Abstract
Purpose
This study aimed to explore the role of the long non-coding RNA (lncRNA) RNA component of mitochondrial RNAase P (RMRP) in sepsis-induced acute kidney injury (AKI).
Materials and Methods
Venous blood was collected from septic patients and healthy people. C57BL/6 mice who underwent cecal ligation and puncture (CLP) were used as in vivo models of septic AKI. Lipopolysaccharide (LPS)-induced HK-2 cells were employed as in vitro models of AKI. Flow cytometry analysis was conducted to detect cell apoptosis. Enzyme-linked immunosorbent assay and Western blot assays were used to detect levels of pro-inflammatory cytokines.
Results
RMRP was upregulated in sera from patients with AKI and in LPS-induced cells. Knockdown of RMRP inhibited cell apoptosis and reduced production of inflammatory factors in LPS-induced cells, as well as alleviated AKI in CLP mice. RMRP facilitated inflammation by activating NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome. We found that microRNA 206 (miR-206) binds with and is negatively regulated by RMRP: miR-206 directly targets the 3′ untranslated region of DEAD-box helicase 5 (DDX5) and negatively regulates DDX5 expression. By binding with miR-206, RMRP upregulated DDX5 expression. Rescue assays revealed that overexpression of DDX5 counteracted the effect of RMRP inhibition on cell apoptosis and inflammatory response in LPS-induced cells.
Conclusion
The lncRNA RMRP contributes to sepsis-induced AKI through upregulation of DDX5 in a miR-206 dependent manner and through activation of NLRP3 inflammasome. This novel discovery may provide a potential strategy for treating AKI.
Keywords: RMRP, miR-206, DDX5, sepsis-induced AKI, NLRP3 inflammasome
INTRODUCTION
Sepsis, a systemic inflammatory response syndrome resulting from bacterial, fungal, or viral infections, can result in organ dysfunction, shock, and even death,1 and has become a leading cause of death in intensive care units.2 Acute kidney injury (AKI), a common and severe complication of sepsis, is characterized by sudden or chronic renal dysfunction,3 and severe sepsis accounts for about 50% of all AKI cases.4,5
Long non-coding RNAs (lncRNAs), a subgroup of non-coding RNAs (ncRNAs) of more than 200 nucleotides in length, are unable to encode proteins.6 Increasing research has indicated that lncRNAs exert crucial effects on biological processes, including cell proliferation, differentiation, apoptosis, inflammation, and fibrosis.7 Some lncRNAs, including NEAT1, TUG1, and HOTAIR, have been identified as biomarkers for sepsis-induced AKI.8,9 Recently, the lncRNA RNA component of mitochondrial RNAase P (RMRP) has been found to participate in various diseases, such as acute leukemias, acute myocardial infarction, and rheumatoid arthritis.10,11,12 Interestingly, RMRP has been reported to modulate cardiomyocyte apoptosis in lipopolysaccharide (LPS)-induced septic mice.13 However, the role of RMRP in sepsis-induced AKI has not been investigated.
LncRNAs have been revealed to serve as competitive endogenous RNAs (ceRNAs) in many diseases. The ceRNA network refers to the competitive binding of lncRNAs with microRNAs (miRNAs) to suppress the silencing effect of miRNAs on target messenger RNAs (mRNAs).14 MiRNAs, another class of ncRNAs with a length of 19–25 nucleotides, are encoded by the genome of higher eukaryotes.15 MiRNAs have been widely reported to be involved in sepsis-induced AKI: for instance, miR-21-3p affects the metabolism of renal tubular epithelial cells via the AKT/CDK2-FOXO1 pathway in sepsis-induced AKI.16 MiR-107 aggravates tubular cell injury by promoting secretion of tumor necrosis factor-α (TNF-α) in endothelial cells in patients with septic AKI.17 Increased levels of microRNA 206 (miR-206) have been revealed to be associated with enhanced regeneration and decreased fibrosis of dystrophic muscles.18 MiR-206 targets the 3'-untranlated regions of SOD1 to increase reactive oxygen species levels and aggravate pulmonary inflammatory responses in asthmatic mice.19 MiR-206 attenuates neuropathic pain in rats via the MEK/ERK pathway by targeting BDNF.20 However, the role of miR-206 in sepsis-induced AKI remains uninvestigated.
DEAD-box helicase 5 (DDX5) protein, also known as p68, is a member of superfamily 2 helicases and has been reported to regulate inflammation and apoptosis.21,22 As an example, knockdown of DDX5 has been shown to selectively inhibit the phosphorylation of NF-κB (Ser 311) subunit and to reduce protein levels of anti-apoptotic factor Bcl-2.23 However, the role of DDX5 remains unclear in sepsis-induced AKI. In our study, we aimed to investigate the role of RMRP in sepsis-induced AKI. At first, we analyzed the expression of RMRP in patients with sepsis. Then, we investigated the biological functions and regulatory networks of RMRP during the progression of sepsis-induced AKI. We discovered that RMRP aggravates sepsis-induced AKI by binding with miR-206 to upregulate DDX5 and by activating NLRP3 inflammasome.
MATERIALS AND METHODS
Patients and sample collection
Septic patients (including severe sepsis and septic shock; n=48) with AKI and healthy individuals (n=25) from the Affiliated Hospital of Nantong University (Nantong, Jiangsu, China) were recruited in this study. Septic patients were defined using American College of Chest Physicians/Society of Critical Care Medicine criteria.24,25 Patients with urinary tract infection or patients who had received anti-sepsis treatment were excluded from our study. Venous blood was collected from septic patients and healthy people, and preserved by anticoagulation with 1 mL of edathamil (EDTA-K2, Solarbio, Beijing, China). Serum was obtained by centrifugation after coagulation by 4 mL of EDTA-K2 and stored at −20℃. Each patient provided written informed consent in advance of the study, and the study was approved by the ethics committee of the Affiliated Hospital of Nantong University (Jiangsu) (IRB No. 2018-063).
