Abstract
Bronchopulmonary dysplasia is a severe and long-term pulmonary disease in premature infants. Hyperoxia-induced acute lung injury plays a critical role in bronchopulmonary dysplasia. Resveratrol is a polyphenolic phytoalexin and a natural agonist of Sirtuin 1. Many studies have shown that resveratrol has a protective effect on hyperoxia-induced lung damage, but its specific protective mechanism is still not clear. Further exploration of the possible protective mechanism of resveratrol was the main goal of this study. In this study, human alveolar epithelial cells were used to establish a hyperoxia-induced acute lung injury cell model, and resveratrol (Res or R), the Sirtuin 1 activator SRT1720 (S) and the Sirtuin 1 inhibitor EX-527 (E) were administered to alveolar epithelial cells, which were then exposed to hyperoxia to investigate the role of Res in mitochondrial function and apoptosis. We divided human alveolar epithelial cells into the following groups: (1) the control group, (2) hyperoxia group, (3) hyperoxia+Res20 group, (4) hyperoxia+Res20+E5 group, (5) hyperoxia+Res20+E10 group, (6) hyperoxia+S2 group, (7) hyperoxia+S2+E5 group, and (8) hyperoxia+S2+E10 group. Hyperoxia-induced cell apoptosis and mitochondrial dysfunction were alleviated by Res and SRT1720. Res and SRT1720 upregulated Sirtuin 1, PGC-1α, NRF1, and TFAM but decreased the expression of acetyl-p53 in human alveolar epithelial cells that were exposed to hyperoxia. These findings revealed that Res may alleviated hyperoxia-induced mitochondrial dysfunction and apoptosis in alveolar epithelial cells through the SIRT1/PGC-1a signaling pathway. Thus, Sirtuin 1 upregulation plays an important role in lung protection.
Keywords: Bronchopulmonary dysplasia, resveratrol, SRT1720, EX-527, apoptosis, mitochondrial dysfunction
Impact statement
With the progression of medical treatment in premature infants, the incidence of BPD has not decreased; thus, it is important to find new treatment and prevention strategies for BPD. This study aimed to provide experimental evidence for the potential application of resveratrol to protect the lungs during hyperoxia. Our study revealed that resveratrol may alleviate hyperoxia-induced mitochondrial dysfunction and apoptosis in alveolar epithelial cells through the SIRT1/PGC-1a signaling pathway. Resveratrol-induced SIRT1 upregulation is involved in lung protection. This study revealed the possible mechanism of resveratrol's lung protection in hyperoxia and provides a theory for the future application of resveratrol in the clinic.
Introduction
Oxygen therapy is widely used for hypoxic conditions for various reasons, especially in neonates. Hyperoxia can cause a variety of side effects,1–4 and the lung is usually the first organ to be damaged. Bronchopulmonary dysplasia (BPD) is a severe and long-term pulmonary disease in premature infants that affects as many as 25% of extreme preterm newborn infants.5 Long-term and high-concentration oxygen exposure is recognized as the main pathogenic factor in the occurrence of BPD.6 However, with the advancement of medical treatment in preterm infants, the survival rate of preterm infants has greatly improved, and the prevalence of BPD has not decreased; therefore, it is important to find new treatment and prevention strategies for BPD.
Sirtuin 1 (SIRT1), a NAD+-dependent protein deacetylase, modulates many essential metabolic processes, including apoptosis, inflammation, oxidative stress, aging, and mitochondrial regulation, via deacetylation of substrate proteins, such as p53, nuclear factor-κB (NF-κB), STAT3, and peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1α).7–11 Deacetylation of SIRT1 may affect the transactivation function of p53, thereby reducing cell cycle arrest and apoptosis.12 The expression of acetyl-p53 (Ac-p53) increases in hyperoxia.13 SIRT1 also regulates mitochondrial function and biosynthesis by interacting with PGC1α.14,15 Resveratrol (3,5,4ʹ-trihydroxy-trans-stilbene, Res, or R) is a polyphenolic compound and is reported to have a wide range of biological activities, including anti-inflammation, antioxidation, anti-apoptosis, and anti-fibrosis.16,17 Many studies have reported that in respiratory diseases, Res exerts its protective effect by regulating SIRT1, reactive oxygen species (ROS) production and apoptosis.13,18 SRT1720 (S) is a selective SIRT1 activating agent.19,20 EX-527 (SEN0014196, E) is an effective, selective SIRT1 inhibitor that effectively inhibits SIRT1 deacetylase activity and thus restores global acetylation levels.21 Res and SRT1720 can stimulate PGC-1α to remodel the metabolism, especially in skeletal muscle.22–24
Mitochondria provide energy for cell survival and function, which are very important in all cell types. In addition to participating in energy metabolism, increasing evidence shows that mitochondria are also involved in nonenergy metabolism, including lipid synthesis, calcium signaling, cell death, and metabolism, by changing mitochondrial protein expression, structure, and function.25–28 Mitochondria also regulate cell apoptosis by promoting the release of proapoptotic mediators.29–33 Studies have shown that mitochondrial dysfunction is related to respiratory diseases, including airway inflammation, pulmonary fibrosis, and BPD.34,35 Under oxidative stress conditions, the mitochondrial genome is a cellular target, such as with acute lung injury (ALI) or pneumonia.36 Mitochondrial DNA (mtDNA) is more vulnerable to oxidative stress than nuclear DNA.37,38 Mitochondrial transcription factor A (TFAM) is considered essential for the stability and transcription of mtDNA.39,40 Studies have shown that TFAM can regulate mitochondrial function by interacting with PGC-1α and nuclear respiratory factor 1 (NRF1).41
However, it is still unclear how Res exerts its protective effect during hyperoxia-induced lung injury. We hypothesized that Res plays a protective role in hyperoxic damage by alleviating mitochondrial dysfunction and that the SIRT1/PGC-1α signaling pathway may be involved. Hence, we developed a hyperoxia model to study the possible mechanism by which Res protects alveolar epithelial cells.
Materials and methods
Cell culture and hyperoxia model
Human alveolar epithelial cells (HPAEpiCs) were used in this experiment (Shanghai Institute of Cell Biology, Chinese Academy Sciences). HPAEpiCs were cultured in Dulbecco's modified Eagle medium (DMEM, high glucose, Invitrogen, Shanghai, China) at 37°C in a 5% CO2 atmosphere.42 The medium included fetal bovine serum (10%, Beyotime, Shanghai, China), penicillin (100 U/mL), and streptomycin (100 μg/mL). Hyperoxia model: 100% CO2 and 100% O2 were mixed in a 5:95 ratio using an air mixing apparatus. Cells were exposed to hyperoxia (inlet mixture gas contained O2 (950 mL/L) and CO2 (50 mL/L) at a speed of 3 L/minutes for 10 minutes) and then cultured in a humidified incubator (5% CO2, 37°C) for 24 h. The oxygen concentration was dynamically measured using a ML-IICB digital intelligent oxygen.
