Abstract
Background: In this study, we aimed to determine the global prevalence, chronological order of symptom appearance, and mortality rates with regard to hemorrhagic and ischemic stroke in patients with coronavirus disease 2019 (COVID-19) and to discuss possible pathogeneses of hemorrhagic and ischemic stroke in individuals with the disease.
Methods: We searched the PubMed, Scopus, and Web of Science databases for relevant articles published up to November 8, 2020. Data regarding study characteristics, hemorrhagic stroke, ischemic stroke, and COVID-19 were retrieved in accordance with the PRISMA guidelines. The Newcastle-Ottawa scale was used to assess the quality of the eligible studies. The pooled prevalence and mortality rate of hemorrhagic and ischemic stroke were calculated.
Results: The pooled estimate of prevalence of hemorrhagic stroke was 0.46% (95% CI 0.40%–0.53%; I 2=89.81%) among 67,155 COVID-19 patients and that of ischemic stroke was 1.11% (95% CI 1.03%–1.22%; I 2=94.07%) among 58,104 COVID-19 patients. Ischemic stroke was more predominant (incidence: 71.58%) than hemorrhagic stroke (incidence: 28.42%) in COVID-19 patients who experienced a stroke. In COVID-19 patients who experienced a stroke, hospital admission with respiratory symptoms was more commonly reported than that with neurological symptoms (20.83% for hemorrhagic stroke and 5.51% for ischemic stroke versus 6.94% for hemorrhagic stroke and 5.33% for ischemic stroke, respectively). The pooled mortality rate of COVID-19 patients who experienced a hemorrhagic and ischemic stroke was 44.72% (95% CI 36.73%–52.98%) and 36.23% (95% CI 30.63%–42.24%), respectively.
Conclusions: Although the occurrence of hemorrhagic and ischemic stroke is low, the mortality rates of both stroke types in patients with COVID-19 are concerning, and therefore, despite several potential pathogeneses that have been proposed, studies aimed at definitively elucidating the mechanisms of hemorrhagic and ischemic stroke in individuals with COVID-19 are warranted.
PROSPERO registration: CRD42020224470 (04/12/20)
Keywords: COVID-19, haemorrhagic stroke, ischemic stroke, meta-analysis, pathogenesis, SARS-CoV-2, systematic review
Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has become a global human pandemic that is believed to have begun in late December 2019. The disease quickly spread to 217 countries, infected more than 82 million individuals, and has caused more than 1.8 million deaths as of December 30, 2020 1. Moreover, the second wave of this pandemic remains ongoing in various countries 2. Numerous treatment strategies and drugs have been proposed 3– 5; however, a definitive therapy or treatment for COVID-19 has not yet been announced by the World Health Organization 6. SARS-CoV-2 is a novel coronavirus, which is reported to have originated initially from an animal source 7. The mortality rate of SARS-CoV-2 infection is the lowest among the infections caused by other members of the coronavirus family that have previously infected humans, including severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) 8, 9. However, SARS-CoV-2 has a higher reproduction rate (R 0) and thus a higher transmission rate than SARS-CoV and MERS-CoV 10.
The majority of individuals infected with SARS-CoV-2 are generally asymptomatic, although the most common symptoms of COVID-19 include dry cough, fever, dyspnea, chest pain, headache, and muscle ache 11, 12. The issue of hypercoagulability-related thrombotic vascular events in those infected with SARS-CoV-2 is emerging 13, 14. Evidence suggests that COVID-19 patients may experience increased rates of thromboembolism, as high as 15%–26% 15. In addition, another concern is the increased risk of a hemorrhagic stroke among COVID-19 patients 16– 18. The World Stroke Organization has reported that COVID-19 increases the risk for an ischemic stroke by approximately 5% (95% confidence interval (CI) 2.8%–8.7%) 19. Other possible explanations for the occurrence of ischemic stroke in COVID-19 patients include reduced angiotensin (ANG) (1-7) synthesis 20, cardioembolism 21, 22, hyperviscosity 23, 24, and an induced hypercoagulative state 25, 26. Discussions surrounding the possible mechanism(s) underlying hemorrhagic stroke in COVID-19 patients have included the expression of angiotensin-converting enzyme 2 (ACE2), immunity, inflammation, endothelial dysfunction at the blood-brain-barrier (BBB), aging, stress, and anxiety 27.
The aims of our present study were to determine the global incidence of ischemic and hemorrhagic stroke in patients with COVID-19; determine the mortality rate of ischemic and hemorrhagic stroke in individuals with COVID-19; assess the frequency of symptoms that lead to hospital admission among COVID-19 patients who have experienced an ischemic or a hemorrhagic stroke; assess the risk factors for ischemic and hemorrhagic stroke in COVID-19; assess the association between ischemic and hemorrhagic stroke and the severity of COVID-19; and assess the association between ischemic and hemorrhagic stroke and mortality in COVID-19. In addition, we also sought to propose possible pathogeneses of ischemic and hemorrhagic stroke in individuals with SARS-CoV-2 infection.
Methods
Registration and protocol
This systematic review and meta-analysis identified the stroke proportion among COVID-19 confirmed cases. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) recommendation to search electronic databases (see completed checklist 28) 29. The protocol of this study was registered in PROSPERO ( CRD42020224470) on 4 th December 2020.
Eligibility criteria of studies
The inclusion criteria were articles written in English which identified stroke as a comorbidity among randomly sampled COVID-19 cases. All case reports, case series, editorials, reviews, commentaries, and studies in targeted specific groups, such as in children were excluded.
Information sources and search strategy
The systematic searches were conducted in three databases ( PubMed, Scopus, and Web of Science) to identify the potential articles as of November 8 th, 2020. The search criteria were as follows. Scopus (TITLE("SARS-CoV-2" OR "COVID-19" OR "Wuhan coronavirus" OR "Wuhan virus" OR "novel coronavirus" OR "nCoV" OR "severe acute respiratory syndrome coronavirus 2" OR "coronavirus disease 2019 virus" OR "2019-nCoV" OR "2019 novel coronavirus" OR "severe acute respiratory syndrome coronavirus 2" OR "coronavirus" OR "coronaviruses" OR "SARS 2" OR "2019-nCoV acute respiratory disease" OR "novel coronavirus pneumonia" OR "COVID") AND ALL("Stroke " OR "cerebrovascular disorders" OR "brain ischemia" OR "brain haemorrhage" OR "cerebrovascular accident" OR "intracerebral haemorrhage" OR "subarachnoid haemorrhage" OR "transient ischemic attack" OR "brain attack" OR "cerebral embolism" OR "cerebral thrombosis" OR "cerebral haemorrhage" OR "cerebrovascular insult" OR "intraparenchymal haemorrhage" OR "intraventricular haemorrhage" OR "cerebral hypoperfusion" OR "brain infarct" OR "cerebral infarct"). Web of Science (TITLE("SARS-CoV-2" OR "COVID-19" OR "Wuhan coronavirus" OR "Wuhan virus" OR "novel coronavirus" OR "nCoV" OR "severe acute respiratory syndrome coronavirus 2" OR "coronavirus disease 2019 virus" OR "2019-nCoV" OR "2019 novel coronavirus" OR "severe acute respiratory syndrome coronavirus 2" OR "coronavirus" OR "coronaviruses" OR "SARS 2" OR "2019-nCoV acute respiratory disease" OR "novel coronavirus pneumonia" OR "COVID") AND ALL=("stroke" OR "cerebrovascular Disorders" OR "brain ischemia" OR "brain haemorrhage" OR "cerebrovascular accident" OR "intracerebral haemorrhage" OR "subarachnoid haemorrhage" OR "transient ischemic attack" OR "brain attack" OR "cerebral embolism" OR "cerebral thrombosis" OR "cerebral haemorrhage" OR "cerebrovascular insult" OR "intraparenchymal haemorrhage" OR "intraventricular haemorrhage" OR "cerebral hypoperfusion" OR "brain infarct" OR "cerebral infarct"). PubMed (TITLE("SARS-CoV-2" OR "COVID-19" OR "Wuhan coronavirus" OR "Wuhan virus" OR "novel coronavirus" OR "nCoV" OR "severe acute respiratory syndrome coronavirus 2" OR "coronavirus disease 2019 virus" OR "2019-nCoV" OR "2019 novel coronavirus" OR "severe acute respiratory syndrome coronavirus 2" OR "coronavirus" OR "coronaviruses" OR "SARS 2" OR "2019-nCoV acute respiratory disease" OR "novel coronavirus pneumonia" OR "COVID") AND ("stroke " OR "cerebrovascular Disorders" OR "brain ischemia" OR "brain haemorrhage" OR "cerebrovascular accident" OR "intracerebral haemorrhage" OR "subarachnoid haemorrhage" OR "transient ischemic attack" OR "brain attack" OR "cerebral embolism" OR "cerebral thrombosis" OR "cerebral haemorrhage" OR "cerebrovascular insult" OR "intraparenchymal haemorrhage" OR "intraventricular haemorrhage" OR "cerebral hypoperfusion" OR "brain infarct" OR "cerebral infarct").
