Abuse of cocaine is a major societal problem that crosses the boundaries of user age, sex, race, and ethnicity. Cocaine use has surged since 2011, and nearly one million individuals in the United States initiate cocaine use annually.1 The financial impact of cocaine use in the United States includes costs associated with healthcare services, lost work productivity, and contact with the criminal justice system. Weekly use of cocaine, particularly in older adults, carries a high risk of cerebral and cardiovascular events, with 6800 deaths attributed to cocaine toxicity in 2015.2 The acute cardiovascular effects of cocaine are attributed primarily to block of catecholamine reuptake into central and peripheral sympathetic neurons. The elevated circulating and synaptic norepinephrine increases heart rate and contractility and mediates vasoconstriction and hypertension. Acute norepinephrine-independent effects of cocaine relate to its effect on proteins involved in cardiac signaling, including voltage-gated sodium, calcium and potassium channels, and calsequestrin.3,4 Ultimately, cocaine toxicity may trigger lethal cardiac arrhythmias, myocardial infarction, and stroke.
In addition to acute cardiovascular effects, cocaine use also is implicated in the development of long-term and adverse cardiovascular consequences, including aortic stiffness and hypertension.5 The molecular pathways by which cocaine contributes to these diseases are poorly understood, but recognition of the pathways may allow for therapeutic intervention to lower cardiovascular risk in patients with a history of cocaine use. In this regard, Zhu et al6 provide an intriguing study in this issue of Hypertension, which identifies a cocaine-responsive microRNA in vascular smooth muscle cells (VSMCs) that mediates the development of aortic stiffness and hypertension in mice. For their studies, Zhu et al6 injected mice with cocaine (20 mg/kg IP body weight) for 10 consecutive days. It should be noted that they used a unit dose of cocaine within the range of doses typically used to elicit behavioral effects of locomotor stimulation and conditioned place preference in mice, at least 5-fold less than the median lethal dose. Cocaine methiodide (CM), a peripherally restricted cocaine analog, was administered at the same dose to distinguish the central nervous system versus non-central nervous system effects of cocaine on cardiovascular parameters. Saline-treated animals were used as controls. Relying on tail cuff measurements obtained 1 hour after cocaine treatment, the authors determined that diastolic and systolic blood pressure, but not heart rate, progressively increased over the 10-day course of treatment with either cocaine or CM. Ultrasound estimates of pulse wave velocity revealed increased aortic stiffness 2 days after the last injection of cocaine or CM, suggesting a persisting impact of cocaine on vascular function that did not rely on central nervous system effects. Fluorescent staining of aortic cryosections indicated elevated reactive oxygen species (ROS) in aorta from cocaine or CM-exposed mice compared with saline controls.
Subsequently, the authors detected a loss of the antioxidant enzyme, Me1 (malic enzyme 1), in aorta of cocaine- and CM-treated mice. Using miRNA target prediction programs, Me1 was identified as a target of the microRNA, miR-30c-5p. Initially, the authors relied on primary cultures of mouse aortic VSMCs to establish whether miR-30c-5p mediated the cocaine-induced loss of Me1 expression. Accordingly, when miR-30c-5p was overexpressed in VSMCs, or the cells were treated with cocaine, Me1 levels decreased and ROS increased in the VSMCs. Although it is surprising that additional cocaine treatment further potentiated loss of Me1 in VSMCs overexpressing miR-30c-5p, cocaine addition to VSMCs transduced with an miR-30c-5p-antagomir failed to downregulate Me1, suggesting that miR-30c-5p was the critical mediator of Me1 loss. Because miRNAs usually target multiple transcripts, the authors also transduced VSMCs with 3′UTR (untranslated region)-deleted Me1, which is resistant to miR-30c-5p. The Me1 expression level in these cells was unaffected by the addition of cocaine, and ROS elevation was abrogated. The fact that cocaine-induced generation of ROS in VSMCs was abolished by an antagomir of miR-30c-5p or by deleting the 3′UTR of Me1 support a critical role of the miR-30c-5p·Me1 axis in cocaine-induced upregulation of ROS in VSMCs.
In a final set of studies, Zhu et al6 returned to systemic cocaine administration in the mouse to determine whether the finding of cocaine-responsive miR-30c-5p·Me1·ROS signaling in VSMCs translates to the in vivo situation and to establish a link between this pathway and the development of aortic stiffness and hypertension. Indeed, mice receiving a smooth muscle cell-specific promoter (SM22α)-driven antago-miR of miR-30c-5p exhibited upregulation of Me1 and reduction of ROS in the aortas of cocaine-treated mice, and these animals showed attenuation of cocaine-induced hypertension. Additionally, treatment of mice with the antioxidant, N-acetyl cysteine, also dampened cocaine-induced elevations of aortic ROS, aortic stiffness, and blood pressure without affecting Me1 expression, supporting an upstream role of Me1 in ROS generation. It should be noted that additional studies will be needed to verify the presence of similar cocaine-responsive signaling in VSMCs of resistance vessels and to evaluate whether the cocaine-responsive miR-30c-5p·Me1·ROS signaling pathway directly influences vascular reactivity in addition to arterial stiffness. Identification of the precise mechanism by which cocaine increases miRNA-30c-5p in VSMCs also is delegated to future studies.
