Abstract
Copper depletion is associated with myocardial ischemic infarction, in which copper metabolism MURR domain 1 (COMMD1) is increased. The present study was undertaken to test the hypothesis that the elevated COMMD1 is responsible for copper loss from the ischemic myocardium, thus worsening myocardial ischemic injury. Mice (C57BL/6J) were subjected to left anterior descending coronary artery permanent ligation to induce myocardial ischemic infarction. In the ischemic myocardium, copper reduction was associated with a significant increase in the protein level of COMMD1. A tamoxifen-inducible, cardiomyocyte -specific Commd1 knockout mouse (C57BL/6J) model (COMMD1CMC▲/▲) was generated using the Cre-LoxP recombination system. COMMD1CMC▲/▲ and wild-type littermates were subjected to the same permanent ligation of left anterior descending coronary artery. At the 7th day after ischemic insult, COMMD1 deficiency suppressed copper loss in the heart, along with preservation of vascular endothelial growth factor and vascular endothelial growth factor receptor 1 expression and the integrity of the vascular system in the ischemic myocardium. Corresponding to this change, infarct size of ischemic heart was reduced and myocardial contractile function was well preserved in COMMD1CMC▲/▲ mice. These results thus demonstrate that upregulation of COMMD1 is at least partially responsible for copper efflux from the ischemic heart. Cardiomyocyte-specific deletion of COMMD1 helps preserve the availability of copper for angiogenesis, thus suppressing myocardial ischemic dysfunction.
Keywords: Myocardial ischemia, copper efflux, COMMD1, cardiac function
Impact statement
Copper depletion is critically involved in the pathogenesis of myocardial ischemic infarction. It was unknown, however, what causes copper loss from the heart in response to ischemia although it was found that COMMD1 is upregulated in ischemic hearts. This study shows that cardiomyocyte-specific conditional deletion of COMMD1 significantly prevents copper loss in the ischemic myocardium, along with a preservation of the myocardial contractile function. Therefore, COMMD1 upregulation is involved in copper efflux from ischemic heart and inhibition of COMMD1 would potentially become an alternative therapeutic approach for the treatment of myocardial ischemic injury.
Introduction
Previous studies demonstrated that myocardial ischemic insult leads to copper (Cu) efflux from the heart along with pathogenesis of myocardial infarction and dysfunction.1–6 Cu homeostasis is tightly regulated by a series of Cu transporters and chaperone proteins involved in Cu uptake, intracellular trafficking, and efflux.7 Interestingly, Cu loss from the ischemic hearts was not related to changes in any known Cu efflux chaperones, such as ATP7A and ATP7B, but was associated with an upregulation of copper metabolism MURR domain 1 (COMMD1) in the heart.1
COMMD1 is a Cu transport chaperone, and is involved in the Cu excretion from liver to bile.8–10 The mutations of Commd1 led to Cu toxicosis of liver in Bedlington terries,11 and liver-specific knockdown of Commd1 in mice led to hepatic Cu accumulation.12 Increased COMMD1 protein level was associated with a decrease in hepatic Cu concentrations in lipopolysaccharides-induced liver injury.13 Therefore, COMMD1 plays an important role for Cu excretion from hepatocytes to bile. COMMD1 was also identified in cardiac tissue although its function in the heart is not clear.1,11,14 It was interesting to observe that COMMD1 was upregulated in response to myocardial ischemia,1 and it is pivotal to know whether the elevation of COMMD1 is involved in Cu efflux from the ischemic hearts.
Cu, as an essential element, is involved in a variety of biological processes in the myocardium, which are essential for cardiac metabolism and function.7,15–21 In particular, Cu is required for HIF-1 transcriptional complex formation22–25 and the subsequent binding of the complex to the HRE sequence of a subset of target genes, particularly the angiogenic genes, although it does not influence either the production or the stability of HIF-1α protein.23–27 In addition, Cu is an important component of cytochrome c oxidase (CcO) and superoxide dismutase 1 (SOD1), which are involved in mitochondrial energy metabolism7,28 and antioxidant activities.29 Dietary Cu restriction leads to cardiac hypertrophy transitionally to heart failure.30 With the deletion of Cu, the capillary density is reduced,31,32 and the structure and function of myocardial mitochondria are disordered in the failing heart.17 Cu supplementation replenishes cardiac Cu content and improves structural integrity and contractile function in hypertrophic hearts induced not only by dietary Cu deficiency, but also by ascending aortic constriction.33,34 Clinical and experimental studies have demonstrated the link between myocardial ischemic infarction and Cu depletion.6,35 It is thus interesting to know if prevention of Cu loss from the heart can suppress myocardial ischemic injury.
To address whether COMMD1 upregulation is involved in Cu loss from ischemic hearts, and consequently, if prevention of Cu loss can suppress myocardial ischemic injury, the present study was undertaken to use a cardiac-specific COMMD1 knockout (COMMD1CMC▲/▲) mouse model to define the cause-and-effect relationship between COMMD1 and cardiac Cu loss in response to ischemic insult. We attempted to test the hypothesis that maintaining Cu homeostasis by reducing Cu loss via depletion of COMMD1 suppresses myocardial ischemic injury.
