Abstract
Purpose
To determine whether the prognostic impact of lymph node ratio (LNR), defined as the ratio between the number of positive lymph nodes and removed lymph nodes, differs between open and minimally invasive surgical approaches for radical hysterectomy (RH) in node-positive, early-stage cervical cancer.
Materials and Methods
We retrospectively identified 2009 International Federation of Gynecology and Obstetrics stage IB1-IIA2 patients who underwent primary type C RH between 2010 and 2018. Among them, only those with pathologically proven lymph node metastases who received adjuvant radiation therapy were included. The prognostic significance of LNR was investigated according to open surgery and minimally invasive surgery (MIS).
Results
In total, 55 patients were included. The median LNR (%) was 9.524 (range, 2.083–62.500). Based on receiver operating characteristic curve analysis, the cut-off value for LNR (%) was determined as 8.831. Overall, patients with high LNR (≥8.831%; n=29) showed worse disease-free survival (DFS) than those with low LNR (<8.831%, n=26) (p=0.027), whereas no difference in overall survival was observed. Multivariate analyses adjusting for clinicopathologic factors revealed that DFS was adversely affected by both MIS [adjusted hazard ratio (HR), 8.132; p=0.038] and high LNR (adjusted HR, 10.837; p=0.045). In a subgroup of open surgery cases, LNR was not associated with disease recurrence. However, in a subgroup of MIS cases, high LNR was identified as an independent poor prognostic factor for DFS (adjusted HR, 14.578; p=0.034).
Conclusion
In patients with node-positive, early-stage cervical cancer, high LNR was associated with a significantly higher disease recurrence rate. This relationship was further consolidated among patients who received MIS RH.
Keywords: Cervical cancer, radical hysterectomy, minimally invasive surgery, laparoscopic surgery, lymph node ratio, recurrence
INTRODUCTION
Cervical cancer, the most common gynecologic malignancy, was estimated to account for 569847 new cancer cases and 311365 cancer deaths worldwide in 2018, ranking as the fourth for both incidence and mortality among all female cancers.1 For the treatment of early-stage cervical cancer, primary radical hysterectomy (RH) via minimally invasive surgery (MIS) has been widely performed. However, MIS RH has faced criticism since the Laparoscopic Approach to Carcinoma of the Cervix (LACC) trial.2 This phase III randomized controlled trial was the first to report higher recurrence and mortality rates in the patients with early-stage cervical cancer who underwent MIS RH, compared to those who underwent open RH. Subsequent retrospective studies and a recent meta-analysis also reported inferior survival outcomes from MIS RH.3,4,5,6 Thus, it is necessary to investigate which factors make the prognosis after MIS RH worse.
The status of regional lymph nodes is a strong prognostic factor and has been incorporated in staging systems for many cancers. In cervical cancer, it was not until 2018 that the revised International Federation of Gynecology and Obstetrics (FIGO) staging system included pelvic and/or para-aortic lymph node metastasis.7 Previously, patients who were initially thought to have stage IB-IIA disease were often found to have lymph node metastasis after primary surgical treatment consisting of RH and pelvic lymph node dissection. Such patients with node-positive, early-stage cervical cancer are recommended to receive adjuvant radiation therapy (RT) because of a considerable risk of recurrence.8,9 In light of the LACC trial, lymph node status might become more crucial for determining postoperative care and predicting prognosis for cervical cancer patients.
Lymph node ratio (LNR), defined as the ratio between the number of positive lymph nodes and removed lymph nodes, is known to be an independent prognostic factor for survival in patients with gastric cancer,10 esophageal cancer,11 colorectal cancer,12,13 and non-small cell lung cancer.14 In cervical cancer, previous studies have reported that high LNR is associated with poor overall survival (OS) or both poor OS and disease-free survival (DFS).15,16,17,18,19 Although some studies have reported the number or log odds of metastatic lymph nodes, rather than LNR, as a more reliable prognostic factors for DFS,20,21 ratiobased nodal assessment seems to be more objective and useful because it offsets variations in the number of regional lymph nodes in each individual and reflects the extent or severity of lymph node metastasis.
Nevertheless, previous studies on LNR in cervical cancer show considerable heterogeneity among the studies, hindering clinical utilization of LNR: the most significant differences are in study populations, such as stage or disease status, and the specific cut-off values of LNR. Moreover, no studies have taken into account the surgical approach, open surgery or MIS, when investigating prognostic significance of LNR, so far. Accordingly, we aimed to determine whether the impact of LNR on survival outcomes differs by the surgical approach in patients with node-positive, early-stage cervical cancer.
