Abstract
Background
Freezing of gait (FOG) is arguably the most disabling motor symptom experienced with Parkinson’s disease (PD), but treatments are extremely limited due to our poor understanding of the underlying mechanisms. Three cortical domains are postulated in recent research (ie, the cognitive, limbic, and sensorimotor domains), thus, treatments targeting these mechanisms of FOG may potentially be effective. Cognitive training, cognitive behavioral therapy (CBT, a well-known anxiety intervention), and proprioceptive training may address the cognitive, limbic, and sensorimotor domains, respectively.
Objective
To investigate whether these 3 treatments could improve functional outcomes of FOG.
Methods
In a single-blind, randomized crossover design, 15 individuals with PD and FOG were randomized into different, counterbalanced orders of receiving the interventions. Each consisted of eight 1-hour sessions, twice weekly for 4 weeks. FOG severity was assessed as the primary outcome using a novel gait paradigm that was aimed at evoking FOG when the cognitive, limbic, or sensorimotor domains were independently challenged.
Results
FOG severity significantly improved after the cognitive intervention, with strong trends toward improvement specifically in the baseline and cognitive-challenge assessment conditions. CBT, as the anxiety intervention, resulted in significantly worse FOG severity. In contrast, proprioceptive training significantly improved FOG severity, with consistent trends across all conditions.
Conclusions
The cognitive and proprioceptive treatments appeared to improve different aspects of FOG. Thus, either of these interventions could potentially be a viable treatment for FOG. However, although the results were statistically significant, they could be sensitive to the relatively small number of participants in the study. Considering the significant results together with nonsignificant trends in both FOG and gait measures, and given equal time for each intervention, proprioceptive training produced the most consistent indications of benefits in this study. (clinicaltrials.gov NCT03065127).
Keywords: Parkinson’s disease, freezing of gait, cognitive function, proprioception, anxiety
Introduction
Freezing of gait (FOG) is a highly disabling motor symptom that is commonly experienced by individuals with Parkinson’s disease (PD). However, despite its negative consequences and prevalence, there is currently no effective treatment for FOG and it does not respond well to the treatments currently used for PD, such as dopamine replacement or deep brain stimulation.1-3 Therefore, investigation of adjunct therapies is highly warranted. In consideration of the most common triggers of FOG (ie, darkness, dual-tasking, fear, etc), it is likely that these triggers may be related to underlying cognitive, limbic, and proprioceptive mechanisms. The cognitive contribution to freezing is hypothesized to be due to an over-demand of cognitive resources that are being used to control walking and complete a secondary task, thus leading to a freezing episode.5-7 The limbic system’s influence on freezing results from a greater limbic load (ie, from anxiety or fear) depleting processing resources required for movement control, which leads to a freezing episode.8,9 Sensorimotor dysfunction due to proprioceptive impairments has also been hypothesized to induce freezing episodes, where the absence of visual feedback prevents the ability override faulty proprioceptive feedback.10-13 Hence, treatment in these 3 domains may be among the most effective strategies for alleviating FOG. These types of therapies have been investigated in other clinical populations (ie, PD nonfreezers and neurologically healthy individuals, as described below), although none have explored the therapeutic benefits to FOG.
To improve the cognitive mechanisms of FOG, cognitive training may be utilized as a therapy. Several studies involving individuals with PD have investigated this type of treatment, and have demonstrated improvements in general cognitive function,14-19 as well as in the specific executive function domains, which may be responsible for FOG (ie, set-shifting ability and inhibitory control).15-17 Interestingly, a study conducted by Paris et al,16 which utilized a computerized cognitive training program (SmartBrain) for 45-minute sessions, 3 times weekly over 4 weeks, demonstrated significant improvements in the greatest number of cognitive outcomes, including set-shifting ability and inhibitory control. These findings demonstrated the efficacy of the cognitive training programs on individuals with PD. However, the functional benefits to motor performance or severity of FOG have yet to be investigated, up until the current study.
To tackle the limbic contribution to FOG, treatment of the underlying anxiety influences of FOG with well-established techniques such as cognitive behavioral therapy (CBT) may be warranted. Studies utilizing CBT have been effective in reducing anxiety in individuals with PD as measured by the State and Trait Anxiety Inventory, the Depression, Anxiety, Stress Scale, and a reduction in anxiety medication use (selective serotonin reuptake inhibitors [SSRIs] and tricyclic antidepressants [TCAs] for anxiety).20-23 However, it is important to note that these studies utilized CBT targeting both depression and anxiety symptoms. There are currently no studies which have investigated CBT designed for anxiety exclusively, therefore, it is unclear whether CBT only for anxiety could be effective in PD. Furthermore, the effects of CBT on outcome measures related to motor function or FOG have not yet been demonstrated, although may be effective in ameliorating FOG behavior, given the contribution of anxiety to FOG.4,8 Importantly, CBT targeting anxiety specifically may be most efficient and beneficial since triggers of anxiety have been demonstrated to elicit freezing episodes.8,9
Proprioceptive training could potentially be a viable treatment option for FOG, given the contribution of proprioceptive deficits to FOG episodes.10-13 It is hypothesized that increasing the accuracy of proprioception (in the absence of vision) as a result of training may improve sensorimotor processing, thus mitigating potential triggers, and decreasing the probability of, FOG episodes from occurring. It has been suggested that training involving active movements is superior to training only involving passive movements.24 Studies investigating this type of training are limited, and only one intervention study has been conducted in individuals with PD.25 All participants improved in wrist proprioceptive thresholds and 4 of the 5 participants demonstrated improvements in wrist movement precision as a result of the training.25 This study demonstrated that proprioception has potential to be trained in individuals with PD, however, this training was only conducted specifically in the wrist joint which may not transfer to improvements in gait. Thus, multijoint proprioceptive training in the upper and lower limbs, such as the methods used by Hocherman26 and Jan et al,27 could yield the greatest functional and transferable benefit to freezers. These studies utilized an active target-matching task and demonstrated improvements in target reproduction accuracy in neurologically health adults. This type of training in both upper and lower limbs has yet to be investigated in FOG, and assessed on functional outcome measures, such as gait.