Material and methods
Animal and animal care
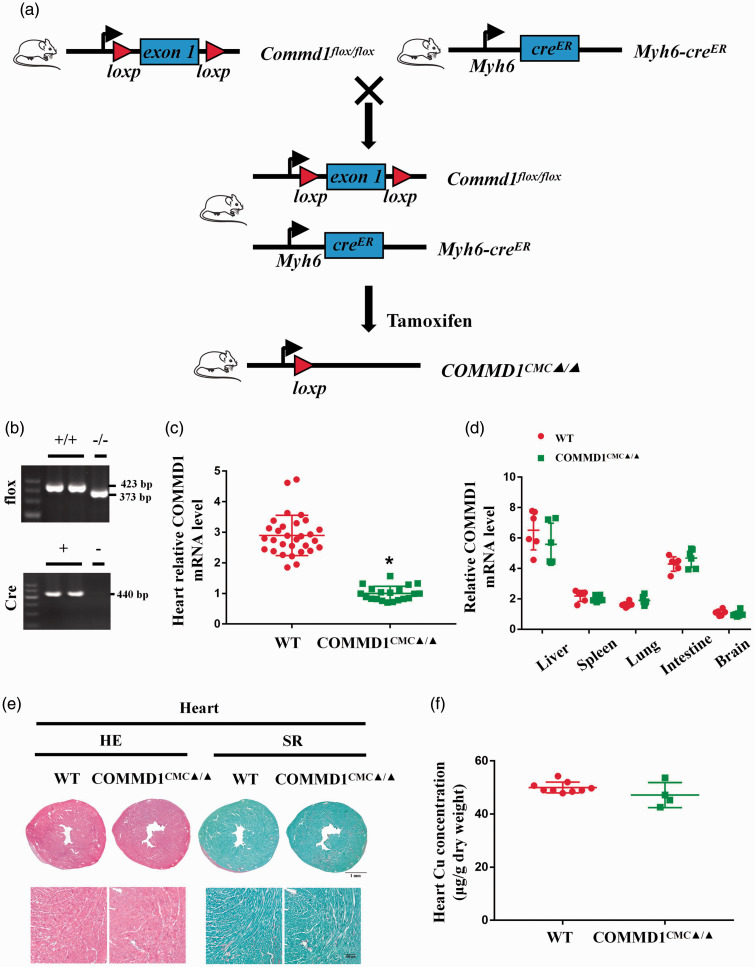
All animal procedures were approved by the Institution Animal Care and Use Committee (IACUC) at Sichuan University West China Hospital, following the guidelines of the US National Institutes of Health (NIH). Myh6-creER mice (Stock No: 005650) and Commd1flox/flox mice (Stock No: 018419) in a C57BL/6 background were purchased from the Jackson Laboratory. Detailed information regarding the generation of cardiomyocyte-specific Commd1 knockout mice (COMMD1CMCs▲/▲) is available in Figure 2(a). Male and female Myh6-creER; Commd1flox/flox mice (six to seven weeks old) were intraperitoneally injected tamoxifen for five times (0.1 g/kg/day, T5648, Sigma) and Myh6-creER negative; Commd1flox/flox littermates (WT) were subjected to the same treatment with tamoxifen. COMMD1CMCs▲/▲ and WT mice, aged eight to nine weeks old, were then used for experiments. This study included 200 mice, 45 of which were non-transgenic mice, 86 of which were Myh6-creER negative; Commd1flox/flox mice and 69 of which were Myh6-creER; Commd1flox/flox mice. Mice were housed in individually ventilated cages under standard laboratory condition (22 ± 2°C, humidity 40–70%, 12 h dark/light cycles) and fed ad libitum access to standard rodent diet with copper content of 6 mg · kg−1 (5C02, LabDiet) and deionized water.
Figure 2.
Generation and characterization of cardiomyocyte-specific Commd1 knockout mice. (a) Generation of cardiomyocyte-specific Commd1 knockout mice. (b) PCR analysis of Commd1flox and Cre genes in tails. (c) RT-PCR analysis of Commd1 expression in cardiac tissues, WT: n = 30; COMMD1CMC▲/▲: n = 20. (d) RT-PCR analysis of Commd1 expression in liver, spleen, lung, intestine, and brain, n = 6 per group. (e) Images of HE and SR staining of heart sections after three weeks of tamoxifen induction, scale bar = 1 mm for the upper panel and 100 µm for the lower panel. (f) AAS analysis of copper concentrations in the cardiac tissues three weeks after tamoxifen induction, WT: n = 9; COMMD1CMC▲/▲: n = 4. Data were expressed as mean ± SD and analyzed using Student's t-test, *P < 0.05 versus WT control. (A color version of this figure is available in the online journal.)
Genotype identification
Genotyping of mice was performed by a standard PCR method using Commd1flox specific primer as recommended by the Jackson Laboratory (oIMR14759: 5′-TTGTGAGCTGATTGGGTGTG-3′; oIMR14760: 5′-CCAGCCTGGATTACACAGAGA-3′) and Cre specific primer as recommended by the Jackson Laboratory (oIMR3798: 5′-AGGTGGACCTGATCATGGAG-3′; oIMR8346: 5′-ATACCGGAG ATCATGCAAGC-3′).
Mouse model of myocardial ischemic infarction
Non-transgenic mice (eight to nine weeks old, male) were subject to permanent ligation of the left anterior descending (LAD) coronary artery for different time (one, four, and seven days) or sham operation without ligation as described in our previous studies.36 COMMD1CMC▲/▲ and WT mice (eight to nine weeks old; male and female) were subjected to permanent ligation of the LAD coronary artery for seven days or sham operation without ligation as described in our previous studies.36 Briefly, mice were subjected to endotracheal intubation after anesthesia with 4% isoflurane (R510-22, RDW Life Science) mixed with 0.5 L/min 100% O2. Then, anesthesia was maintained with 2% isoflurane mixed with 0.5 L/min 100% O2 during surgery. Heart of mouse was exposed via the left 3rd and 4th intercostal thoracotomy and the LAD artery of mice was ligated permanently with a 7/0 monofilament suture. Immediate and 4 h postoperative limb lead ECGs were performed and the elevation of the ST segment indicated successful occlusion of LAD. The success rate of LAD coronary artery ligation was about 94%.
Echocardiography
Transthoracic echocardiography was performed on sedated mice with 1% isoflurane mixed with 0.5 L/min 100% O2 using an 11.5 MHZ transducer (Vivid 7 Dimension, GE) to evaluate the heart functions at seven days post-MI as previously described.37 Left-ventricular internal end-diastolic diameter (LVIDd), left-ventricular internal end systolic-diameter (LVIDs), left-ventricular end-diastolic volume (EDV), and left-ventricular end-systolic volume (ESV) were quantified by digitally recorded two-dimensional short-axis M-mode tracings at the papillary muscle level.38 Fractional shortening (FS) was calculated as FS = (LVIDd – LVIDs)/LVIDd × 100%, and ejection fraction (EF) was calculated as EF = (EDV – ESV)/EDV × 100%. All echocardiographic measurements were performed by a single-blinded investigator.
The collection of blood and tissues
Mice were euthanized with isoflurane inhalation (4%) followed by cervical dislocation. Approximately 200 µL blood was collected through submandibular vein under the same anesthesia above. After clotting, the blood was centrifuged at 3500 r/min for 15 min at 20°C to separate serum, and then the serum was stored at −80°C for copper concentrations assay. The tissues were harvested and flushed with precooled saline until fluids were colorless. Heart tissues were separated into two parts: ischemic area (IA) and remote area (RA) as described in our previous studies,19 then the different areas of hearts were stored at liquid nitrogen for qRT-PCR analysis and Western blotting, and −80°C for copper concentrations assay. Other heart tissues were embedded in OCT gel (Leica, German) and frozen in liquid nitrogen, then the hearts were cut into 5 µm sections for immunofluorescent staining. The other heart tissues were fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned at 2.5 µm sections for histopathological staining. The other organ tissues were preserved in liquid nitrogen for qRT-PCR analysis and in −80°C for copper concentrations assay, including liver, spleen, lung, intestine, and brain.