MATERIALS AND METHODS
Study population
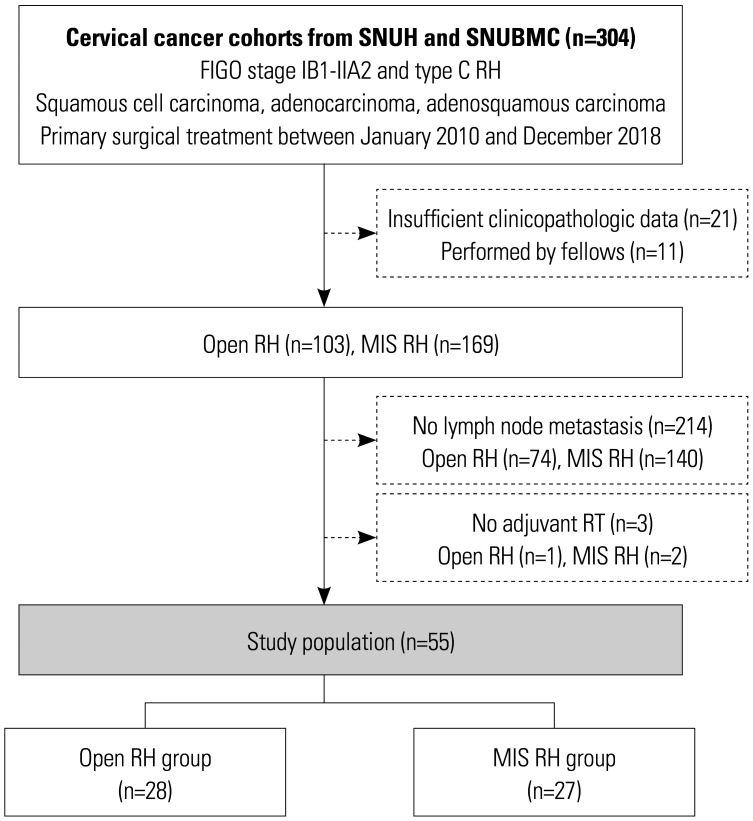
From the cervical cancer cohorts of two institutional hospitals, we identified consecutive patients with 2009 FIGO stage IB1-IIA2 cervical cancer who underwent primary type C RH according to the Querleu-Morrow classification between January 2010 and December 2018.22,23 Patients were excluded if they: 1) had histologic types other than squamous cell carcinoma, usual type adenocarcinoma, and adenosquamous carcinoma; 2) received neoadjuvant chemotherapy; or 3) had insufficient clinicopathologic data. RH cases that were performed by fellows were also excluded. Based on these criteria, 103 and 169 patients were identified to receive open RH and MIS RH, respectively. For the study purpose, we further excluded 214 patients without pathologically proven lymph node metastasis, and 3 patients who did not receive adjuvant RT despite the presence of lymph node metastasis. Consequently, 55 patients (28 for open RH and 27 for MIS RH) were set as study population (Fig. 1).
Fig. 1. Flow diagrams depicting the selection of the study population. FIGO, International Federation of Gynecology and Obstetrics; RH, radical hysterectomy; MIS, minimally invasive surgery; RT, radiation therapy.
Data collection
During the study period, all surgical procedures were performed by faculty members who had completed their gynecologic oncology fellowship training. Before the LACC trial, the surgical approach was decided upon the surgeons' preference. However, after the LACC trial, it was chosen through a comprehensive consultation with patients.
Reviewing medical records and pathologic reports, we extracted data regarding clinicopathologic characteristics (e.g., age, histologic type, FIGO stage, conization, pelvic and paraaortic lymphadenectomy, high-risk factors, lymphovascular space invasion, depth of stromal invasion, and tumor size measured by pathological examination) and specific adjuvant treatment [e.g., adjuvant RT only, concurrent chemoradiation therapy (CCRT), or consolidation chemotherapy].
At our institutions, patients with cervical cancer routinely undergo pre-operative imaging studies, such as computed tomography (CT) scans, magnetic resonance imaging (MRI), and whole-body 18F-fluorodeoxyglucose positron emission tomography/CT (PET/CT). Each patient's pre-operative lymph node status was evaluated by reviewing all available pre-operative imaging studies.
In terms of survival outcome, we defined DFS as the length of time between the date of primary RH and the date of disease progression, and we defined OS as the length between the date of primary RH and the date of cancer-related death or the end of the study. Surveillance methods between the two institutions did not differ: computed tomography scanning was routinely performed every 3 to 4 months for the first 2 years, regardless of the surgical approach. Disease progression was assessed according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1.24
Statistical analysis
Each patient's LNR (%) was calculated as follows: the number of positive lymph nodes was divided by the total number of retrieved lymph nodes and multiplied by 100. Receiver operating characteristic (ROC) curve analysis was performed to determine the cut-off values of LNR for predicting recurrence within 3 years after surgery. The prognostic significance of LNR was investigated according to two surgical approaches, open surgery and MIS.
We compared the patients' clinicopathologic characteristics between the open RH and MIS RH groups and between the high and low LNR groups. Student's t- or Mann-Whitney U-tests were used for comparisons of continuous variables, and Pearson's chi-squared or Fisher's exact tests were used for categorical variables. To compare the survival of the two groups, we used the Kaplan-Meier method with the log-rank test. In multivariate analyses, we included variables that significantly differed between groups and those that are generally well known to affect survival outcomes, and conducted a stepwise variable selection process. We calculated adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) using Cox proportional hazards regression models. All statistical analyses were performed using SPSS statistical software (version 25.0; IBM Corp., Armonk, NY, USA). A p value less than 0.05 was considered statistically significant.