Data from the articles and the supplementary materials were extracted. Reference lists from the eligible articles were retrieved for further relevant studies.
Study selection and data extraction
EndNote X9 (Thompson Reuters, Philadelphia, PA, USA) was used to import all titles and abstracts of the identified articles, and duplicated records were removed. Potentially eligible articles were identified through screening of the titles and abstracts. The full texts of the resulting studies were then thoroughly reviewed by two authors (HAM and MI) and the eligibility of each study was decided. Any disagreements between the investigators were solved by consulting with another investigator (MF).
Data extraction
Collected information included study characteristics (author, study site, study design), number of patients with ischemic stroke or haemorrhagic stroke in COVID-19 cases and their mortality cases, the chronological order of patient admission to hospital based on symptoms (COVID-19 first, ischemic or haemorrhagic stroke first) and the COVID-19 characteristics (number of patients, severity, and mortality).
Role of the funding source
This study received no external funding.
Outcomes
The primary outcomes were: (a) global incidence of ischemic and haemorrhagic stroke in COVID-19 patients; (b) mortality rate of ischemic and haemorrhagic stroke in COVID-19; (c) the frequency of symptoms related to hospital admission among COVID-19 patients with ischemic or haemorrhagic stroke; (d) risk factors of ischemic and haemorrhagic stroke in COVID-19; (e) association of ischemic and haemorrhagic stroke with COVID-19 severity; and (f) association of ischemic and haemorrhagic stroke with mortality of COVID-19. The possible pathogenesis mechanisms of ischemic and haemorrhagic stroke in SARS-CoV-2 infection were also explained in this review.
Data synthesis
The global prevalence of ischemic stroke was calculated as the number of COVID-19 patients who experienced ischemia divided by the total number of COVID-19 patients with or without ischemic or hemorrhagic stroke, expressed as frequency (%) and 95% CI. The frequency of symptoms that led to hospital admission was calculated as the total number of COVID-19 patients presenting with either respiratory or neurological symptoms first, divided by the total number of ischemic stroke cases among COVID-19 patients, and expressed as percentage and 95% CI. The mortality rate was calculated as the number of deaths of COVID-19 patients who experienced ischemic stroke divided by the total number of COVID-19 patients who experienced ischemic stroke. The same calculations were performed for hemorrhagic stroke.
Risk of bias assessment
The Newcastle-Ottawa scale (NOS) 30 was used to critically assess the quality of the studies included in the meta-analysis. The Q test was used to evaluate the heterogeneity and potential publication bias of the data gathered from the studies.
Statistical analysis
The association between ischemic and hemorrhagic stroke and the occurrence of COVID-19 was calculated and expressed as the cumulative odds ratio and 95% CI using the Z test; differences with p < 0.05 were considered to be statistically significant. Heterogeneity among studies was assessed using the Q test, and heterogeneous data were analyzed using a random effects model. Publication bias was assessed using Egger’s test and funnel plots (p < 0.05 was considered to indicate potential for publication bias). The data were analyzed using Review Manager version 5.3 (The Cochrane Collaboration) 31.
Results
Study eligibility
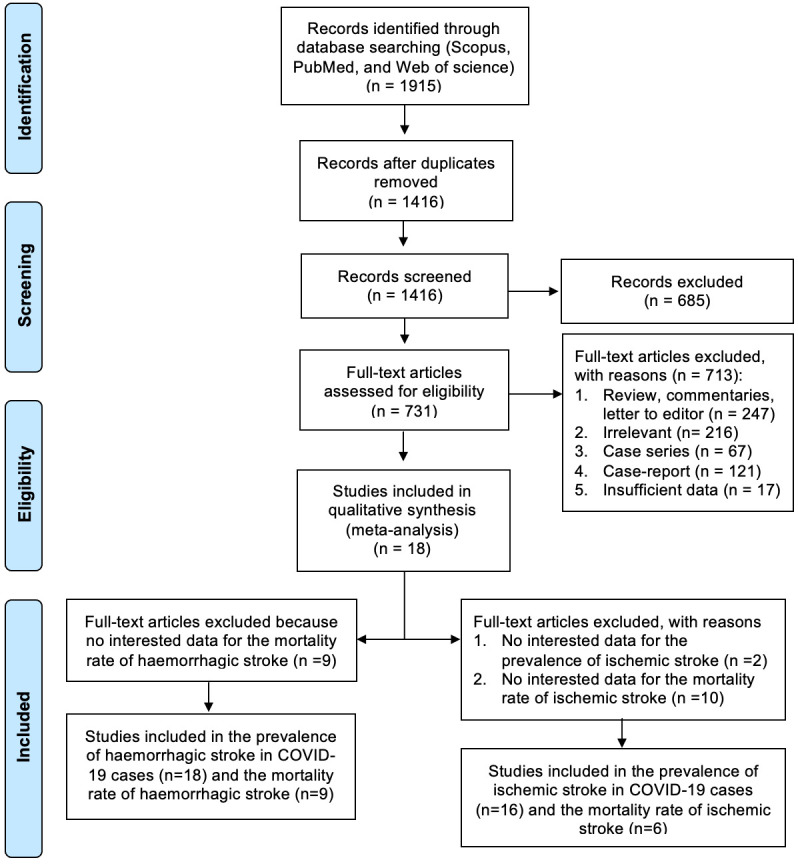
A total of 1915 articles were retrieved via a literature search, with 1416 citations remaining after the duplicates were removed. An additional 685 articles were excluded after screening the titles and abstracts, leaving 731 studies ( Figure 1), the full texts of which were reviewed for eligibility, with an additional 713 excluded. Exclusions included reviews, irrelevant studies, case series, case reports, and studies with insufficient data. This process resulted in 18 studies being included in the final analysis.
Figure 1. Flowchart of the literature search according to PRISMA.
All 18 studies were included in the meta-analysis to calculate the global prevalence of hemorrhagic stroke in COVID-19 patients and the frequency of symptoms leading to hospital admission 32– 49. Data from nine studies were included to calculate the mortality rate of hemorrhagic stroke in COVID-19 patients 32, 33, 35– 37, 40, 45, 48, 49, while the other nine studies did not report relevant data. The 18 studies and the prevalence of hemorrhagic stroke reported in each of them are summarized in Table 1.
Table 1. The prevalence of haemorrhagic stroke among COVID-19 patients around the globe.
