertension | |
| Alemtuzumab | ||
| Pertuzumab | Breast, gastric, esophageal | Heart failure, cardiomyopathy |
| Trastuzumab | ||
| Small-molecule tyrosine kinase inhibitors | ||
| Axitinib | Renal | Hypertension |
| Cabozantinib | Thyroid, renal | Hypertension, arterial and venous thromboembolism |
| Cetuximab | Lung, pancreatic, colorectal | Arterial and venous thromboembolism |
| Crizotinib | Lung | Bradyarrhythmia, QT prolongation |
| Dabrafenib | Melanoma | QT prolongation |
| Dasatinib | Leukemia, gastrointestinal stromal tumor | QT prolongation, pleural effusion, pulmonary hypertension |
| Erlotinib | Lung, pancreatic, colorectal | Arterial and venous thromboembolism |
| Ibrutinib | Lymphoma | Atrial fibrillation, hypertension, bleeding |
| Imatinib | Leukemia, gastrointestinal stromal tumor, myelodysplastic syndrome, melanoma, mastocytosis, sarcoma | Edema |
| Osimertinib | Lung | Atrial fibrillation, heart failure, cardiomyopathy. QT prolongation |
| Pazopanib | Renal, sarcoma, thyroid | Hypertension, bradyarrhythmia, heart failure, cardiomyopathy |
| Ponatinib | Leukemia | Heart failure, cardiomyopathy, hypertension, ischemia, arterial and venous thromboembolism |
| Ramucirumab | Colorectal, gastric, lung | Hypertension |
| Regorafenib | Colorectal, gastrointestinal stromal tumor | Hypertension |
| Sorafenib | Hepatocellular, renal, thyroid | Hypertension, heart failure, cardiomyopathy, ischemia |
| Sunitinib | Renal, thyroid, sarcoma, gastrointestinal stromal tumor, pancreatic neuroendo-crine tumor | Hypertension, heart failure, cardiomyopathy, arterial and venous thromboembolism |
| Trametinib | Melanoma | Heart failure, cardiomyopathy, bradyarrhythmia, QT prolongation, arterial and venous thromboembolism, hypertension |
| Vandetanib | Thyroid | Hypertension, QT prolongation |
| Vemurafenib | Melanoma | Hypertension, QT prolongation |
| Ziv-aflibercept | Colorectal | Hypertension |
| Immune checkpoint inhibitors | Melanoma, lung, kidney, bladder, head and neck, lymphoma | Myocarditis, arrhythmia/sudden cardiac death, cardiomyopathy, vasculitis, pericarditis |
PD-1 inhibitors
|
||
PD-L1 inhibitors
|
||
CTLA-4 inhibitor
|
||
| Proteasome inhibitors | ||
| Bortezomib | Multiple myeloma, mantle cell lymphoma | Hypertension |
| Carfilzomib | Multiple myeloma | Hypertension, heart failure, cardiomyopathy, arterial and venous thromboembolism, acute coronary syndrome |
| mTOR inhibitors | ||
| Everolimus | Breast, pancreas | Hypertension |
| Temsirolimus | Renal | Hypertension |
| Immunomodulatory drugs | Multiple myeloma | Venous thromboembolism |
| Lenalidomide | ||
| Pomalidomide | ||
| Thalidomide | ||
| Histone deacetylase inhibitors | Lymphoma | QT prolongation |
| Belinostat | ||
| Vorinostat | ||
| Endocrine therapy | ||
Selective estrogen receptor modulators
|
Breast | Venous thromboembolism, QT prolongation |
Aromatase inhibitors
|
Breast | Venous thromboembolism, hypertension, hyperlipidemia |
Antiandrogens
|
Prostate | Hypertension, hyperlipidemia, atherosclerosis |
CYP17 inhibitor
|
Prostate | Hypertension, myocardial infarction |
| Chimeric antigen receptor T-cell therapy | B-cell acute lymphoblastic leukemia (refractory or relapse), large B-cell lymphoma (refractory or relapse) | Tachycardia, arrhythmia, hypotension, hypertension, heart failure, cytokine release syndrome |
| Axicabtagene ciloleucel | ||
| Tisagenlecleucel | ||
| Miscellaneous | ||
| Ribociclib | Breast | QT prolongation |
| Arsenic trioxide | Leukemia | QT prolongation |
| Tretinoin | Leukemia | Heart failure, cardiomyopathy |