Copper concentration measurement
As described in our previous study,19 lyophilized tissues and serum were digested with nitric acid (HNO3, Sigma) overnight at 60°C. Copper concentrations were determined by graphite furnace atomic absorption spectrophotometry (ICE3500, Thermo) and normalized by serum volume or dry weight of tissue samples.
Western blotting analysis
The protein level of COMMD1 was determined by Western blotting. Total protein of each sample was isolated using TRIzol regent (15596–026, Invitrogen) following the manufacturer’s instruction. The protein concentrations were determined by a BCA protein assay kit (Thermo). Equal amounts of 30 µg proteins were separated by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-rad). Membranes were blocked in 5% nonfat milk for 1 h at room temperature, then incubated with primary antibody (anti-COMMD1, sc-166248, Santa cruz; anti-COMMD1, ab5174, Abclonal) for overnight at 4°C and the appropriate secondary antibody for 1 h at 37°C. The blot was visualized using a chemiluminescence HRP substrate (Milipore) and analyzed by IPP 6.0 software.
Immunofluorescence analysis
Frozen sections were fixed by 4% paraformaldehyde at room temperature for 15 min and incubated with 2% BSA at 37°C for 1 h, then sections were incubated with the primary antibody (anti-COMMD1, sc-166248, Santa cruz; anti-CD31 antibody, AF3286, RD) at 37°C for 1 h and subsequently at 4°C overnight. After washed with PBS, sections were incubated with the fluorescent secondary antibody respectively for 1.5 h at 37°C and the nucleus was labeled by DAPI at room temperature for 5 min. After washed with PBS, sections were examined under a confocal microscope (ECLIPSE Ti A1, Nikon).
Histopathological analysis
As described in our previous study, the heart tissues were placed in 4% paraformaldehyde, dehydrated, and paraffin embedded. Then the tissues were cut into 2.5 µm sections, which were stained with hematoxylin-eosin (HE) or Sirius red (SR). Overview of heart sections was collected with a Nikon Eclipse 80i microscope connected to a video camera.
Reverse transcription and qRT-PCR
Total RNA of each sample was isolated using TRIzol regent (15596–026, Invitrogen) following the manufacturer’s instruction. cDNA was synthesized by reverse transcribing 1.0 µg RNA using a Prime Script™ RT regent kit (RR037A, TaKaRa). The converted cDNA was amplified using a SYBR Premix Ex Taq™ II (TaKaRa) with the primers for COMMD1, vascular endothelial growth factor (VEGF), and vascular endothelial growth factor receptor 1 (VEGFR1). TBP served as a loading control. The primer sequences were designed using Primer-BLAST of NCBI and synthesized by Invitrogen (Table 1).
Table 1.
qRT-PCR primer sequences for mouse.
| Primer | Forward sequence (5ʹ to 3ʹ) | Reverse sequence (5ʹ to 3ʹ) |
|---|---|---|
| COMMD1 | AACGCCTTTCACGGACACTC | AGTCCTCTCATCTTCGCCAG |
| VEGF | AGGCTGCTGTAACGATGAAG | TCTCCTATGTGCTGGCTTTG |
| VEGFR-1 | GGCTCCTTCTAACTCTCTTCATC | GGAACTTCATCTGGGTCCATAA |
| TBP | GTTTGGCTAGGTTTCTGCGG | CCATGAAATAGTGATGCTGGGC |
Statistical analysis
All data were presented as mean ± SD, and n represents the number of mice. Statistical analyses were performed using the GraphPad Prism 7.0 software. Data were analyzed by two tailed Student’s t-test, one-way ANOVA with Tukey's multiple comparisons test, or two-way ANOVA with Tukey’ s multiple comparison test where appropriate. P < 0.05 was considered statistically significant.
Results
Changes of copper concentrations and COMMD1 in ischemic hearts
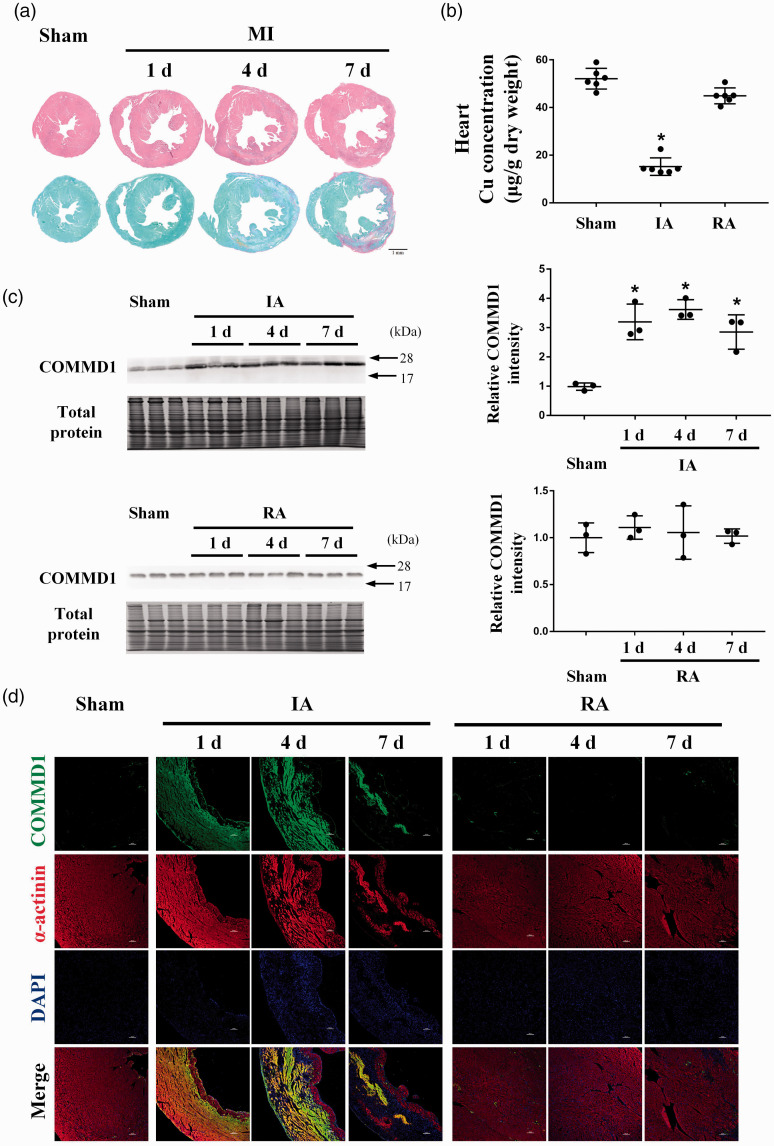
Histopathological analyses showed that the ischemic area was located in the left ventricular anterior wall after LAD ligation (Figure 1(a)). Myocardial cell death and massive collagen deposition were observed in the ischemic myocardium at the 7th day after LAD ligation (Figure 1(a)). Cu content was decreased in the ischemic area (IA) compared to the sham-operated control (Figure 1(b)). Concomitantly, COMMD1 protein levels were increased at the 1st day after LAD ligation and retained at the same high level at the 4th and 7th day in the ischemic area (Figure 1(c)). There was no difference in COMMD1 protein levels between the remote area (RA) and the sham-operated control at all the time points (Figure 1(c)). Immunofluorescence analysis by co-staining α-actinin (cardiomyocyte marker) and COMMD1 showed that the increased COMMD1 was mainly located in cardiomyocytes in the ischemic area (Figure 1(d)).