| Study Design | Country | NOS | COVID-19 | Chronology of the symptoms | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|
| Haemorrhagic
Stroke |
Total
COVID-19 |
Not
clear (%) |
Respiratory
symptoms first (%) |
Neurologic
symptoms first (%) |
|||||
| Case | Death | ||||||||
| Retrospective cohort | US | 8 | 35 | 16 | 5227 | 35 (16.2) | - | - | 32 |
| Retrospective cohort | US | 8 | 33 | 14 | 3824 | - | 29 (13.4) | 4 (1.85) | 33 |
| Prospective cross-
sectional |
Spain | 7 | 1 | - | 2000 | - | 1 (0.46) | - | 34 |
| Retrospective cohort | Spain | 8 | 5 | 2 | 1683 | - | 4 (1.85) | 1 (0.46) | 35 |
| Retrospective cohort | US | 8 | 9 | 5 | 3218 | - | 5 (2.31) | 4 (1.85) | 36 |
| Retrospective cohort | UEA | 8 | 12 | 4 | 591 | 12 (5.55) | - | - | 37 |
| Retrospective cohort | Turkey | 7 | 2 | - | 239 | 2 (0.93) | - | - | 38 |
| Retrospective cohort | US | 8 | 14 | - | 10,596 | 14 (6.48) | - | - | 39 |
| Retrospective cohort | China | 8 | 1 | 1 | 219 | - | 1 (0.46) | - | 40 |
| Retrospective cohort | US | 8 | 1 | - | 509 | 1 (0.46) | - | - | 41 |
| Retrospective cohort | Italy | 7 | 2 | - | 213 | 2 (0.93) | - | - | 42 |
| Retrospective cohort | US | 8 | 8 | - | 650 | - | 2 (0.93) | 6 (2.78) | 43 |
| Retrospective cohort | Italy | 8 | 11 | - | 1760 | 11 (5.09) | - | - | 44 |
| Retrospective cohort | UK | 8 | 14 | 4 | 3403 | 14 (6.48) | - | - | 45 |
| Retrospective cohort | Multi-
national |
8 | 27 | - | 17,799 | 27 (12.5) | - | - | 46 |
| Retrospective cohort | US | 7 | 3 | - | 90 | - | 3 (1.39) | - | 47 |
| Retrospective cohort | Multi-
national |
8 | 28 | 16 | 14,483 | 28
(12.96) |
- | - | 48 |
| Retrospective cohort | China | 7 | 10 | 4 | 651 | 10 (4.63) | - | - | 49 |
| Total | 216 | 66 | 67,155 | 156/216
(72.2) |
45/216
(20.83) |
15/216
(6.94) |
|||
NOS = Newcastle-Ottawa scale score, COVID-19 = coronavirus disease 19
Only 16 studies were included in the meta-analysis to calculate the global prevalence of ischemic stroke in COVID-19 patients and the frequency of symptoms leading to hospital admission 34– 49. Data from six studies were included to calculate the mortality rate of ischemic stroke in COVID-19 patients 35– 37, 40, 48, 49, as no relevant data was reported in the remaining 12 studies. The prevalences of ischemic stroke reported in each of these studies are summarized in Table 2.
Table 2. The prevalence of ischemic stroke among COVID-19 patients around the globe.
| Study design | Country | NOS | COVID-19 | Chronology of the symptoms | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ischemic
stroke |
Total
COVID-19 |
Not clear
(%) |
Respiratory
symptoms (%) |
Neurology
symptoms (%) |
|||||
| Case | Death | ||||||||
| Prospective cross-
sectional |
Spain | 7 | 10 | - | 2000 | - | 6 (1.10) | 4 (0.74) | 34 |
| Retrospective cohort | Spain | 8 | 17 | 5 | 1683 | 17 (3.13) | - | - | 35 |
| Retrospective cohort | US | 8 | 26 | 10 | 3218 | - | 9 (1.65) | 17 (3.13) | 36 |
| Retrospective cohort | UEA | 8 | 19 | 4 | 591 | 19 (3.49) | - | - | 37 |
| Retrospective cohort | Turkey | 7 | 7 | - | 239 | 7 (1.29) | - | - | 38 |
| Retrospective cohort | US | 8 | 72 | - | 10,596 | 72 (13.2) | - | - | 39 |
| Retrospective cohort | China | 8 | 10 | 5 | 219 | - | 7 (1.29) | 3 (0.55) | 40 |
| Retrospective cohort | US | 8 | 7 | - | 509 | 7 (1.29) | - | - | 41 |
| Retrospective cohort | Italy | 7 | 2 | - | 213 | 2 (0.37) | - | - | 42 |
| Retrospective cohort | US | 8 | 12 | - | 650 | - | 7 (1.29) | 5 (0.92) | 43 |
| Retrospective cohort | Italy | 8 | 37 | - | 1760 | 37 (6.80) | - | - | 44 |
| Retrospective cohort | UK | 8 | 6 | - | 3403 | 6 (1.10) | - | - | 45 |
| Retrospective cohort | Multi-
national |
8 | 123 | - | 17,799 | 123 (22.6) | - | - | 46 |
| Retrospective cohort | US | 7 | 1 | - | 90 | - | 1 (0.18) | - | 47 |
| Retrospective cohort | Multi-
national |
8 | 156 | 54 | 14,483 | 156 (28.68) | - | - | 48 |
| Retrospective cohort | China | 7 | 39 | 18 | 651 | 39 (7.17) | - | - | 49 |
| Total | 544 | 96 | 58,104 | 485/544
(89.1) |
30/544 (5.5) | 29/544 (5.3) | |||
NOS = Newcastle-Ottawa scale score, COVID-19 = coronavirus disease 19
Prevalence of hemorrhagic and ischemic stroke in COVID-19 patients
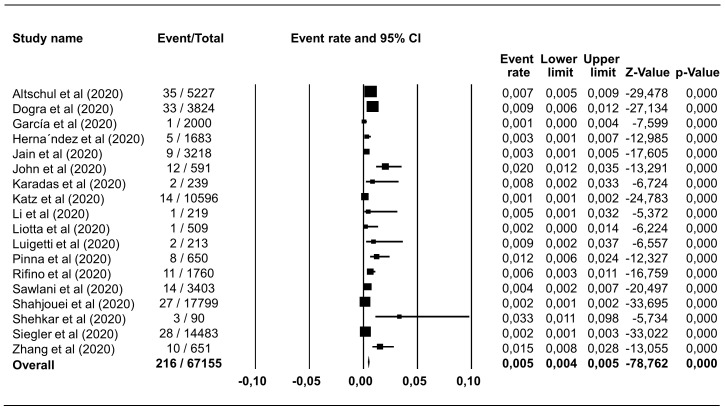
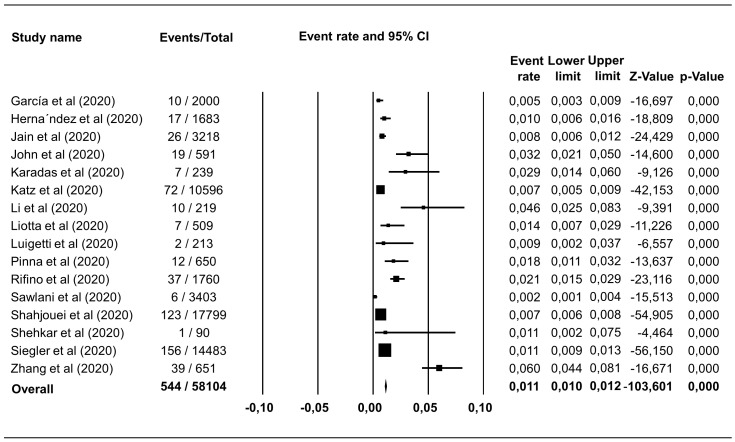
Hemorrhagic stroke was reported in 18 studies including 67,155 COVID-19 patients, with a pooled estimate of prevalence of 0.46% (95% CI 0.40%–0.53%), I 2=89.81% ( Figure 2). Ischemic stroke was identified in 544 of 58,104 COVID-19 patients in 16 studies, which corresponded to a pooled prevalence estimate of 1.11% (95% CI 1.03%–1.22%), I 2=94.07% ( Figure 3). The incidence of hemorrhagic and ischemic stroke in COVID-19 patients was 28.42% (216/760) and 71.58% (544/760), respectively.
Figure 2. Forest plot of the prevalence of haemorrhagic stroke in COVID-19 patients.
The pooled estimate of haemorrhagic stroke prevalence is 0.46% with 95% CI 0.40%–0.53%, p<0.0001; p-value for Egger and heterogeneity is 0.882 and <0.0001, respectively with I 2 89.81%.
Figure 3. Forest plot of the prevalence of ischemic stroke in COVID-19 patients.
The pooled estimate of ischemic stroke prevalence is 1.11% with 95% CI 1.03%–1.22%, p<0.0001; p-value for Egger and heterogeneity is 0.730 and <0.0001, respectively with I 2 94.07%.
Among COVID-19 patients who experienced hemorrhagic stroke, 45 of 216 patients (20.83%; 95% CI 15.41%–26.24%) complained of respiratory symptoms before neurological symptoms, while neurological symptoms preceded respiratory symptoms in 15 of 216 patients (6.94%; 95% CI 3.55%–10.33%). No clear onset of either neurological or respiratory symptoms in COVID-19 patients was reported in 11 studies (156/216, 72.22%) ( Table 1).
Among COVID-19 patients who experienced ischemic stroke, 30 of 544 patients (5.51%; 95% CI 3.59%–7.43%) experienced respiratory symptoms before neurological symptoms, while 29 of 544 patients (5.33%; 95% CI 3.44%–7.21%) complained of neurological symptoms before respiratory symptoms. No clear onset of either neurological or respiratory symptoms in COVID-19 patients was reported in 11 studies (485/544, 89.15%) ( Table 2).