Thus, the aim of the present study was to compare cognitive (computerized cognitive training), limbic (CBT), and proprioceptive (upper and lower limb target-matching) therapies in individuals with FOG, to determine which has the greatest therapeutic benefits and greatest improvements in FOG functional outcomes.
Methods
The current study was a prospective, single-blind, randomized crossover design. Following baseline assessments, participants were block randomized using computer number generation into six groups with a counterbalanced order of interventions. Allocation of intervention sequence was not disclosed to participants. Participants completed all 3 interventions over 3 separate phases in the study. A 2-week washout period occurred between each intervention phase in order to prevent carryover effects. We checked for carryover effects across the washout period by using the Wilcoxon signed-rank test to compare the pretest FOG scores following each intervention (after the washout period) versus no previous intervention, and we found no significant differences (P > .1). The complete flow of participants through the study is presented in Supplemental Figure 1 (see Supplemental Material). All data collection was completed at the Movement Disorders Research and Rehabilitation Centre at Wilfrid Laurier University (MDRC; Waterloo, Ontario).
Participant recruitment was completed through the Movement Disorders Research and Rehabilitation Centre participant database at Wilfrid Laurier University (MDRC; Waterloo, Ontario) from September 2016 to April 2017. Inclusion criteria consisted of a diagnosis of PD and classification as a “freezer” (ie, experiences FOG), and either gender. FOG (freezer) status was determined from a prior clinical assessment by a movement disorders specialist. Individuals were excluded if they were diagnosed with a neurological disease other than PD or scored 19 or lower on the Montreal Cognitive Assessment assessed prior to trial commencement, unable to walk at least 10 m unassisted, and unable to understand verbal instructions in English. The sample size was determined by availability of participants. This study was approved by the Research Ethics Board at Wilfrid Laurier University. All participants provided written informed consent prior to beginning the study in accordance with the Declaration of Helsinki. This study is registered as a National Clinical Trial (clinicaltrials.gov NCT03065127). The 3 interventions each involved eight 1-hour sessions occurring twice weekly for 4 weeks. Participants completed the training while on dopaminergic medication.
Computerized Cognitive Training
This treatment entailed computer-aided cognitive training using the web-based version of the SmartBrain tool. Sessions were supervised by trained personnel and completed on an HP Pavilion g6 laptop with a mouse. The program contained 13 activities aiming to stimulate specific aspects of cognitive and executive function, which are known to potentially contribute to FOG episodes (set shifting/mental flexibility, inhibitory control, and attention). The difficulty level of each activity increased as participants progressed.
Limbic Training (Cognitive Behavioral Therapy)
CBT focusing on anxiety was conducted in one-on-one sessions with a trained psychotherapy masters student supervised by a faculty member who was an experienced clinical psychotherapist. The CBT treatment in the current study was designed using the recommendations of CBT for PD by Egan et al.20 The content of the treatment included psychoeducation, attention refocusing/cognitive shifting, behavioral activation, thought diaries, grief therapy, and behavioral experiments. Treatment activities were individualized to each participant.
Proprioceptive Training
Proprioceptive training involved a target matching task utilizing self-defined active movements and was completed in the upper and lower limbs separately, similar to the protocols used in previous studies.22,23 For the upper limb target-reaching task, participants were seated in front of a table. Ten numbered targets were marked along the surface, with 5 targets placed symmetrically on the right and left sides of the participants’ midline. At the start of each trial, participants placed their hand at the “origin” located at the midline at the bottom of the surface. Participants first viewed the single target, and once blindfolded, were instructed to reach toward the target without sliding their arm, aiming to touch the center of the target with their fingertip. The blindfold was immediately removed once the reach was completed to allow participants to view their error in joint angle production. This process was repeated for each target, and participants attempted to complete 5 rounds of the 10 targets in right and left upper limbs in a serial practice order. The lower limb target matching protocol was identical, where participants were seated with the targets placed at their feet and instructed to touch the center of the target with their toe without sliding the foot. See Supplemental Figure 2 in Supplementary Material for a visual representation of the task.
Outcome Measures
Outcome measures were collected by assessors blinded to group allocation.
Outcomes of Treatment Efficacy
The Trail-Making Test, Stroop Test, Parkinson Anxiety Scale (PAS), and passive joint-angle matching were included to verify that the cognitive, limbic, and proprioceptive interventions were successful in accomplishing the expected improvements to the specific domains based on previous studies. These procedures are described in the Supplementary Material. Additionally, research has shown that FOG is associated with greater disease severity.1,28-30 Therefore, an assessment of motor symptom severity using the UPDRS-III (Unified Parkinson’s Disease Rating Scale–III) was included. A blinded movement disorders specialist (QJA) performed the appraisal.