Establishment of a sepsis model
The septic AKI model was developed as described before.26 C57BL/6 mice (Vital River Co. Ltd, Beijing, China; male, 20–25 g, n=40) were housed independently at 20–25℃ in 50% humidity and were randomly divided into four groups (n=10 in each group): sham group, cecal ligation and puncture (CLP) group, CLP+AAV-sh-NC group, and CLP+AAV-sh-RMRP group. In the CLP group, mice were anesthetized by intraperitoneal injection of 2% sodium pentobarbital (80 mg/kg). Then, the mice were placed on the laboratory bench, and the abdomen was then shaved and disinfected with 70% isopropanol. Subsequently, an incision (1.5 cm) was made at the midline of the mice abdomen to fully expose the cecum. Next, sterile sewing silk (no. 4) was used to ligature the cecum (1 cm away from the cecum tail). Afterwards, the blind end was perforated by a 20-gauge needle, and little feces were squeezed. Lastly, we sutured the abdominal incision after restoring the cecum to the abdominal cavity, and the mice were moved to a thermostatic blanket for rewarming. The mice in the sham group underwent a similar operation without CLP. All experimental procedures were conducted in accordance with the instructions of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Adeno-associated virus
Adeno-associated virus (AAV) (serotype 2) containing the sequences of short hairpin RNA (sh-RMRP) or short hairpin negative control (sh-NC) were bought from Vigene Biosciences (Shanghai, China) and injected (1012 v.g/mL) into mice via the tail vein. The mice were sacrificed 28 days after injection. Finally, the left kidneys were dissected, and blood was collected for biochemical assays.
Cell treatment and transfection
Apoptosis or necrosis of proximal tubule epithelial cells is the most common cause of AKI. LPS-induced human proximal tubule tubular epithelial cells, HK-2 cells, are widely used in in vitro models of septic AKI.27 HK-2 cell lines were bought from BeNa Culture Collection (Beijing, China) and cultivated in Dulbecco's Modified Eagle's Medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/mL of penicillin, and 100 µg/mL of streptomycin (Sigma, Saint Louis, MO, USA) at 37℃ in 5% CO2. For cell treatment, HK-2 cells were treated with 100 ng/mL of LPS for 24 h, and HK-2 cells in the control group were treated with same dose of cell culture medium (Con). For cell transfection, sh-RMRP with sh-NC as a control, miR-206 mimics with NC mimics as a control, and pcDNA3.1/DDX5 (DDX5) with empty pcDNA3.1 as a control were transfected or co-transfected into HK-2 cells by Lipofectamine 2000 following the manufacturer's instructions. All plasmids were obtained from GenePharma (Shanghai, China).
Renal function measurement
The concentrations of blood urea nitrogen (BUN) and serum creatinine in the sera of mice were, respectively, detected by a Hitachi 7060 automatic biochemistry analyzer (Hitachi, Tokyo, Japan) and a creatinine assay kit (BioAssay Systems, Hayward, CA, USA) according to the manufacturer's instructions.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The extraction of total RNA from HK-2 cells and kidney tissue samples was performed using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). The RevertAid First Strand cDNA Synthesis Kit (K1622, Thermo Scientific, Waltham, MA, USA) and TaqMan microRNA assay kit (Applied Biosystems, Waltham, MA, USA) were used for reverse transcription. qPCR was carried out with the SYBR-Green PCR Master Mix kit (Applied Biosystems) or TaqMan miRNA assay kit (Applied Biosystems). The relative expression levels of RNAs were calculated with the 2–ΔΔCt method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and RNU6 (U6) served as control genes. The primer sequences were as follows:
RMRP: 5′-ACT CCA AAG TCC GCC AAG A-3′ (forward), 5′-TGC GTA ACT AGA GGG AGC TGA C-3′ (reverse); miR-206: 5′-CGG GCT TGT GGA ATG GTA AGC-3′ (forward), 5′-GCT TCG GCA GCA CAT ATA CTA AAA T-3′ (reverse); DDX5: 5′-GGC CTG ATC ACA GAA CCA TT-3′ (forward), 5′-ACC ACC CTT ATT CCC AAA CC-3′ (reverse); U6: 5′-CGC TTC ACG AAT TTG CGT GTC AT-3′ (forward), 5′-ATG GAA CGC TTC ACG A-3′ (reverse); and GAPDH: 5′-TAT GAT GAT ATC AAG AGG GTA GT-3′ (forward), 5′-TGT ATC CAA ACT CAT TGT CAT AC-3′ (reverse).
Hematoxylin-eosin staining
Tissue samples were isolated and fixed in 10% formalin for 48 h and then embedded in paraffin. Sections (4 µm) containing renal tissues were prepared from the wax blocks and were stained with hematoxylin and eosin. Pathological changes in mice kidneys were analyzed using a light microscope as described previously.28
Enzyme-linked immunosorbent assay
Protein levels of TNF-α, interleukin-6 (IL-6), and interleukin-1β (IL-1β) in the supernatants of HK-2 cells or kidney tissues of mice in each group were measured with enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions.
Apoptosis assay
After transfection for 48 h, cells were collected in a flow tube and centrifuged at 1000 r/min for 5 min, after which the supernatant was removed. In accordance with the instructions of the Annexin-V fluorescein isothiocyanate (FITC) cell apoptosis detection kit (Sigma), cells in each flow tube were added with 150 µL of binding buffer and 5 µL of Annexin-V-FITC and mixed. Then, the cells were cultured at room temperature for 15 min in the dark. Next, 100 µL of binding buffer and 5 µL of propidium iodide (PI; Sigma) were added to the cells and mixed. Cell apoptosis was detected by flow cytometry (FACSCanto II, BD Biosciences, Hercules, CA, USA).
Western blot analysis
Cells or kidney tissue samples were lysed with lysis buffer containing protease inhibitors. Equal amounts of protein were dissolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to polyvinylidene fluoride membranes. After being blocked with 5% nonfat milk, the membranes were immunoblotted with primary antibodies against B-cell lymphoma-2 (Bcl-2), B-cell lymphoma-Associated X (Bax), cleaved cysteinyl aspartate specific proteinase (caspase)-1, Apoptosis associated speck-like protein containing caspase recruitment domain (ASC), Recombinant Human NACHT, LRR and PYD domains-containing protein 3 (NLRP3), DDX5, and GAPDH at a dilution of 1:1000 overnight at 4℃. Following washing, the membranes were exposed to horseradish peroxidase-conjugated goat anti-rabbit (1:5000, Abcam, Cambridge, UK) secondary antibodies, and signals were detected with the enhanced chemiluminescence detection system. All antibodies mentioned above were purchased from Abcam (Cambridge).