Cell counting kit-8 assay
After the cells reached 50–60% confluence, the cells were incubated with several different concentrations of Res (5 μM, 10 μM, 20 μM, 50 μM, 100 μM, 150 μM, 200 μM, 250 μM), SRT1720 (0.5 μM, 1 μM, 2 μM, 4 μM, 6 μM, 8 μM), and EX-527 (1 μM, 2.5 μM, 5 μM, 10 μM, 20 μM, 50 μM, 100 μM), and three wells were used for each concentration. Cell proliferation was measured after 24 h by WST-8 assay (Cell Counting Kit-8, Dojindo, Kumamoto, Japan). The absorbance was measured with a microplate reader (450 nm).
Research groups
Based on the results of CCK8 and ROS detection and reference related research, we finally determined the drug concentration of Res (20 μM), SRT1720 (2 μM), and EX-527 (5 μM and 10 μM). The cells were divided into the following groups after they reached 50 to 60% confluence: (1) a control group in which the cells were exposed to normoxia (21% O2), (2) hyperoxia group in which the cells were exposed to hyperoxia, (3) hyperoxia+Res20 group in which 20 μM Res (DESITE, Chengdu, China) was added and the cells were exposed to hyperoxia, (4) hyperoxia+Res20+E5 group in which 20 μM Res and 5 μM EX-527 (MCE, New Jersey, US) were added and the cells were exposed to hyperoxia, (5) hyperoxia+Res20+E10 group in which 20 μM Res and 10 μM EX-527 were added and the cells were exposed to hyperoxia, (6) hyperoxia+S2 group in which 2 μM SRT1720 (Selleck, Houston, TX, USA) was added, and the cells were exposed to hyperoxia (7) hyperoxia+S2+E5 group in which 2 μM SRT1720 and 5 μM EX-527 were added and the cells were exposed to hyperoxia, and (8) the hyperoxia+S2+E10 group in which 2 μM SRT1720 and 10 μM EX-527 were added and the cells were exposed to hyperoxia. All cell cultures were cultured in a humidified incubator (5% CO2, 37°C) for 24 h and then harvested for the next steps.
Measurement of intracellular total ROS
The cells were incubated in 2′,7′-dichlorofluorescein diacetate (DCHF-DA) (Beyotime, Shanghai, China) medium for 20 min. After cell incubation, the cells were washed, and serum‐free high glucose DMEM was used. Fluorescence was measured with a fluorescence enzyme labeling instrument.
Measurement of mitochondrial ROS by MitoSOX staining
First, 1 μM of MitoSOX™ (Invitrogen, Shanghai, China) working solution and 10 mg/l of Hoechst 33342 (Solarbio, Beijing, China) working solution were used. The cells were washed twice with Hank's solution and then incubated with the configured Mito SOX™ working solution for 20 min (37°C). This process was carried out in a dark room. After incubation, the cells were washed again with Hank’s solution. The cells were then incubated in the prepared Hoechst 33342 working solution for 10 min (37°C) in the dark. Finally, a fluorescence microscope was used for observation and image collection.
Measurement of mitochondrial membrane potential
The JC-1 Kit (Solarbio, Beijing, China) was used to evaluate the mitochondrial membrane potential in the cells. The JC‐1 dying working solution was prepared according to the manufacturer's instructions. The cells were incubated with the JC-1 staining working solution in the dark for 20 min (37°C). Finally, a fluorescence microscope was used for observation and image collection. The average fluorescence intensity of red or green fluorescence was evaluated by ImageJ software. The membrane potential is represented by the ratio of red/green fluorescence intensity, and a decrease in the ratio means that the membrane potential is relatively low.
Flow cytometry analysis of apoptosis
FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen, Franklin Lakes, NJ) was used to measure cell apoptosis, and apoptotic cells were quantitated using a flow cytometer (Thermo Fisher Scientific).
Western blot
After treatment for 24 h as described before, the cells were lysed with lysis buffer (4°C), followed by protein concentration detection using a BCA Protein Assay kit (Beyotime, Shanghai, China). The proteins were separated by SDS-PAGE with the same amount of proteins for each lane (30 µg/lane). The separated proteins were transferred to PVDF membranes. The membranes were blocked with 5% (w/v) dry nonfat milk and incubated with the corresponding primary antibodies against SIRT1 (1:1000, Abcam, UK, ab110304), acetyl-p53 (1:1000, Abcam, UK, ab183544), PGC-1α (1:1000, Cell Signaling, USA, #2178), NRF1 (1:1000, Cell Signaling, USA, #46743), TFAM (1:1000, Cell Signaling, USA, #8076), and GAPDH (1:15000, Proteintech, USA, Cat no: 10494–1-AP) at 4°C with gentle shaking overnight. Goat anti-rabbit IgG-HRP (Beyotime, Shanghai, China) and goat anti-mouse IgG-HRP (Beyotime, Shanghai, China) were used as secondary antibodies, and these secondary antibodies were added to the membranes. GAPDH (Proteintech, Chicago, USA) was used as an internal control. Finally, the electrochemiluminescence (ECL) system was adopted for band detection.
Statistical analysis
SPSS 19.0 statistical software was used to perform statistical analysis. The data are expressed as the mean ± standard deviation (SD) of four independent experiments and statistically analyzed using one-way analysis of variance (ANOVA) or a nonparametric test (Kruskal–Wallis). Statistical significance was set at P < 0.05.