Ethics statement
This retrospective cohort study was conducted after approval from the Institutional Review Boards of Seoul National University Hospital (No. J-1911-003-1074) and Seoul National University Boramae Medical Center (No. 20190213/20-2019-7/032). The study was performed in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived.
RESULTS
The clinicopathologic characteristics of all patients are presented in Table 1. The open RH and MIS RH groups showed similar mean ages, histologic types, and FIGO 2009 stage. Among the study population (n=55), 45 (81.8%) received all three imaging modalities, while 5 (9.1%), 2 (3.6%), and 3 (5.5%) received both CT and PET/CT, both CT and MRI, and CT only, respectively. Half of the patients (n=28, 50.9%) were suspected of having lymph node metastasis pre-operatively, and pathologic examination confirmed the presence of metastatic lymph nodes. Meanwhile, all of the other patients (n=27, 49.1%) who showed no involvement of lymph nodes in pre-operative imaging studies were identified to have pathologic lymph node metastasis. Such pre-operative lymph node status was similar between the open RH and MIS RH groups (p=0.688). Both groups showed similar proportions of pre-operative conization and para-aortic lymphadenectomy. On pathologic examination, no differences were observed between the groups, except parametrial invasion, which was more frequent in the open RH group, compared to the MIS RH group (57.1% vs. 22.2%; p=0.008). After surgery, all patients received CCRT for adjuvant treatment. Subsequent consolidation chemotherapy was performed significantly less in the open RH group (17.9% vs. 44.4%; p=0.033).
Table 1. Clinicopathologic Characteristics of the Study Population.
| Characteristics | All (n=55, %) | Open RH (n=28, %) | MIS RH (n=27, %) | p value | |
|---|---|---|---|---|---|
| Age (yr) | 0.055 | ||||
| Median (range) | 52.6 (32.4–76.6) | 55.9 (32.6–75.6) | 45.7 (32.4–76.6) | ||
| Surgical approach | N/A | ||||
| Open | 28 (50.9) | 28 (100.0) | 0 | ||
| Laparoscopy | 23 (41.8) | 0 | 23 (85.2) | ||
| Robot-assisted surgery | 4 (7.3) | 0 | 4 (14.8) | ||
| Histologic type | 0.357 | ||||
| Squamous cell carcinoma | 50 (90.9) | 24 (85.7) | 26 (96.3) | ||
| Adenocarcinoma | 4 (7.3) | 3 (10.7) | 1 (3.7) | ||
| Adenosquamous carcinoma | 1 (1.8) | 1 (3.6) | 0 | ||
| 2009 FIGO stage | |||||
| IB | 39 (70.9) | 18 (64.3) | 21 (77.8) | 0.271 | |
| IIA | 16 (29.1) | 10 (35.7) | 6 (22.2) | ||
| IB1 | 25 (45.5) | 8 (28.6) | 17 (63.0) | 0.060 | |
| IB2 | 14 (25.5) | 10 (35.7) | 4 (14.8) | ||
| IIA1 | 6 (10.9) | 3 (10.7) | 3 (11.1) | ||
| IIA2 | 10 (18.2) | 7 (25.0) | 3 (11.1) | ||
| Pre-operative conization | 7 (12.7) | 2 (7.1) | 5 (18.5) | 0.206 | |
| Pre-operative LN evaluation* | 0.688 | ||||
| No involvement | 27 (49.1) | 13 (46.4) | 14 (51.9) | ||
| Suspicious for LN metastasis | 28 (50.9) | 15 (53.6) | 13 (48.1) | ||
| Para-aortic LN removal | 0.246 | ||||
| No | 41 (74.5) | 19 (67.9) | 22 (81.5) | ||
| Sampling/biopsy/dissection | 14 (25.5) | 9 (32.1) | 5 (18.5) | ||
| Pathologic cervical mass size, mm | |||||
| Mean±SD | 48.5±17.8 | 48.6±15.8 | 48.4±20.0 | 0.973 | |
| <20 | 2 (3.6) | 0 | 2 (7.4) | 0.341 | |
| ≥20 and <40 | 15 (27.3) | 8 (28.6) | 7 (25.9) | ||
| ≥40 | 38 (69.1) | 20 (71.4) | 18 (66.7) | ||
| Parametrial involvement | 22 (40.0) | 16 (57.1) | 6 (22.2) | 0.008 | |
| Resection margin involvement | 5 (9.1) | 1 (3.6) | 4 (14.8) | 0.147 | |
| 2018 FIGO stage | >0.999 | ||||
| IIIC1 | 51 (92.7) | 26 (92.9) | 25 (92.6) | ||
| IIIC2 | 4 (7.3) | 2 (7.1) | 2 (7.4) | ||
| LNs | |||||
| Removed LNs, median (range) | 24.0 (8–62) | 24.5 (12–48) | 24.0 (8–62) | 0.418 | |
| Positive LNs, median (range) | 2.0 (1–19) | 2.0 (1–14) | 2.0 (1–19) | 0.903 | |
| LNR, % | |||||
| Median (range) | 9.524 (2.083–62.500) | 8.452 (2.083–48.276) | 12.500 (2.381–62.500) | 0.556 | |
| Adjuvant treatment | 0.033 | ||||
| CCRT | 38 (69.1) | 23 (82.1) | 15 (55.6) | ||
| CCRT followed by chemotherapy | 17 (30.9) | 5 (17.9) | 12 (44.4) | ||
CCRT, concurrent chemoradiation therapy; FIGO, International Federation of Gynecology and Obstetrics; LN, lymph node; LNR, lymph node ratio; MIS, minimally invasive surgery; N/A, not applicable; RH, radical hysterectomy; SD, standard deviation.