Figure 1.
The changes of copper concentration and COMMD1 in the ischemic heart. (a) Overview of HE and SR staining of heart sections at the 1st, 4th, and 7th day after myocardial ischemia, scale bar = 1 mm. (b) AAS analysis of copper concentrations in different portion of heart at the 7th day after myocardial ischemia, n = 6 for each group. (c) Western blot analysis of the protein level of COMMD1 in different portion of heart at the 1st, 4th, and 7th day after myocardial ischemia, n = 3 for each group. (d) Immunofluorescence images showing COMMD1 (green) and cardiomyocytes (α-actinin, red) in different portion of heart at the 1st, 4th, and 7th day after LAD ligation, DAPI stain (blue) labels nuclei, scale bar = 100 µm. Data were expressed as mean ± SD and analyzed using one-way ANOVA with Tukey's multiple comparisons test, *P < 0.05 versus Sham control. IA: infarct area; RA: remote area. (A color version of this figure is available in the online journal.)
Cardiac-specific and conditional Commd1 knockout mice
A tamoxifen-inducible, cardiomyocyte (CMC)-specific Commd1 knockout mouse model (COMMD1CMC▲/▲) was produced as described in Figure 2(a). Analyses of Commd1 gene and its mRNA expression confirmed that the allele was specifically and effectively deleted in cardiomyocytes in the knockout mouse model (Figure 2(b) and (c)). The expression of COMMD1Commd1 in other tissues was not affected by this tamoxifen-inducible cardiac deletion of Commd1 (Figure 2(d)). There were no significant changes observed in COMMD1CMC▲/▲ mice in comparison to wild type (WT) mice, as determined by histological examination and Cu measurement (Figure 2(e) and (f)).
COMMD1 deletion suppresses Cu loss from the ischemic myocardium
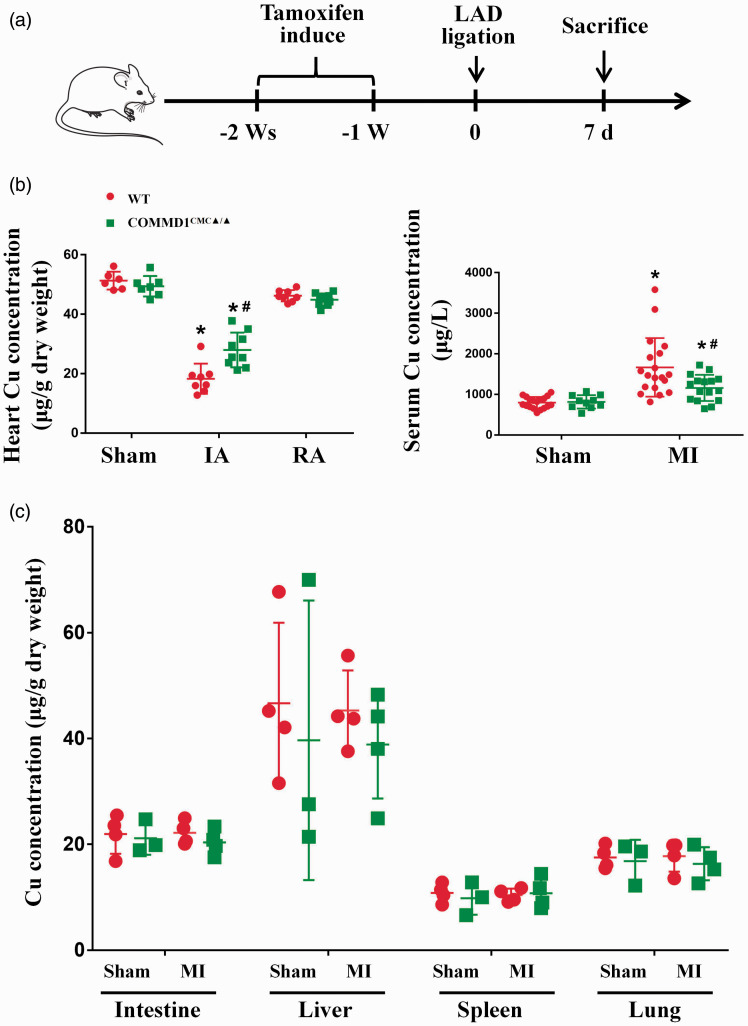
Both WT and COMMD1CMC▲/▲ mice were subjected to ischemic insult by coronary artery ligation (Figure 3(a)). At seventh day after myocardial ischemia, Cu concentrations in the ischemic area of WT mouse hearts were decreased by 2.8 folds, while this decrease in the COMMD1 knockout mice was only 1.7 folds (Figure 3(b)). Correspondingly, serum Cu concentrations were increased in both WT and COMMD1CMC▲/▲ mice, but this increase was much higher in WT mice than in COMMD1CMC▲/▲ mice (Figure 3(c)). There were no significant changes in Cu concentrations in other tissues regardless of differences in genotype or surgical operation in these mice (Figure 3(d)).
Figure 3.
Suppressed copper loss from the COMMD1 deficient ischemic heart. (a) Schematic diagram of animal study design. (b) AAS analysis of copper concentrations in different portion of heart at the 7th day after myocardial ischemia, WT-Sham: n = 6; COMMD1CMC▲/▲-Sham: n = 7; WT-IA: n = 8; COMMD1CMC▲/▲-IA: n = 9; WT-RA: n = 8; COMMD1CMC▲/▲-RA: n = 9. (c) AAS analysis of copper concentrations in serum at the 7th day after myocardial ischemia, WT-Sham: n = 18; COMMD1CMC▲/▲-Sham: n = 10; WT-MI: n = 19; COMMD1CMC▲/▲-MI: n = 16. (d) AAS analysis of copper concentrations in noncardiac tissues at the 7th day after myocardial ischemia, WT-Sham: n = 4; COMMD1CMC▲/▲-Sham: n = 3; WT-MI: n = 4; COMMD1CMC▲/▲-MI: n = 4. Data were expressed as mean ± SD and analyzed using two-way ANOVA with Tukey's multiple comparisons test, *P < 0.05 versus Sham control and #P < 0.05 versus WT control. IA: infarct area; RA: remote area. (A color version of this figure is available in the online journal.)