Results
Cytotoxicity of res, SRT1720, and EX-527 on HPAEpiCs in vitro
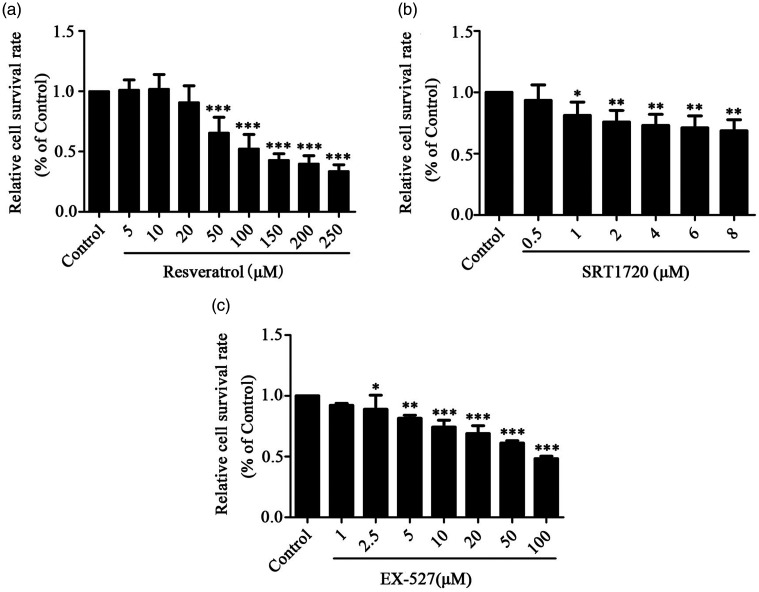
We added Res, SRT1720 and EX-527 drugs in different concentrations to HPAEpiCs, and then CCK8 was used for drug cytotoxicity detection. All three drugs showed dose-dependent cytotoxicity, as shown in Figure 1. Next, different concentrations of Res (IC50, 119 μM), SRT1720 (IC50, 32 μM), and EX-527 (IC50, 93 μM) were added to HPAEpiCs before hyperoxia exposure, and the subsequent drug concentration of the experiment was explored by detecting ROS. We found that Res 20 μM and SRT1720 2 μM could not only reduce the production of ROS, but also had little effect on the inhibition of cell growth, as shown in Figure 2(a) and (c). Based on previous studies,43–45 we chose Res 20 μM and SRT1720 2 μM as the concentrations used in this study. EX-527 can promote the production of ROS at 5 μM and 10 μM, and has little effect on the growth inhibition of cells, as shown in Figure 2(b). At the same time, we also refer to previous related studies.45,46 Therefore, we chose 5 μM and 10 μM as the concentrations used in this study.
Figure 1.
Effects of Res, SRT1720 and EX-527 on the viability of HPAEpiC cells. (a) Res inhibits the growth of HPAEpiC cells in a dose-dependent manner. (b) SRT1720 inhibits the growth of HPAEpiC cells in a dose-dependent manner. (c) EX-527 inhibits the growth of HPAEpiC cells in a dose-dependent manner. Data from four independent experiments. *P < 0.05; **P < 0.01; and ***P < 0.001.
Figure 2.
The effects of Res, SRT1720, and EX-527 on the total ROS of HPAEpiC cells. (a) Effects of Res at different concentrations on the total ROS of HPAEpiC cells induced by hyperoxia. (b) Effects of EX-527 at different concentrations on the total ROS of HPAEpiC cells induced by hyperoxia. (c) Effects of SRT1720 at different concentrations on the total ROS of HPAEpiC cells induced by hyperoxia. (d) Effects of Res and EX-527 on the total ROS of HPAEpiC cells induced by hyperoxia. (e) Effects of SRT1720 and EX-527 on the total ROS of HPAEpiC cells induced by hyperoxia. Data from four independent experiments. *P < 0.05; **P < 0.01; and ***P < 0.001.
Res reduced the total ROS of alveolar epithelial cells exposed to hyperoxia
As shown in Figure 2(a), the total ROS levels were significantly increased in the hyperoxia group compared with the control group, indicating that total ROS production was increased under hyperoxic conditions. Res significantly decreased the hyperoxia-induced total ROS increase in the HPAEpiC cells. Figure 2(b) shows that compared with the hyperoxia group, EX-527 significantly increased the total ROS in the HPAEpiC cells. As shown in Figure 2(c), compared with the hyperoxia group, SRT1720 significantly reduced the increase in total ROS that was induced by hyperoxia. As shown in Figure 2(d), the ROS level of the hyperoxia+Res20 group was significantly lower than that of the hyperoxia group. In the hyperoxia+Res20+E5 and hyperoxia+Res20+E10 groups, in which the cells were pretreated with EX-527 and Res and then exposed to hyperoxia, the levels of total ROS were significantly increased compared with those of the hyperoxia + Res20 group. However, there was no significant difference in ROS levels under different EX-527 concentrations. SRT1720 (Figure 2(e)) demonstrated similar results to Res. The ROS level in the hyperoxia+S2+E10 group was higher than that in the hyperoxia+S2+E5 group.
Res reduced mtROS production in alveolar epithelial cells that were exposed to hyperoxia
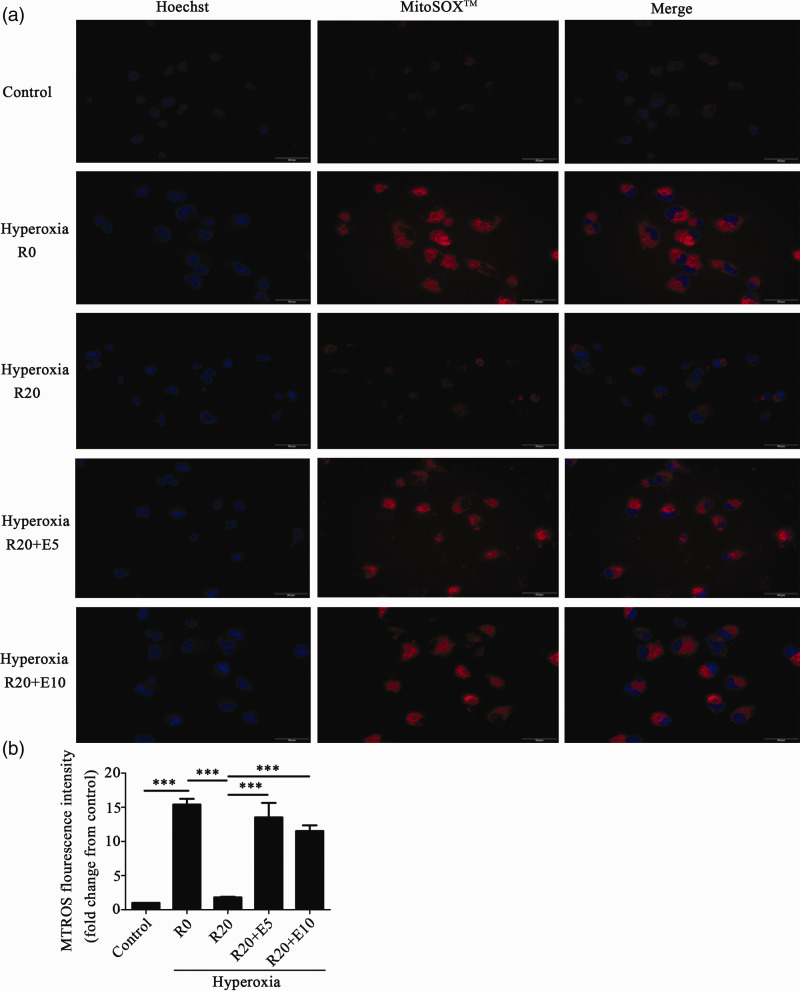
We also used MitoSOX™ to detect the production of mtROS. As shown in Figure 3, the levels of mtROS were significantly increased in the hyperoxia group compared to those in the control group, indicating that exposure to hyperoxia increased the production of mtROS. In the hyperoxia+Res20 group, the level of mtROS was lower than that in the hyperoxia group with no Res treatment. In the hyperoxia+Res20+E5 and hyperoxia+Res20+E10 groups, the levels of mtROS were significantly increased with no statistically significant difference between the two groups. As shown in Figure S1, SRT1720 and Res had similar results.