Luciferase reporter assay
Wild-type (Wt) or mutant (Mut) binding sequences of RMRP or DDX5 3'UTR on miR-206 were subcloned into pmirGLO dual-luciferase vector to construct RMRP-Wt, RMRP-Mut, DDX5-Wt, and DDX5-Mut vectors. Then, wild-type or mutant pmir-GLO-RMRP or pmirGLO-DDX5 was transfected with miR-206 mimics or NC mimics. After 48 h, luciferase activity was detected by the dual luciferase reporter assay system (Promega, Madison, WI, USA). The vectors used in this assay were obtained from GenePharma (Shanghai, China).
Statistical analysis
All statistical analyses were conducted utilizing SPSS software (version 19.0; IBM, Corp., Armonk, NY, USA). The data are presented as means±standard deviations. Unpaired Student's t test was employed to compare differences between groups. One-way analysis of variance was conducted for comparisons of more than two groups. Only p values less than 0.05 were considered significant.
RESULTS
Knockdown of RMRP inhibits cell apoptosis and inflammation
RMRP reportedly prevents the apoptosis of cardiomyocytes in LPS-induced septic mice via the miR-1-5p/hsp70 axis.13 To assess the role of RMRP in sepsis-induced AKI, we collected sera from septic patients with AKI and from healthy individuals, as well as established cell models by treating HK-2 cells with LPS. According to the results of RT-qPCR, RMRP expression was upregulated in the sera of septic patients with AKI, compared with that from healthy individuals (Fig. 1A). Similarly, LPS stimulation increased RMRP expression in HK-2 cells (Fig. 1B). Transfection of sh-RMRP significantly reduced RMRP levels in LPS-induced HK-2 cells (Fig. 1C). Functionally, flow cytometry assay demonstrated that cell apoptosis rates increased upon treatment with LPS, and knockdown of RMRP reversed the effect induced by LPS on cell apoptosis (Fig. 1D). The results of Western blot showed that LPS-induced increases in Bax expression and decreases in Bcl-2 expression were counteracted by knockdown of RMRP in HK-2 cells (Fig. 1E). Finally, ELISA suggested that LPS stimulation increased the levels of TNF-α, IL-6, and IL-1β, and these results were restored by knockdown of RMRP (Fig. 1F). These findings demonstrated that knockdown of RMRP inhibits cell apoptosis and the production of inflammatory factors in in vitro models of sepsis-induced AKI.
Fig. 1. Knockdown of RMRP inhibits cell apoptosis and inflammation. (A) RT-qPCR analysis of the expression of RMRP in sera from healthy people (n=25) and sepsis patients with AKI (n=48). (B) The expression of RMRP in LPS-induced HK-2 cells was confirmed by RT-qPCR analysis. (C) RT-qPCR analysis was conducted to evaluate the knockdown efficiency of RMRP in LPS-induced HK-2 cells. (D) Flow cytometry analysis was carried out to explore cell apoptosis rates. (E) The protein levels of Bax and Bcl-2 were analyzed by Western blotting. (F) ELISA was used to detect the levels of TNF-α, IL-6, and IL-1β in HK-2 cells. *p<0.05. RT-qPCR, reverse transcription quantitative polymerase chain reaction; LPS, lipopolysaccharide; RMRP, RNA component of mitochondrial RNAase P; DDX5, DEAD-box helicase 5; NLRP3, NACHT, LRR and PYD domains-containing protein 3; Bax, B-cell lymphoma-Associated X; Bcl-2, B-cell lymphoma-2; ELISA, enzyme-linked immunosorbent assay; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-1β, interleukin-1β.
RMRP promotes inflammation by activating NLRP3 inflammasome
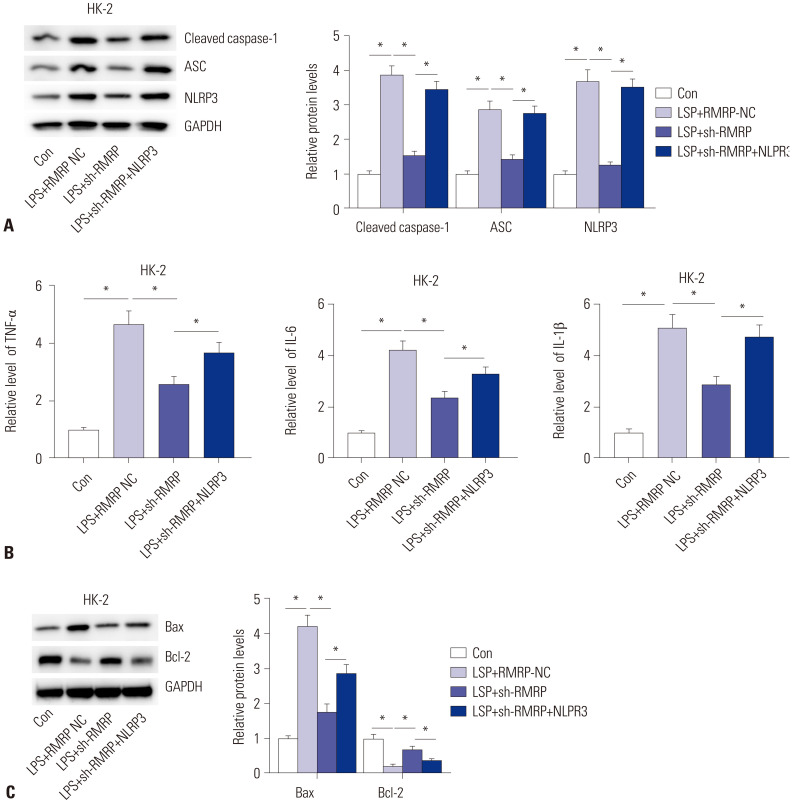
Considering that NLRP3 inflammasome is closely associated with the production of inflammatory factors, we then probed the relationship between RMRP and NLRP3 inflammasome. The protein levels of NLRP3 inflammasome components, including NLRP3, ASC, and cleaved caspase-1, in LPS-induced HK-2 cells were detected. The result showed that knockdown of RMRP decreased protein levels of NLRP3, ASC, and cleaved caspase-1, and such effects were counteracted by NLRP3 overexpression (Fig. 2A). Moreover, results from ELISA also demonstrated that knockdown of RMRP elicited decreases in TNF-α, IL-6, and IL-1β, which were rescued by overexpression of NLRP3 (Fig. 2B). Finally, the sh-RMRP-induced decreases in Bax and increases in Bcl-2 were counteracted by overexpression of NLRP3 (Fig. 2C). These findings revealed that RMRP promotes inflammation by activating NLRP3 inflammasome in an in vitro model of sepsis-induced AKI.