*Imaging study-based.
The median number of total nodes examined was 24.0 (range, 8–62) and was similar between the open RH and MIS RH group (p=0.556). No difference in LNR (%) was observed between the open RH and MIS RH groups (median, 8.452 vs. 12.500; p=0.556). In ROC curve analysis, the area under the curve of LNR for prediction of 3-year disease recurrence was 0.748 (95% CI, 0.578–0.918; p=0.015), and 8.831% was determined as the optimal threshold (Supplementary Fig. 1, only online). The sensitivity and specificity for the use of this value were 81.8% and 63.6%, respectively. We divided the patients into two groups: LNR-high (≥8.831%; n=29) vs. LNR-low (<8.831%; n=26).
Between the LNR-high and -low groups, no differences in patient age, surgical approach, histologic type, and FIGO 2009 stage were observed (Supplementary Table 1, only online). Imaging study-based lymph node evaluation results and proportions of pre-operative conization, para-aortic lymphadenectomy, and pathologic risk factors were also similar between the two groups. Similar proportions of patients in the LNR-high and -low groups received consolidation chemotherapy after CCRT (31.0% vs. 30.8%; p=0.983).
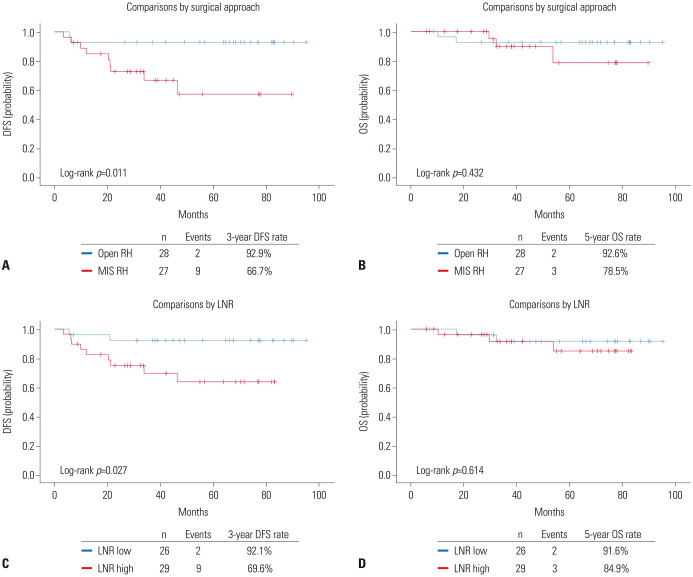
At a median follow-up of 53.7 months, the MIS RH group showed significantly higher disease recurrence than the open RH group (3-year DFS rate, 66.7% vs. 92.9; p=0.011), whereas no difference in OS was observed (p=0.432) (Fig. 2A and B). In terms of LNR, the LNR-high group showed significantly worse DFS than the LNR-low group (3-year DFS rate, 69.6% vs. 92.1%; p=0.027), while similar OS was observed between the two groups (p=0.614) (Fig. 2C and D). In multivariate analysis adjusting for clinicopathologic variables, both MIS (aHR, 8.132; 95% CI, 1.126–58.730; p=0.038) and LNR ≥8.831% (aHR, 10.837; 95% CI, 1.054–111.364; p=0.045) were identified as independent poor prognostic factors for DFS (Table 2), but not for OS (Supplementary Table 2, only online).
Fig. 2. Survival outcomes of study population according to surgical approach (upper) and LNR (lower). (A and C) DFS. (B and D) OS. DFS, disease-free survival; OS, overall survival; RH, radical hysterectomy; MIS, minimally invasive surgery; LNR, lymph node ratio.
Table 2. Factors associated with Disease-Free Survival.
| Characteristics | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | Adjusted HR | 95% CI | p value | ||
| Age (yr) | ≥50 vs. <50 | 0.296 | 0.078–1.117 | 0.072 | 0.138 | 0.015–1.270 | 0.080 |
| 2009 FIGO stage | IIA vs. IB | 0.474 | 0.102–2.195 | 0.340 | |||
| Pre-operative conization | Yes vs. No | 1.669 | 0.358–7.785 | 0.514 | |||
| Para-aortic LN removal | Yes vs. No | 0.539 | 0.116–2.501 | 0.430 | 0.011 | 0.000–0.971 | 0.049 |
| Cervical mass size by pathology, mm | ≥40 vs. <40 | 2.086 | 0.450–9.662 | 0.347 | 3.360 | 0.624–18.102 | 0.158 |
| Parametrial involvement | Yes vs. No | 1.275 | 0.389–4.181 | 0.688 | 15.861 | 1.546–162.734 | 0.020 |
| Resection margin involvement | Yes vs. No | 4.626 | 1.216–17.591 | 0.025 | 6.491 | 0.426–98.833 | 0.178 |
| Adjuvant treatment | CCRT+Chemo vs. CCRT | 0.886 | 0.234–3.349 | 0.858 | |||
| LNR, % | 1.045 | 1.011–1.079 | 0.009 | ||||
| LNR, % | ≥8.831 vs. <8.831 | 4.788 | 1.031–22.231 | 0.046 | 10.837 | 1.054–111.364 | 0.045 |
| Surgical approach | MIS vs. Open surgery | 5.978 | 1.272–28.108 | 0.024 | 8.132 | 1.126–58.730 | 0.038 |
CCRT, concurrent chemoradiation therapy; Chemo, chemotherapy; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; LN, lymph node; HR, hazard ratio; LNR, lymph node ratio; MIS, minimally invasive surgery.