Figure 3.
Res reduced mtROS production in HPAEpiC cells that were exposed to hyperoxia. (a) The effects of Res on mtROS induced by hyperoxia. The blue fluorescence represents the nucleus, while the red fluorescence represents mtROS (400× magnification, Scale bar: 50 μm). (b) The relative level of mtROS compared to the control group. Data from four independent experiments. *P < 0.05; **P< 0.01; and ***P< 0.001.(A color version of this figure is available in the online journal.)
Res alleviated the decrease in the mitochondrial membrane potential of alveolar epithelial cells when exposed to hyperoxia
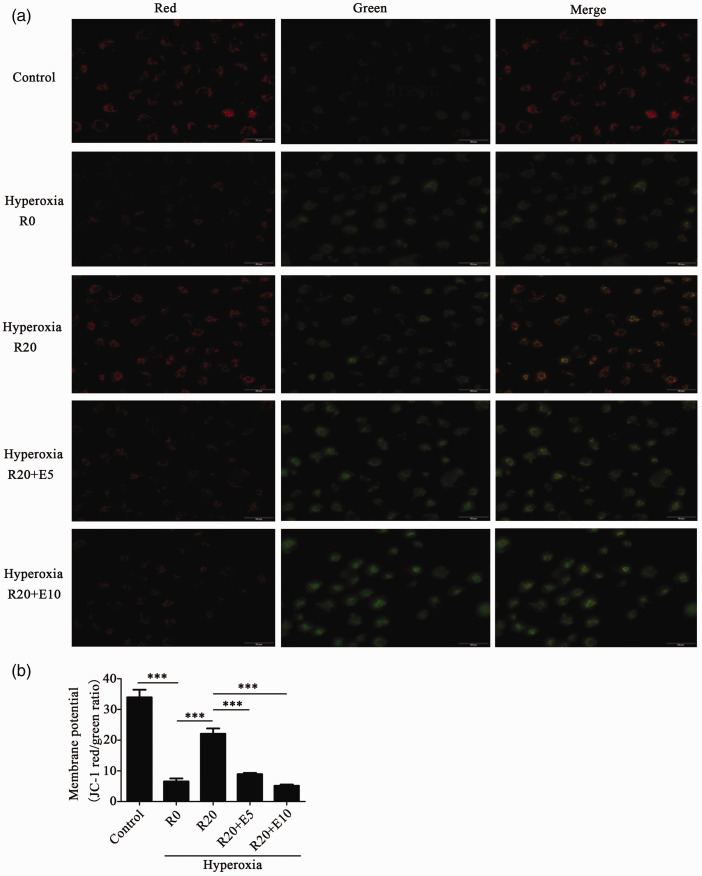
The decreased mitochondrial membrane potential is important in the early stage of apoptosis. The JC-1 Kit was used to measure the mitochondrial membrane potential. As shown in Figure 4, the mitochondrial membrane potential was significantly decreased in the hyperoxia group compared with the control group. In the hyperoxia+Res20 group, the level of mitochondrial membrane potential was higher than that in the hyperoxia group with no Res. In the hyperoxia+Res20+E5 and hyperoxia+Res20+E10 groups, the levels of mitochondrial membrane potential were decreased with no statistically significant difference between the two groups. Additionally, SRT1720 and Res showed similar results, as shown in Figure S2.
Figure 4.
Res alleviated the decrease in mitochondrial membrane potential of alveolar epithelial cells when exposed to hyperoxia. (a) The effects of Res on the decrease in mitochondrial membrane potential in HPAEpiC cells induced by hyperoxia (400× magnification, Scale bar: 50 μm). (b) The red/green ratio of each group. Data from four independent experiments. *P < 0.05; **P< 0.01; and ***P< 0.001. (A color version of this figure is available in the online journal.)
Res reduced the apoptosis of alveolar epithelial cells that were exposed to hyperoxia
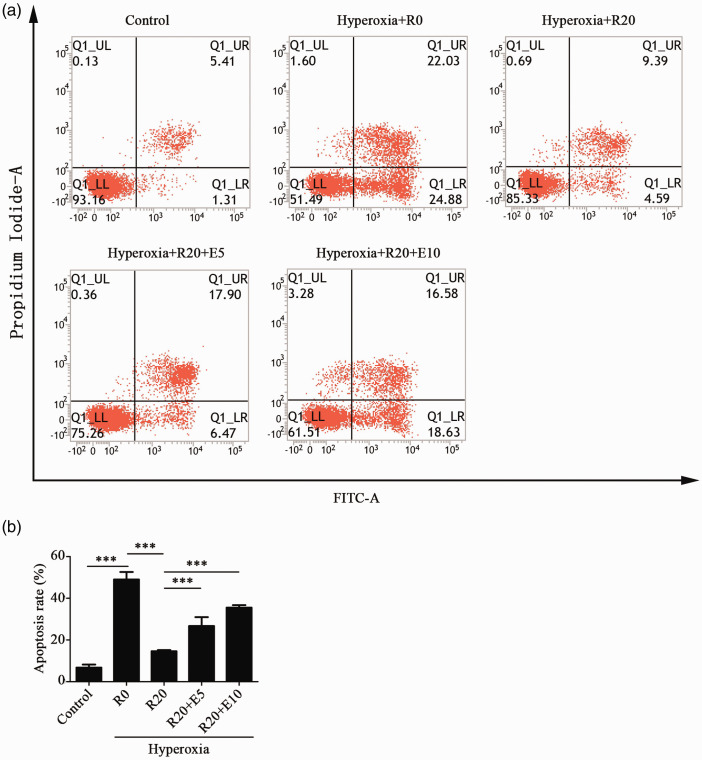
As shown in Figure 5, the apoptotic rate in the hyperoxia group was significantly increased compared with that of the control group. The apoptotic rate of the hyperoxia+Res20 group was significantly decreased compared with that of the hyperoxia group. Compared with the hyperoxia+Res20 group, the apoptotic rates of the hyperoxia+Res20+E5 and hyperoxia+Res20+E10 groups were significantly increased with no statistically significant difference between the two groups. As shown in Figure S3, SRT1720 and Res demonstrated similar results.
Figure 5.
Res reduced the apoptosis of alveolar epithelial cells that were exposed to hyperoxia. (a) The effects of Res and EX-527 on apoptosis of HPAEpiC cells induced by hyperoxia. (b) The apoptotic rates of each group. Data from four independent experiments. *P < 0.05; **P< 0.01; and ***P< 0.001. (A color version of this figure is available in the online journal.)