QT interval and arrhythmia monitoring strategies are constantly evolving. Recent publications provide helpful guidance in monitoring for the risk of ventricular arrhythmia caused by hydroxychloroquine-azithromycin treatment for COVID-19.111,112 Although a standard 12-lead ECG is the most accurate way to assess the QT interval and cardiac arrhythmias, resource utilization and management are important considerations in the setting of COVID-19. Acquisition of a 12-lead ECG requires both personnel and equipment exposure. As such, the number of requested ECGs should be limited, and only a small number of technicians and ECG machines should be designated for patients with COVID-19 to limit exposure. For inpatients, telemetry monitoring may be considered for those deemed high risk based on a validated risk score for drug-associated QT interval prolongation.113 There is also a need for alternative tools for QT monitoring considering the tremendous cost and resource utilization required for currently recommended QT interval monitoring with serial 12-lead ECGs, typically in triplicate.114 For example, many wearable devices and other digital technology, such as the Apple Inc smartwatch, can provide single-lead ECG data with excellent fidelity. The KardiaMobile-6L, developed by AliveCor for atrial fibrillation detection, just received emergency clearance from the US Food and Drug Administration for use as a device for QT interval monitoring of patients with COVID-19. This tool and the BodyGuardian Heart (Preventice Solutions) may be very important devices to ensure cardiac safety and also to maintain the currently required social distancing mandates.110
Recommendation
All patients with cancer who are being initiated on therapies associated with QT interval prolongation require a baseline 12-lead ECG. Any subsequent monitoring that is necessary can be accurately and safely performed with topical devices that allow monitoring of arrhythmias and QT interval prolongation. Nonessential medications should be eliminated, and electrolytes should be fastidiously replaced if those issues are present.
Cancer Therapies With Risk of Thromboembolism
The characteristics of cancer disease, the type of anticancer therapy, and coexisting diseases, including CVD, are important risk factors for venous thromboembolism.115 Pulmonary embolism remains a leading cause of mortality in clinical oncology. SARS–CoV-2 appears to be an additional risk factor for thromboembolic complications (including arterial microthrombi), and D-dimer (>1 μg/mL) is one of the most important predictors for fatal outcomes.116–120 Inflammation, endothelial activation, and injury are the most likely mechanisms. The clinical difficulty is that pulmonary embolism, pulmonary infarction, and SARS–CoV-2 may have the same symptoms—shortness of breath, cough, and even fever—and they can coexist.17
Recommendations
In C-O patients with confirmed SARS–CoV-2 who are immobilized in bed, primary antithrombotic prophylaxis should be used.
In C-O patients with dyspnea, cough, and fever, diagnostics for the exclusion of SARS–CoV-2 and pulmonary embolism should be performed.
In patients with cancer who have confirmed pulmonary embolism, SARS–CoV-2 should be excluded.
In patients with cancer who have pneumonia and confirmed SARS–CoV-2, evaluation by computed tomography should include an assessment for pulmonary embolism.
Low-molecular-weight heparins appear to be the first choice for antithrombotic treatment; new oral anticoagulants may be considered if there are not important drugdrug interactions with other planned treatments.
Concluding Statements
In a matter of a few months, COVID-19 has had an unimaginable impact on human lives and local, national, and global economies. The pace and number of studies in this area have been unprecedented, and so has the rapidly evolving knowledge base. Specific recommendations for clinical practice will continue to evolve in the weeks and months to come. The current general observations emphasize that patients with CVD and/or cancer are among the highest risk groups for poor outcomes, especially if they contract SARS–CoV-2. Proper awareness and strategies need to be in place to manage these patients optimally and thoughtfully to ensure a safe environment. Patients who are being treated for cancer represent a complex and fragile population. The management of their comorbidities, and of CVD in particular, becomes an absolute priority, especially during COVID-19. As a result of this pandemic, the need for a proactive and integrated approach to C-O care has never been more important than it is today. It is the intent of this consensus document to provide a meaningful structure to achieve a truly integrated and safe approach for the benefit of our patients.