Freezing of Gait Outcomes
Gait Assessment
This paradigm aimed to present FOG-evoking conditions that maximized cognitive, limbic, and proprioceptive processing mechanisms that were likely to influence gait and FOG measures. Participants walked across a walkway measuring 9.75 m (length) × 0.3 m (width) marked by lines on the floor. Participants were equipped with active infrared light emitting diodes (IREDs) on the following locations: xiphoid process, bilateral lateral malleoli, and bilateral fifth metatarsals to allow recording of kinematic data with 8 Optotrak cameras (Northern Digital, NDI) at a frequency of 100 Hz. The primary outcome measures were the frequency and total duration of FOG episodes in each trial. However, since it is difficult to elicit FOG events in an experimental setting, it was important to also investigate changes in gait that do not result in FOG episodes but might be indicative of an impending FOG episode. Thus, spatiotemporal aspects of gait excluding FOG episodes (gait velocity, step length, step length variability [CV], step time, and step time variability [CV], percentage of time in double support, and percentage of double support time variability [CV]) were also analyzed. These parameters have been demonstrated to be associated with FOG, and typically become abnormal in the periods prior to an FOG episode.31-33
All gait data (FOG and spatiotemporal parameters) were analyzed in Matlab 9.2 (Math Works Inc). Signals were smoothed with a 7-Hz low-pass, fourth-order, zero-lag Butterworth filter. Freezing was identified from velocity of the xiphoid process. Similar to Cowie et al,34 FOG episodes were identified when the velocity dropped below 10% of normal. FOG offset was marked only when velocity rose above 25% of normal. While the results depend somewhat on these threshold values, this approach allowed for the inclusion of periods of festination, in which “full blown” akinetic FOG episodes did not occur. This analysis did not include any initiation FOG (ie, FOG episodes that occurred when the participant attempted to initiate walking). Since FOG episodes did not occur in every trial, the total number of FOG episodes that occurred across 3 trials, and mean duration across the 3 trials was analyzed (rather than each trial separately).
Gait assessments involved 3 conditions that aimed to challenge the cognitive, sensorimotor, and limbic domains independently, in addition to a single-task (baseline) condition. Three blocks of the 4 randomized conditions were completed for a total of 12 trials. These conditions are outlined below.
Single-task (BL)
Participants were instructed to walk across the walkway at a self-selected pace with no manipulations to the gait task.
Cognitive challenge (COG)
An auditory digit monitoring dual-task condition was used to challenge cognitive processing by diverting attention from walking. A 12-second audio track played a stream of numbers ranging from 1 to 9 presented in random order in each trial. The interstimulus interval between each announced number ranged from 100 to 1000 ms and was also randomized in order to prevent gait synchronization with the audio track. Participants were instructed to walk at a comfortable pace while mentally counting the number of times that 2 previously specified numbers (eg, 2s and 4s, or 3s and 4s) were announced among the stream of numbers. At the end of each trial, participants responded with the number of times they heard the target numbers. If the audio track ended before the participant reached the end of the walkway, they were instructed to retain their response until the walking task was complete. No feedback on performance of the secondary task was given to the participant at any time. This protocol has been used previously by Pieruccuni-Faria et al35 and has been verified to interfere with cognitive processing. This type of secondary task was selected since it does not include any motor components, thus allowing for the dual-task to be isolated to the cognitive domain.35
Limbic challenge (LIM)
This condition aimed to induce anxiety by increasing postural threat. Participants walked, without a suspension harness, across an elevated walkway (similar to a balance beam) measuring 9 m (length) × 0.3 m (width) × 0.6 m (height). This height has also been used in previous studies to increase postural threat in healthy older adults.36,37
Proprioceptive challenge (PROP)
This condition aimed to increase the reliance on proprioceptive feedback in the absence of vision while walking, thus challenging the sensorimotor domain.11 Participants were instructed to walk at a comfortable pace through a completely darkened room. The room was free of immediate obstacles in order to prevent collision or the fear of a potential collision from interfering with the proprioceptive manipulation. After completion of each trial, the lights were turned on to allow participants to safely return to the starting position, and to prevent acclimation to the darkness. This protocol was selected over other conventional methods (ie, unstable balance tasks) since this method increases reliance on proprioceptive feedback while allowing assessment conditions to remain consistent.
New Freezing of Gait Questionnaire (NFOGQ)
This questionnaire provided a self-report measure of frequency and duration of FOG episodes. This tool has been validated and proven to be highly reliable in individuals with PD, as well as assessing treatment interventions for FOG.38
Results
Seventeen individuals with Parkinson’s disease that experienced FOG completed pre-post assessment and randomization into the study. Participant demographics are presented in Table 1. Following randomization, 2 individuals were unable to make the necessary time commitment and withdrew from the study (Supplemental Figure 1 in supplementary material). Two additional individuals withdrew after commencement of the study (1 could not meet the remaining time commitment, 1 had a previous medical condition exacerbation). An intention-to-treat analysis was performed. No adverse events were reported during this study. Specific details on outcomes of treatment efficacy are available in the supplementary material. In brief, there were no significant effects in the trail-making test, Stroop Test, PAS, UPDRS-III scores, NFOGQ scores, or secondary task error during the dual-task gait assessment. Passive upper limb joint-angle matching significantly improved in the 60° condition and 30° condition after proprioceptive training.
Table 1.
Participant Demographics Presented as Group Means (Standard Deviation in Parentheses).
| Number (male/female) | Age, y | Disease duration, y | UPDRS-III | MoCA | LED | Attendance (%) | ||
|---|---|---|---|---|---|---|---|---|
| Cognitive training | CBT | Proprioceptive training | ||||||
| 15 (11/4) | 74 (6.22) | 10.78 (6.89) | 24.9 (7.18) | 24.66 (3.37) | 1456.58 (1010.17) | 96.43 (7.64) | 97.5 (5.17) | 97.11 (5.48) |
Abbreviations: UPDRS-III, Unified Parkinson’s Disease Rating Scale–III; MoCA, Montreal Cognitive Assessment; LED, Levodopa Equivalent Dose; CBT, cognitive behavioral therapy.