COMMD1 deletion preserves myocardial contractile function and reduces the infarct size
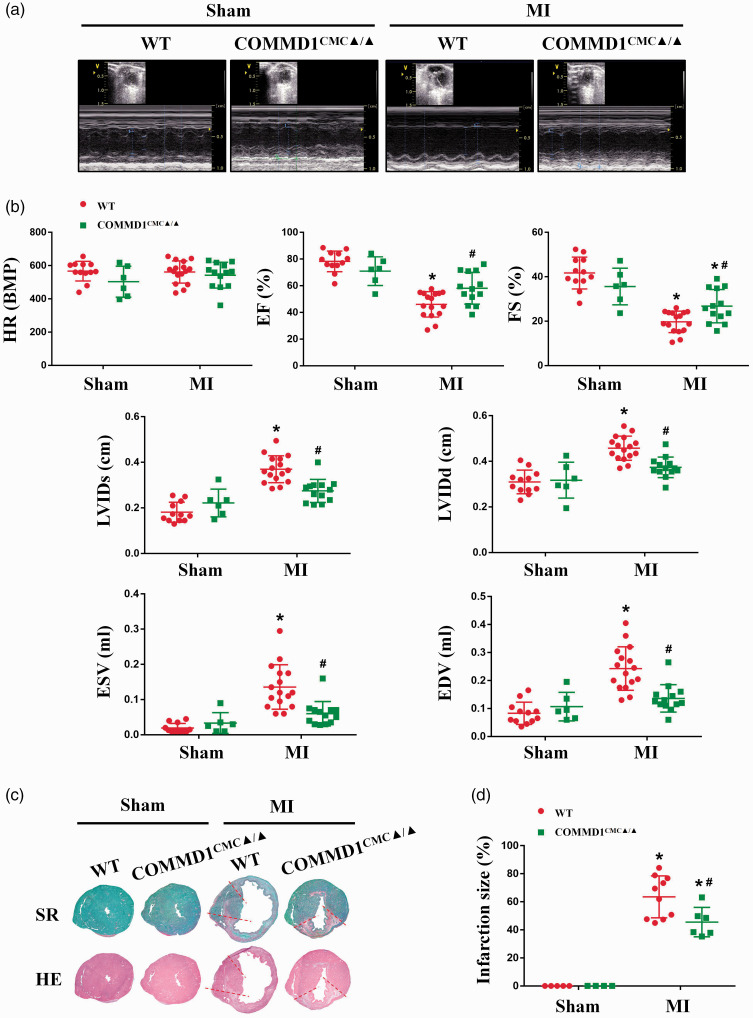
Corresponding to Cu depletion, myocardial contractile function was impaired by ischemic insult, but the extent of this cardiac dysfunction in the COMMD1CMC▲/▲ mice was much less than in the WT mice, as measured by differential changes in EF and FS, as well as in EDV, ESV, LVIDd, and LVIDs in these mice (Figure 4(a) and (b)). Consistently, the infarct size in the COMMD1CMC▲/▲ mice was much smaller than in the WT mice (Figure 4(c) and (d)).
Figure 4.
Preservation of myocardial contractile function in the COMMD1 deficient ischemic heart. (a) The representative images of echocardiography on the papillary muscles at the 7th day after LAD ligation. (b) Heart rate (HR), parameters of ejection fraction (EF%), fractional shortening (FS%), left ventricular end systolic diameter (LVIDs), left ventricular end diastolic diameter (LVIDd), end systolic volume (ESV), and end diastolic volume (EDV) measured by echocardiography, WT-Sham, n = 12; COMMD1CMC▲/▲-Sham, n = 6; WT-MI, n = 16; COMMD1CMC▲/▲-MI, n = 13. (c) Pathological examination of infarct size of hearts at the 7th day after LAD ligation. (d) The quantitative analysis of infarct size of ventricular cross-sections, WT-Sham, n = 5; COMMD1CMC▲/▲-Sham, n = 4; WT-MI, n = 10; COMMD1CMC▲/▲-MI, n = 6. Data were expressed as mean ± SD and analyzed using two-way ANOVA with Tukey's multiple comparisons test, *P < 0.05 versus Sham control and #P < 0.05 versus WT control. (A color version of this figure is available in the online journal.)
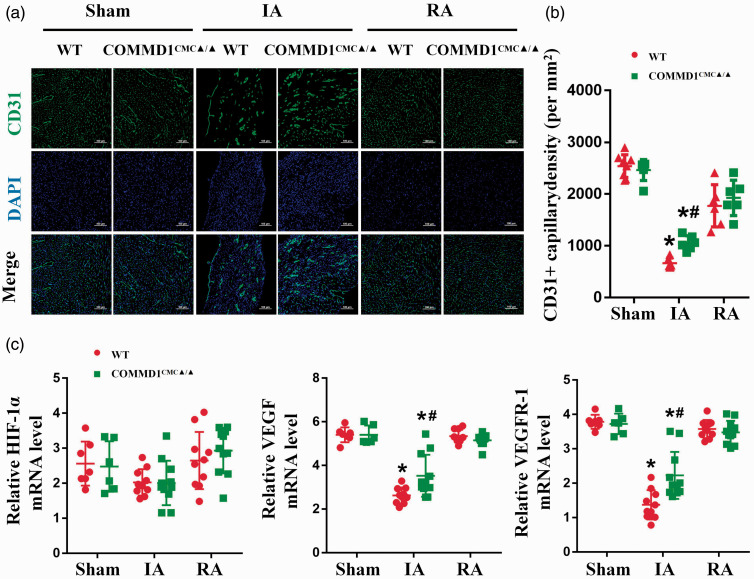
COMMD1 deletion preserves capillary density in the ischemic myocardium
Given Cu plays an essential role in HIF-1-mediated myocardial angiogenesis, preserving the loss of Cu from the ischemic heart would be beneficial for the vascular remodeling after ischemic injury. We thus examined the changes in capillary density in COMMD1CMC▲/▲ mice seven days after LAD ligation. A significant reduction of capillary density was observed in the ischemic myocardium compared with the sham-operated controls in WT mice, which was remarkably prevented in COMMD1CMC▲/▲ mice (Figure 5(a) and (b)).
Figure 5.