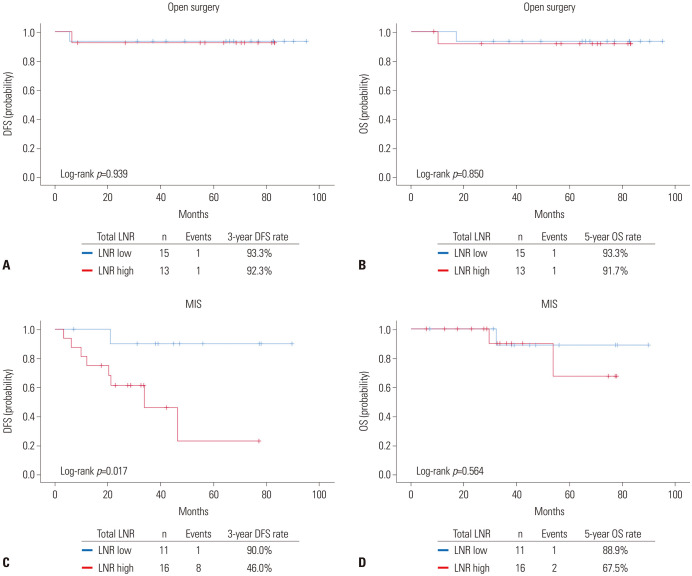
Subsequent survival analyses were performed according to the surgical approach. In patients who underwent open RH (n=28), no differences in DFS (p=0.939) and OS (p=0.850) were observed between the LNR-high and -low groups (Fig. 3A and B). However, owing to the small number of events, we could not conduct further multivariate analyses adjusting for clinicopathologic variables.
Fig. 3. Comparisons of survival outcomes between LNR-high and LNR-low groups in open surgery (upper) and MIS (lower). (A and C) DFS. (B and D) OS. DFS, disease-free survival; OS, overall survival; MIS, minimally invasive surgery; LNR, lymph node ratio.
In patients who underwent MIS RH (n=27), the MIS RH group showed significantly worse DFS than the open RH group (3-year DFS rate, 46.0% vs. 90.0%; p=0.017), while the two groups showed similar OS (p=0.564) (Fig. 3C and D). In multivariate analysis adjusting for patient age, para-aortic lymphadenectomy, cervical mass size, and parametrial invasion, LNR ≥8.831% was identified as an independent poor prognostic factor for DFS (aHR, 14.578; 95% CI, 1.225–173.437; p=0.034) (Table 3).
Table 3. Factors Associated with Disease-Free Survival in Patients Who Underwent MIS.
| Variables | Test | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | Adjusted HR | 95% CI | p value | ||
| Age (yr) | ≥50 vs. <50 | 0.435 | 0.090–2.100 | 0.300 | 0.267 | 0.044–1.612 | 0.150 |
| 2009 FIGO stage | IIA vs. IB | 0.810 | 0.166–3.941 | 0.794 | |||
| Pre-operative conization | Yes vs. No | 1.742 | 0.337–8.996 | 0.507 | |||
| Para-aortic LN removal | Yes vs. No | 1.049 | 0.215–5.106 | 0.953 | 0.115 | 0.005–2.633 | 0.176 |
| Cervical mass size by pathology, mm | ≥40 vs. <40 | 1.672 | 0.346–8.075 | 0.522 | 4.013 | 0.653–24.668 | 0.134 |
| Parametrial involvement | Yes vs. No | 1.852 | 0.442–7.769 | 0.399 | 8.506 | 0.611–118.469 | 0.111 |
| Resection margin involvement | Yes vs. No | 4.307 | 1.021–18.175 | 0.047 | |||
| Adjuvant treatment | CCRT+Chemo vs. CCRT | 0.697 | 0.173–2.805 | 0.612 | |||
| LNR, % | 1.106 | 1.043–1.173 | 0.001 | ||||
| LNR, % | ≥8.831 vs. <8.831 | 8.797 | 1.070–72.315 | 0.043 | 14.578 | 1.225–173.437 | 0.034 |
CCRT, concurrent chemoradiation therapy; Chemo, chemotherapy; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; LN, lymph node; HR, hazard ratio; LNR, lymph node ratio; MIS, minimally invasive surgery.