FOG Outcome Measures
FOG Frequency and Duration
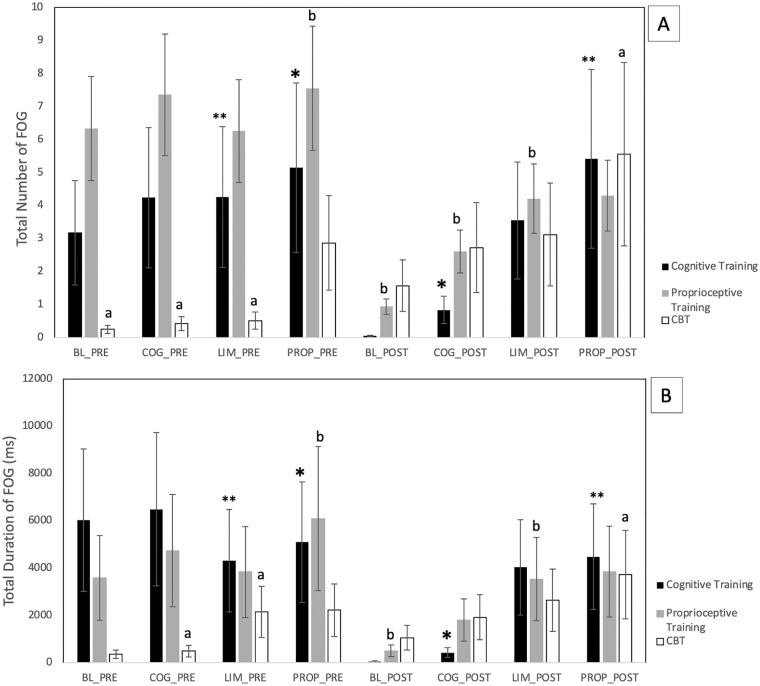
Cognitive treatment led to significant main-effect improvements in both FOG frequency, χ2(7) = 18.06, P = .011, and total duration of FOG, χ2(7) = 18.25, P = .01 (Figure 1A and B). There were trends toward improvement within some of the individual assessment conditions, particularly the baseline and cognitive assessment conditions. However, post hoc testing did not reveal significant pre-post differences within these individual conditions.
Figure 1.
Total frequency (A) and duration (B) of freezing of gait (FOG) episodes in all gait assessment conditions at pre and post cognitive training, proprioceptive training, and cognitive behavioral therapy (CBT) interventions. Post hoc Wilcoxon signed-rank tests also showed certain statistically significant pairwise differences (P < .05), marked with *, **, a, and b. However, these comparisons were across different test conditions, so they are not clinically meaningful. None of the pre-post differences in individual test conditions was significant.
CBT led to significant worsening of both FOG frequency, χ2(7) = 24.43, P = .0009 (Figure 1A), and duration of FOG episodes, χ2(7) = 22.26, P = .002 (Figure 1B). In addition to these main effects, both FOG frequency and duration were individually higher after CBT in every assessment condition. However, the pre-post differences within each assessment condition were not statistically significant in post hoc testing.
In contrast, proprioceptive training led to significant main-effect improvements in both FOG frequency, χ2(7) = 19.29, P = .007) (Figure 1A), and FOG duration, χ2(7) = 21.63, P = .003 (Figure 1B). Furthermore, there were trends toward improvement in both FOG frequency and duration, consistent across every assessment condition. However, none of the differences within assessment conditions was significant individually, in post hoc testing.
Five of the 15 participants in the current study did not exhibit FOG during the assessments, raising the possibility that the results excessively reflected certain participants. For this reason, we calculated additional metrics that were less sensitive to changes in individual participants’ FOG frequency and duration. First, we counted the numbers of participants with increasing versus decreasing FOG frequency and total duration following each intervention. Second, we calculated the mean relative change in FOG frequency and total duration, as a fraction of the mean FOG frequency and duration for each participant. With regard to proprioceptive training, all 4 of these metrics were consistent with the improvement suggested by the statistical analysis. More participants improved than got worse (4 vs 2 for both FOG frequency and duration, with no change in the other 7 participants that completed this part of the study). The average relative changes were also improvements for both FOG frequency and duration. However, for the cognitive and CBT interventions, these additional metrics were not consistent with the statistical results. For the cognitive intervention, all of these metrics were inconsistent with the statistical results. For CBT, 1 was consistent, 1 was inconsistent, and 2 were tied (equal numbers of participants had increased and decreased FOG and duration).
Spatiotemporal Measures
Spatiotemporal measures included gait velocity, step length, step-length variability, step time, step-time variability, double-support time, and double-support variability. While freezing directly affects some of these (eg, gait speed), calculation of these measures excluded frozen periods, to provide relatively independent information. Overall, despite the interventions’ effects on FOG, they had no significant impact on any of the spatiotemporal measures.
Instead, significant differences among these measures were overwhelmingly related to assessment condition and trial number, rather than to intervention. For example, spatiotemporal measures were generally better (eg, faster gait, less variability) in the baseline and cognitive assessment conditions than in the proprioceptive-challenge and limbic-challenge conditions. Further details of the results on each parameter are shown in the Supplementary Material.
To supplement the analysis of statistical significance, we also calculated the average gait velocity of each participant, across all assessment conditions, before and after each intervention. Consistent with the lack of statistical significance, gait velocities changed less than 1% of the mean after the cognitive and CBT interventions. However, gait velocity was 6.6% higher following proprioceptive training. This could either reflect a random fluctuation, or a functionally significant change that we did not have sufficient statistical power to detect.
Discussion
The aim of the current study was to investigate and compare three types of interventions for the remediation of FOG in individuals with PD. To our knowledge, this is the first study to investigate therapies that targeted the potential underlying mechanisms of FOG in individuals with FOG.
Effects of Training on Freezing of Gait
There were significant improvements in both FOG frequency and duration with the proprioceptive training intervention. Consistent with these main-effect improvements, both FOG frequency and duration were lower after proprioceptive training in every assessment condition (ie, the baseline condition, and the cognitive, limbic, and proprioceptive challenge conditions), although these differences were not individually significant. Relatedly, more individual participants had decreased than increased FOG frequency and duration following proprioceptive training. These results were also consistent with a nonsignificant trend toward increased gait velocity following proprioceptive training.