DISCLOSURES:
Tomas G. Neilan is supported by the National Institutes of Health/National Heart, Lung, and Blood Institute (R01HL137562, R01HL130539, and K24HL150238)] and in part through a kind gift from A. Curtis Greer and Pamela Kohlberg. Joerg Herrmann is supported by the National Institutes of Health/National Cancer Institute (R01CA233601) and the Florida Heart Research Foundation. Daniel Lenihan reports grants from Myocardial Solutions, Inc, and personal fees from Acorda, Bristol Myers Squibb, Clementia, Lilly, and Roche outside the submitted work. Susan Dent reports grants from Novartis US and personal fees from Novartis Canada outside the submitted work. Anju Nohria reports grants from Amgen and personal fees from Takeda Oncology outside the submitted work. Joshua D. Mitchell reports research support from Pfizer outside the submitted work. Michael G. Fradley reports personal fees from Medtronic and Takeda outside the submitted work. Alexander R. Lyon reports grants and personal fees from Pfizer and Servier; and personal fees from Amgen, Boehringer Ingelheim, Brainstorm Inc, Bristol Myers Squibb, Clinigen Group, Eisai Ltd, Eli Lilly, Ferring Pharmaceuticals, Heartfelt Technologies Ltd, iOWNA, Myocardial Solutions, Novartis, Roche, and Takeda, outside the submitted work. Teresa López-Fernández reports personal fees and conference support from Amgen, Bayer, Daiichi-Sankyo, Incyte, iQUONE, Janssen, Merck Sharpe & Dohme, Pfizer, and TEVA outside the submitted work. Rupal O’Quinn reports grants from Northwest Imaging Forums and personal fees from Guidepoint outside the submitted work. Sebastian Szmit reports personal fees, nonfinancial support, and other support from Bristol Myers Squibb; personal fees and other support from Pfizer; and personal fees from Amgen, Angelini, AstraZeneca, Bayer, Berlin-Chemie, Clinigen, Janssen-Cilag, Polpharma, Roche, and TEVA outside the submitted work. Giuseppe Curigliano reports travel support from Roche and personal fees from AstraZeneca, Bristol Myers Squibb, Daichii-Sankyo, Ellipsis, Novartis, Lilly, Merck, and Novartis outside the submitted work. Tomas G. Neilan reports personal fees from AbbVie, Bristol Myers Squibb, H3-Biomedicine, Intrinsic Imaging, Parexel Imaging, and Syros Pharmaceuticals outside the submitted work. Joerg Herrmann reports personal fees from Amgen, Ariad Pharmaceuticals, and Bristol Myers Squibb outside the submitted work. The remaining authors made no disclosures.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 2.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia Y, Jin R, Zhao J, Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. Published online June 18, 2020. doi: 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 10.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvi RM, Frigault MJ, Fradley MG, et al. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). J Am Coll Cardiol. 2019;74:3099–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. [DOI] [PubMed] [Google Scholar]
- 13.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. Published online March 16, 2020. doi: 10.1093/eurheartj/ehaa290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hua A, O’Gallagher K, Sado D, Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020;41:2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Fan Y, Lu Z. Experiences and lesson strategies for cardiology from the COVID-19 outbreak in Wuhan, China, by ‘on the scene’ cardiologists. Eur Heart J. 2020;41:1788–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danzi GB, Loffi M, Galeazzi G, Gherbesi D. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. Published online May 14, 2020. doi: 10.7326/M20-2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wichmann D, Sperhake JP, Lutgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. Published online May 6, 2020. doi: 10.7326/M20-2003 [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganatra S, Hammond SP, Nohria A. The novel coronavirus disease (COVID-19) threat for patients with cardiovascular disease and cancer. JACC CardioOncol. 2020;2:350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6: 1108–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARSCOV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov. 2020; 10:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogado J, Obispo B, Pangua C, et al. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin Transl Oncol. Published online May 25, 2020. doi: 10.1007/s12094-020-02381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garassino MC, Whisenant JG, Huang LC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee LYW, Cazier JB, Starkey T, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battershill PM. Influenza pandemic planning for cancer patients. Curr Oncol. 2006;13:119–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25: e936–e945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mauri D, Kamposioras K, Tolia M, Alongi F, Tzachanis D, International Oncology Panel and European Cancer Patient Coalition Collaborators. Summary of international recommendations in 23 languages for patients with cancer during the COVID-19 pandemic. Lancet Oncol. 2020;21:759–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ball S, Ghosh RK, Wongsaengsak S, et al. Cardiovascular toxicities of immune checkpoint inhibitors: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:1714–1727. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann J, Yang EH, Iliescu CA, et al. Vascular toxicities of cancer therapies: the old and the new—an evolving avenue. Circulation. 2016;133:1272–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khosravani H, Rajendram P, Notario L, Chapman MG, Menon BK. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke. 2020;51:1891–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Circulation. 2020;21:e823–e831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skulstad H, Cosyns B, Popescu BA, et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382: 970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. [DOI] [PubMed] [Google Scholar]