Mortality rate of hemorrhagic and ischemic stroke in COVID-19 patients
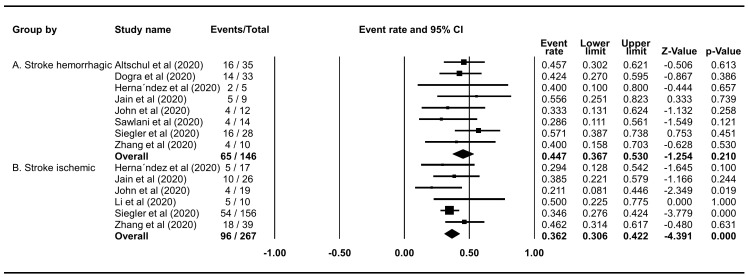
The mortality rate of COVID-19 patients who experienced hemorrhagic stroke was 44.72% (95% CI 36.73%–52.98%), and the rate was slightly lower in those who experienced ischemic stroke (36.23%; 95% CI 30.63%–42.24%) ( Figure 4).
Figure 4. Forest plot of the mortality rate of haemorrhagic and ischemic stroke in COVID-19 patients.
( A) The pooled mortality rate of haemorrhagic stroke is 44.72% (95% CI 36.73%–52.98%, p=0.2099); heterogeneity p=0.7343, I 2=0%, and Egger’s p=<0.0001. ( B) The pooled mortality rate of ischemic stroke is 36.23% (95% CI 30.63%–42.24%, p<0.0001); heterogeneity p=0.438, I 2 0%, and Egger’s p<0.0001.
Association between hemorrhagic and ischemic stroke and the severity and mortality of COVID-19
There was a lack of data regarding the association between hemorrhagic and ischemic stroke and the severity of the disease and mortality rate of patients with COVID-19. One study reported that 63% of COVID-19 patients who experienced ischemic stroke required admission to the intensive care unit (ICU) 50.
Discussion
Hemorrhagic stroke in COVID-19 patients
The cumulative incidence of hemorrhagic stroke in COVID-19 patients in the present study was 0.3% (216 of 67,155). This result is lower than a previously reported incidence (0.7%; 95% CI 0.50%–0.9%) 51. The incidence of hemorrhagic stroke among all stroke types in COVID-19 patients fluctuated, with 12.2% in May 2020 52, 9.7% in June 2020 53, 17.2% in July 2020 54, 11.6% in September 2020 55, and 28.42% in the present systematic review.
Among 216 COVID-19 patients who experienced hemorrhagic stroke, 20.83% were admitted to a hospital owing to respiratory symptoms and developed a brain hemorrhage during hospitalization, while 6.94% (15/216) were admitted owing to neurological symptoms. The disease course is important for predicting the severity of the systemic disease as COVID-19 in the former group was more severe with abnormal vital sign(s), elevated inflammatory and coagulopathy markers, altered mental status, and the patients likely required mechanical ventilation and ICU care 43. Diffuse microhemorrhages have been observed previously in COVID-19 patients, via brain imaging, and such microhemorrhages are scattered mostly in the juxtacortical white matter, corpus callosum, and brain stem 56– 61. The fatality rate of COVID-19 patients who experienced hemorrhagic stroke was 44.72% in our study, which is slightly lower than the rate reported previously (48.6%) 51.
Ischemic stroke in COVID-19 patients
The pooled prevalence of ischemic stroke among COVID-19 patients in our systematic review was 0.94% (544 of 58,104). This result is much lower than that described in another report in which acute ischemic stroke was observed in 1.6% of the patients who presented with or were hospitalized owing to COVID-19 62. Another study also reported increased incidence of ischemic stroke among COVID-19 patients compared to that in the non-COVID-19 group (81.4% vs. 74.6%) 63. The incidence of ischemic stroke in our investigation was significantly lower compared with that in another study (71.58% vs. 87.4%, respectively) 55.
COVID-19 patients who were admitted with respiratory symptoms and later experienced acute brain infarct comprised 5.51% (30 of 544) of the sample. Meanwhile, 5.33% were admitted owing to neurological symptoms related to ischemic stroke and had a positive result on real-time polymerase chain reaction (RT-PCR) testing for SARS-CoV-2 after screening; there was no clear explanation for hospital admission in the remainder (89.15%). This result is lower than that reported in a previous study, in which ischemic stroke was the reason for hospital admission in 26% of the COVID-19 patients 62.
The cause of ischemic stroke is multifactorial in those with SARS-CoV-2 infection, and it may be due to systemic embolization and diffuse microvascular thrombosis (attributed to a significant increase in prothrombotic factors) 53. In our systematic review, the mortality rate of COVID-19 patients who experienced ischemic stroke was 36.23%. This rate is higher than that in two previous studies, which reported inpatient mortality rates of 32% and 22.8% 50. among COVID-19 patients who experienced ischemic stroke.
Possible pathogenesis of hemorrhagic stroke in COVID-19 patients
Hemorrhagic stroke is caused by the rupture of cerebral vessels, leading to the extravasation of blood components into the surrounding brain tissue. However, the molecular mechanism by which SARS-CoV-2 infection causes hemorrhagic stroke remains unclear. The ACE2 receptor occupied by the virus appears to be the primary culprit, which then induces subsequent damage to host cells 64. Dysfunction of the ACE2 receptor is linked to the elevation of Ang II levels. Ang II is produced from Ang I, and this reaction is catalyzed by the action of ACE. Ang II-related effects are generated after being bound to the AT1 receptor. To counteract the dangerous effects caused by the excessive level of ACE/Ang II/AT1R axis, the ACE2/Ang (1-7)/Mas axis is activated 65.
SARS-CoV-2 infection increases Ang II levels. SARS-CoV-2 uses the ACE2 receptor as a portal to enter host cells 66. Along with a protease, i.e., TMPRSS2, this receptor assists the virus in infecting cells 66. Viral occupation of the ACE2 receptor affects the normal physiological function of the receptor, which is to degrade Ang II, resulting in the accumulation of Ang II in the blood. Elevated Ang II levels are associated with damage linked to the occurrence of hemorrhagic stroke 67.
A previous study proposed four modes of action used by Ang II to exert its effects (i.e., direct impact on the vascular system) causing vasoconstriction, promotion of platelet aggregation, increased free radical production, and a reduction in insulin sensitivity 68. These actions of Ang II are associated with the occurrence of hemorrhagic stroke. Vasoconstriction is a vital physiological alteration that occurs in hypertension, which is recognized as one of the major risk factors for hemorrhagic stroke 67. Ang II is also related to the activation of thrombogenic factors. This may explain the elevation of D-dimer levels, which are monitored in COVID-19 patients, particularly in those with severe infection 69, 70. Consequently, the activation of a procoagulant state may induce a hemorrhagic stroke 71. Ang II is also known to inhibit the PI3K/AKT signaling pathway, which regulates the secretion of insulin, leading to lowered insulin sensitivity 68, 72, which is another risk factor for hemorrhagic stroke. A study using a rat model confirmed that diabetes could degrade tight junction (TJ) proteins mediated by the action of matrix metalloproteinases (MMPs) 73. The correlation between junctional disruption, MMPs, and hemorrhagic stroke is described in the next section.
SARS-CoV-2 infection causes a cytokine storm that induces degradation of the extracellular matrix. When SARS-CoV-2 infects the body, the immune system produces massive amounts of pro-inflammatory cytokines in response. An excessive amount of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-6, has been reported in most COVID-19 patients 74. This phenomenon—known as the “cytokine storm”—results in the failure of multiple organs and contributes to COVID-19-related death. Ang II is strongly linked to the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which is responsible for the development of oxidative stress, a condition that has been known to be correlated with the excessive production of pro-inflammatory cytokines 67, 75. An in vitro study using a human BBB model revealed that the spike protein of SARS-CoV-2 could elevate the levels of IL-1β and IL-6 76. Several mechanisms have been proposed to explain the role of these cytokines in weakening vessel walls and the subsequent increase in the risk of hemorrhagic stroke, including its effect in the degradation of the extracellular matrix (ECM), which is the primary structure responsible for maintaining the integrity of vascular endothelial cells.