FOG frequency and duration were also significantly reduced after the cognitive intervention, although this result was less consistent across assessment conditions, with substantial improvements only in the baseline and cognitive challenge conditions. In contrast, there was significant worsening, with consistent trends across assessment conditions, with the anxiety-targeting CBT intervention. Despite the statistical significance in these results, there was high variance in each condition, suggesting that individual outcomes may be hard to predict. These results were also not consistent with supplementary metrics, including the numbers of participants with individual improvements, and the mean changes in FOG frequency and duration normalized to each participant’s mean. Importantly, as this supplemental analysis provides context for the main results, the inconsistency across results for the cognitive and limbic interventions suggests that any changes in FOG severity may be attributed to random fluctuations in individual participants’ performance and thus may represent a spurious change. An important caveat in this study is the relatively small sample size, which may have allowed a small number of participants to skew the results. To investigate this, individual participants’ results were inspected post hoc. Visual inspection of FOG frequency and duration revealed that changes were indeed driven by a few participants, while over half the participants did not freeze in each assessment condition. A further limitation is that we used CBT designed for anxiety, so these results may not generalize to CBT designed for depression.
No significant changes were found for the subjective assessment of FOG severity (NFOGQ). There were also no statistically or clinically significant changes in UPDRS-III scores in any of the three interventions, although there was a non-significant trend toward improvement following proprioceptive training. It is unclear why interventions that affected FOG in the lab did not lead to parallel improvements in the NFOGQ. The improvements may have been more prominent in the lab setting, or the measures used in the lab may have been more sensitive. Another possibility is that the follow-up period was not long enough and thus changes were not adequately captured by the NFOGQ, as this outcome measure examines performance in the past four weeks.
Effects of Training on Spatiotemporal Gait Properties
None of the interventions led to significant changes in any spatiotemporal measure, including gait velocity, step length, step-length variability, step time, step-time variability, double-support time, and double-support variability. Importantly, while FOG directly affects properties such as gait speed, these measures excluded freezing periods, and thus independently assessed changes in gait during nonfreezing periods.
Previous studies have demonstrated that gait variability is related to cognitive dual-task interference on gait.31,39 Furthermore, increases specifically in step length variability are associated with poorer executive function and dementia,35,40-42 and have been correlated with gray matter integrity, particularly in the hippocampus and anterior cingulate gyrus (which are involved in memory and executive functioning, respectively43,44) in older adults.45 Thus, an improvement in variability of step length following cognitive training would not have been surprising, but none was found.
Previous research has demonstrated that reducing sensory information available during locomotion (ie, challenging the sensorimotor domain) in individuals with PD causes an increase in demands on cognitive processing.35 Therefore, an improvement in step length variability with cognitive training might have been expected during the proprioceptive challenge condition. There was in fact a trend in this direction, but it was not significant.
After completion of CBT, pairwise comparisons suggested a significant improvement in step length variability only in the first trial of the proprioceptive walking assessment condition. Previous studies support that heightened anxiety caused an increase in step-to-step variability5,8,39; therefore, an improvement in step length variability might be an indication that anxiety was reduced as a result of CBT. Interestingly, anxiety has been shown to interfere with sensorimotor processing while walking in individuals with PD,12 which is consistent with improvement in the proprioceptive challenge condition. Alternatively, this might also suggest that the proprioceptive challenge utilized in the current study (ie, walking in complete darkness) was influencing not only the sensorimotor domain but potentially also anxiety (possibly from fear of postural instability).11 However, while this result was statistically significant, visual inspection of the data suggested that it was due to a spuriously large variability in the CBT pretest. There should be no systematic differences in pretest scores across interventions, so this statistically significant result appears to be a false positive.
Previous research has suggested that individuals with PD attempt to modify temporal aspects of gait (eg, step time) in order to increase proprioceptive feedback to adapt to inaccurate basal ganglia processing.46 Perhaps related to this, there were many significant differences in spatiotemporal measures across assessment conditions. These results are consistent with a previous study, which demonstrated that when postural threat is greater (ie, increasing anxiety), individuals with PD adapt by increasing stride time to reduce the risk of instability.47
Effects of Training on Domain-Specific Measures
The primary objective of this study was to evaluate the effects of interventions that had previously been well-established to improve to influence the cognitive, limbic and proprioceptive domains on FOG and spatiotemporal measures of gait, but we also measured the effects of each intervention on the associated domain, that is the effect of cognitive training on cognitive function, CBT on anxiety, and proprioceptive training on proprioception.
Contrary to our expectation, participants did not significantly improve on any of the outcome measures of cognitive function (ie, Trail-Making Test and Stroop Test) after the cognitive training intervention, although percentage change trended toward improvements in both measures. These results conflict with previous findings by Paris et al,16 which used the same software in individuals with PD and demonstrated improvements in several cognitive measures, including the Trail-Making Test and Stroop Test. This may be due to differences in the training protocols. The total time using Smartbrain in the Paris et al study was 9 hours and included additional cognitive homework exercises. However, in the current study, the intervention time was kept at 8 hours and excluded the home exercises in order to keep the duration of all interventions equal. It is possible that the lack of significant findings in the current study are due to insufficient treatment duration. Furthermore, individuals with a Montreal Cognitive Assessment score less than 19 were excluded from this study to ensure participants comprehended instructions. Thus, it is unknown whether there were ceiling effects in treatment outcomes, and if individuals with more severe cognitive deficits could show greater improvements to this treatment.
Similarly, there were no significant improvements in the PAS after CBT, although percentage change showed an improvement. The current study matched the guidelines for the minimum time required for a CBT intervention in individuals with PD to be effective,20 yet improvements were not significant. It is possible that this timing may not have been sufficient, as the current study used only the minimum amount of time recommended. This also conflicts with previous studies using CBT in PD which demonstrated significant effects with a longer treatment period (one 2-hour session per week over 8 weeks).23 Another possible explanation is that the outcome measures used in previous studies have not been validated in a PD population. The outcome measure selected for the current study (PAS) has been validated in PD48; however, sensitivity to change has not yet been evaluated. Therefore, it is also possible that the PAS was not sensitive enough to detect a change from the intervention.