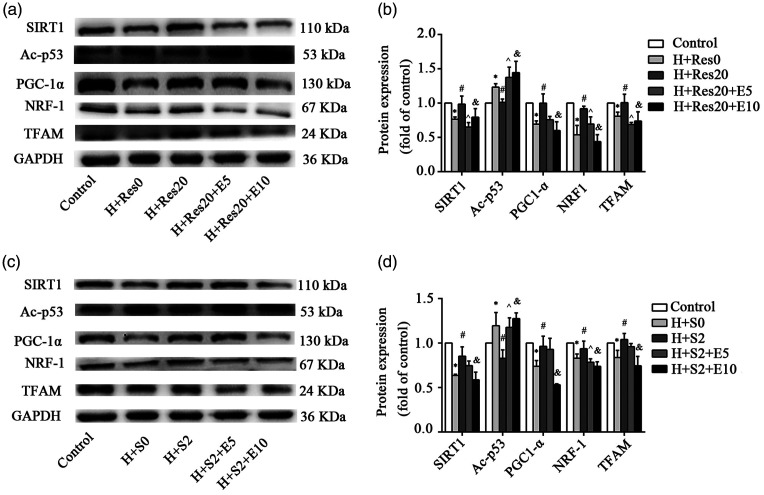
Res upregulated the expression of SIRT1, PGC-1α, NRF1, and TFAM in alveolar epithelial cells and downregulated Ac-p53 when exposed to hyperoxia
As shown in Figure 6(a) and (b), the expression levels of SIRT1, PGC-1α, NRF1, and TFAM were decreased, and the expression of Ac-p53 was increased in the hyperoxia group compared with that of the control group. In the hyperoxia+Res20 group, the expression levels of SIRT1, PGC-1α, NRF1 and TFAM were higher, while the expression of Ac-p53 was lower than that in the hyperoxia group with no Res. In the hyperoxia+Res20+E5 and hyperoxia+Res20+E10 groups, the expressions of SIRT1, PGC-1α, NRF1, and TFAM were decreased, and the expression of Ac-p53 was increased. The expression of PGC-1α and NRF1 in the hyperoxia+Res20+E10 group decreased more significantly than that in the hyperoxia+Res20+E5 group. As shown in Figure 6(c) and (d), SRT1720 and Res demonstrated similar results. The changes in SIRT1, PGC-1α, and TFAM expression were more obvious in the hyperoxia+Res20+E10 group.
Figure 6.
Res and SRT1720 upregulated the expression of SIRT1, PGC-1α, NRF1, and TFAM in alveolar epithelial cells and downregulated Ac-p53 when exposed to hyperoxia. (a, b) The effects of Res on the protein expression of SIRT1, PGC-1α, NRF1, TFAM, and Ac-p53 in HPAEpiC cells during hyperoxia. Data from four independent experiments. *: Control group versus hyperoxia (H+R0) group, P < 0.05; #: Hyperoxia (H+R0) group versus hyperoxia+Res20 (H+R20) group, P < 0.05; ^: Hyperoxia+Res (H+R20) group versus hyperoxia+Res20 + 5 μM EX-527 (H+R20+E5) group, P < 0.05; &: Hyperoxia+Res20 (H+R20) group versus hyperoxia+Res20 + 5 μM EX-527 (H+R20+E5) group, P < 0.05. (c, d) The effects of SRT1720 on the protein expression of SIRT1, PGC-1α, NRF1, TFAM and Ac-p53 in HPAEpiC cells during hyperoxia. Data from four independent experiments. *: Control group versus hyperoxia (H+S0) group, P < 0.05; #: Hyperoxia (H+S0) group versus hyperoxia+SRT1720 (H+S2) group, P < 0.05; ^: Hyperoxia+SRT1720 (H+S2) group versus hyperoxia+SRT1720 + 5 μM EX-527 (H+S2+E5) group, P < 0.05; &: Hyperoxia+SRT1720 (H+S2) group versus hyperoxia+SRT1720 + 10 μM EX-527 (H+S2+E10) group, P < 0.05.
Overall, the results of this study suggested that hyperoxia exposure can increase total ROS and mtROS production, decrease mitochondrial membrane potential, downregulate SIRT1, PGC-1α, NRF1, and TFAM, upregulate Ac-p53, and induce apoptosis and mitochondrial dysfunction in human alveolar epithelial cells. Pretreatment with Res or SRT1720 decreased the total ROS and mtROS production, increased the mitochondrial membrane potential, upregulated SIRT1, PGC-1α, NRF1, and TFAM, and downregulated Ac-p53 to alleviate hyperoxia-induced apoptosis and mitochondrial dysfunction. The results were in contrast to those of the Res or SRT1720 pretreatment groups when the cells were pretreated with EX-527 before Res or SRT1720 treatment.
Discussion
BPD has a serious impact on the growth of children, and can affect their respiratory systems, nervous systems, movement, and hearing. The impact of the respiratory system is most obvious, as it is manifested in childhood and even after reaching adulthood. Incidence of respiratory diseases, respiratory medicine usage, hospital re-admission rate, incidence of pulmonary hypertension, and abnormal lung function are significantly increased. Due to the severe adverse effects of BPD, it is very important to conduct early intervention for premature infants with high-risk factors of BPD. In the past, many studies have explored the prevention and treatment of BPD, including vitamin A, glucocorticoids and caffeine, which are administered shortly after the birth of premature infants. Therefore, in this study, resveratrol was added before hyperoxia exposure to explore the preventive effect of resveratrol on BPD.
Increasing evidence has indicated that hyperoxia can cause a variety of side effects.1–4 Excessive ROS and inflammation induced by hyperoxia lead to lung injury during the development of BPD.6,47 Excessive ROS, which are produced under hyperoxic conditions, are considered to be the main cause of lung injury. Hyperoxia causes excessive production of ROS, resulting in lung endothelial and epithelial cell damage, destruction of the alveolar-capillary barrier, increased lung permeability, and activation of the release of pro-inflammatory factors.48
Res has many pharmacological activities, including anti-inflammation, antioxidation, anti-apoptosis, and anti-fibrosis.16 Previous studies have shown that Res alleviated apoptosis induced by hyperoxia via ROS reduction both in vitro and in vivo.17,18 A recent study showed that hyperoxia promoted the production of ROS and NO, enlarged the Bax/Bcl‐2 ratio, activated the mitochondria-dependent apoptotic pathway, and thus induced the apoptosis of pulmonary epithelial cells. This indicates that the mitochondrial-dependent apoptotic pathway is involved in hyperoxia injury.49 In the study of hyperoxia-induced lung injury, SRT1720 may play a similar role as Res.19,20 EX-527 can effectively inhibit SIRT1 deacetylase activity and thus restore global acetylation levels.21 In this study, we further showed that after Res pretreatment, the total ROS and mtROS decreased significantly, and SRT1720 produced similar results as Res. However, after pretreatment with EX-527 and Res (or SRT1720), the total ROS and mtROS were significantly increased. These data indicate that Res and SRT1720 can inhibit the increase in intracellular ROS induced by hyperoxia, while EX-527 can eliminate the inhibition of Res and SRT1720 on the increase in intracellular ROS induced by hyperoxia in which mitochondria play an important role.