Detailed characteristics of the 11 patients with recurrence (nine for MIS RH and two for open RH) are presented in Supplementary Table 3 (only online). Median LNR (%) values were 22.222 and 9.365 in the MIS RH and open RH groups, respectively. Consolidation cisplatin and fluorouracil chemotherapy and extended RT (boost on the para-aortic area) were administered to 3 (27.3%) and 4 (36.4%) patients, respectively. In terms of recurrence sites, the pelvis was the most common site (7/11, 63.6%), followed by retroperitoneal LNs (4/11, 36.4%), distant sites (3/11, 27.3%), and abdomen (2/11, 18.2%). Of the 5 patients with distant and/or abdominal metastasis, only one received consolidation chemotherapy after completion of adjuvant CCRT. Of the 4 patients with nodal recurrence, none received either extended RT or consolidation chemotherapy.
DISCUSSION
In this study, we investigated the impact of LNR on survival outcomes in node-positive, early-stage cervical cancer according to surgical approach. Overall, patients with high LNR had significantly worse DFS, compared with low LNR. This relationship was further consolidated among patients who received MIS RH.
LNR combines information on the number of positive lymph nodes and the total number of resected lymph nodes, and may better stratify patients with regard to prognosis. Consistent with the literature,15,16,17,18,19,25 our study also confirmed pathologic LNR as a predictor of survival in cervical cancer. The cut-off values for the LNR-high group in cervical cancer differed for each previous study, ranging from 5% to 40%,18,19 and most used 10%.15,16,17 The cut-off value of our study also falls within this range: unlike previous studies, we determined the cut-off value for all of these patients through ROC curve analysis, and it was 8.831%. Our data provide useful prognostic information to physicians and patients, allowing the selection of patients for more aggressive adjuvant therapy. However, living in the era of post-LACC trial, we cannot help but consider the surgical approach in interpreting the study results.
Like the LACC trial and subsequent retrospective studies, we also found inferior survival outcomes from MIS RH, compared with open RH. While conducting the current study, we expected similar survival outcomes between the open RH and MIS RH groups, because all patients received adjuvant CCRT due to lymph node metastasis and because CCRT might reduce the risk of recurrence from MIS RH. Instead, the 3-year DFS rate of the MIS RH group was 66.7%, surprisingly low, compared to historical values.26,27,28 Adjuvant CCRT seems to be insufficient to overcome adverse effects from MIS RH in patients with node-positive, early-stage cervical cancer. Considering that higher disease recurrence was observed in the LNR-high group than the LNR-low group in patients who received MIS RH, whereas no difference was observed between the two groups in patients who received open RH, poor prognosis of high LNR seems to be aggravated by MIS RH.
In detail, adjuvant CCRT may not neutralize the MIS-specific adverse effects suggested by various researchers, such as tumor spillage and dissemination promoted by the use of uterine manipulators, intracorporeal colpotomy, and a prolonged steep Trendelenburg position.29,30 In addition, we hypothesize that the cancer tissues of each anatomical site are disturbed and fragmented by the procedures of MIS RH, resulting in further adverse effects on survival outcomes beyond the therapeutic effects from adjuvant CCRT. For example, existing metastatic pelvic/para-aortic lymph nodes may be broken down into gross or microscopic sizes more frequently during laparoscopic or robotic lymphadenectomy than open lymphadenectomy. During removal of resected lymph nodes, unless they were surrounded by an endo-bag, cancer cells within lymph nodes may fall off more common in MIS than in open surgery. This is supported by our findings that abdominopelvic recurrence occurred in 5 out of 9 patients with recurrence in the MIS RH group despite adjuvant CCRT. Moreover, patients with higher LNR values tend to have more cancer cells in lymph nodes, thus chances that there are more broken and scattered cancer tissues/cells in the abdominopelvic cavity will be higher. Unless en bloc resection of the involved structures or delicate removal of all fragmented tissue was performed at the time of the primary surgery, such adverse effects from the MIS RH would hardly be reversed by conventional adjuvant treatment. Further proof-of-concept studies are required to support our hypothesis.
Interestingly, while previous studies reported significant associations between high LNR and poor OS,15,16,17,18,19 the current study showed similar OS between the LNR-high and -low groups both in the study population and patients who underwent MIS RH. No difference in OS, despite a significantly higher recurrence rate in the LNR-high group, might stem from the following reasons: 1) In the step of study population selection, we only included patients who received adjuvant RT in accordance with current clinical practice guidelines. By excluding patients who did not receive adjuvant RT, the overall prognosis would have improved. 2) Although the median observation period of the current study was 53.7 months, it seems not long enough to conduct a fair survival analysis. Also, the study population was small (n=55). Combined together, death events for each group were relatively fewer and might result in no difference in OS; and 3) recurrent cases were successfully salvaged by second-line treatment, regardless of the LNR. Further large-scale prospective cohort studies might solve both issues in the near future. Further large-scale prospective cohort studies might solve these issues in the near future.