Degradation of the ECM caused by MMPs increases BBB permeability, promotes extravasation of blood components, and contributes to hemorrhagic brain injury 77. Many studies have reported that TNF-α can induce the production of MMPs, which are proteolytic enzymes that degrade the ECM. For example, a study reported that TNF-α administered intravenously to mice produced an elevation in the MMP-9 levels, followed by a significant increase in BBB permeability 78. Another study demonstrated that MMP-3 expression was upregulated in porcine choroid plexus epithelial cells, which was followed by a reduction in transepithelial electrical resistance, indicating decreased cellular tightness 79. Another pro-inflammatory cytokine, IL-1β, is also involved in the induction of MMPs, resulting in the destruction of the ECM. The expression and activity of MMP-2 in cardiac microvascular endothelial cells were induced by IL-1β 80. After experimenting with chondrocytes, a study has also revealed that IL-1β exposure leads to MMP-1 upregulation 81. This action is suggested to involve various signaling pathways (i.e., ERK1/2, JNKs) and protein kinase C (PKC) 80– 82. Studies have also reported the upregulation of MMP expression and activity in various models after exposure to IL-6. This cytokine increases MMP-9 activity during aortic aneurysms and ruptures in mice 83. MMP-2 and MMP-9 levels have also been found to be increased in COVID-19 patients, which is consistent with the elevation of IL-6 expression in patients with lymphoma 84. A STAT3 signaling pathway has been proposed as the pathway used by IL-6 to upregulate MMPs 83, 85.
Moreover, the impairment of ECM caused by those pro-inflammatory cytokines may be significantly associated with the action of reactive oxygen species (ROS), such as superoxide and singlet oxygen, and reactive nitrogen species, such as nitrogen oxide and peroxynitrite 86, 87. A study found that TNF-α and IL-6 administration in human brain microvascular endothelial cells (HBMVEC) induced increased levels of ROS 75. Oxidative stress-related BBB disruption leading to the incidence of stroke is strongly related to the activation of MMPs 88– 91.
Interestingly, MMPs could alter the regulation of junctional proteins. A study using a rat model demonstrated that TJ damage in cerebral vessels was mediated by MMP-2 and MMP-9, and that this action could be inhibited by the MMP inhibitor BB-1101 92. A study found that the degradation of occludin, a transmembrane protein of the TJ, in BBB model bEnd3 monolayer was mediated by MMP-2 93, while another study confirmed that MMP-9 mediated the destruction of TJ protein in a BBB model hCMEC/D3 94.
SARS-CoV-2 infection induces a cytokine storm that causes disturbance in junctional protein formation. Elevation of cytokine levels caused by SARS-CoV-2 infection could weaken vessel walls and ultimately increase the incidence of hemorrhagic stroke by impairing cellular junctional proteins, which is also the primary structure responsible for maintaining vascular endothelial cell integrity. The integrity of vascular endothelial cells is, in large part, determined by the presence of junctional proteins. In general, three major junctions are located in the BBB: TJs, adherens junctions (AJs), and gap junctions 95. Any disturbances occurring in any of these structures will ultimately lead to vascular endothelial dysfunction. A previous study proposed that serum levels of TJ proteins may be used to predict the incidence of a hemorrhagic event(s) following ischemic stroke 96.
Disruption of junctional proteins could be caused by pro-inflammatory cytokines. Using bEnd.3 endothelial cells as the BBB model, a previous study demonstrated that TNF-α and IL-6 exposure produced a significant increase in cellular permeability, which could be attributed to the decreased expression of ZO-1 and claudins 97. These findings are supported by a study investigating primary cerebral microvessels isolated from sheep, which revealed that 100 ng/mL of IL-6 reduced the expression of occludin 98. The expression of cadherin, occludin, and claudin-5 led to a dose-dependent decrease in a HBMVEC model after treatment with TNF-α and IL-6 75. Using human umbilical vein endothelial cells (HUVECs), another study reported that the expression of occludin and E-cadherin was downregulated following exposure to interferon (IFN)-γ 99. TNF-α treatment to HUVECs caused a change in localization of claudin-5 and JAM-A, while this cytokine also reduced the expression of occludin 100.
Damage to junctional proteins could also be associated with the disruption of polarity complex proteins. Polarity proteins work by regulating many aspects of cellular differentiation and proliferation, including junctional protein formation and localization 101, 102. Although the understanding of polarity complexes is mainly supported by a wide range of experiments involving epithelial cells, the complexes also play pivotal roles in endothelial cells 103, 104. Thus, impairments to polarity complexes could subsequently result in damage to transmembrane and cytoplasmic junctional proteins. For example, PATJ knockdown Caco2 cells affect the localization of occludin and ZO-3 in TJ formation 105. Another study reported that VE-cadherin, the major transmembrane protein of AJ, connected to Pals1 during the formation of vascular lumen indicating the specific role of this polarity protein in regulating junctional formation in endothelial cells 106. Interestingly, SARS-CoV-2 has been suggested to interact with the Pals1 protein in host cells through its envelope (E) protein 107. A study demonstrated that another betacoronavirus—SARS—also uses this mode of interaction with host cells 108.
The action of cytokines in downregulating junctional proteins could be mediated by the activation of NADPH oxidase 75. This enzyme is one of the main sources of ROS in the vascular system, along with mitochondrial enzymes and xanthin oxidase 109. It should be noted that the activation of NADPH oxidase is also linked to endothelial dysfunction leading to COVID-19-related thrombotic events 110. It has been proposed that SARS-CoV-2 induces a thrombosis event by stimulating various tissue factors that are dependent on the activation of NADPH oxidase following its attack on endothelial cells 111.
Collectively, hemorrhagic stroke in COVID-19 patients may be associated with the elevation of Ang II levels, which is an event subsequent to SARS-CoV-2 occupation of the ACE2 receptor. The cytokine storm is also responsible for the degradation of some important components of cerebral vessels, such as MMPs and TJ, triggering cerebral vascular rupture.
Possible pathogeneses of ischemic stroke in COVID-19 patients
SARS-CoV-2 infection could cause ischemic stroke through the induction of a hypercoagulative state, endothelial injury, cytokine storm, and/or cardiogenic embolism 21. Dysfunction of endothelial cells (induced by SARS-CoV-2 infection) may increase thrombin formation and fibrinolysis 112. Coagulopathy due to a thrombosis event has been observed in COVID-19 patients, with elevated D-dimer and fibrinogen, although with no significant prolonged prothrombin time and activated partial thromboplastin time 25, 26, 113. Increased fibrinogen levels also contribute to hyperviscosity, which is consistently found in COVID-19 patients, in whom viscosity varies between 1.9 to 4.2 centipoise (normal range, 1.4–1.8 centipoise) 23. Hyperviscosity is not only caused by increases in fibrinogen level, but also by the cytokine storm, which plays an important role in increasing viscosity levels in those with COVID-19 by inducing the excessive release of IL-6 and TNF-α 114, 115.
Systemic inflammation, which activates the complement pathway, induces the excessive release of inflammatory cytokines, causing venous thromboembolism by platelets and also inducing a hypercoagulative state 116– 118. This hypercoagulability could lead to macro- and microthrombus formation, which ultimately leads to cerebrovascular incidents 119, 120.
The ACE2 receptor also plays an important role in the neurological manifestations of SARS-CoV-2 infection. ACE2 converts Ang II into ANG (1-7), which plays an essential role as a neuroprotector. Administration of ANG (1-7) in animal models resulted in a decrease in neurological deficits and infarct size in rats with ischemic stroke 121, 122. Therefore, in COVID-19, the SARS-CoV-2 spike protein binds to the ACE2 receptor, resulting in decreased ANG (1-7) synthesis 123. Cardioembolic (19.21%) and atherothrombotic (7.39%) events have also been reported to contribute to the etiology of ischemic stroke in COVID-19 patients 124.
Conclusion
Although the prevalence of hemorrhagic and ischemic stroke is low in COVID-19 patients, this systematic review may increase awareness among clinicians regarding the potentially high mortality rate of individuals with this infection who experience a stroke, especially those with severe infection.
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Reporting guidelines
Figshare: PRISMA checklist for ‘Hemorrhagic and ischemic stroke in patients with coronavirus disease 2019: Incidence, risk factors, and pathogenesis - A systematic review and meta-analysis’, https://doi.org/10.6084/m9.figshare.13513509 28.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgement
Authors would like to thank HT Editorial Service in assisting during manuscript writing processes.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 3 approved]
Ethics statement
Not required.