- 44.Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348: 1967–1976. [DOI] [PubMed] [Google Scholar]
- 45.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook TM. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic—a narrative review. Anaesthesia. 2020;75:920–927. [DOI] [PubMed] [Google Scholar]
- 48.Ma QX, Shan H, Zhang HL, Li GM, Yang RM, Chen JM. Potential utilities of mask-wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. Published online March 31, 2020. doi: 10.1002/jmv.25805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak. J Am Coll Cardiol. 2020;75:3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szekely Y, Lichter Y, Taieb P, et al. The spectrum of cardiac manifestations in coronavirus disease 2019 (COVID-19)—a systematic echocardiographic study. Circulation.. Published online May 29, 2020. doi: 10.1161/circulationaha.120.047971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seraphim A, Westwood M, Bhuva AN, et al. Advanced imaging modalities to monitor for cardiotoxicity. Curr Treat Options Oncol. 2019;20:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. [DOI] [PubMed] [Google Scholar]
- 53.Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 7):vii155–vii166. [DOI] [PubMed] [Google Scholar]
- 54.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. [DOI] [PubMed] [Google Scholar]
- 55.Lenihan DJ, Stevens PL, Massey M, et al. The utility of point-of-care biomarkers to detect cardiotoxicity during anthracycline chemotherapy: a feasibility study. J Card Fail. 2016;22:433–438. [DOI] [PubMed] [Google Scholar]
- 56.Cornell RF, Ky B, Weiss BM, et al. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J Clin Oncol. 2019;37:1946–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 58.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 59.Feola M, Garrone O, Occelli M, et al. Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int J Cardiol. 2011;148:194–198. [DOI] [PubMed] [Google Scholar]
- 60.Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. [DOI] [PubMed] [Google Scholar]
- 61.Demissei BG, Hubbard RA, Zhang L, et al. Changes in cardiovascular biomarkers with breast cancer therapy and associations with cardiac dysfunction. J Am Heart Assoc. 2020;9:e014708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goel S, Liu J, Guo H, et al. Decline in left ventricular ejection fraction following anthracyclines predicts trastuzumab cardiotoxicity. JACC Heart Fail. 2019;7:795–780. [DOI] [PubMed] [Google Scholar]
- 63.Demissei BG, Finkelman BS, Hubbard RA, et al. Detailed phenotyping reveals distinct trajectories of cardiovascular function and symptoms with exposure to modern breast cancer therapy. Cancer. 2019;125:2762–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bouwer NI, Liesting C, Kofflard MJM, et al. NT-proBNP correlates with LVEF decline in HER2-positive breast cancer patients treated with trastuzumab. Cardiooncology. 2019;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ponde N, Bradbury I, Lambertini M, et al. Cardiac biomarkers for early detection and prediction of trastuzumab and/or lapatinib-induced cardiotoxicity in patients with HER2-positive early-stage breast cancer: a NeoALTTO sub-study (BIG 1–06). Breast Cancer Res Treat. 2018;168:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zardavas D, Suter TM, Van Veldhuisen DJ, et al. Role of troponins I and T and N-terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early-stage human epidermal growth factor receptor 2-positive breast cancer receiving trastuzumab: a herceptin adjuvant study cardiac marker substudy. J Clin Oncol. 2017;35:878–884. [DOI] [PubMed] [Google Scholar]
- 67.Thavendiranathan P, Amir E. Left ventricular dysfunction with trastuzumab therapy: is primary prevention the best option? J Clin Oncol. 2017;35:820–825. [DOI] [PubMed] [Google Scholar]
- 68.Kitayama H, Kondo T, Sugiyama J, et al. High-sensitive troponin T assay can predict anthracycline- and trastuzumab-induced cardiotoxicity in breast cancer patients. Breast Cancer. 2017;24:774–782. [DOI] [PubMed] [Google Scholar]
- 69.Altundag K Robust predictive markers are needed for early detection of trastuzumab-related cardiac dysfunction in breast cancer. Breast Cancer. 2017;24:794. [DOI] [PubMed] [Google Scholar]
- 70.Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107:1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goel S, Simes RJ, Beith JM. Exploratory analysis of cardiac biomarkers in women with normal cardiac function receiving trastuzumab for breast cancer. Asia Pac J Clin Oncol. 2011;7:276–280. [DOI] [PubMed] [Google Scholar]
- 74.Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–2270. [DOI] [PubMed] [Google Scholar]