Preserved expression of angiogenic factors and capillary density in the COMMD1 deficient ischemic myocardium. (a) Immunofluorescence images of CD31+ capillaries (green) in different portion of heart at the 7th day after LAD ligation. DAPI stain (blue) labels nuclei, scale bar = 100 µm. (b) Quantification analysis of capillary density, WT-Sham, n = 8; COMMD1CMC▲/▲-Sham, n = 6; WT-IA, n = 6; COMMD1CMC▲/▲-IA, n = 6; WT-RA, n = 6; COMMD1CMC▲/▲-RA, n = 6. (c) RT-PCR analysis of HIF-1α, VEGF, and VEGFR-1 in different portion of heart at the 7th day after LAD ligation, WT-Sham, n = 7; COMMD1CMC▲/▲-Sham, n = 6; WT-IA, n = 10; COMMD1CMC▲/▲-IA, n = 11; WT-RA, n = 10; COMMD1CMC▲/▲-RA, n = 11. Data were expressed as mean ± SD and analyzed using two-way ANOVA with Tukey's multiple comparisons test, *P < 0.05 versus Sham control and #P < 0.05 versus WT control. IA: infarct area; RA: remote area. (A color version of this figure is available in the online journal.)
To further understand the potential mechanism underlying the increased capillaries in the ischemic heart after COMMD1 deletion, we performed qRT-PCR analysis to determine the changes in the expression of HIF-1-regulated angiogenic genes, such as VEGF and VEGFR-1. Correspondingly, the mRNA levels of VEGF and VEGFR-1 were dramatically decreased in the ischemic myocardium, but were partially preserved after COMMD1 knockout. COMMD1 deletion had no significant effect on the expression of HIF-1α (Figure 5(c)).
Discussion
Myocardial ischemia is accompanied by Cu depletion in the heart.35 The present study followed our previous observation that an increase in COMMD1 in the ischemic myocardium was associated with Cu efflux4 to define the role of COMMD1 in Cu loss in the ischemic heart. The results here demonstrated that cardiomyocyte-specific deletion of COMMD1 indeed partially prevented Cu loss in the heart in response to ischemic insult. This Cu retention was associated with partial preservation of cardiac contractile function with no sex difference. Therefore, the elevation of COMMD1 levels in ischemic heart is at least partially responsible for Cu efflux from the ischemic heart.
COMMD1 is a rate-limiting factor in hepatic Cu excretion through its interaction with a Cu transporting P-type ATPase (ATP7B) in the liver.12 In addition, COMMD1 interacts with another Cu transporting P-type ATPase (ATP7A) involved in Cu transport along the gastro-intestinal tract and across the blood–brain barrier.39 In the liver and other tissues, the elevation of COMMD1 is a response to the increase in Cu concentrations,40 and in most cases it is along an increase in ATP7A or ATP7B.10,41 The present study showed that COMMD1 is also involved in Cu efflux in the heart, although this takes place under the pathological condition of myocardial ischemia. Both ATP7A and ATP7B are present in the heart,1,42,43 but their expression was not responsive to ischemic insult.1 Thus, the increase in COMMD1 was not accompanied by a parallel increase in either ATP7A or ATP7B.1 It was also noted that in contrast to the liver and other organs, the elevation of COMMD1 in the heart was not associated with Cu elevation, but rather with ischemic insult, representing another mechanism for COMMD1 upregulation in myocardium. How does ischemia trigger the elevation of COMMD1 in the heart would be an interesting topic for future studies.
Cardiomyocyte-specific deletion of COMMD1 diminished the loss of Cu from the ischemic heart, underlining the role of COMMD1 in Cu efflux from the heart in response to ischemic insult. However, it appears that Cu efflux from the heart is related to multiple factors as indicated by the fact that COMMD1 deletion only partially preserved Cu levels in the ischemic heart. In our previous studies, it was observed that ischemia leads to overproduction of homocysteine in cardiomyocytes, which forms a Cu-homocysteine complex, excreting from cells.44,45 The combination of COMMD1 elevation and homocysteine overproduction would exert an additive action on Cu efflux from ischemic heart.
Cu restriction exaggerates ischemic injury to the heart. This was clearly demonstrated in the present study, as indicated by the fact that reduced Cu loss by COMMD1 deletion indeed preserved myocardial contractile function under the ischemic condition, accompanied by an increased capillary density in the ischemic heart. As a key transcription mediator, HIF-1 is a master regulator of angiogenesis.23 Cu is required for the transactivation of HIF-1-regulated angiogenic gene, such as VEGF and VEGFR-1.26,27 The results here showed that the expression of HIF-1α was not affected by Cu status, agreeing with previous observation,23–27 but the expressions of VEGF and VEGFR-1 were elevated with the preservation of Cu in ischemic heart in COMMD1 deletion mice. Thus, the increased capillary density in the ischemic heart is probably ascribed to the preservation of the expression of HIF-1-regulated angiogenic factors, such as VEGF and VEGFR-1. Despite of the Cu-mediated myocardial angiogenesis, Cu reservation by COMMD1 deletion may also protect cardiac function via preserving the activity of Cu-dependent enzymes involved in mitochondrial energy metabolism and antioxidant activities, such as CcO and SOD1. However, this needs to be further investigated in the future.
In addition to COMMD1-mediated Cu loss in the ischemic myocardium, the direct interaction between COMMD1 and HIF-1α would also make an important contribution to enhancing ischemic injury to the heart. It has been shown that COMMD1 directly binds to the amino terminus of HIF-1α, preventing its dimerization with HIF-1β and subsequent transcriptional activity.46 Mouse embryos deficient in COMMD1 showed increased HIF-1α protein stability and upregulation of HIF-1-regulated genes.47 Conversely, overexpression of COMMD1 in human cell lines inhibited HIF-1 activity.47 It thus can be speculated that COMMD1 elevation would directly inhibit HIF-1 activity in the ischemic myocardium. However, the defective HIF-1 transcriptional activity may not be ascribed to the direct interaction between COMMD1 and HIF-1 in the ischemic myocardium. We observed here the level of COMMD1 was elevated at the first day after myocardium ischemia induced by LAD ligation, agreeing with a previous report.48 But neither HIF-1 activity nor myocardial angiogenesis was inhibited during the acute phase of myocardial ischemia (less than four days after LAD ligation).49 On the other hand, the time course of Cu loss from the ischemic myocardium was in parallel with the suppression of angiogenesis in the later phase myocardial ischemia.1 Therefore, the adverse effect of COMMD1 on HIF-1 activity would most likely result from COMMD1-mediated Cu efflux instead of from its direct interaction with HIF-1 in the ischemic myocardium.
In conclusion, the results obtained here demonstrate that the elevation of COMMD1 is partially responsible for Cu efflux from the ischemic myocardium. Cardiomyocyte-specific deletion of COMMD1 thus helped preserve the availability of Cu to some critical biological processes such as HIF-1 transcriptional activity in the ischemic myocardium, defensing myocardial structural and functional damage by ischemic insult. It is important to understand how ischemia causes COMMD1 elevation in the heart in future studies.