References
- 1. Worldometers: COVID-19 coronavirus pandemic.[cited 2020 November 14]. Reference Source [Google Scholar]
- 2. Fan G, Yang Z, Lin Q, et al. : Decreased Case Fatality Rate of COVID-19 in the Second Wave: A study in 53 countries or regions. Transbound Emerg Dis. 2020. 10.1111/tbed.13819 [DOI] [PubMed] [Google Scholar]
- 3. Frediansyah A, Nainu F, Dhama K, et al. : Remdesivir and its antiviral activity against COVID-19: A systematic review. Clin Epidemiol Glob Health. 2021;9:123–127. 10.1016/j.cegh.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frediansyah A, Tiwari R, Sharun K, et al. : Antivirals for COVID-19: a critical review. Clin Epidemiol Glob Health. 2021;9:90–98. 10.1016/j.cegh.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mudatsir M, Yufika A, Nainu F, et al. : Antiviral Activity of Ivermectin Against SARS-CoV-2: An Old-Fashioned Dog with a New Trick—A Literature Review. Sci Pharm. 2020;88(3):36. 10.3390/scipharm88030036 [DOI] [Google Scholar]
- 6. WHO: Solidarity clinical trial for COVID-19 treatments. 2020. Reference Source [Google Scholar]
- 7. Harapan H, Itoh N, Yufika A, et al. : Coronavirus disease 2019 (COVID-19): A literature review. J Infect Public Health. 2020;13(5):667–673. 10.1016/j.jiph.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhama K, Patel SK, Pathak M, et al. : An update on SARS-CoV-2/COVID-19 with particular reference to its clinical pathology, pathogenesis, immunopathology and mitigation strategies. Travel Med Infect Dis. 2020;37:101755. 10.1016/j.tmaid.2020.101755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dhama K, Khan S, Tiwari R, et al. : Coronavirus Disease 2019-COVID-19. Clin Microbiol Rev. 2020;33(4):e00028–20. 10.1128/CMR.00028-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petersen E, Koopmans M, Go U, et al. : Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20(9):e238–e244. 10.1016/S1473-3099(20)30484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Wang Y, Chen Y, et al. : Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92(6):568–76. 10.1002/jmv.25748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. : Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rothstein A, Oldridge O, Schwennesen H, et al. : Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020;51(9):e219–e22. 10.1161/STROKEAHA.120.030995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dorgalaleh A, Dabbagh A, Tabibian S, et al. : Patients with Congenital Bleeding Disorders Appear to be Less Severely Affected by SARS-CoV-2: Is Inherited Hypocoagulability Overcoming Acquired Hypercoagulability of Coronavirus Disease 2019 (COVID-19)?Thieme Medical Publishers. Semin Thromb Hemost. 2020;46(7):853–855. 10.1055/s-0040-1713435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malas MB, Naazie IN, Elsayed N, et al. : Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. 10.1016/j.eclinm.2020.100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poyiadji N, Shahin G, Noujaim D, et al. : COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology. 2020;296(2):E119–E120. 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muhammad S, Petridisa A, Cornelius JF, et al. : Letter to editor: Severe brain haemorrhage and concomitant COVID-19 Infection: A neurovascular complication of COVID-19. Brain Behav Immun. 2020;87:150–151. 10.1016/j.bbi.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al Saiegh F, Ghosh R, Leibold A, et al. : Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. 2020;91(8):846–848. 10.1136/jnnp-2020-323522 [DOI] [PubMed] [Google Scholar]
- 19. Qureshi AI, Abd-Allah F, Al-Senani F, et al. : Management of acute ischemic stroke in patients with COVID-19 infection: Report of an international panel. Int J Stroke. 2020;15(5):540–554. 10.1177/1747493020923234 [DOI] [PubMed] [Google Scholar]
- 20. Sardu C, Gambardella J, Morelli M, et al. : Is COVID-19 an Endothelial Disease? Clinical and Basic Evidence. Preprints. 2020;2020040204. 10.20944/preprints202004.0204.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Markus HS, Brainin M: COVID-19 and stroke—A global World Stroke Organization perspective. Int J Stroke. 2020;15(4):361–4. 10.1177/1747493020923472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lodigiani C, Iapichino G, Carenzo L, et al. : Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maier CL, Truong AD, Auld SC, et al. : COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. 2020;395(10239):1758–1759. 10.1016/S0140-6736(20)31209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen G, Wu D, Guo W, et al. : Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang N, Bai H, Chen X, et al. : Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Helms J, Tacquard C, Severac F, et al. : High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–98. 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johansson K, Jansson JH, Johansson L, et al. : D-dimer is associated with first-ever intracerebral hemorrhage: A nested case-control study. Stroke. 2018;49(9):2034–2039. 10.1161/STROKEAHA.118.021751 [DOI] [PubMed] [Google Scholar]
- 28. Harapan H: Hemorrhagic and ischemic stroke in patients with coronavirus disease 2019: Incidence, risk factors, and pathogenesis - A systematic review and meta-analysis. figshare.Journal contribution.2021. 10.6084/m9.figshare.13513509.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stang A: Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 31. Cochrane T: Review Manager (RevMan) 5.3.Copenhagen: The Nordic Cochrane Centre.2008;373. Reference Source [Google Scholar]
- 32. Altschul DJ, Unda SR, de La Garza Ramos R, et al. : Hemorrhagic presentations of COVID-19: Risk factors for mortality. Clin Neurol Neurosurg. 2020;198:106112. 10.1016/j.clineuro.2020.106112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dogra S, Jain R, Cao M, et al. : Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29(8):104984. 10.1016/j.jstrokecerebrovasdis.2020.104984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. García-Moncó JC, Cabrera-Muras A, Collía-Fernández A, et al. : Neurological reasons for consultation and hospitalization during the COVID-19 pandemic. Neurol Sci. 2020;41(11):3031–8. 10.1007/s10072-020-04714-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hernández-Fernández F, Sandoval Valencia H, Barbella-Aponte RA, et al. : Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143(10):3089–3103. 10.1093/brain/awaa239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jain R, Young M, Dogra S, et al. : COVID-19 related neuroimaging findings: A signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. 10.1016/j.jns.2020.116923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. John S, Hussain SI, Piechowski-Jozwiak B, et al. : Clinical characteristics and admission patterns of stroke patients during the COVID 19 pandemic: A single center retrospective, observational study from the Abu Dhabi, United Arab Emirates. Clin Neurol Neurosurg. 2020;199:106227. 10.1016/j.clineuro.2020.106227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karadaş Ö, Öztürk B, Sonkaya AR: A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol Sci. 2020;41(8):1991–1995. 10.1007/s10072-020-04547-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Katz JM, Libman RB, Wang JJ, et al. : Cerebrovascular Complications of COVID-19. Stroke. 2020;51(9):e227–e231. 10.1161/STROKEAHA.120.031265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Y, Li M, Wang M, et al. : Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5(3):279–284. 10.1136/svn-2020-000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liotta EM, Batra A, Clark JR, et al. : Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7(11):2221–2230. 10.1002/acn3.51210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Luigetti M, Iorio R, Bentivoglio AR, et al. : Assessment of neurological manifestations in hospitalized patients with COVID-19. Eur J Neurol. 2020;27(11):2322–8. 10.1111/ene.14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pinna P, Grewal P, Hall JP, et al. : Neurological manifestations and COVID-19: Experiences from a tertiary care center at the Frontline. J Neurol Sci. 2020;415:116969. 10.1016/j.jns.2020.116969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rifino N, Censori B, Agazzi E, et al. : Neurologic manifestations in 1760 COVID-19 patients admitted to Papa Giovanni XXIII Hospital, Bergamo, Italy. J Neurol. 2020;1–8. 10.1007/s00415-020-10251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sawlani V, Scotton S, Nader K, et al. : COVID-19-related intracranial imaging findings: a large single-centre experience. Clin Radiol. 2021;76(2):108–116. 10.1016/j.crad.