Alveolar epithelial cell apoptosis is regarded as a significant feature in hyperoxia-induced acute lung injury.50,51 One of the protective mechanisms of Res in respiratory diseases is anti-apoptosis.16 Previous studies have shown that Res alleviated apoptosis induced by hyperoxia both in vitro and in vivo.17,18 The decrease in mitochondrial membrane potential is an early feature of apoptosis. The expression of SIRT1 changes with changes in oxygen content. SIRT1 is a deacetylase that regulates many important metabolic processes by interacting with substrate proteins, including p53, NF-κB, STAT3, and PGC1-α. In our previous in vitro research, it was shown that hyperoxia induces a decrease in SIRT1 expression, and the use of resveratrol can increase SIRT1 expression.18,52 Our animal experiments showed that resveratrol may reduce apoptosis by stimulating SIRT1.13 Therefore, we used resveratrol, a SIRT1 agonist to further study the role of SIRT1 in hyperoxia. In this study, the mitochondrial membrane potential was shown to be significantly decreased in the hyperoxia group compared with the control group, while pretreatment with Res, such as SRT1720, mitigated hyperoxia-induced mitochondrial membrane potential declination. Flow cytometry was used to evaluate the apoptotic rates of each group. The results showed that the apoptotic rate of the hyperoxia group was significantly increased compared with that of the control group, while pretreatment with Res or SRT1720 decreased the apoptotic rate. However, when pretreated with EX-527 before Res or SRT1720 treatment, the apoptotic rate was increased. The SRT1720 and Res groups demonstrated similar results. In terms of protein expression, the use of Res and SRT1720 can offset the downregulation of SIRT1 expression and upregulation of acetyl-p53 expression induced by hyperoxia, while the effect of Res and SRT1720 can be alleviated by EX-527. We can conclude that hyperoxia can induce cell apoptosis, and the activation of SIRT1 can alleviate this change, which is consistent with our previous experiments.
Mitochondria play an important role in cell function, including lipid synthesis, calcium signaling, cell death, and metabolism, by changing the mitochondrial protein expression, structure, and function.25–28 Therefore, the maintenance of normal mitochondrial function remains crucial to cell life. Under oxidative stress conditions, the mitochondrial genome has always been considered a cellular target.15 PGC-1α and its downstream transcription factors NRF1 and TFAM are involved in the regulation of mitochondrial function, and TFAM is essential for mtDNA stabilization and transcription.39,40 SIRT1 can interact with PGC-1α to regulate mitochondrial function and biosynthesis.13,14 In our study, mtROS and mitochondrial membrane potential were decreased when exposed to hyperoxia, and the expression levels of PGC-1α, NRF1, and TFAM were decreased, suggesting that hyperoxia caused damage to human alveolar epithelial cells via mitochondrial dysfunction. When pretreated with Res or SRT1720, the expressions of PGC-1α, NRF1, and TFAM increased, while the expressions of these proteins decreased when they were pretreated with EX-527 before Res or SRT1720 treatment, accompanied by changes in mtROS and the mitochondrial membrane potential. Resveratrol can improve hyperoxia-induced mitochondrial dysfunction, and the SIRT1/PGC-1a signaling pathway may be involved in this protective effect.
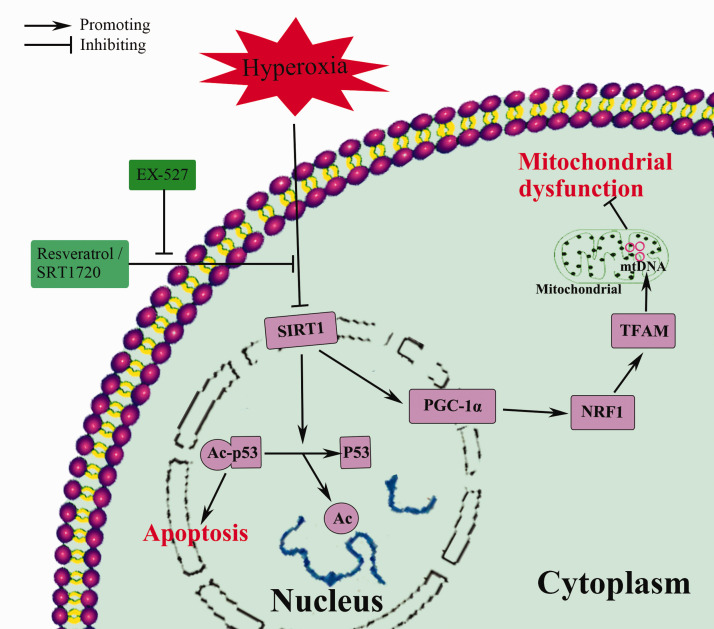
In conclusion, this study suggests that hyperoxia-induced apoptosis and mitochondrial dysfunction in alveolar epithelial cells can be alleviated by pretreatment with Res and may occur through the SIRT1/PGC-1a signaling pathway. Res-induced SIRT1 upregulation was involved in lung protection (Figure 7). However, our research was limited to the cell level, and we did not further study the relationship between SIRT1 and PGC-1a. Therefore, in future experiments, we will further study the SIRT1/PGC-1a signaling pathway and conduct animal experiments, including gene knockout, to further explore the protective effect of resveratrol. On the other hand, although resveratrol has many benefits, due to its low bioavailability and instability, it is currently mainly used in cell and animal research, with only a few clinical studies. Therefore, improving the bioavailability of resveratrol and turning to clinical research will also be a focus for future research.
Figure 7.
Protective mechanism of resveratrol against hyperoxia-induced cell damage. (A color version of this figure is available in the online journal.)
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220975106 for Resveratrol alleviates alveolar epithelial cell injury induced by hyperoxia by reducing apoptosis and mitochondrial dysfunction by Xiaodan Zhu, Fan Wang, Xiaoping Lei and Wenbin Dong in Experimental Biology and Medicine
ACKNOWLEDGMENTS
We thank the Infection and Immunity Laboratory for technical assistance.
