The new vaccines against SARS-CoV-2 are novel in terms of specificity, their wide dissemination across the global population and the inclusion of newly licensed mRNA platforms. We discuss here how the approved vaccines trigger innate immunity to promote durable immunological memory and consider the future implications of protecting populations with these vaccines.
Subject terms: Vaccines, SARS-CoV-2
This Comment outlines how the recently licensed vaccines for COVID-19 activate innate immune mechanisms to promote immune memory to SARS-CoV-2. The authors also consider future challenges that could limit vaccine efficacy.
The global SARS-CoV-2 pandemic has caused significant loss of life, profound disruption to lives and livelihoods, and widespread economic, sociological and psychological damage. Severe COVID-19 involving acute respiratory distress syndrome (ARDS), multi-organ failure and death remains the most serious threat from infection, but long-term sequelae from mild disease have also been reported. The high transmissibility, presence of asymptomatic carriers and emergence of new variants have had a prolonged effect on the global population for the past year and counting. Vaccination constitutes the most promising path back to ‘normal life’; here, we discuss how the newly approved vaccines can mobilize innate and adaptive immune responses, implications for their durability, and ongoing and future challenges for protecting the population.
Approved vaccine formulations
Significant advances in cutting edge vaccine technologies over the past decade have resulted in two main types of SARS-CoV-2 vaccines now being approved for emergency use — an unprecedented achievement in modern medical science. The approved vaccines developed by Pfizer and Moderna use mRNA technology and lipid nanoparticle (LNP) delivery systems, while the approved formulations by AstraZeneca, Johnson and Johnson and Gam-COVID-vac (Sputnik V) contain DNA delivered within non-replicating recombinant adenovirus (AdV) vector systems1–4. Both the mRNA and AdV vaccines encode production of the SARS-CoV-2 spike (S) protein, which is the primary target for neutralizing antibodies generated from natural infection and for therapeutic monoclonal antibodies1. To date, results from the phase III clinical trials showed that both the Pfizer/BioNTech (BNT162b2) and Moderna (mRNA-1273) mRNA vaccines achieved 90–95% efficacy in protecting against COVID-19 (refs1,2), while the AdV vaccines (ChAdOx1 nCoV-19) and Gam-COVID-vac (Sputnik V) showed protection at a slightly lower efficacy (average 70% and 91%, respectively)3,4. Both vaccine types generate significant neutralizing antibody titres and virus-specific T cell responses as measured in blood 2–4 weeks post inoculation5,6. These trials, which collectively involved more than 100,000 participants, provide compelling rationale for expedient and widespread vaccination of the global population. While the AdV vaccine platform has been licensed for Ebola, the mRNA vaccine platform represents a newly licensed formulation. Thus, we still have much to learn about how these vaccines mobilize the immune response, the durability of protection and how to further optimize them to protect against new variants, strains and disease manifestations.
Triggering innate and adaptive responses
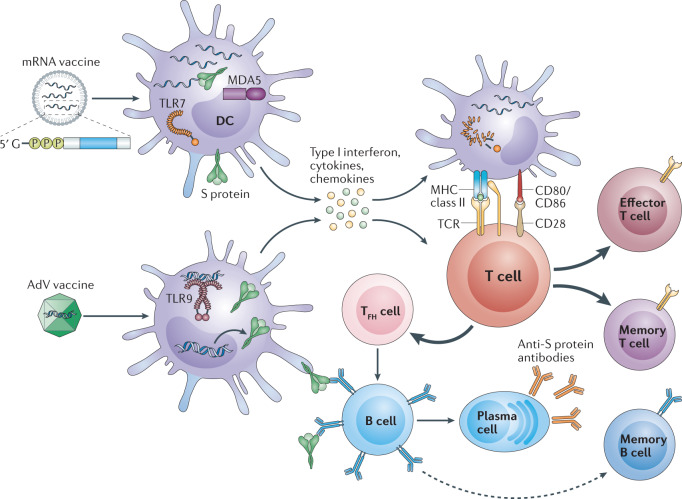
To stimulate adaptive immunity, a vaccine requires a pathogen-specific immunogen as well as an adjuvant — the latter stimulates the innate immune system and provides the necessary second signal for T cell activation. An optimal adjuvant stimulates innate immunity without inducing systemic inflammation that could elicit severe side effects. For mRNA vaccines, the mRNA can serve as both immunogen (encoding the viral protein) and adjuvant, owing to intrinsic immunostimulatory properties of RNA. Upon entry into cells, single-stranded RNA (ssRNA) and double-stranded RNA (dsRNA) are recognized by various endosomal and cytosolic innate sensors that form a critical part of the innate immune response to viruses. Endosomal Toll-like receptors (TLR3 and TLR7) bind to ssRNA in the endosome, while components of the inflammasome such as MDA5, RIG-I, NOD2 and PKR bind to ssRNA and dsRNA in the cytosol, resulting in cellular activation, and production of type I interferon and multiple inflammatory mediators7 (Fig. 1). The current vaccines contain purified, in vitro-transcribed single-stranded mRNA with modified nucleotides to reduce binding to TLR and immune sensors, thus limiting excessive production of type I interferon and its inhibitory function on cellular translation (see ref.7). The LNP carrier further protects the mRNA, can target delivery to lymphatics and promote protein translation in lymph nodes (LNs)7. Once in the LN, the LNP is engulfed by dendritic cells (DCs), which subsequently produce and present the antigen to T cells for activation of the adaptive immune response.
Fig. 1. How mRNA and adenovirus vector vaccines elicit immunity to SARS-CoV-2.