Collectively, as shown in the Figure, Zhu et al6 provide compelling data to suggest that cocaine exposure stimulates miR-30C-5p in VSMCs, which downregulates Me1, elevates ROS, and contributes to the development of hypertension and aortic stiffness in mice. However, the association between cocaine use and enduring cardiovascular disease is not fully resolved because vascular parameters were obtained just 2 days after final cocaine administration. Also, because the authors used tail-cuff recordings instead of direct blood pressure measurement, they captured only limited blood pressure values. Additionally, the utilization of a single dose of cocaine precluded an assessment of dose-dependence in cocaine’s ability to promote aortic stiffness and hypertension in mice and the full extent to which the drug may elicit these persistent, adverse changes. Finally, it should be recognized that the C57BL/6 mice used for in vivo experiments may have different sensitivity to the cardiovascular effects of cocaine compared with other mouse strains. Strain differences in sensitivity to cocaine have been extensively demonstrated with regard to its behavioral and convulsant effects and pharmacokinetic profile.7–11 Thus, the results of Zhu et al6 may not readily translate to other murine strains, or the dose of cocaine may have to be modified. Finally, assuming that the preclinical findings of Zhu et al6 extend to human cocaine users, the development of an miRNA-based therapy to mitigate the development of hypertension in these individuals still would face many challenges, including target and tissue specificity. Regardless, a recent clinical report indicating a distinct profile of VSMC-modulating miRNAs in peripheral blood mononuclear cells from humans with essential hypertension12 raises the possibility that normalizing the expression level and function of distinct miRNAs represents a potential antihypertensive strategy for patients.
Figure.
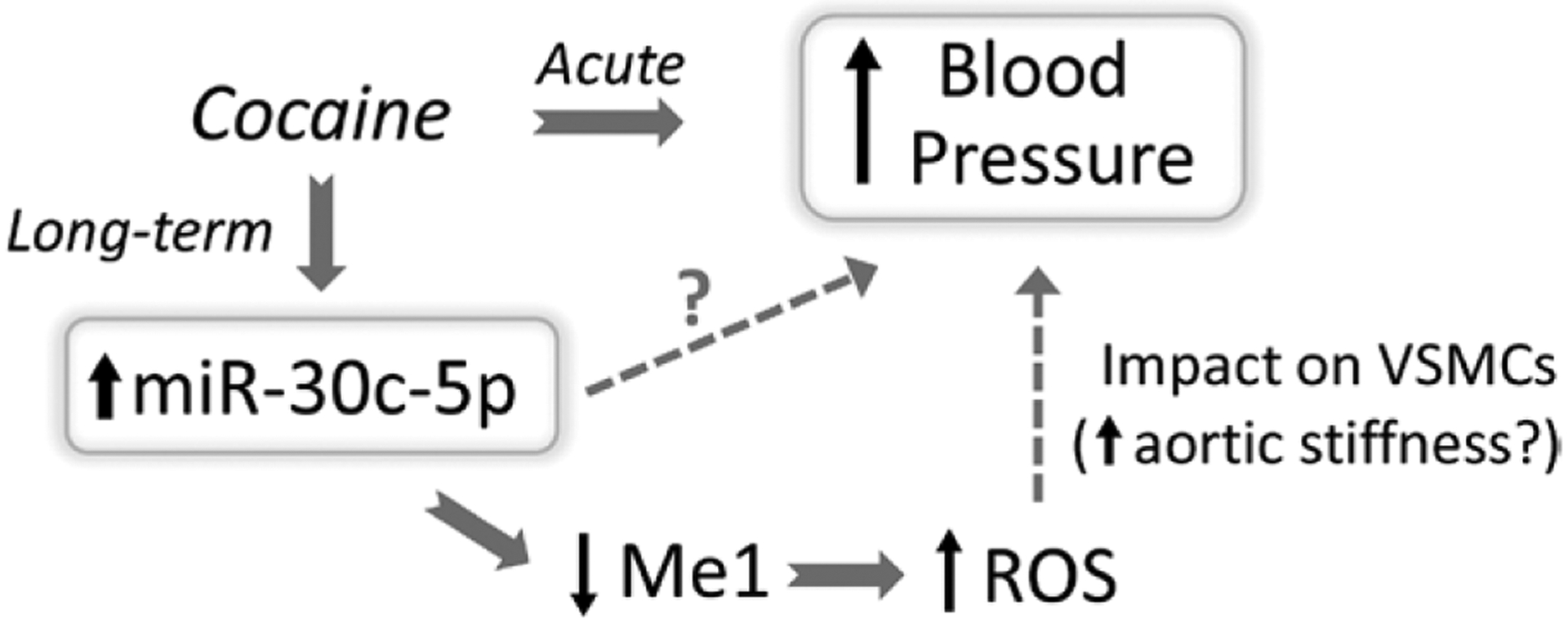
The molecular pathway by which cocaine use may contribute to the development of hypertension. Distinct from cocaine’s acute hypertensive effect, Zhu et al6 propose a cocaine-responsive miRNA (miR-30c-5p) in vascular smooth muscle cells (VSMCs), which may contribute to the development of chronic hypertension in a rat model of daily cocaine administration. The authors suggest that stimulation of miR-30c-5p in VSMCs by cocaine downregulates the antioxidant molecule, Me1 (malic enzyme 1), resulting in elevated reactive oxygen species (ROS). The generation of ROS in VSMCs was associated with enhanced aortic stiffness and hypertension. The authors acknowledge that miR-30c-5p may modulate the expression of other transcripts in VSMCs that elevate ROS and promote the long-term elevation of blood pressure.