Fig. 2. RMRP promotes inflammation by activating the NLRP3 inflammasome. (A) Western blot analysis revealed the protein levels of NLRP3, ASC, and cleaved caspase-1. (B) ELISA was used to detect the levels of TNF-α, IL-6, and IL-1β in HK-2 cells. (C) Western blot analysis determined the protein levels of Bax and Bcl-2 in HK-2 cells. *p<0.05. NLRP3, NACHT, LRR and PYD domains-containing protein 3; ASC, apoptosis associated speck-like protein containing caspase recruitment domain; caspase, cysteinyl aspartate specific proteinase; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-1β, interleukin-1β; Bax, B-cell lymphoma-Associated X; Bcl-2, B-cell lymphoma-2.
Knockdown of RMRP alleviates AKI in CLP mice
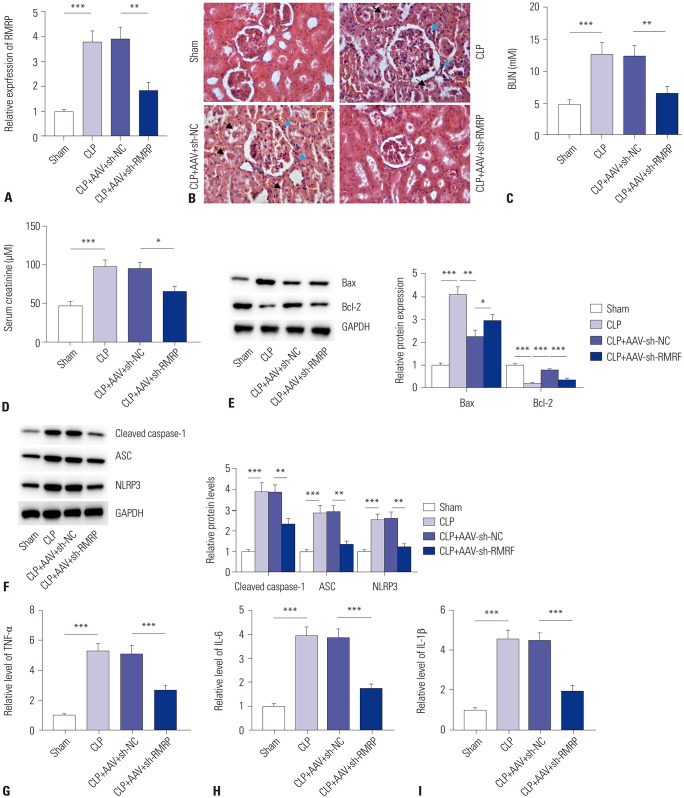
To clarify the effects of RMRP on AKI in vivo, we established septic mouse models by CLP operation. As shown in Fig. 3A, the levels of RMRP were significantly reduced by injection of AAV-sh-RMRP into the kidney tissues of mice. Light microscopy revealed that knockdown of RMRP alleviated tubular cell swelling and inflammatory cell infiltration in the kidney tissues of CLP mice (Fig. 3B). Assessment of renal function in the mice revealed that knockdown of RMRP decreased BUN and serum creatinine levels in the sera of CLP mice (Fig. 3C, D). Moreover, knockdown of RMRP triggered increases in Bax protein levels and decreases in Bcl-2 protein levels in the kidney tissues of CLP mice (Fig. 3E). Furthermore, we found that the protein levels of NLRP3, ASC, and cleaved caspase-1 were reduced by inhibition of RMRP in kidney tissues of CLP mice, suggesting that NLRP3 inflammasome was inactivated by knockdown of RMRP (Fig. 3F). Lastly, ELISA indicated that the levels of TNF-α, IL-6, and IL-1β in kidney tissues of CLP mice decreased by knockdown of RMRP (Fig. 3G-I). All of these data suggested that knockdown of RMRP alleviates AKI in a CLP mouse model.
Fig. 3. Knockdown of RMRP alleviates AKI in CLP mice. (A) RT-qPCR analysis was carried out to evaluate the knockdown efficiency of sh-RMRP in renal tissues of CLP mice. (B) H&E staining was conducted to assess degrees of kidney injury in mice (the black arrow indicates inflammatory cell infiltration, and the blue arrow indicates tubular cell swelling). (C and D) Statistical analysis of levels of BUN and serum creatinine in the sera of sham mice and CLP mice. (E and F) Western blot was conducted to examine protein levels of Bax, Bcl-2, NLRP3, ASC, and cleaved caspase-1 in renal tissues of sham mice and CLP mice. (G-I) Levels of TNF-α, IL-6, and IL-1β in kidney tissues of sham mice and CLP mice were determined by ELISA. *p<0.05, **p<0.01, ***p<0.001. RT-qPCR, reverse transcription quantitative polymerase chain reaction; RMRP, RNA component of mitochondrial RNAase P; AKI, acute kidney injury; BUN, blood urea nitrogen; CLP, cecal ligation and puncture; H&E, hematoxylin-eosin; RT-qPCR, reverse transcription quantitative polymerase chain reaction; RMRP, RNA component of mitochondrial RNAase P; NLRP3, NACHT, LRR and PYD domains-containing protein 3; Bax, B-cell lymphoma-Associated X; Bcl-2, B-cell lymphoma-2; ASC, apoptosis associated speck-like protein containing caspase recruitment domain; caspase, cysteinyl aspartate specific proteinase; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-1β, interleukin-1β; ELISA, enzyme-linked immunosorbent assay.