Footnotes
AUTHORS’ CONTRIBUTIONS: XZ mainly designed the research, analyzed the data and prepared the figures; FW mainly performed the experiments and drafted the manuscript. XZ, FW, XL, and WD approved the final version of the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: This work was supported by the National Natural Science Foundation of China (No 81571480 to Wen-bin Dong).
ORCID iD: Xiaodan Zhu https://orcid.org/0000-0003-2717-4396
Supplemental Material: Supplementary material for this article is available online.
References
- 1.Nakagawa M, Nishizaki N, Endo A, Someya T, Saito Y, Mizutani A, Hara T, Murano Y, Sakuraya K, Hara S, Umino D, Hirano D, Fujinaga S, Ohtomo Y, Shimizu T. Impaired nephrogenesis in neonatal rats with oxygen-induced retinopathy. Pediatr Int 2017; 59:704–10 [DOI] [PubMed] [Google Scholar]
- 2.Goss KN, Kumari S, Tetri LH, Barton G, Braun RK, Hacker TA, Eldridge MW. Postnatal hyperoxia exposure durably impairs right ventricular function and mitochondrial biogenesis. Am J Respir Cell Mol Biol 2017; 56:609–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endesfelder S, Weichelt U, Strauss E, Schlor A, Sifringer M, Scheuer T, Buhrer C, Schmitz T. Neuroprotection by caffeine in hyperoxia-induced neonatal brain injury. Int J Mol Sci 2017; 18:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou HC, Chen CM. Neonatal hyperoxia disrupts the intestinal barrier and impairs intestinal function in rats. Exp Mol Pathol 2017; 102:415–21 [DOI] [PubMed] [Google Scholar]
- 5.Jobe AH. Animal models, learning lessons to prevent and treat neonatal chronic lung disease. Front Med 2015; 2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buczynski BW, Maduekwe ET, O'Reilly MA. The role of hyperoxia in the pathogenesis of experimental BPD. Semin Perinatol 2013; 37:69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Ma X, Ouyang T, Chen H, Lin J, Liu J, Xiao Y, Yu J, Huang Y. SIRT1 reverses senescence via enhancing autophagy and attenuates oxidative stress-induced apoptosis through promoting p53 degradation. Int J Biol Macromol 2018; 117:225–34 [DOI] [PubMed] [Google Scholar]
- 8.Singh V, Ubaid S. Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation. Inflammation 2020; 43:1589–98 [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Wang J, Zhao A, Li J. SIRT1 activation inhibits hyperglycemia-induced apoptosis by reducing oxidative stress and mitochondrial dysfunction in human endothelial cells. Mol Med Rep 2017; 16:3331–8 [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Hu Z, Fu Q, Song X, Cui Q, Jia R, Zou Y, He C, Li L, Yin Z. Resveratrol mitigates lipopolysaccharide-mediated acute inflammation in rats by inhibiting the TLR4/NF-kappaBp65/MAPKs signaling Cascade. Sci Rep 2017; 7:45006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng T, Lu Y. SIRT1 protects human lens epithelial cells against oxidative stress by inhibiting p53-Dependent apoptosis. Curr Eye Res 2016; 41:1068–75 [DOI] [PubMed] [Google Scholar]
- 12.Abdolvahabi Z, Nourbakhsh M, Hosseinkhani S, Hesari Z, Alipour M, Jafarzadeh M, Ghorbanhosseini SS, Seiri P, Yousefi Z, Yarahmadi S, Golpour P. MicroRNA-590-3P suppresses cell survival and triggers breast cancer cell apoptosis via targeting sirtuin-1 and deacetylation of p53. J Cell Biochem 2019; 120:9356–68 [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Lei X, Wang J, Dong W. Protective effects of resveratrol on hyperoxia-induced lung injury in neonatal rats by alleviating apoptosis and ROS production. J Matern Fetal Neonatal Med 2020; 33:4150–8 [DOI] [PubMed] [Google Scholar]
- 14.Zhang T, Chi Y, Kang Y, Lu H, Niu H, Liu W, Li Y. Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC-1alpha mediated attenuation of mitochondrial oxidative stress. J Cell Physiol 2019; 234:5033–43 [DOI] [PubMed] [Google Scholar]
- 15.Fang WJ, Wang CJ, He Y, Zhou YL, Peng XD, Liu SK. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1alpha deacetylation. Acta Pharmacol Sin 2018; 39:59–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulashekar M, Stom SM, Peuler JD. Resveratrol's potential in the adjunctive management of cardiovascular disease, obesity, diabetes, alzheimer disease, and cancer. J Am Osteopath Assoc 2018; 118:596–605 [DOI] [PubMed] [Google Scholar]
- 17.Zhu XD, Lei XP, Dong WB. Resveratrol as a potential therapeutic drug for respiratory system diseases. Drug Des Devel Ther 2017; 11:3591–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Dong WB, Lei XP, Li QP, Zhang LY, Zhang LP. Resveratrol suppresses hyperoxia-induced nucleocytoplasmic shuttling of SIRT1 and ROS production in PBMC from preterm infants in vitro. J Matern Fetal Neonatal Med 2018; 31:1142–50 [DOI] [PubMed] [Google Scholar]
- 19.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007; 450:712–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP, Stein RL. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem 2010; 285:32695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gertz M, Fischer F, Nguyen GT, Lakshminarasimhan M, Schutkowski M, Weyand M, Steegborn C. Ex-527 inhibits sirtuins by exploiting their unique NAD+-dependent deacetylation mechanism. Proc Natl Acad Sci U S A 2013; 110:E2772–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006; 127:1109–22 [DOI] [PubMed] [Google Scholar]
- 23.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 2008; 8:347–58 [DOI] [PubMed] [Google Scholar]
- 24.Svensson K, Schnyder S, Albert V, Cardel B, Quagliata L, Terracciano LM, Handschin C. Resveratrol and SRT1720 elicit differential effects in metabolic organs and modulate systemic parameters independently of skeletal muscle peroxisome proliferator-activated receptor gamma Co-activator 1alpha (PGC-1alpha). J Biol Chem 2015; 290:16059–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet 2009; 43:95–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Picca A, Lezza AM. Regulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions: useful insights from aging and calorie restriction studies. Mitochondrion 2015; 25:67–75 [DOI] [PubMed] [Google Scholar]