In summary, Zhu et al6 report a cocaine-responsive miRNA in VSMCs, which seems to contribute to the development of vascular stiffness and hypertension in mice subjected to daily cocaine administration. The findings add to our growing knowledge of the diverse miRNA signaling pathways in VSMCs that influence blood pressure and contribute to cardiovascular pathologies. Moreover, the findings support the concept that repeated exposure to cocaine can elicit long-term and detrimental changes in cardiovascular function, which are distinct from its acute toxicity. Finally, the findings of Zhu et al6 lend scientific impetus to the concept that health professionals should obtain a drug history to ascertain cocaine use as a risk factor for cardiovascular disease. Patients should be aware of the consequences of chronic cocaine use within the larger framework of cardiovascular disease and realize that using cocaine not only carries immediate risk for a cardiovascular event but also may cause long-term consequences for cardiovascular health that persist after ending drug use.
Footnotes
Disclosures
None.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1.John WS, Wu LT. Trends and correlates of cocaine use and cocaine use disorder in the United States from 2011 to 2015. Drug Alcohol Depend. 2017;180:376–384. doi: 10.1016/j.drugalcdep.2017.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. NCHS Data on Drug-poisoning Deaths, CDC. 2017. https://www.cdc.gov/nchs/data/factsheets/factsheet_drug_poisoning.pdf.
- 3.O’Leary ME, Hancox JC. Role of voltage-gated sodium, potassium and calcium channels in the development of cocaine-associated cardiac arrhythmias. Br J Clin Pharmacol. 2010;69:427–442. doi: 10.1111/j.1365-2125.2010.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez EJ, Hayes RP, Barr JT, Lewis KM, Webb BN, Subramanian AK, Nissen MS, Jones JP, Shelden EA, Sorg BA, Fill M, Schenk JO, Kang C. Potential role of cardiac calsequestrin in the lethal arrhythmic effects of cocaine. Drug Alcohol Depend. 2013;133:344–351. doi: 10.1016/j.drugalcdep.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozor R, Grieve SM, Buchholz S, Kaye S, Darke S, Bhindi R, Figtree GA. Regular cocaine use is associated with increased systolic blood pressure, aortic stiffness and left ventricular mass in young otherwise healthy individuals. PLoS One. 2014;9:e89710. doi: 10.1371/journal.pone.0089710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu W, Wang H, Wei J, Sartor GC, Bao MM, Pierce CT, Wahlestedt CR, Dykxhoorn DM, Dong C. Cocaine exposure increases blood pressure and aortic stiffness via the miR-30c-5p–malic enzyme 1–reactive oxygen species pathway. Hypertension. 2018;71:752–760. doi: 10.1161/HYPERTENSIONAHA.117.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azar MR, Acar N, Erwin VG, Barbato GF, Morse AC, Heist CL, Jones BC. Distribution and clearance of cocaine in brain is influenced by genetics. Pharmacol Biochem Behav. 1998;59:637–640. [DOI] [PubMed] [Google Scholar]
- 8.Kuzmin A, Johansson B. Reinforcing and neurochemical effects of cocaine: differences among C57, DBA, and 129 mice. Pharmacol Biochem Behav. 2000;65:399–406. doi: 10.1016/S0091-3057(99)00211-7. [DOI] [PubMed] [Google Scholar]
- 9.Marley RJ, Witkin JM, Goldberg SR. Genetic factors influence changes in sensitivity to the convulsant properties of cocaine following chronic treatment. Brain Res. 1991;542:1–7. [DOI] [PubMed] [Google Scholar]
- 10.Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology (Berl). 2005;181:327–336. doi: 10.1007/s00213-005-2259-6. [DOI] [PubMed] [Google Scholar]
- 11.Reith ME, Selmeci G. Cocaine binding sites in mouse striatum, dopamine autoreceptors, and cocaine-induced locomotion. Pharmacol Biochem Behav. 1992;41:227–230. doi: 10.1016/0091-3057(92)90087-V. [DOI] [PubMed] [Google Scholar]
- 12.Kontaraki JE, Marketou ME, Zacharis EA, Parthenakis FI, Vardas PE. Differential expression of vascular smooth muscle-modulating microRNAs in human peripheral blood mononuclear cells: novel targets in essential hypertension. J Hum Hypertens. 2014;28:510–516. doi: 10.1038/jhh.2013.117. [DOI] [PubMed] [Google Scholar]