Participants significantly improved on some of the passive joint-angle matching tasks after completion of proprioceptive training, confirming that this intervention was effective in improving proprioception. Similar to previous findings,25 it remains unclear whether improvements in proprioception translate to overall improvements in gait. While there were no significant improvements in spatiotemporal parameters of gait, there were trends toward improvements in some of these parameters. Furthermore, significant main effects on FOG frequency and duration following proprioceptive training suggest that there could be benefits to some aspects of gait. Thus, it is unclear from these results how improvement in proprioception related to changes in gait. Additionally, the proprioceptive assessment used in the current study only involved joint angle matching of the upper extremity, while the proprioceptive challenge condition in the gait assessment likely involved proprioception of the entire body, especially the lower extremities. Therefore, it is unclear whether the inconsistency of results was due to the mismatch in assessments. Future studies should utilize a more consistent assessment method.
Notably, despite a lack of significant changes in the cognitive and anxiety scores, each of the interventions significantly affected FOG measures. This might suggest that the FOG measures were simply more sensitive indicators of changes in these domains, or that the actual effects of the interventions were somewhat narrower than the scope of the assessments. The lack of significant changes in some of these measures may also suggest that more extensive training is warranted. Alternatively, it may be possible that while the cognitive and anxiety interventions might have been previously demonstrated to improve specific domains in PD, perhaps in more severe PD patients who experience FOG these improvements are more difficult to achieve. Thus, more extensive training might also lead to better domain-specific improvements and more importantly even greater changes in FOG measures. For example, while cognitive training appeared to have little benefit in the proprioceptive and limbic assessment conditions, more extensive cognitive training might be effective. Furthermore, each of the interventions demonstrated a trend toward improvement in domain-specific outcome measures, which could be an indication that each was effective, although further scrutiny is required regarding optimal frequency, intensity, and timing. Thus, each of these interventions should nevertheless be carefully considered for treatment of FOG.
Conclusion
Evidence from the current study suggests that both cognitive and proprioceptive training have the potential to improve both FOG frequency and total duration. Therefore, each of these interventions might be employed as a viable treatment option for FOG, but it may be that individualized treatment approaches are necessary. For example, individuals with greater cognitive impairment might potentially benefit from a greater focus on cognitive treatment, although the current study did not specifically investigate this possibility. With 8 hours of training in each category, proprioceptive training gave the most consistent indications of improvements in FOG severity across assessment conditions. In some cases, perhaps, combinations of these therapies are warranted, and this requires further investigation. Results for each type of intervention may also provide some insight into the underlying mechanism of gait impairments and FOG episodes. Finally, despite the statistical significance of the FOG severity results, further work is needed to confirm them with larger numbers of participants.
Supplemental Material
Supplemental material, sj-JPG-1-nnr-10.1177_1545968321992331 for Investigating Therapies for Freezing of Gait Targeting the Cognitive, Limbic, and Sensorimotor Domains by Rebecca Chow, Bryan P. Tripp, Daniel Rzondzinski and Quincy J Almeida in Neurorehabilitation and Neural Repair
Supplemental material, sj-jpg-2-nnr-10.1177_1545968321992331 for Investigating Therapies for Freezing of Gait Targeting the Cognitive, Limbic, and Sensorimotor Domains by Rebecca Chow, Bryan P. Tripp, Daniel Rzondzinski and Quincy J Almeida in Neurorehabilitation and Neural Repair
Supplemental material, sj-png-3-nnr-10.1177_1545968321992331 for Investigating Therapies for Freezing of Gait Targeting the Cognitive, Limbic, and Sensorimotor Domains by Rebecca Chow, Bryan P. Tripp, Daniel Rzondzinski and Quincy J Almeida in Neurorehabilitation and Neural Repair
Acknowledgments
We gratefully acknowledge the assistance of the lab team and patients at the Movement Disorders Research and Rehabilitation Centre, Ontario, Canada for their contribution to this research.
Footnotes
Author Contributions: Research project: (A) Conception, RC; (B) Organization, RC, QJA, DR; (C) Execution, RC, QJA, BT, DR. Statistical analysis: (A) Design, RC, QJA; (B) Execution, RC, BT, (C) Review and critique, QJA. Manuscript: (A) Writing of the first draft, RC; (B) Review and critique, QJA, BT.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Canada Foundation for Innovation (QJA). However, the funding sources did not play any role in the design, collection, analysis and interpretation of the data, or preparation of the manuscript. Full financial disclosures of all authors for the past year: Canadian Foundation for Innovation (QJA)
ORCID iD: Quincy J Almeida  https://orcid.org/0000-0002-0806-4397
https://orcid.org/0000-0002-0806-4397
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website along with the online version of this article.