MiR-206 directly binds with RMRP
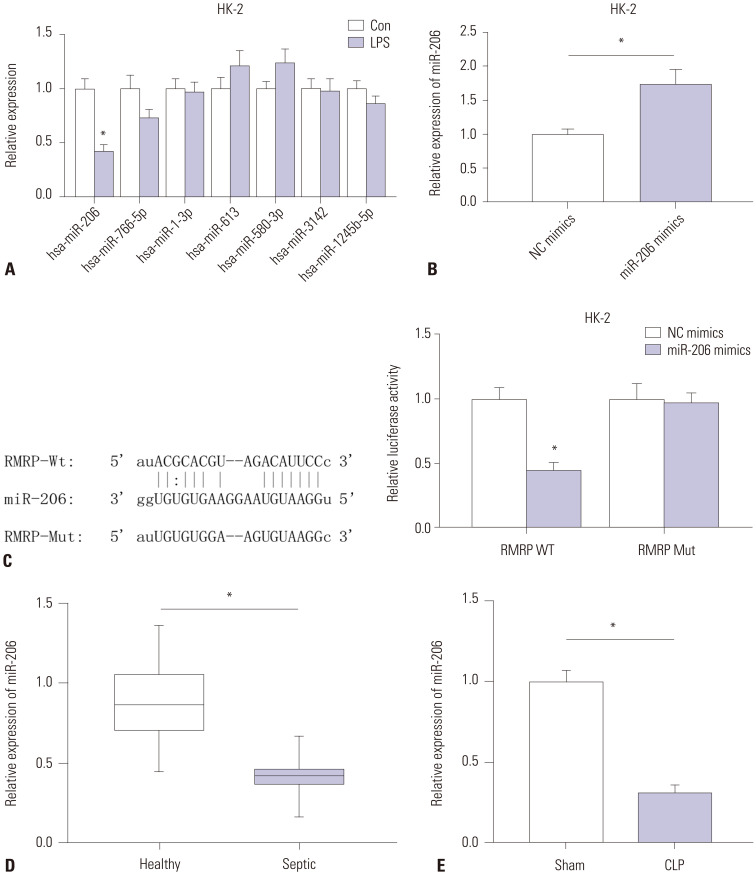
Increasing research has indicated that lncRNA can compete with mRNA for miRNA.14 Additionally, lncRNA RMRP has been identified to act as a ceRNA.11 Hence, we hypothesized that RMRP may also act in the same manner in sepsis-induced AKI. Searching the starBase website, we found that seven miRNAs (miR-206, miR-766-5p, miR-1-3p, miR-613, miR-580-3p, miR-3142, and miR-1245b-5p) harbored binding sites on RMRP. Subsequent RT-qPCR analysis showed that only miR-206 was downregulated in response to LPS stimulation in HK-2 cells (Fig. 4A). Therefore, we chose miR-206 for the following experiment. As shown in Fig. 4B, miR-206 levels were significantly overexpressed by transfection of miR-206 mimics in HK-2 cells. We constructed wild-type and mutant pmirGLORMRP plasmids that were co-transfected with miR-206 mimics or NC mimics into HK-2 cells. We discovered that luciferase activity of pmirGLO-RMRP-Wt was significantly decreased by miR-206 mimics, while no significant change was observed in pmirGLO-RMRP-Mut groups (Fig. 4C), indicating that miR-206 can bind with RMRP at the predicted sites. Furthermore, RT-qPCR analysis revealed that miR-206 expression was downregulated in sera from septic patients with AKI, compared to that from healthy individuals (Fig. 4D). Compared to sham mice, CLP mice exhibited downregulated miR-206 expression in kidney tissues (Fig. 4E).
Fig. 4. MiR-206 can directly bind with RMRP. (A) RT-qPCR analysis was conducted to detect the expression of seven candidate miRNAs. (B) RT-qPCR analysis was conducted to evaluate the overexpression efficiency of miR-206 mimics. (C) The binding sequences were predicted by starBase, and the relationship between miR-206 and RMRP was confirmed by luciferase reporter assay. (D and E) RT-qPCR was performed to detect miR-206 levels in the sera of sepsis patients with AKI (compared to healthy individuals) and in kidney tissues of CLP mice (compared to sham mice). *p<0.05. RMRP, RNA component of mitochondrial RNAase P; RT-qPCR, reverse transcription quantitative polymerase chain reaction; AKI, acute kidney injury; CLP, cecal ligation and puncture.
DDX5 is a target of miR-206
Next, we sought to identify the target mRNA of miR-206. With the assistance of bioinformatics tools, eight potential mRNAs were screened (Fig. 5A). Among eight candidate targets, only DDX5 was upregulated after LPS stimulation in HK-2 cells (Fig. 5B). Moreover, luciferase reporter assay showed that miR-206 mimics weakened the luciferase activity of pmirGLO-RMRP-Wt vector, and the luciferase activity of pmirGLO-RMRP-Mut vector showed no significant change in response to miR-206 mimics (Fig. 5C). We also found that miR-206 overexpression reduced DDX5 protein levels in HK-2 cells (Fig. 5D). RT-qPCR analysis revealed higher levels of DDX5 mRNA in septic patients with AKI, compared to that in health individuals (Fig. 5E). Furthermore, Western blot analysis confirmed that DDX5 was upregulated in renal tissues of CLP mice (Fig. 5F). Altogether, the results demonstrated that DDX5 acts as a target of miR-206.