- 75.Dang CT, Yu AF, Jones LW, et al. Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol. 2016;34:1030–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014; 15:1063–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Touyz RM, Herrmann SMS, Herrmann J. Vascular toxicities with VEGF inhibitor therapies-focus on hypertension and arterial thrombotic events. J Am Soc Hypertens. 2018;12:409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Witteles RM. Biomarkers as predictors of cardiac toxicity from targeted cancer therapies. J Card Fail. 2016;22:459–464. [DOI] [PubMed] [Google Scholar]
- 80.Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1:72–78. [DOI] [PubMed] [Google Scholar]
- 81.Chari A, Stewart AK, Russell SD, et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2018;2:1633–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li W, Garcia D, Cornell RF, et al. Cardiovascular and thrombotic complications of novel multiple myeloma therapies: a review. JAMA Oncol. 2017;3:980–988. [DOI] [PubMed] [Google Scholar]
- 83.Gertz MA, Dispenzieri A, Sher T. Pathophysiology and treatment of cardiac amyloidosis. Nat Rev Cardiol. 2015;12:91–102. [DOI] [PubMed] [Google Scholar]
- 84.Kumar SK, Gertz MA, Dispenzieri A. Validation of Mayo Clinic staging system for light chain amyloidosis with high-sensitivity troponin. J Clin Oncol. 2019;37:171–173. [DOI] [PubMed] [Google Scholar]
- 85.Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brown SA, Ray JC, Herrmann J. Precision cardio-oncology: a systems-based perspective on cardiotoxicity of tyrosine kinase inhibitors and immune checkpoint inhibitors. J Cardiovasc Transl Res. 2020;13:402–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Awadalla M, Mahmood SS, Groarke JD, et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol. 2020;75:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L, Awadalla M, Mahmood SS, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. 2020;41:1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. [DOI] [PubMed] [Google Scholar]
- 91.Hu JR, Florido R, Lipson EJ, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115:854–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Neilan TG, Rothenberg ML, Amiri-Kordestani L, et al. Myocarditis associated with immune checkpoint inhibitors: an expert consensus on data gaps and a call to action. Oncologist. 2018;23:874–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herrmann J Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. 2020;17: 474–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ganatra S, Carver JR, Hayek SS, et al. Chimeric antigen receptor T-cell therapy for cancer and heart: JACC Council perspectives. J Am Coll Cardiol. 2019;74: 3153–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ascierto PA, Fox BA, Urba WJ, et al. Insights from immuno-oncology: the Society for Immunotherapy of Cancer statement on access to IL-6-targeting therapies for COVID-19. J Immunother Cancer. 2020;9:e000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herrmann J Vascular toxic effects of cancer therapies. Nat Rev Cardiol. 2020;17:503–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395: 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang XJ, Qin JJ, Cheng X, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. Published online June 24, 2020. doi: 10.1016/j.cmet.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gupte M, Boustany-Kari CM, Bharadwaj K, et al. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295:R781–R788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Palmer BR, Jarvis MD, Pilbrow AP, et al. Angiotensin-converting enzyme 2 A1075G polymorphism is associated with survival in an acute coronary syndromes cohort. Am Heart J. 2008;156:752–758. [DOI] [PubMed] [Google Scholar]
- 105.Fan Z, Wu G, Yue M, et al. Hypertension and hypertensive left ventricular hypertrophy are associated with ACE2 genetic polymorphism. Life Sci. 2019;225:39–45. [DOI] [PubMed] [Google Scholar]
- 106.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 107.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rico-Mesa JS, White A, Anderson AS. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr Cardiol Rep. 2020;22:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Castelletti S, Dagradi F, Goulene K, et al. A wearable remote monitoring system for the identification of subjects with a prolonged QT interval or at risk for drug-induced long QT syndrome. Int J Cardiol. 2018;266:89–94. [DOI] [PubMed] [Google Scholar]
- 111.Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for drug interactions on QTc in exploratory COVID-19 (coronavirus disease 2019) treatment. Circulation. 2020;141:e906–e907. [DOI] [PubMed] [Google Scholar]
- 112.Sapp JL, Alqarawi W, MacIntyre CJ, et al. Guidance on minimizing risk of drug-induced ventricular arrhythmia during treatment of COVID-19: a statement from the Canadian Heart Rhythm Society. Can J Cardiol. 2020;36: 948–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Menna P, Salvatorelli E, Minotti G. Cancer drugs and QT prolongation: weighing risk against benefit. Expert Opin Drug Saf. 2017;16:1099–1102. [DOI] [PubMed] [Google Scholar]
- 115.Connolly GC, Francis CW. Cancerassociated thrombosis. Hematology Am Soc Hematol Educ Program. 2013;2013: 684–691. [DOI] [PubMed] [Google Scholar]
- 116.Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. Published online May 27, 2020. doi: 10.1097/CCM.0000000000004458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020; 18:1559–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]