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shahjouei S, Naderi S, Li J, et al. : Risk of stroke in hospitalized SARS-CoV-2 infected patients: A multinational study. EBioMedicine. 2020;59:102939. 10.1016/j.ebiom.2020.102939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shekhar R, Sheikh AB, Suriya SS, et al. : Neurological Complications Among Native Americans with COVID-19: Our Experience at a Tertiary Care Academic Hospital in the U.S. J Stroke Cerebrovasc Dis. 2020;29(12):105260. 10.1016/j.jstrokecerebrovasdis.2020.105260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Siegler JE, Cardona P, Arenillas JF, et al. : Cerebrovascular events and outcomes in hospitalized patients with COVID-19: The SVIN COVID-19 Multinational Registry. Int J Stroke. 2020;1747493020959216. 10.1177/1747493020959216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang LJ, Sun WW, Wang YJ, et al. : Clinical Course and Mortality of Stroke Patients With Coronavirus Disease 2019 in Wuhan, China. Stroke. 2020;51(9):2674–82. 10.1161/STROKEAHA.120.030642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cagnazzo F, Arquizan C, Derraz I, et al. : Neurological manifestations of patients infected with the SARS-CoV-2: a systematic review of the literature. J Neurol. 2020;1–10. 10.1007/s00415-020-10285-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheruiyot I, Sehmi P, Ominde B, et al. : Intracranial hemorrhage in coronavirus disease 2019 (COVID-19) patients. Neurol Sci. 2020;1–9. 10.1007/s10072-020-04870-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bhatia R, Pedapati R, Komakula S, et al. : Stroke in Coronavirus Disease 2019: A Systematic Review. J Stroke. 2020;22(3):324–35. 10.5853/jos.2020.02264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee KW, Yusof Khan AHK, Ching SM, et al. : Stroke and Novel Coronavirus Infection in Humans: A Systematic Review and Meta-Analysis. Front Neurol. 2020;11(1196):579070. 10.3389/fneur.2020.579070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Siow I, Lee KS, Zhang JJY, et al. : Stroke as a Neurological Complication of COVID-19: A Systematic Review and Meta-Analysis of Incidence, Outcomes and Predictors. J Stroke Cerebrovasc Dis. 2020;30(3):105549. 10.1016/j.jstrokecerebrovasdis.2020.105549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nannoni S, de Groot R, Bell S, et al. : Stroke in COVID-19: A systematic review and meta-analysis. Int J Stroke. 2020;1747493020972922. 10.1177/1747493020972922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vattoth S, Abdelhady M, Alsoub H, et al. : Critical illness-associated cerebral microbleeds in COVID-19. Neuroradiol J. 2020;33(5):374–6. 10.1177/1971400920939229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gupta NA, Lien C, Iv M: Critical illness-associated cerebral microbleeds in severe COVID-19 infection. Clin Imaging. 2020;68:239–41. 10.1016/j.clinimag.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fitsiori A, Pugin D, Thieffry C, et al. : COVID-19 is Associated with an Unusual Pattern of Brain Microbleeds in Critically Ill Patients. J Neuroimaging. 2020;30(5):593–7. 10.1111/jon.12755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cannac O, Martinez-Almoyna L, Hraiech S: Critical illness-associated cerebral microbleeds in COVID-19 acute respiratory distress syndrome. Neurology. 2020;95(11):498–499. 10.1212/WNL.0000000000010537 [DOI] [PubMed] [Google Scholar]
- 60. Dixon L, McNamara C, Gaur P, et al. : Cerebral microhaemorrhage in COVID-19: a critical illness related phenomenon? Stroke Vasc Neurol. 2020;5(4):315–322. 10.1136/svn-2020-000652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fitsiori A, Pugin D, Thieffry C, et al. : COVID-19 is Associated with an Unusual Pattern of Brain Microbleeds in Critically Ill Patients. J Neuroimaging. 2020;30(5):593–597. 10.1111/jon.12755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Merkler AE, Parikh NS, Mir S, et al. : Risk of Ischemic Stroke in Patients With Coronavirus Disease 2019 (COVID-19) vs Patients With Influenza. JAMA Neurol. 2020;77(11):1366–72. 10.1001/jamaneurol.2020.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Benussi A, Pilotto A, Premi E, et al. : Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020;95(7):e910–e920. 10.1212/WNL.0000000000009848 [DOI] [PubMed] [Google Scholar]
- 64. Spence JD, de Freitas GR, Pettigrew LC, et al. : Mechanisms of Stroke in COVID-19. Cerebrovasc Dis. 2020;49(4):451–8. 10.1159/000509581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Regenhardt RW, Bennion DM, Sumners C: Cerebroprotective action of angiotensin peptides in stroke. Clin Sci (Lond). 2014;126(3):195–205. 10.1042/CS20130324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hoffmann M, Kleine-Weber H, Schroeder S, et al. : SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–80.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Qureshi AI, Abd-Allah F, Al-Senani F, et al. : Management of acute ischemic stroke in patients with COVID-19 infection: Report of an international panel. Int J Stroke. 2020;15(5):540–54. 10.1177/1747493020923234 [DOI] [PubMed] [Google Scholar]
- 68. Dandona P, Dhindsa S, Ghanim H, et al. : Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007;21(1):20–7. 10.1038/sj.jhh.1002101 [DOI] [PubMed] [Google Scholar]
- 69. Paliogiannis P, Mangoni AA, Dettori P, et al. : D-Dimer Concentrations and COVID-19 Severity: A Systematic Review and Meta-Analysis. Front Public Health. 2020;8:432. 10.3389/fpubh.2020.00432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yu HH, Qin C, Chen M, et al. : D-dimer level is associated with the severity of COVID-19. Thromb Res. 2020;195:219–225. 10.1016/j.thromres.2020.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. An SJ, Kim TJ, Yoon BW: Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update. J Stroke. 2017;19(1):3–10. 10.5853/jos.2016.00864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang X, Liu G, Guo J, et al. : The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14(11):1483–96. 10.7150/ijbs.27173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hawkins BT, Lundeen TF, Norwood KM, et al. : Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat:contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50(1):202–11. 10.1007/s00125-006-0485-z [DOI] [PubMed] [Google Scholar]
- 74. Song P, Li W, Xie J, et al. : Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. 10.1016/j.cca.2020.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rochfort KD, Collins LE, Murphy RP, et al. : Downregulation of Blood-Brain Barrier Phenotype by Proinflammatory Cytokines Involves NADPH Oxidase-Dependent ROS Generation: Consequences for Interendothelial Adherens and Tight Junctions. PLoS One. 2014;9(7):e101815. 10.1371/journal.pone.0101815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, et al. : The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol Dis. 2020;146:105131. 10.1016/j.nbd.2020.105131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lapchak PA, Wu Q: Vascular Dysfunction in Brain Hemorrhage: Translational Pathways to Developing New Treatments from Old Targets. J Neurol Neurophysiol. 2011;2011:S1–e001. 10.4172/2155-9562.S1-e001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tsuge M, Yasui K, Ichiyawa T, et al. : Increase of tumor necrosis factor-alpha in the blood induces early activation of matrix metalloproteinase-9 in the brain. Microbiol Immunol. 2010;54(7):417–24. 10.1111/j.1348-0421.2010.00226.x [DOI] [PubMed] [Google Scholar]
- 79. Zeni P, Doepker E, Schulze-Topphoff U, et al. : MMPs contribute to TNF-alpha-induced alteration of the blood-cerebrospinal fluid barrier in vitro. Am J Physiol Cell Physiol. 2007;293(3):C855–64. 10.1152/ajpcell.00470.2006 [DOI] [PubMed] [Google Scholar]
- 80. Mountain DJ, Singh M, Menon B, et al. : Interleukin-1beta increases expression and activity of matrix metalloproteinase-2 in cardiac microvascular endothelial cells: role of PKCalpha/beta1 and MAPKs. Am J Physiol Cell Physiol. 2007;292(2):C867–75. 10.1152/ajpcell.00161.2006 [DOI] [PubMed] [Google Scholar]
- 81. Raymond L, Eck S, Mollmark J, et al. : Interleukin-1 beta induction of matrix metalloproteinase-1 transcription in chondrocytes requires ERK-dependent activation of CCAAT enhancer-binding protein-beta. J Cell Physiol. 2006;207(3):683–8. 10.1002/jcp.20608 [DOI] [PubMed] [Google Scholar]
- 82. Bauer J, Huy C, Brenmoehl J, et al. : Matrix metalloproteinase-1 expression induced by IL-1β requires acid sphingomyelinase. FEBS Lett. 2009;583(5):915–20. 10.1016/j.febslet.2009.02.008 [DOI] [PubMed] [Google Scholar]