Fig. 5. DDX5 is a target of miR-206. (A) Venn diagram depicts the number of candidate target genes of miR-206. (B) Expression of candidate mRNAs in LPS-induced HK-2 cells was detected by RT-qPCR. (C) Binding sequences were predicted by TargetScan, and the combination between miR-206 and DDX5 3'UTR was confirmed by luciferase reporter assay. (D) Western blot analysis was conducted to measure DDX5 protein levels in the condition of miR-206 overexpression. (E) RT-qPCR to detect the mRNA levels of DDX5 in sera from septic patients with AKI (compared to healthy individuals). (F) Western blot analysis was conducted to detect protein expression of DDX5 in kidney tissues in CLP mice (compared to sham mice). *p<0.05. DDX5, DEAD-box helicase 5; RT-qPCR, reverse transcription quantitative polymerase chain reaction; LPS, lipopolysaccharide; UTR, untranslated region; AKI, acute kidney injury; CLP, cecal ligation and puncture.
RMRP facilitates cell apoptosis and inflammation by upregulating DDX5
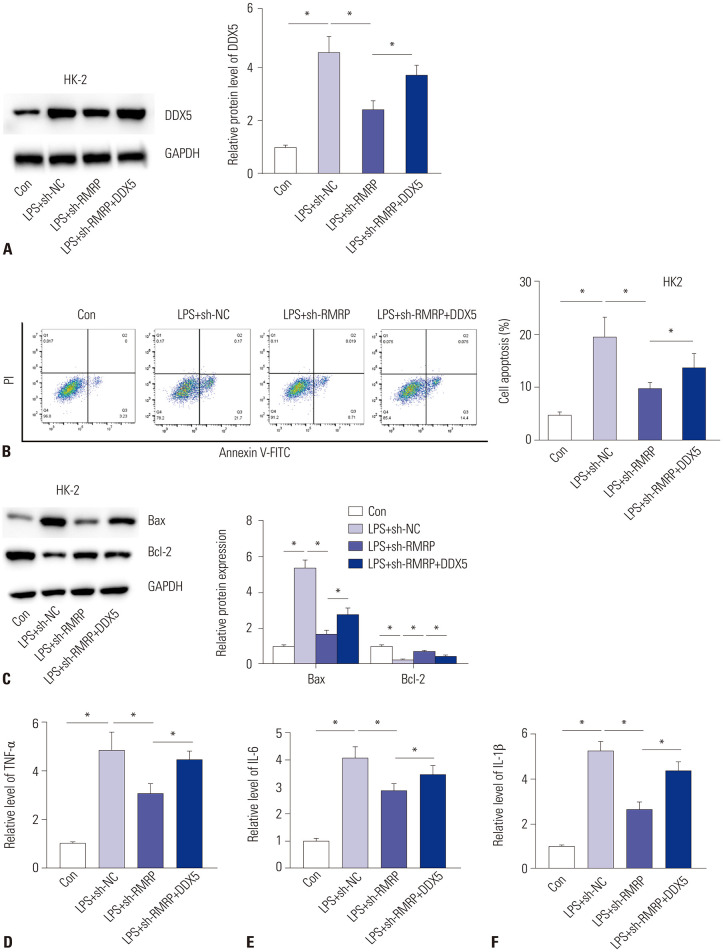
To investigate whether RMRP affects inflammation and cell apoptosis by regulating DDX5 in LPS-induced HK-2 cells, rescue assays were performed. At first, the transfection of pcDNA3.1/DDX5 significantly increased the protein levels of DDX5 (Fig. 6A) in RMRP-silenced HK-2 cells after stimulation of LPS. Furthermore, we detected cell apoptosis by flow cytometry analysis. The results revealed that knockdown of RMRP inhibited cell apoptosis, and overexpression of DDX5 reversed this effect (Fig. 6B). Western blot analysis disclosed that sh-RMRP-mediated deceases in Bax and increases in Bcl-2 were neutralized by overexpression of DDX5 (Fig. 6C). Subsequently, the suppressive effects of sh-RMRP on concentrations of TNF-α, IL-6, and IL-1β were rescued by transfection of pcDNA3.1/DDX5 (Fig. 6D-F). Altogether, our findings indicate that RMRP facilitates apoptosis and inflammation of LPS-induced HK-2 cells by upregulation of DDX5 and by activation of NLRP3 inflammasome (Supplementary Fig. 1, only online).
Fig. 6. RMRP facilitates cell apoptosis and inflammation by upregulation of DDX5. (A) Western blot analysis was conducted to detect the expression of DDX5 in different groups. (B) Flow cytometry analysis was conducted to explore cell apoptosis in different groups. (C) Protein levels of Bax and Bcl-2 in different groups in LPS-induced HK-2 cells were detected by Western blot analysis. (D-F) Levels of TNF-α, IL-6, and IL-1β in LPS-induced HK-2 cells were confirmed by ELISA. *p<0.05. RMRP, RNA component of mitochondrial RNAase P; DDX5, DEAD-box helicase 5; Bax: B-cell lymphoma-Associated X; Bcl-2, B-cell lymphoma-2; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-1β, interleukin-1β; ELISA, enzyme-linked immunosorbent assay.
DISCUSSION
AKI is a complex clinical kidney disorder with complicated pathogenesis that can be life-threatening.1 Known factors, such as necrotic tubular obstruction of renal tubules, glomerular vascular thrombosis, inflammatory cell infiltration, endothelial dysfunction, and intrarenal hemodynamic changes, are associated with sepsis-mediated AKI.29 Previous studies claimed that sepsis-mediated AKI displays obvious inflammatory response-mediated injury, generally accompanied with cellular apoptosis.30 Additionally, inflammatory, anti-inflammatory, and immune responses induced by inflammation are involved in the progression of sepsis-induced AKI. The concentrations of pro-inflammatory cytokines, including IL-6, TNF-α, and IL-1β, have been indicated as being related to the severity of sepsis-induced AKI.31
Increasing evidence suggests that lncRNAs play a significant role in inflammatory responses by regulating inflammatory factors. As an example, upregulation of NEAT1 is related to high risk, poor prognosis, and increased levels of proinflammatory cytokines in patients with sepsis.32 In addition, HOTAIR promotes the production of TNF-α by activation of the NF-κB pathway in LPS-induced cardiomyocytes.33 Similarly, in this study, RMRP levels were upregulated in the sera of septic patients with AKI, and knockdown of RMRP inhibited the production of pro-inflammatory cytokines and reduced cell apoptosis in LPS-induced HK-2 cells.