References
- 1. Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734-744. doi: 10.1016/S1474-4422(11)70143-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaamonde Gamo J, Cabello JP, Gallardo Alcaniz MJ, Flores Barragan JM, Carrasco Garcia de Leon S, Ibanez Alonso RE. Freezing of gait unresponsive to dopaminergic stimulation in patients with severe Parkinsonism [in Spanish]. Neurologia. 2010;25:27-31. [PubMed] [Google Scholar]
- 3. Vorovenci RJ, Biundo R, Antonini A. Therapy-resistant symptoms in Parkinson’s disease. J Neural Transm (Vienna). 2016;123:19-30. doi: 10.1007/s00702-015-1463-8 [DOI] [PubMed] [Google Scholar]
- 4. Lewis SJG, Barker RA. A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:333-338. doi: 10.1016/j.parkreldis.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 5. Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of gait and Parkinson’s disease: the effects of dual tasking. J Neurol Neurosurg Psychiatry. 2009;80:347-350. doi: 10.1136/jnnp.2008.157362 [DOI] [PubMed] [Google Scholar]
- 6. Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, Nieuwboer A. Freezing of gait in Parkinson’s disease: the impact of dual-tasking and turning. Mov Disord. 2010;25:2563-2570. doi: 10.1002/mds.23327 [DOI] [PubMed] [Google Scholar]
- 7. Maidan I, Bernad-Elazari H, Gazit E, Giladi N, Hausdorff JM, Mirelman A. Changes in oxygenated hemoglobin link freezing of gait to frontal activation in patients with Parkinson disease: an fNIRS study of transient motor-cognitive failures. J Neurol. 2015;262:899-908. doi: 10.1007/s00415-015-7650-6 [DOI] [PubMed] [Google Scholar]
- 8. Ehgoetz Martens KA, Ellard CG, Almeida QJ. Does anxiety cause freezing of gait in Parkinson’s disease? PLoS One. 2014;9:e106561. doi: 10.1371/journal.pone.0106561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehgoetz Martens KA, Ellard CG, Almeida QJ. Anxiety-provoked gait changes are selectively dopa-responsive in Parkinson’s disease. Eur J Neurosci. 2015;42:2028-2035. doi: 10.1111/ejn.12928 [DOI] [PubMed] [Google Scholar]
- 10. Tan T, Almeida QJ, Rahimi F. Proprioceptive deficits in Parkinson’s disease patients with freezing of gait. Neuroscience. 2011;192:746-752. doi: 10.1016/j.neuroscience.2011.06.071 [DOI] [PubMed] [Google Scholar]
- 11. Ehgoetz Martens KA, Pieruccini-Faria F, Almeida QJ. Could sensory mechanisms be a core factor that underlies freezing of gait in Parkinson’s disease? PLoS One. 2013;8:e62602. doi: 10.1371/journal.pone.0062602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ehgoetz Martens KA, Ellard CG, Almeida QJ. Virtually-induced threat in Parkinson’s: dopaminergic interactions between anxiety and sensory-perceptual processing while walking. Neuropsychologia. 2015;79(pt B):322-331. doi: 10.1016/j.neuropsychologia.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 13. Pereira MP, Gobbi LTB, Almeida QJ. Freezing of gait in Parkinson’s disease: evidence of sensory rather than attentional mechanisms through muscle vibration. Parkinsonism Relat Disord. 2016;29:78-82. doi: 10.1016/j.parkreldis.2016.05.021 [DOI] [PubMed] [Google Scholar]
- 14. Sinforiani E, Banchieri L, Zucchella C, Pacchetti C, Sandrini G. Cognitive rehabilitation in Parkinson’s disease. Arch Gerontol Geriatr Suppl. 2004;38:387-391. doi: 10.1016/j.archger.2004.04.049 [DOI] [PubMed] [Google Scholar]
- 15. Petrelli A, Kaesberg S, Barbe MT, et al. Effects of cognitive training in Parkinson’s disease: a randomized controlled trial. Parkinsonism Relat Disord. 2014;20:1196-1202. doi: 10.1016/j.parkreldis.2014.08.023 [DOI] [PubMed] [Google Scholar]
- 16. París AP, Saleta HG, de la Cruz Crespo Maraver M, et al. Blind randomized controlled study of the efficacy of cognitive training in Parkinson’s disease. Mov Disord. 2011;26:1251-1258. doi: 10.1002/mds.23688 [DOI] [PubMed] [Google Scholar]
- 17. Sammer G, Reuter I, Hullmann K, Kaps M, Vaitl D. Training of executive functions in Parkinson’s disease. J Neurol Sci. 2006;248:115-119. doi: 10.1016/j.jns.2006.05.028 [DOI] [PubMed] [Google Scholar]
- 18. Leung I, Walton C, Hallock H, Lewis SJG, Valenzuela M, Lampit A. Cognitive training in Parkinson disease: a systematic review and meta-analysis. Neurology. 2015;85:1843-1851. doi: 10.1212/WNL.0000000000002145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naismith SL, Mowszowski L, Diamond K, Lewis SJG. Improving memory in Parkinson’s disease: a healthy brain ageing cognitive training program. Mov Disord. 2013;28:1097-1103. doi: 10.1002/mds.25457 [DOI] [PubMed] [Google Scholar]
- 20. Egan SJ, Laidlaw K, Starkstein S. Cognitive behaviour therapy for depression and anxiety in Parkinson’s disease. J Parkinsons Dis. 2015;5:443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feeney F, Egan S, Gasson N. Treatment of depression and anxiety in Parkinson’s disease: a pilot study using group cognitive behavioural therapy. Clin Psychol. 2005;9:31-38. doi: 10.1080/13284200500048240 [DOI] [Google Scholar]
- 22. Troeung L, Egan SJ, Gasson N. A meta-analysis of randomised placebo-controlled treatment trials for depression and anxiety in Parkinson’s disease. PLoS One. 2013;8:e79510. doi: 10.1371/journal.pone.0079510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Troeung L, Egan SJ, Gasson N. A waitlist-controlled trial of group cognitive behavioural therapy for depression and anxiety in Parkinson’s disease. BMC Psychiatry. 2014;14:19. doi: 10.1186/1471-244X-14-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aman JE, Elangovan N, Yeh IL, Konczak J. The effectiveness of proprioceptive training for improving motor function: a systematic review. Front Hum Neurosci. 2015;8:1075. doi: 10.3389/fnhum.2014.01075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elangovan N, Tuite P, Minneapolis JK. Proprioceptive training as a means to enhance sensorimotor function in Parkinson’s disease. Mov Disord. 2016;31(suppl 2):30-31. [Google Scholar]