ACKNOWLEDGEMENTS
We wish to thank Qin Sheng, Qipu Feng, and Joey Wang for technical support.
AUTHORS’ CONTRIBUTIONS: All authors participated in the experimental design, interpretation of the results, and review of the manuscript; CL, YX, KL, and XM were involved in the experimentation; CL performed data analysis; YJK, TW, and CL wrote the manuscript, and YJK edited and approved the final version of the manuscript.
Declaration OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by National Science Foundation of China (grant number 81230004 to YJ Kang).
ORCID iD: Y James Kang https://orcid.org/0000-0001-8449-7904
References
- 1.Li K, Li C, Xiao Y, Wang T, James Kang Y, Featured A. The loss of copper is associated with the increase in copper metabolism MURR domain 1 in ischemic hearts of mice. Exp Biol Med 2018; 243:780–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kodali HP, Pavilonis BT, Schooling CM. Effects of copper and zinc on ischemic heart disease and myocardial infarction: a Mendelian randomization study. Am J Clin Nutr 2018; 108:237–42 [DOI] [PubMed] [Google Scholar]
- 3.Chen A, Li G, Liu Y. Association between copper levels and myocardial infarction: a meta-analysis. Inhal Toxicol 2015; 27:237–46 [DOI] [PubMed] [Google Scholar]
- 4.Berenshtein E, Mayer B, Goldberg C, Kitrossky N, Chevion M. Patterns of mobilization of copper and iron following myocardial ischemia: possible predictive criteria for tissue injury. J Mol Cell Cardiol 1997; 29:3025–34 [DOI] [PubMed] [Google Scholar]
- 5.He W, James Kang Y. Ischemia-induced copper loss and suppression of angiogenesis in the pathogenesis of myocardial infarction. Cardiovasc Toxicol 2013; 13:1–8 [DOI] [PubMed] [Google Scholar]
- 6.Chevion M, Jiang Y, Har-El R, Berenshtein E, Uretzky G, Kitrossky N. Copper and iron are mobilized following myocardial ischemia: possible predictive criteria for tissue injury. Proc Natl Acad Sci U S A 1993; 90:1102–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng L, Han P, Liu J, Li R, Yin W, Wang T, Zhang WJ, James Kang Y. Role of copper in regression of cardiac hypertrophy. Pharmacol Ther 2015; 148:66–84 [DOI] [PubMed] [Google Scholar]
- 8.van de Sluis B, Rothuizen J, Pearson PL, van Oost BA, Wijmenga C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet 2002; 11:165–73 [DOI] [PubMed] [Google Scholar]
- 9.Favier RP, Spee B, Fieten H, van den Ingh T, Schotanus BA, Brinkhof B, Rothuizen J, Penning LC. Aberrant expression of copper associated genes after copper accumulation in COMMD1-deficient dogs. J Trace Elem Med Biol 2015; 29:347–53 [DOI] [PubMed] [Google Scholar]
- 10.Fedoseienko A, Bartuzi P, van de Sluis B. Functional understanding of the versatile protein copper metabolism MURR1 domain 1 (COMMD1) in copper homeostasis. Ann N Y Acad Sci 2014; 1314:6–14 [DOI] [PubMed] [Google Scholar]
- 11.Klomp AEM, van de Sluis B, Klomp LWJ, Wijmenga C. The ubiquitously expressed MURR1 protein is absent in canine copper toxicosis. J Hepatol 2003; 39:703–9 [DOI] [PubMed] [Google Scholar]
- 12.Vonk WIM, Bartuzi P, de Bie P, Kloosterhuis N, Wichers CGK, Berger R, Haywood S, Klomp LW, Wijmenga C, van de Sluis B. Liver-specific Commd1 knockout mice are susceptible to hepatic copper accumulation. PLoS One 2011; 6:29183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han M, Lin Z, Zhang Y. The alteration of copper homeostasis in inflammation induced by lipopolysaccharides. Biol Trace Elem Res 2013; 154:268–74 [DOI] [PubMed] [Google Scholar]
- 14.Burstein E, Hoberg JE, Wilkinson AS, Rumble JM, Csomos RA, Komarck CM, Maine GN, Wilkinson JC, Mayo MW, Duckett CS. COMMD proteins, a novel family of structural and functional homologs of MURR1. J Biol Chem 2005; 280:22222–32 [DOI] [PubMed] [Google Scholar]
- 15.Tapiero H, Townsend DM, Tew KD. Trace elements in human physiology and pathology. Copper. Biomed Pharmacother 2003; 57:386–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medeiros DM. Perspectives on the role and relevance of copper in cardiac disease. Biol Trace Elem Res 2017; 176:10–9 [DOI] [PubMed] [Google Scholar]
- 17.Kopp SJ, Klevay LM, Feliksik JM. Physiological and metabolic characterization of a cardiomyopathy induced by chronic copper deficiency. Am J Physiol 1983; 245:855–66 [DOI] [PubMed] [Google Scholar]
- 18.Xiao Y, Wang T, Song X, Yang D, Chu Q, Kang YJ. Copper promotion of myocardial regeneration. Exp Biol Med 2020; 245:911–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Chen C, Liu Y, Sun X, Ding X, Qiu L, Han PF, James Kang Y. Feature article: trientine selectively delivers copper to the heart and suppresses pressure overload-induced cardiac hypertrophy in rats. Exp Biol Med 2018; 243:1141–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Xiao Y, Liu J, Feng L, Kang YJ. Copper-induced reduction in myocardial fibrosis is associated with increased matrix metalloproteins in a rat model of cardiac hypertrophy. Metallomics 2018; 10:201–8 [DOI] [PubMed] [Google Scholar]
- 21.Prohaska JR, Heller LJ. Mechanical properties of the copper-deficient rat heart. J Nutr 1982; 112:2142–50 [DOI] [PubMed] [Google Scholar]
- 22.Feng W, Ye F, Xue W, Zhou Z, Kang YJ. Copper regulation of hypoxia-inducible factor-1 activity. Mol Pharmacol 2009; 75:174–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huiqi X, Kang YJ. Role of copper in angiogenesis and its medicinal implications. Curr Med Chem 2009; 16:1304–14 [DOI] [PubMed] [Google Scholar]