- 83. Ju X, Ijaz T, Sun H, et al. : IL-6 regulates extracellular matrix remodeling associated with aortic dilation in a fibrillin-1 hypomorphic mgR/mgR mouse model of severe Marfan syndrome. J Am Heart Assoc. 2014;3(1):e000476. 10.1161/JAHA.113.000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kossakowska AE, Edwards DR, Prusinkiewicz C, et al. : Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin's lymphomas. Blood. 1999;94(6):2080–9. [PubMed] [Google Scholar]
- 85. Huang X, Dai S, Dai J, et al. : Luteolin decreases invasiveness, deactivates STAT3 signaling, and reverses interleukin-6 induced epithelial-mesenchymal transition and matrix metalloproteinase secretion of pancreatic cancer cells. Onco Targets Ther. 2015;8:2989–3001. 10.2147/OTT.S91511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Siwik DA, Colucci WS: Regulation of Matrix Metalloproteinases by Cytokines and Reactive Oxygen/Nitrogen Species in the Myocardium. Heart Fail Rev. 2004;9(1):43–51. 10.1023/B:HREV.0000011393.40674.13 [DOI] [PubMed] [Google Scholar]
- 87. Yang C, Hawkins KE, Doré S, et al. : Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. 2019;316(2):C135–c53. 10.1152/ajpcell.00136.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Freeman LR, Keller JN: Oxidative stress and cerebral endothelial cells: regulation of the blood-brain-barrier and antioxidant based interventions. Biochim Biophys Acta. 2012;1822(5):822–9. 10.1016/j.bbadis.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gu Y, Dee CM, Shen J: Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front Biosci (Schol Ed). 2011;3:1216–31. 10.2741/222 [DOI] [PubMed] [Google Scholar]
- 90. Jian Liu K, Rosenberg GA: Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic Biol Med. 2005;39(1):71–80. 10.1016/j.freeradbiomed.2005.03.033 [DOI] [PubMed] [Google Scholar]
- 91. Lakhan SE, Kirchgessner A, Tepper D, et al. : Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol. 2013;4:32. 10.3389/fneur.2013.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yang Y, Estrada EY, Thompson JF, et al. : Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27(4):697–709. 10.1038/sj.jcbfm.9600375 [DOI] [PubMed] [Google Scholar]
- 93. Liu J, Jin X, Liu KJ, et al. : Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32(9):3044–57. 10.1523/JNEUROSCI.6409-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brilha S, Ong CWM, Weksler B, et al. : Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis. Sci Rep. 2017;7(1):16031. 10.1038/s41598-017-16250-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Keep RF, Andjelkovic AV, Xiang J, et al. : Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. J Cereb Blood Flow Metab. 2018;38(8):1255–1275. 10.1177/0271678X18774666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kazmierski R, Michalak S, Wencel-Warot A, et al. : Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients. Neurology. 2012;79(16):1677–85. 10.1212/WNL.0b013e31826e9a83 [DOI] [PubMed] [Google Scholar]
- 97. Voirin AC, Perek N, Roche F: Inflammatory stress induced by a combination of cytokines (IL-6, IL-17, TNF-α) leads to a loss of integrity on bEnd.3 endothelial cells in vitro BBB model. Brain Research. 2020;1730:146647. 10.1016/j.brainres.2020.146647 [DOI] [PubMed] [Google Scholar]
- 98. Cohen SS, Min M, Cummings EE, et al. : Effects of Interleukin-6 on the Expression of Tight Junction Proteins in Isolated Cerebral Microvessels from Yearling and Adult Sheep. Neuroimmunomodulation. 2013;20(5):264–73. 10.1159/000350470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Feng J, Ito M, Ichikawa K, et al. : Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274(52):37385–90. 10.1074/jbc.274.52.37385 [DOI] [PubMed] [Google Scholar]
- 100. Ozaki H, Ishii K, Horiuchi H, et al. : Cutting edge: combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J Immunol. 1999;163(2):553–7. [PubMed] [Google Scholar]
- 101. Assémat E, Bazellières E, Pallesi-Pocachard E, et al. : Polarity complex proteins. Biochim Biophys Acta. 2008;1778(3):614–30. 10.1016/j.bbamem.2007.08.029 [DOI] [PubMed] [Google Scholar]
- 102. Bauer HC, Krizbai IA, Bauer H, et al. : “You Shall Not Pass”—tight junctions of the blood brain barrier. Front Neurosci. 2014;8:392. 10.3389/fnins.2014.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kruse C, Kurz ARM, Pálfi K, et al. : Polarity Protein Scrib Facilitates Endothelial Inflammatory Signaling. Arterioscler Thromb Vasc Biol. 2015;35(9):1954–62. 10.1161/ATVBAHA.115.305678 [DOI] [PubMed] [Google Scholar]
- 104. Lizama CO, Zovein AC: Polarizing pathways: balancing endothelial polarity, permeability, and lumen formation. Exp Cell Res. 2013;319(9):1247–54. 10.1016/j.yexcr.2013.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Michel D, Arsanto JP, Massey-Harroche D, et al. : PATJ connects and stabilizes apical and lateral components of tight junctions in human intestinal cells. J Cell Sci. 2005;118(Pt 17):4049–57. 10.1242/jcs.02528 [DOI] [PubMed] [Google Scholar]
- 106. Brinkmann BF, Steinbacher T, Hartmann C, et al. : VE-cadherin interacts with cell polarity protein Pals1 to regulate vascular lumen formation. Mol Biol Cell. 2016;27(18):2811–21. 10.1091/mbc.E16-02-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. De Maio F, Lo Cascio E, Babini G, et al. : Improved binding of SARS-CoV-2 Envelope protein to tight junction-associated PALS1 could play a key role in COVID-19 pathogenesis. Microbes Infect. 2020;22(10):592–7. 10.1016/j.micinf.2020.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Teoh KT, Siu YL, Chan WL, et al. : The SARS coronavirus E protein interacts with PALS1 and alters tight junction formation and epithelial morphogenesis. Mol Biol Cell. 2010;21(22):3838–52. 10.1091/mbc.E10-04-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rajendran P, Rengarajan T, Thangavel J, et al. : The vascular endothelium and human diseases. Int J Biol Sci. 2013;9(10):1057–69. 10.7150/ijbs.7502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Violi F, Oliva A, Cangemi R, et al. : Nox2 activation in Covid-19. Redox Biol. 2020;36:101655. 10.1016/j.redox.2020.101655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. DiNicolantonio JJ, McCarty M: Thrombotic complications of COVID-19 may reflect an upregulation of endothelial tissue factor expression that is contingent on activation of endosomal NADPH oxidase. Open Heart. 2020;7(1):e001337. 10.1136/openhrt-2020-001337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Schmitt FCF, Manolov V, Morgenstern J, et al. : Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care. 2019;9(1):19. 10.1186/s13613-019-0499-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Guan Wj, Ni Zy, Hu Y, et al. : Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hirano T, Murakami M: COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity. 2020;52(5):731–733. 10.1016/j.immuni.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hu B, Huang S, Yin L: The cytokine storm and COVID-19. J Med Virol. 2020;10:1002. 10.1002/jmv.26232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vazquez-Garza E, Jerjes-Sanchez C, Navarrete A, et al. : Venous thromboembolism: thrombosis, inflammation, and immunothrombosis for clinicians. J Thromb Thrombolysis. 2017;44(3):377–385. 10.1007/s11239-017-1528-7 [DOI] [PubMed] [Google Scholar]
- 117. Bucciarelli P, Martinelli I, Artoni A, et al. : Circulating microparticles and risk of venous thromboembolism. Thromb Res. 2012;129(5):591–7. 10.1016/j.thromres.2011.08.020 [DOI] [PubMed] [Google Scholar]
- 118. Magro C, Mulvey JJ, Berlin D, et al. : Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1–13. 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Qi X, Keith KA, Huang JH: COVID-19 and stroke: A review. Brain Hemorrhages. 2020. 10.1016/j.hest.2020.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bikdeli B, Madhavan MV, Jimenez D, et al. : COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950–2973. 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mecca AP, Regenhardt RW, O’Connor TE, et al. : Cerebroprotection by angiotensin‐(1–7) in endothelin‐1‐induced ischaemic stroke. Exp physiol. 2011;96(10):1084–96. 10.1113/expphysiol.2011.058578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sampaio WO, Nascimento AAS, Santos RAS: Systemic and regional hemodynamic effects of angiotensin-(1– 7) in rats. Am J Physiol Heart Circ Physiol. 2003;284(6):H1985–94. 10.1152/ajpheart.01145.2002 [DOI] [PubMed] [Google Scholar]
- 123. Sweid A, Hammoud B, Bekelis K, et al. : Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int J Stroke. 2020;15(7):733–742. 10.1177/1747493020937189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fraiman P, Junior CG, Moro E, et al. : COVID-19 and Cerebrovascular Diseases: A Systematic Review and Perspectives for Stroke Management. Front Neurol. 2020;11:574694. 10.3389/fneur.2020.574694 [DOI] [PMC free article] [PubMed] [Google Scholar]