- 26. Hocherman S. Proprioceptive guidance and motor planning of reaching movements to unseen targets. Exp Brain Res. 1993;95:349-358. doi: 10.1007/BF00229793 [DOI] [PubMed] [Google Scholar]
- 27. Jan MH, Tang PF, Lin JJ, Tseng SC, Lin YF, Un DH. Efficacy of a target-matching foot-stepping exercise on proprioception and function in patients with knee osteoarthritis. J Orthop Sport Phys Ther. 2008;38:19-25. doi: 10.2519/jospt.2008.2512 [DOI] [PubMed] [Google Scholar]
- 28. Contreras A, Grandas F. Risk factors for freezing of gait in Parkinson’s disease. J Neurol Sci. 2012;320:66-71. doi: 10.1016/j.jns.2012.06.018 [DOI] [PubMed] [Google Scholar]
- 29. Macht M, Kaussner Y, Möller JC, et al. Predictors of freezing in Parkinson’s disease: a survey of 6620 patients. Mov Disord. 2007;22:953-956. doi: 10.1002/mds.21458 [DOI] [PubMed] [Google Scholar]
- 30. Giladi N, Herman T, Hausdorff JMÀ. Freezing of gait in older adults with high level gait disorders: association with impaired executive function. J Neural Transm (Vienna). 2007;114:1349-1353. doi: 10.1007/s00702-007-0772-y [DOI] [PubMed] [Google Scholar]
- 31. Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003;149:187-194. doi: 10.1007/s00221-002-1354-8 [DOI] [PubMed] [Google Scholar]
- 32. Nanhoe-Mahabier W, Snijders AH, Delval A, et al. Walking patterns in Parkinson’s disease with and without freezing of gait. Neuroscience. 2011;182:217-224. doi: 10.1016/j.neuroscience.2011.02.061 [DOI] [PubMed] [Google Scholar]
- 33. Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kaucsik E. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov Disord. 2001;16:1066-1075. doi: 10.1002/mds.1206 [DOI] [PubMed] [Google Scholar]
- 34. Cowie D, Limousin P, Peters A, Hariz M, Day BL. Doorway-provoked freezing of gait in Parkinson’s disease. Mov Disord. 2012;27:492-499. doi: 10.1002/mds.23990 [DOI] [PubMed] [Google Scholar]
- 35. Pieruccini-faria F, Jones JA, Almeida QJ. Brain and cognition motor planning in Parkinson’s disease patients experiencing freezing of gait: the influence of cognitive load when approaching obstacles. Brain Cogn. 2014;87:76-85. doi: 10.1016/j.bandc.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 36. Gage WH, Sleik RJ, Polych MA, McKenzie NC, Brown LA. The allocation of attention during locomotion is altered by anxiety. Exp Brain Res. 2003;150:385-394. doi: 10.1007/s00221-003-1468-7 [DOI] [PubMed] [Google Scholar]
- 37. Brown LA, Doan JB, Whishaw IQ, Suchowersky O. Parkinsonian deficits in context-dependent regulation of standing postural control. Neurosci Lett. 2007;418:292-297. doi: 10.1016/j.neulet.2007.03.040 [DOI] [PubMed] [Google Scholar]
- 38. Nieuwboer A, Rochester L, Herman T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture. 2009;30:459-463. doi: 10.1016/j.gaitpost.2009.07.108 [DOI] [PubMed] [Google Scholar]
- 39. Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248-1256. doi: 10.1111/j.1460-9568.2005.04298.x [DOI] [PubMed] [Google Scholar]
- 40. Nakamura T, Meguro K, Sasaki H. Relationship between falls and stride length variability in senile dementia of the alzheimer type. Gerontology. 1996;42:108-113. doi: 10.1159/000213780 [DOI] [PubMed] [Google Scholar]
- 41. Brach JS, Studenski S, Perera S, VanSwearingen JM, Newman AB. Stance time and step width variability have unique contributing impairments in older persons. Gait Posture. 2008;27:431-439. doi: 10.1016/j.gaitpost.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morris R, Lord S, Lawson RA, et al. Gait rather than cognition predicts decline in specific cognitive domains in early Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 2017;72:1656-1662. doi: 10.1093/gerona/glx071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669-682. doi: 10.1016/0028-3932(79)90042-3 [DOI] [PubMed] [Google Scholar]
- 44. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215-222. doi: 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- 45. Rosso AL, Olson MJ, Yang M, et al. Higher step length variability indicates lower gray matter integrity of selected regions in older adults. Gait Posture. 2014;40:225-230. doi: 10.1016/j.gaitpost.2014.03.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Almeida QJ, Frank JS, Roy EA, et al. An evaluation of sensorimotor integration during locomotion toward a target in Parkinson’s disease. Neuroscience. 2005;134:283-293. doi: 10.1016/j.neuroscience.2005.02.050 [DOI] [PubMed] [Google Scholar]
- 47. Caetano MJD, Gobbi LTB, Sánchez-Arias M del R, Stella F, Gobbi S. Effects of postural threat on walking features of Parkinson’s disease patients. Neurosci Lett. 2009;452:136-140. doi: 10.1016/j.neulet.2009.01.053 [DOI] [PubMed] [Google Scholar]
- 48. Leentjens AFG, Dujardin K, Pontone GM, Starkstein SE, Weintraub D, Martinez-Martin P. The Parkinson Anxiety Scale (PAS): development and validation of a new anxiety scale. Mov Disord. 2014;29:1035-1043. doi: 10.1002/mds.25919 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-JPG-1-nnr-10.1177_1545968321992331 for Investigating Therapies for Freezing of Gait Targeting the Cognitive, Limbic, and Sensorimotor Domains by Rebecca Chow, Bryan P. Tripp, Daniel Rzondzinski and Quincy J Almeida in Neurorehabilitation and Neural Repair
Supplemental material, sj-jpg-2-nnr-10.1177_1545968321992331 for Investigating Therapies for Freezing of Gait Targeting the Cognitive, Limbic, and Sensorimotor Domains by Rebecca Chow, Bryan P. Tripp, Daniel Rzondzinski and Quincy J Almeida in Neurorehabilitation and Neural Repair
Supplemental material, sj-png-3-nnr-10.1177_1545968321992331 for Investigating Therapies for Freezing of Gait Targeting the Cognitive, Limbic, and Sensorimotor Domains by Rebecca Chow, Bryan P. Tripp, Daniel Rzondzinski and Quincy J Almeida in Neurorehabilitation and Neural Repair