In this study, we used the 2009 FIGO staging system, not the latest revision, for the following reasons: First, we were not sure about the clinical situation at that time, so we judged it would be difficult to convert the old stage into the new stage. Instead, we just collected the patients' stages recorded following the 2009 FIGO staging system. Second, we regarded stage IIIC in the 2018 FIGO staging system as a very heterogeneous group. For example, whether the tumor size is large or small and whether the parametrial invasion is present or not, if there is only lymph node metastasis, it is set to 2018 FIGO stage IIIC.7 Therefore, we decided that it would be better to use the 2009 FIGO staging system for describing patient characteristics. Third, many recent studies and on-going clinical trials are still using the 2009 FIGO staging system. Lastly, gynecologic oncologists and physicians are already very familiar with the 2009 FIGO staging system.
The current study has several limitations. First, inevitable issues, such as selection biases, might exist owing to the retrospective design. For example, although the survival benefit from adjuvant chemotherapy after CCRT in node-positive, early-stage cervical cancer has not been proven yet, consolidation chemotherapy was performed significantly less in the open RH group than in the MISH RH group. Second, although this study was a two-institutional cohort study, the sample size might be too small for robust statistical analyses. Third, interinstitutional heterogeneity might exist, especially in surgical techniques, despite the two institutions sharing training programs for gynecologic oncologists. Last, this study only focused on survival outcomes, not investigating other treatment-related indicators, such as complications and quality-of-life outcomes. Nevertheless, this is the first study to report the prognostic significance of LNR according to surgical approach in patients with node-positive, early-stage cervical cancer.
In conclusion, this retrospective cohort study indicates that the percentage of positive lymph nodes is an important prognostic factor for disease recurrence in node-positive, 2009 FIGO stage IB-IIA cervical cancer. LNR ≥8.831% was associated with a significantly higher recurrence rate in patients who received primary surgical treatment, especially that performed by MIS. Routine evaluation of LNR may be useful for the prediction of prognosis and identification of patients who might be beneficial from more aggressive adjuvant treatment and intense surveillance programs. Further large studies validating our study findings are warranted.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (No. HI19C0664).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Se Ik Kim, Tae Hun Kim, and Yong-Sang Song.
- Data curation: Se Ik Kim and Tae Hun Kim.
- Formal analysis: Se Ik Kim and Tae Hun Kim.
- Funding acquisition: Yong-Sang Song.
- Investigation: Se Ik Kim and Tae Hun Kim.
- Methodology: Se Ik Kim and Tae Hun Kim.
- Project administration: Yong-Sang Song.
- Resources: Yong-Sang Song.
- Software: Se Ik Kim and Tae Hun Kim.
- Supervision: Yong-Sang Song.
- Validation: Maria Lee and Hee Seung Kim.
- Visualization: Yong-Sang Song.
- Writing—original draft: Se Ik Kim and Tae Hun Kim.
- Writing—review & editing: all authors.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Receiver operating characteristic curve analysis to determine cut-off values in relation to 3-year disease recurrence. LNR, lymph node ratio; AUC, area under the receiver operating characteristic curve; CI, confidence interval.
Clinicopathologic Characteristics of LNR-High and LNR-Low Groups
Factors Associated with Overall Survival
Detailed Characteristics of the Patients in the Study Population Who Experienced Recurrence
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 3.Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905–1914. doi: 10.1056/NEJMoa1804923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SI, Cho JH, Seol A, Kim YI, Lee M, Kim HS, et al. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1-IIA2 cervical cancer. Gynecol Oncol. 2019;153:3–12. doi: 10.1016/j.ygyno.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Uppal S, Gehrig PA, Peng K, Bixel KL, Matsuo K, Vetter MH, et al. Recurrence rates in patients with cervical cancer treated with abdominal versus minimally invasive radical hysterectomy: a multiinstitutional retrospective review study. J Clin Oncol. 2020;38:1030–1040. doi: 10.1200/JCO.19.03012. [DOI] [PubMed] [Google Scholar]
- 6.Nitecki R, Ramirez PT, Frumovitz M, Krause KJ, Tergas AI, Wright JD, et al. Survival after minimally invasive vs open radical hysterectomy for early-stage cervical cancer: a systematic review and meta-analysis. JAMA Oncol. 2020;6:1019–1027. doi: 10.1001/jamaoncol.2020.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatla N, Berek JS, Cuello Fredes M, Denny LA, Grenman S, Karunaratne K, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145:129–135. doi: 10.1002/ijgo.12749. [DOI] [PubMed] [Google Scholar]
- 8.Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:64–84. doi: 10.6004/jnccn.2019.0001. [DOI] [PubMed] [Google Scholar]