The NLRP3 inflammasome is a cytosolic multiprotein caspase-activated complex platform in innate immunity and is required for the maturation and release of IL-1β and IL-18.34 IL-1β and IL-18 activate their respective receptors present on cells inside and outside kidneys, resulting in the release of other proinflammatory cytokines to initiate inflammatory milieu within the kidney.35 NLRP3 inflammasome is widely implicated in a variety of renal injuries, including acute and chronic kidney disease. In the present study, knockdown of RMRP reduced protein levels of NLRP3, ASC, and cleaved caspase-1, suggesting that NLRP3 inflammasome is inactivated by inhibition of RMRP. Moreover, concentrations of pro-inflammatory cytokines displayed similar alterations in LPS-induced HK-2 cells upon silencing of RMRP. Further in vivo study indicated that knockdown of RMRP alleviates AKI in CLP mice.
Accumulating studies have confirmed that RMRP can act as a ceRNA by competitively binding with miRNA to release mRNA. For instance, RMRP promotes hypoxia-induced injury by targeting the miR-214-5p/p53 axis.11 Knockdown of RMRP inhibits hepatocellular carcinoma progression via regulation of the miR-206/TACR1 axis.36 RMRP has also been indicated to promote cell proliferation of nucleus pulposus by regulating miR-206 expression.37 Likewise, in our study, we also found that RMRP can directly bind with miR-206. Subsequently, we innovatively identified that DDX5 is a direct target of miR-206 and that its expression is inhibited by miR-206. RMRP upregulated DDX5 expression by binding with miR-206 to suppress the inhibitory effects of miR-206 on DDX5. Finally, rescue assay revealed that DDX5 upregulation counteracted the effects of RMRP inhibition on cell apoptosis and inflammation.
In conclusion, our data implicated the upregulation of RMRP in sepsis-induced AKI. The suppression of RMRP appeared to alleviate LPS-induced AKI by inactivating NLRP3 inflammasome and by downregulating DDX5 via miR-206. This novel discovery suggests that RMRP may be a potential diagnostic marker or therapeutic target for sepsis-induced AKI.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Xia Zhang and Zhongwei Huang.
- Data curation: Xia Zhang, Zhongwei Huang, Yan Wang, and Ting Wang.
- Formal analysis: Xia Zhang, Zhongwei Huang, and Jingjing Li.
- Investigation: Xia Zhang and Zhongwei Huang.
- Methodology: Xia Zhang and Zhongwei Huang.
- Project administration: Xia Zhang and Zhongwei Huang.
- Resources: Xia Zhang and Zhongwei Huang.
- Software: Xia Zhang, Zhongwei Huang, and Peipei Xi.
- Supervision: Zhongwei Huang.
- Validation: Xia Zhang.
- Visualization: Xia Zhang and Zhongwei Huang.
- Writing—original draft: Xia Zhang.
- Writing—review & editing: Xia Zhang and Zhongwei Huang.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIAL
Graphical abstract: RMRP promotes an inflammatory response and apoptosis of LPS-induced HK-2 cells by interaction with miR-206 to upregulate DDX5 and by activation of the NLRP3 inflammasome. RMRP, RNA component of mitochondrial RNAase P; LPS, lipopolysaccharide; DDX5, DEAD-box helicase 5; NLRP3, NACHT, LRR and PYD domains-containing protein 3.
References
- 1.Napolitano LM. Sepsis 2018: definitions and guideline changes. Surg Infect (Larchmt) 2018;19:117–125. doi: 10.1089/sur.2017.278. [DOI] [PubMed] [Google Scholar]
- 2.Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22:999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 3.Prowle JR. Sepsis-associated AKI. Clin J Am Soc Nephrol. 2018;13:339–342. doi: 10.2215/CJN.07310717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 5.Cho KS, Ko IK, Yoo JJ. Bioactive compounds for the treatment of renal disease. Yonsei Med J. 2018;59:1015–1025. doi: 10.3349/ymj.2018.59.9.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarroux J, Morillon A, Pinskaya M. History, discovery, and classification of lncRNAs. Adv Exp Med Biol. 2017;1008:1–46. doi: 10.1007/978-981-10-5203-3_1. [DOI] [PubMed] [Google Scholar]
- 7.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Qiu J, Chen B, Lin Y, Chen Y, Xie G, et al. Long non-coding RNA NEAT1 plays an important role in sepsis-induced acute kidney injury by targeting miR-204 and modulating the NF-κB pathway. Int Immunopharmacol. 2018;59:252–260. doi: 10.1016/j.intimp.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Jiang ZJ, Zhang MY, Fan ZW, Sun WL, Tang Y. Influence of lncRNA HOTAIR on acute kidney injury in sepsis rats through regulating miR-34a/Bcl-2 pathway. Eur Rev Med Pharmacol Sci. 2019;23:3512–3519. doi: 10.26355/eurrev_201904_17717. [DOI] [PubMed] [Google Scholar]
- 10.Son HJ, Choi EJ, Yoo NJ, Lee SH. Somatic mutations in long-non-coding RNA RMRP in acute leukemias. Pathol Res Pract. 2019;215:152647. doi: 10.1016/j.prp.2019.152647. [DOI] [PubMed] [Google Scholar]
- 11.Teng Y, Ding M, Wang X, Li H, Guo Q, Yan J, et al. LncRNA RMRP accelerates hypoxia-induced injury by targeting miR-214-5p in H9c2 cells. J Pharmacol Sci. 2020;142:69–78. doi: 10.1016/j.jphs.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Moharamoghli M, Hassan-Zadeh V, Dolatshahi E, Alizadeh Z, Farazmand A. The expression of GAS5, THRIL, and RMRP lncRNAs is increased in T cells of patients with rheumatoid arthritis. Clin Rheumatol. 2019;38:3073–3080. doi: 10.1007/s10067-019-04694-z. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Cai Y, Lai X, Wang Z, Wei S, Tan K, et al. lncRNA RMRP prevents mitochondrial dysfunction and cardiomyocyte apoptosis via the miR-1-5p/hsp70 axis in LPS-induced sepsis mice. Inflammation. 2020;43:605–618. doi: 10.1007/s10753-019-01141-8. [DOI] [PubMed] [Google Scholar]