The two vaccine formulations — mRNA encoding the SARS-CoV-2 spike (S) protein encapsulated in lipid nanoparticles or adenovirus (AdV) vectors encoding the S protein — gain entry into dendritic cells (DCs) at the injection site or within lymph nodes, resulting in production of high levels of S protein. In addition, innate sensors are triggered by the intrinsic adjuvant activity of the vaccines, resulting in production of type I interferon and multiple pro-inflammatory cytokines and chemokines. RNA sensors such as Toll-like receptor 7 (TLR7) and MDA5 are triggered by the mRNA vaccines, and TLR9 is the major double-stranded DNA sensor for the AdV vaccine. The resultant activated DCs present antigen and co-stimulatory molecules to S protein-specific naive T cells, which become activated and differentiated into effector cells to form cytotoxic T lymphocytes or helper T cells. T follicular helper (TFH) cells help S protein-specific B cells to differentiate into antibody-secreting plasma cells and promote the production of high affinity anti-S protein antibodies. Following vaccination, S protein-specific memory T cells and B cells develop and circulate along with high affinity SARS-CoV-2 antibodies, which together help prevent subsequent infection with SARS-CoV-2. TCR, T cell receptor.
The AdV vaccines also contain inherent adjuvant properties, although these reside with the virus particle that encases the DNA encoding the immunogen. Following injection, AdV particles target innate immune cells like DCs and macrophages and stimulate innate immune responses by engaging multiple pattern-recognition receptors including those that bind dsDNA — in particular TLR9 — to induce type I interferon secretion8. Unlike AdV vectors, mRNA vaccines do not engage TLR9, but both vaccine formulations converge on the production of type I interferon (Fig. 1). Type I interferon-producing DCs and other cells that have taken up the vaccine-derived nucleic acids encoding the S protein can deliver both an antigenic and inflammatory signal to T cells in LNs draining the injection site. This activates S protein-specific T cells and mobilizes adaptive immunity against SARS-CoV-2 (Fig. 1).
The ability of mRNA and AdV vaccines to promote intracellular production of S protein along with innate immune responses should prime both CD8+ and CD4+ T cells to differentiate into effector and memory subsets. In particular, vaccine-driven production of type I interferon promotes differentiation of CD4+ and CD8+ effector T cells producing inflammatory and cytotoxic mediators, and CD4+ T follicular helper (TFH) cells, which promote B cell differentiation into antibody-secreting plasma cells (Fig. 1). Both the mRNA and AdV vaccines require two doses spaced 3–4 weeks apart to promote optimal protection and have been associated with mild to moderate side effects, including injection site pain, transient fever and chills, which can be augmented with the second dose. This secondary enhancement of the inflammatory response can derive from short-term changes to innate cells like macrophages through a phenomenon called ‘trained immunity’9, and/or from activation of memory T cells and B cells generated from the initial injection. Type I interferon has been shown to amplify T cell memory and promote B cell differentiation and survival, suggesting vaccine-associated inflammation in the booster can further promote generation and perpetuation of long-term immunological memory.
Durability and future challenges
Preclinical and early results from human trials show that both vaccines generate anti-S protein IgG and virus-specific neutralizing antibody responses for several months post-vaccination5,6, while the T cell data remain to be fully elucidated. This short-term durability is likely sufficient for curtailing the spread of SARS-CoV-2 and beginning the path back to normalcy. However, the global pervasiveness of SARS-CoV-2 along with the emergence of S protein variants could potentially limit vaccine efficacy. Eradication of SARS-CoV-2 from the population may prove challenging, owing to reservoirs within individuals who are not vaccinated and/or in other animal species. New vaccine formulations containing the variant S sequences and additional SARS-CoV-2 proteins could be generated, and annual or semi-annual SARS-CoV-2 vaccines could be given for persisting strains and/or seasonal variants. The mRNA vaccine formulation is ideally suited for repeat or modified vaccination as different mRNAs containing mutant S proteins can be rapidly synthesized and included within the LNP carrier. By contrast, the AdV vector formulation generates AdV-specific immunity, which can limit efficacy of repeated boosters owing to immune-mediated clearance of the vector.
The unprecedented mass and simultaneous vaccination of the global population will undoubtedly reveal heterogeneity in vaccination responses and some individuals may not generate robust antibody responses or be protected. Immunity to respiratory viruses can be mediated by tissue-resident memory T (TRM) cells that are established in the lung during the initial infection and retained as non-circulating populations that mediate protective responses in situ upon viral re-challenge10. TRM cells can be generated from site-specific vaccination with attenuated viral vaccine formulations10. It would be interesting to determine whether intranasal delivery of mRNA vaccines can promote TRM cells and protection in the lung. The development of self-replicating mRNA vaccines (which mimic viral replication) may also enhance protective T cell immunity. Such alterations in formulation and delivery route could be used to optimize the vaccines according to immune status and age.
In conclusion, the SARS-CoV-2 pandemic has accelerated the licensing of promising vaccine formulations that provide hope for fortifying our immune systems against the current and future emerging pandemics.
Competing interests
The authors declare no competing interests.
Contributor Information
John R. Teijaro, Email: teijaro@scripps.edu
Donna L. Farber, Email: df2396@cumc.columbia.edu
References
- 1.Baden LR, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logunov DY, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widge AT, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 2021;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahin U, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 7.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug. Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayedahmed EE, Elkashif A, Alhashimi M, Sambhara S, Mittal SK. Adenoviral vector-based vaccine platforms for developing the next generation of influenza vaccines. Vaccines. 2020;8:574. doi: 10.3390/vaccines8040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao Y, et al. Induction of autonomous memory alveolar macrophages requires t cell help and is critical to trained immunity. Cell. 2018;175:1634–1650. doi: 10.1016/j.cell.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Paik DH, Farber DL. Anti-viral protective capacity of tissue resident memory T cells. Curr. Opin. Virol. 2020;46:20–26. doi: 10.1016/j.coviro.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]