- 27.De Stefani D, Rizzuto R, Pozzan T. Enjoy the trip: calcium in mitochondria back and forth. Annu Rev Biochem 2016; 85:161–92 [DOI] [PubMed] [Google Scholar]
- 28.Diebold L, Chandel NS. Mitochondrial ROS regulation of proliferating cells. Free Radic Biol Med 2016; 100:86–93 [DOI] [PubMed] [Google Scholar]
- 29.Lerner CA, Sundar IK, Rahman I. Mitochondrial redox system, dynamics, and dysfunction in lung inflammaging and COPD. Int J Biochem Cell Biol 2016; 81:294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cloonan SM, Choi AM. Mitochondria in lung disease. J Clin Invest 2016; 126:809–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pouwels SD, Hesse L, Faiz A, Lubbers J, Bodha PK, Ten Hacken NH, van Oosterhout AJ, Nawijn MC, Heijink IH. Susceptibility for cigarette smoke-induced DAMP release and DAMP-induced inflammation in COPD. Am J Physiol Lung Cell Mol Physiol 2016; 311:L881–L92 [DOI] [PubMed] [Google Scholar]
- 32.Piantadosi CA, Suliman HB. Mitochondrial dysfunction in lung pathogenesis. Annu Rev Physiol 2017; 79:495–515 [DOI] [PubMed] [Google Scholar]
- 33.Nakahira K, Hisata S, Choi AM. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal 2015; 23:1329–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rangarajan S, Bernard K, Thannickal VJ. Mitochondrial dysfunction in pulmonary fibrosis. Ann Am Thorac Soc 2017; 14:S383–S88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Wang W, Zhu B, Wang X. Epithelial mitochondrial dysfunction in lung disease. Adv Exp Med Biol 2017; 1038:201–17 [DOI] [PubMed] [Google Scholar]
- 36.Schumacker PT, Gillespie MN, Nakahira K, Choi AM, Crouser ED, Piantadosi CA, Bhattacharya J. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol 2014; 306:L962–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fathi H, Ebrahimzadeh MA, Ziar A, Mohammadi H. Oxidative damage induced by retching; antiemetic and neuroprotective role of sambucus ebulus L. Cell Biol Toxicol 2015; 31:231–9 [DOI] [PubMed] [Google Scholar]
- 38.Kim KC, Lee IK, Kang KA, Kim HS, Kang SS, Hyun JW. Baicalein (5,6,7-trihydroxyflavone) reduces oxidative stress-induced DNA damage by upregulating the DNA repair system. Cell Biol Toxicol 2012; 28:421–33 [DOI] [PubMed] [Google Scholar]
- 39.Maniura-Weber K, Goffart S, Garstka HL, Montoya J, Wiesner RJ. Transient overexpression of mitochondrial transcription factor A (TFAM) is sufficient to stimulate mitochondrial DNA transcription, but not sufficient to increase mtDNA copy number in cultured cells. Nucleic Acids Res 2004; 32:6015–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu HL, Zhang ZX, Chen CS, Cai C, Zhao JP, Wang X. Effects of mitochondrial potassium channel and membrane potential on hypoxic human pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 2010; 42:661–6 [DOI] [PubMed] [Google Scholar]
- 41.Lu X, Zhang L, Li P, Wang J, Li R, Huang Y, Wu M, Zhou H, Li Y, Wei S, Li K, Li H, Zhou X, Zhao Y, Xiao X. The protective effects of compatibility of aconiti lateralis radix praeparata and zingiberis rhizoma on rats with heart failure by enhancing mitochondrial biogenesis via Sirt1/PGC-1alpha pathway. Biomed Pharmacother 2017; 92:651–60 [DOI] [PubMed] [Google Scholar]
- 42.Wu T, Zhang S, Liang X, He K, Wei T, Wang Y, Zou L, Zhang T, Xue Y, Tang M. The apoptosis induced by silica nanoparticle through endoplasmic reticulum stress response in human pulmonary alveolar epithelial cells. Toxicol in Vitro 2019; 56:126–32 [DOI] [PubMed] [Google Scholar]
- 43.Yang Q, Xu E, Dai J, Liu B, Han Z, Wu J, Zhang S, Peng B, Zhang Y, Jiang Y. A novel long noncoding RNA AK001796 acts as an oncogene and is involved in cell growth inhibition by resveratrol in lung cancer. Toxicol Appl Pharmacol 2015; 285:79–88 [DOI] [PubMed] [Google Scholar]
- 44.Shukla S, Sharma A, Pandey VK, Raisuddin S, Kakkar P. Concurrent acetylation of FoxO1/3a and p53 due to sirtuins inhibition elicit bim/PUMA mediated mitochondrial dysfunction and apoptosis in berberine-treated HepG2 cells. Toxicol Appl Pharmacol 2016; 291:70–83 [DOI] [PubMed] [Google Scholar]
- 45.Liang D, Zhuo Y, Guo Z, He L, Wang X, He Y, Li L, Dai H. SIRT1/PGC-1 pathway activation triggers autophagy/mitophagy and attenuates oxidative damage in intestinal epithelial cells. Biochimie 2020; 170:10–20 [DOI] [PubMed] [Google Scholar]
- 46.Sabir MS, Khan Z, Hu C, Galligan MA, Dussik CM, Mallick S, Stone AD, Batie SF, Jacobs ET, Whitfield GK, Haussler MR, Heck MC, Jurutka PW. SIRT1 enzymatically potentiates 1,25-dihydroxyvitamin D3 signaling via vitamin D receptor deacetylation. J Steroid Biochem Mol Biol 2017; 172:117–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Natarajan V, Ha AW, Dong Y, Reddy NM, Ebenezer DL, Kanteti P, Reddy SP, Usha Raj J, Lei Z, Maienschein-Cline M, Arbieva Z, Harijith A. Expression profiling of genes regulated by sphingosine kinase1 signaling in a murine model of hyperoxia induced neonatal bronchopulmonary dysplasia. BMC Genomics 2017; 18:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy SP, Hassoun PM, Brower R. Redox imbalance and ventilator-induced lung injury. Antioxid Redox Signal 2007; 9:2003–12 [DOI] [PubMed] [Google Scholar]
- 49.Zou D, Li J, Fan Q, Zheng X, Deng J, Wang S. Reactive oxygen and nitrogen species induce cell apoptosis via a mitochondria-dependent pathway in hyperoxia lung injury. J Cell Biochem 2019; 120:4837–50 [DOI] [PubMed] [Google Scholar]
- 50.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008; 295:L379–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caser EB, Zandonade E, Pereira E, Gama AM, Barbas CS. Impact of distinct definitions of acute lung injury on its incidence and outcomes in brazilian ICUs: prospective evaluation of 7,133 patients*. Crit Care Med 2014; 42:574–82 [DOI] [PubMed] [Google Scholar]
- 52.Tan F, Dong W, Lei X, Liu X, Li Q, Kang L, Zhao S, Zhang C. Attenuated SUMOylation of sirtuin 1 in premature neonates with bronchopulmonary dysplasia. Mol Med Rep 2018; 17:1283–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220975106 for Resveratrol alleviates alveolar epithelial cell injury induced by hyperoxia by reducing apoptosis and mitochondrial dysfunction by Xiaodan Zhu, Fan Wang, Xiaoping Lei and Wenbin Dong in Experimental Biology and Medicine