- 9.Cibula D, Pötter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie Meder C, et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother Oncol. 2018;127:404–416. doi: 10.1016/j.radonc.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Lemmens VE, Dassen AE, van der Wurff AA, Coebergh JW, Bosscha K. Lymph node examination among patients with gastric cancer: variation between departments of pathology and prognostic impact of lymph node ratio. Eur J Surg Oncol. 2011;37:488–496. doi: 10.1016/j.ejso.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Bhamidipati CM, Stukenborg GJ, Thomas CJ, Lau CL, Kozower BD, Jones DR. Pathologic lymph node ratio is a predictor of survival in esophageal cancer. Ann Thorac Surg. 2012;94:1643–1651. doi: 10.1016/j.athoracsur.2012.03.078. [DOI] [PubMed] [Google Scholar]
- 12.Costi R, Beggi F, Reggiani V, Riccò M, Crafa P, Bersanelli M, et al. Lymph node ratio improves TNM and Astler-Coller’s assessment of colorectal cancer prognosis: an analysis of 761 node positive cases. J Gastrointest Surg. 2014;18:1824–1836. doi: 10.1007/s11605-014-2591-4. [DOI] [PubMed] [Google Scholar]
- 13.Fortea-Sanchis C, Martínez-Ramos D, Escrig-Sos J. The lymph node status as a prognostic factor in colon cancer: comparative population study of classifications using the logarithm of the ratio between metastatic and nonmetastatic nodes (LODDS) versus the pN-TNM classification and ganglion ratio systems. BMC Cancer. 2018;18:1208. doi: 10.1186/s12885-018-5048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Lin Z, Lyu M, Chen N, Liao H, Wang Z, et al. Prognostic value of lymph node ratio in non-small-cell lung cancer: a meta-analysis. Jpn J Clin Oncol. 2020;50:44–57. doi: 10.1093/jjco/hyz120. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Zhang L, Tian J, Fu X, Ren X, Hao Q. Significance of the absolute number and ratio of metastatic lymph nodes in predicting postoperative survival for the International Federation of Gynecology and Obstetrics stage IA2 to IIA cervical cancer. Int J Gynecol Cancer. 2013;23:157–163. doi: 10.1097/IGC.0b013e3182778bcf. [DOI] [PubMed] [Google Scholar]
- 16.Polterauer S, Hefler L, Seebacher V, Rahhal J, Tempfer C, Horvat R, et al. The impact of lymph node density on survival of cervical cancer patients. Br J Cancer. 2010;103:613–616. doi: 10.1038/sj.bjc.6605801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demirci S, Ozsaran Z, Ozsaran A, Yavas F, Demircioglu B, Hanhan M, et al. Evaluation of treatment results and prognostic factors in early-stage cervical carcinoma patients treated with postoperative radiotherapy or radiochemotherapy. Eur J Gynaecol Oncol. 2012;33:62–67. [PubMed] [Google Scholar]
- 18.Joo JH, Kim YS, Nam JH. Prognostic significance of lymph node ratio in node-positive cervical cancer patients. Medicine (Baltimore) 2018;97:e11711. doi: 10.1097/MD.0000000000011711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aslan K, Meydanli MM, Oz M, Tohma YA, Haberal A, Ayhan A. The prognostic value of lymph node ratio in stage IIIC cervical cancer patients triaged to primary treatment by radical hysterectomy with systematic pelvic and para-aortic lymphadenectomy. J Gynecol Oncol. 2020;31:e1. doi: 10.3802/jgo.2020.31.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon J, Eom KY, Kim IA, Kim JS, Kim YB, No JH, et al. Prognostic value of log odds of positive lymph nodes after radical surgery followed by adjuvant treatment in high-risk cervical cancer. Cancer Res Treat. 2016;48:632–640. doi: 10.4143/crt.2015.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon J, Eom KY, Kim YS, Park W, Chun M, Lee J, et al. The prognostic impact of the number of metastatic lymph nodes and a new prognostic scoring system for recurrence in early-stage cervical cancer with high risk factors: a multicenter cohort study (KROG 15-04) Cancer Res Treat. 2018;50:964–974. doi: 10.4143/crt.2017.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynaecol Obstet. 2014;125:97–98. doi: 10.1016/j.ijgo.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Querleu D, Morrow CP. Classification of radical hysterectomy. Lancet Oncol. 2008;9:297–303. doi: 10.1016/S1470-2045(08)70074-3. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Fleming ND, Frumovitz M, Schmeler KM, dos Reis R, Munsell MF, Eifel PJ, et al. Significance of lymph node ratio in defining risk category in node-positive early stage cervical cancer. Gynecol Oncol. 2015;136:48–53. doi: 10.1016/j.ygyno.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 27.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 28.Lee TS, Kang SB, Kim YT, Park BJ, Kim YM, Lee JM, et al. Chemoradiation with paclitaxel and carboplatin in high-risk cervical cancer patients after radical hysterectomy: a Korean Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 2013;86:304–310. doi: 10.1016/j.ijrobp.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 29.Tewari KS. Minimally invasive surgery for early-stage cervical carcinoma: interpreting the laparoscopic approach to cervical cancer trial results. J Clin Oncol. 2019;37:3075–3080. doi: 10.1200/JCO.19.02024. [DOI] [PubMed] [Google Scholar]
- 30.Park JY, Nam JH. How should gynecologic oncologists react to the unexpected results of LACC trial? J Gynecol Oncol. 2018;29:e74. doi: 10.3802/jgo.2018.29.e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Receiver operating characteristic curve analysis to determine cut-off values in relation to 3-year disease recurrence. LNR, lymph node ratio; AUC, area under the receiver operating characteristic curve; CI, confidence interval.
Clinicopathologic Characteristics of LNR-High and LNR-Low Groups
Factors Associated with Overall Survival
Detailed Characteristics of the Patients in the Study Population Who Experienced Recurrence