- 14.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 16.Lin Z, Liu Z, Wang X, Qiu C, Zheng S. MiR-21-3p plays a crucial role in metabolism alteration of renal tubular epithelial cells during sepsis associated acute kidney injury via AKT/CDK2-FOXO1 Pathway. Biomed Res Int. 2019;2019:2821731. doi: 10.1155/2019/2821731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Zhang Z, Wang J, Miao H. MiR-107 induces TNF-α secretion in endothelial cells causing tubular cell injury in patients with septic acute kidney injury. Biochem Biophys Res Commun. 2017;483:45–51. doi: 10.1016/j.bbrc.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Sandonà M, Consalvi S, Tucciarone L, De Bardi M, Scimeca M, Angelini DF, et al. HDAC inhibitors tune miRNAs in extracellular vesicles of dystrophic muscle-resident mesenchymal cells. EMBO Rep. 2020;21:e50863. doi: 10.15252/embr.202050863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Xu J, Liu H, Li J, Hao H. PM2.5 inhibits SOD1 expression by up-regulating microRNA-206 and promotes ROS accumulation and disease progression in asthmatic mice. Int Immunopharmacol. 2019;76:105871. doi: 10.1016/j.intimp.2019.105871. [DOI] [PubMed] [Google Scholar]
- 20.Sun W, Zhang L, Li R. Overexpression of miR-206 ameliorates chronic constriction injury-induced neuropathic pain in rats via the MEK/ERK pathway by targeting brain-derived neurotrophic factor. Neurosci Lett. 2017;646:68–74. doi: 10.1016/j.neulet.2016.12.047. [DOI] [PubMed] [Google Scholar]
- 21.Son DJ, Jung YY, Seo YS, Park H, Lee DH, Kim S, et al. Interleukin-32α inhibits endothelial inflammation, vascular smooth muscle cell activation, and atherosclerosis by upregulating timp3 and reck through suppressing microRNA-205 biogenesis. Theranostics. 2017;7:2186–2203. doi: 10.7150/thno.18407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng W, Chen G, Jia H, He X, Jing Z. DDX5 RNA helicases: emerging roles in viral infection. Int J Mol Sci. 2018;19:1122. doi: 10.3390/ijms19041122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka K, Tanaka T, Nakano T, Hozumi Y, Yanagida M, Araki Y, et al. Knockdown of DEAD-box RNA helicase DDX5 selectively attenuates serine 311 phosphorylation of NF-κB p65 subunit and expression level of anti-apoptotic factor Bcl-2. Cell Signal. 2020;65:109428. doi: 10.1016/j.cellsig.2019.109428. [DOI] [PubMed] [Google Scholar]
- 24.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101:1481–1483. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 25.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 26.Sun S, Wang J, Wang J, Wang F, Yao S, Xia H. Maresin 1 mitigates sepsis-associated acute kidney injury in mice via inhibition of the NF-κB/STAT3/MAPK pathways. Front Pharmacol. 2019;10:1323. doi: 10.3389/fphar.2019.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sureshbabu A, Patino E, Ma KC, Laursen K, Finkelsztein EJ, Akchurin O, et al. RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI Insight. 2018;3:e98411. doi: 10.1172/jci.insight.98411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu C, Qi D, Sun JF, Li P, Fan HY. Rhein prevents endotoxin-induced acute kidney injury by inhibiting NF-κB activities. Sci Rep. 2015;5:11822. doi: 10.1038/srep11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med. 2008;36:S198–S203. doi: 10.1097/CCM.0b013e318168ccd5. [DOI] [PubMed] [Google Scholar]
- 30.Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891. doi: 10.1136/bmj.k4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skube SJ, Katz SA, Chipman JG, Tignanelli CJ. Acute kidney injury and sepsis. Surg Infect (Larchmt. 2018;19:216–224. doi: 10.1089/sur.2017.261. [DOI] [PubMed] [Google Scholar]
- 32.Huang Q, Huang C, Luo Y, He F, Zhang R. Circulating lncRNA NEAT1 correlates with increased risk, elevated severity and unfavorable prognosis in sepsis patients. Am J Emerg Med. 2018;36:1659–1663. doi: 10.1016/j.ajem.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Wu H, Liu J, Li W, Liu G, Li Z. LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of LPS-induced sepsis mice by activating NF-κB pathway. Biochem Biophys Res Commun. 2016;471:240–246. doi: 10.1016/j.bbrc.2016.01.117. [DOI] [PubMed] [Google Scholar]
- 34.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang P, Huang J, Li Y, Chang R, Wu H, Lin J, et al. Exogenous carbon monoxide decreases sepsis-induced acute kidney injury and inhibits NLRP3 inflammasome activation in rats. Int J Mol Sci. 2015;16:20595–20608. doi: 10.3390/ijms160920595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hongfeng Z, Andong J, Liwen S, Mingping B, Xiaowei Y, Mingyong L, et al. lncRNA RMRP knockdown suppress hepatocellular carcinoma biological activities via regulation miRNA-206/TACR1. J Cell Biochem. 2020;121:1690–1702. doi: 10.1002/jcb.29404. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Peng L, Gong X, Zhang X, Sun R, Du J. LncRNA-RMRP promotes nucleus pulposus cell proliferation through regulating miR-206 expression. J Cell Mol Med. 2018;22:5468–5476. doi: 10.1111/jcmm.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graphical abstract: RMRP promotes an inflammatory response and apoptosis of LPS-induced HK-2 cells by interaction with miR-206 to upregulate DDX5 and by activation of the NLRP3 inflammasome. RMRP, RNA component of mitochondrial RNAase P; LPS, lipopolysaccharide; DDX5, DEAD-box helicase 5; NLRP3, NACHT, LRR and PYD domains-containing protein 3.