- 24.Qiu L, Ding X, Zhang Z, Kang YJ. Copper is required for cobalt-induced transcriptional activity of hypoxia-inducible factor-1. J Pharmacol Exp Ther 2012; 342:561–7 [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Qiu L, Lin C, Yang H, Fu H, Li R, Kang YJ. Copper-dependent and -independent hypoxia-inducible factor-1 regulation of gene expression. Metallomics 2014; 6:1889–93 [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Zhang W, Wu Z, Yang Y, Kang YJ. Copper levels affect targeting of hypoxia-inducible factor 1α to the promoters of hypoxia-regulated genes. J Biol Chem 2018; 293:14669–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z, Zhang W, Kang YJ. Copper affects the binding of HIF-1α to the critical motifs of its target genes. Metallomics 2019; 11:429–38 [DOI] [PubMed] [Google Scholar]
- 28.Zuo X, Xie H, Dong D, Jiang N, Zhu H, Kang YJ. Cytochrome c oxidase is essential for copper-induced regression of cardiomyocyte hypertrophy. Cardiovasc Toxicol 2010; 10:208–15 [DOI] [PubMed] [Google Scholar]
- 29.Faiz M, Acarin L, Peluffo H, Villapol S, Castellano B, González B. Antioxidant Cu/Zn SOD: expression in postnatal brain progenitor cells. Neurosci Lett 2006; 401:71–6 [DOI] [PubMed] [Google Scholar]
- 30.Elsherif L, Ortines RV, Saari JT, Kang YJ. Congestive heart failure in copper-deficient mice. Exp Biol Med 2003; 228:811–7 [DOI] [PubMed] [Google Scholar]
- 31.Hinkel R, Trenkwalder T, Kupatt C. Gene therapy for ischemic heart disease. Expert Opin Biol Ther 2011; 11:723–37 [DOI] [PubMed] [Google Scholar]
- 32.Tyagi SC. Vasculogenesis and angiogenesis: extracellular matrix remodeling in coronary collateral arteries and the ischemic heart. J Cell Biochem 1997; 65:388–94 [PubMed] [Google Scholar]
- 33.Elsherif L, Wang L, Saari JT, Kang YJ. Regression of dietary copper restriction-induced cardiomyopathy by copper repletion in mice. J Nutr 2004; 134:855–60 [DOI] [PubMed] [Google Scholar]
- 34.Jiang Y, Reynolds C, Xiao C, Feng W, Zhou Z, Rodriguez W, Tyagi SC, Eaton JW, Saari JT, Kang YJ. Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J Exp Med 2007; 204:657–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klevay LM. Copper and ischemic heart disease. Biol Trace Elem Res 1983; 5:245–55 [DOI] [PubMed] [Google Scholar]
- 36.Cheng X, Hou J, Liu J, Sun X, Sheng Q, Han P, Kang YJ. Safety evaluation of sevoflurane as anesthetic agent in mouse model of myocardial ischemic infarction. Cardiovasc Toxicol 2017; 17:150–6 [DOI] [PubMed] [Google Scholar]
- 37.Ferferieva V, Van den Bergh A, CP, Jasaityte R, La Gerche A, Rademakers F, Herijgers P, D'hooge J. Assessment of strain and strain rate by two-dimensional speckle tracking in mice: comparison with tissue Doppler echocardiography and conductance catheter measurements. Eur Heart J 2012; 14:765–73 [DOI] [PubMed] [Google Scholar]
- 38.Gueret P, Meerbaum S, Zwehl W, Wyatt HL, Davidson RM, Uchiyama T, Corday E. Two-dimensional echocardiographic assessment of left ventricular stroke volume: experimental correlation with thermodilution and cineangiography in normal and ischemic states. Cathet Cardiovasc Diagn 1981; 7:247–58 [DOI] [PubMed] [Google Scholar]
- 39.Phillips-Krawczak CA, Singla A, Starokadomskyy P, Deng Z, Osborne DG, Li H, Dick CJ, Gomez TS, Koenecke M, Zhang JS, Dai H, Sifuentes-Dominguez LF, Geng LN, Kaufmann SH, Hein MY, Wallis M, McGaughran J, Gecz J., Sluis Bv, billadeau DD, burstein E. COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A. Mol Biol Cell 2015; 26:91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts EA, Lau CHF, TRd S, Yang S. Developmental expression of Commd1 in the liver of the Jackson toxic milk mouse. Biochem Biophys Res Commun 2007; 363:921–5 [DOI] [PubMed] [Google Scholar]
- 41.Vonk WIM, de Bie P, Wichers CGK, van den Berghe PVE, van der Plaats R, Berger R, Wijmenga C, Klomp LW, van de Sluis B. The copper-transporting capacity of ATP7A mutants associated with menkes disease is ameliorated by COMMD1 as a result of improved protein expression. Cell Mol Life Sci 2012; 69:149–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang SP, Liu H, Amarsingh GV, Cheung CC, Hogl S, Narayanan U, Zhang L, McHarg S, Xu JS, Gong DM, Kennedy J, Barry B, Choong YS, Phillips AR, Cooper GJ. Diabetic cardiomyopathy is associated with defective myocellular copper regulation and both defects are rectified by divalent copper chelation. Cardiovasc Diabetol 2014; 13:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chun H, Catterton T, Kim H, Lee J, Kim B-E. Organ-specific regulation of ATP7A abundance is coordinated with systemic copper homeostasis. Sci Rep 2017; 7:12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang YJ. Copper and homocysteine in cardiovascular diseases. Pharmacol Ther 2011; 129:321–31 [DOI] [PubMed] [Google Scholar]
- 45.Zuo X, Dong D, Sun M, Xie H, Kang YJ. Homocysteine restricts copper availability leading to suppression of cytochrome C oxidase activity in phenylephrine-treated cardiomyocytes. PLoS One 2013; 8:67549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van de Sluis B, Mao X, Zhai Y, Groot AJ, Vermeulen JF, van der Wall E, van Diest PJ, Hofker MH, Wijmenga C, Klomp LW, Cho KR, Fearon ER, Vooijs M, Burstein E. COMMD1 disrupts HIF-1alpha/beta dimerization and inhibits human tumor cell invasion. J Clin Invest 2010; 120:2119–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van de Sluis B, Muller P, Duran K, Chen A, Groot AJ, Klomp LW, Liu PP, Wijmenga C. Increased activity of hypoxia-inducible factor 1 is associated with early embryonic lethality in Commd1 null mice. Mol Cell Biol 2007; 27:4142–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wildman REC, Medeiros DM, Jenkins J. Comparative aspects of cardiac ultrastructure, morphometry, and electrocardiography of hearts from rats fed restricted dietary copper and selenium. Biol Trace Elem Res 1994; 46:51–66 [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi K, Maeda K, Takefuji M, Kikuchi R, Morishita Y, Hirashima M, Murohara T. Dynamics of angiogenesis in ischemic areas of the infarcted heart. Sci Rep 2017; 7:7156. [DOI] [PMC free article] [PubMed] [Google Scholar]